Abstract
Objective
To determine the relation of stressful life events to metabolic control.
Design
We interviewed adolescents with type 1 diabetes (n = 132; average age at enrollment = 12 years) annually for five years.
Measures
Each year we administered measures of stressful life events, psychological distress, and self-care behavior. We downloaded data from blood glucose meters, and obtained measures of metabolic control (hemoglobin A1c) from medical records.
Results
Using longitudinal growth curve modeling, stressful life events predicted greater psychological distress, poorer self-care behavior, and worse metabolic control in both cross-sectional and longitudinal (lagged) analyses. Cross-sectionally, many of these relations were stronger among older than younger adolescents. Self-care behavior partly mediated this association.
Conclusion
Stressful life events are related to poor metabolic control—especially for older adolescents. A primary mechanism appears to be a lack of good self care.
Keywords: type 1 diabetes, metabolic control, adolescence, stressful life events
Adolescence is a period in development marked by significant biological, psychological, and social changes (Holmbeck, Friedman, Abad, & Jandasek, 2006). Adolescents face multiple challenges and transitions, including puberty, changes in schools, investment in peer and romantic relationships, and shifts in relationships with family (Graber, 2004; Holmbeck et al., 2006). Each of these transitions and challenges has the potential to become sources of stress. In fact, stressful life events across multiple domains have been shown to increase over the course of adolescence (Byrne, Davenport, & Mazanov, 2007; Brooks-Gunn, 1991; Ge, Lorenz, Conger, Elder, & Simons, 1994; Larson & Ham, 1993). This increase in stressful life events has been associated with increases in depression, anxiety, low self-esteem, and behavioral problems (Allison, Adlaf, Ialomiteanu, & Rehm, 1999; Brooks-Gunn, 1991; Byrne et al., 2007; Byrne & Mazanov, 2003; Johnson et al., 2002; Stevens, Murphy, & McKnight, 2003).
Stress is a particularly important domain of study among adolescents with type 1 diabetes because metabolic control deteriorates over the course of adolescence (Greening, Stoppelbein, Konishi, Jordan, & Moll, 2007; Leonard, Jang, Savik & Plumbo, 2005), and stress could play a role in this deterioration. There are only a handful of studies among adolescents that examine the relation of stressful life events to blood glucose control.
One way in which the relation of stress to blood glucose has been examined is in the laboratory. Laboratory studies tend to expose people to brief stressors, such as challenging video games, and examine effects on blood glucose. In an older study of 31 adolescents with type 1 diabetes, neither of two laboratory stressors were associated with blood glucose changes (Delamater et al., 1988). Reviews of the literature typically conclude that the impact of laboratory stressors on blood glucose is not consistent across studies (Goetsch & Wiebe, 1995; Kramer, Ledolter, Manos, & Bayless, 2000). One limitation of these studies is that a variety of stressors have been used and individual differences in perceptions of stress have not been examined. Some stressors may be more relevant and engaging to some individuals than others.
Survey studies remedy this deficiency, in part, by allowing individuals to define what is stressful. Survey studies tend to find relations between reports of stressful life events and poor metabolic control (Delamater, 1992; Goetsch & Wiebe, 1995; Kramer et al., 2000). Studies of adolescents with type 1 diabetes have linked poor metabolic control to a measure of chronic life stress (Hanson, Henggeler, & Burghen, 1987), a life-events measure completed at the end of a 2-week camp (Kager & Holden, 1992), and a measure of perceived stress (Balfour, White, Schiffrin, Dougherty, & Dufreshne, 1993). There are exceptions, however. One study of adolescents with type 1 diabetes showed no relation between a life events checklist and metabolic control (Smith, Mauseth, Palmer, Pecoraro, & Wenet, 1991).
One limitation of survey studies is that they are typically cross-sectional in nature, limited to a single assessment of stress and a single assessment of metabolic control. A study of adolescents that measured daily stress for three consecutive days did not find links between average daily stress and metabolic control (Hanson & Pichert, 1986). A study of adolescents that examined the relation of life events to metabolic control for 6 consecutive years using random coefficient modeling found that the number of negative life events was marginally related to poor metabolic control (Goldston, Kovacs, Obrosky, & Iyengar, 1995). Although these two studies employed longitudinal designs, neither study examined links of stress to changes in metabolic control.
Limitations of Previous Research
Taken collectively, the evidence for a relation between stress and metabolic control among adolescents is tenuous. The studies conducted to date have a number of weaknesses. First, only a handful of studies have focused on adolescents. Second, many of these studies have employed small sample sizes. Third, the majority of studies are cross-sectional, examining the relation of a single measure of stress and a single measure of metabolic control. Fourth, with the exception of one study, researchers have failed to examine whether relations of stress to blood glucose are the same for males and females. There is some evidence that girls are more responsive to stress during adolescence than boys (Byrne & Mazanov, 2003; Ge et al., 1994; Shih, Eberhart, Hammen, & Brennan, 2006; Stevens et al., 2003). One study of adolescents with diabetes showed that interpersonal conflict was more strongly related to psychological distress and poor metabolic control for girls than boys (Helgeson, Lopez, & Kamarck, 2009). Finally, only a handful of studies have attempted to identify the mechanism linking stress to metabolic control. Before turning to the present study, we examine this issue.
Explanations for Link of Stress to Glucose Control
There are two primary explanations for why stressful life events would have a negative effect on metabolic control. First, stress may be directly related to metabolic control through its effect on the neuroendocrine system; that is, it may directly alter blood glucose levels. Stressful life events are thought to activate the sympathetic nervous system which leads to the release of stress hormones (i.e., epinephrine, cortisol). Stress hormones increase glucose production in the liver, inhibit insulin secretion in the pancreas, and/or decrease the insulin response to glucose (i.e., increase insulin resistance; Goetsch & Wiebe, 1995; Kramer et al., 2000). Relatedly, stressful life events might increase psychological distress which then has these metabolic effects.
Second, stressful life events may influence metabolic control indirectly by detracting from self-care behavior. Evidence on the relation of stress to self-care behavior among adolescents is mixed. One study linked stressful life events to poor self-care (Goldston et al., 1995), whereas another did not (Hanson et al., 1987). Studies that have tested whether self-care mediates the relation of stress to metabolic control also are not clear. One study of children ages 8–13 found that serious noncompliance accounted for part of the relation of life events to poor metabolic control (Goldston et al., 1995), whereas two older studies of adolescents found that adherence did not mediate this relation (Hanson & Pichert, 1986; Hanson et al., 1987). Thus, it is not a surprise that a literature review concluded there is little evidence that self-care behavior accounts for much of the stress-blood glucose relation (Kramer et al., 2000).
It also is possible that the two pathways are linked. Although research has not linked neuroendocrine function to adherence, psychological distress has been linked to poor adherence (e.g., Kiviniemi, Voss-Humke, & Seifert, 2007; Korbel, Wiebe, Berg, & Palmer, 2007).
The Present Study
We enrolled a group of children with type 1 diabetes during early adolescence. We administered a measure of stressful life events and assessed metabolic control on an annual basis for 5 years. Thus, we examined whether the relation between stressful life events and metabolic control was affected by age. A previous study showed that stressful life events were more strongly related to negative affect for older than younger adolescents (Larson & Ham, 1993). Because we had five waves of data, we conducted both cross-sectional and longitudinal (i.e., lagged) analyses. Cross-sectional analyses reveal whether two variables are simultaneously related across the multiple waves of assessment, but they do not reveal anything about the causal direction of that relation. Longitudinal analyses begin to address causality by revealing whether stressful life events are associated with changes in outcomes over time. We also examined whether relations to metabolic control held across males and females. We examined two potential mechanisms of the link between stress and metabolic control—psychological distress and self-care behavior. We had multiple measures of each and examined these variables as important outcomes in and of themselves as well as potential mediators of the stress-blood glucose relation. Researchers have called for more longitudinal field studies to link changes in stress to changes in blood glucose and to examine individual differences in these relations (Kramer et al., 2000). The proposed study met these challenges.
Method
Participants
We enrolled 132 adolescents with type 1 diabetes (70 girls, 62 boys) in the study. Adolescents were eligible to participate in the study if they were in the 5th, 6th, or 7th grade; were attending Children’s Hospital of XXXX diabetes center; had been diagnosed with diabetes for more than one year; and did not have another major chronic illness (e.g., cancer, rheumatoid arthritis).1 This information was provided by parents and later verified by medical records. Age at study start ranged from 10.73 to 14.21, with a mean of 12.10 years. The majority of children (80%) were ages 11 and 12. Males and females were of a similar age. Length of illness ranged from 1 to 13 years (M = 4.91, SD = 2.96). The percentage of children using an insulin pump was 26% at study start and increased over each of the next four years (35%, 44%, 50%, 48%); the rest of the children were using injections. The majority of participants were white (93%), 2% were African American, 1% was Asian, 1% was American Indian, and 3% were mixed races. These figures are consistent with the diabetes population seen at Children’s Hospital (8% minority), which draws from a largely suburban and partly rural area. The four factor Hollingshead index (1975) of social status (mother and father education and occupation) revealed an average family score of 41.97 (SD = 11.05), which reflects the lower end of technical workers, medium business, and minor professionals.
Procedure
The study was approved by the appropriate Institutional Review Boards. Letters of invitation were sent to all adolescents with diabetes who were approximately 11–13 years of age and attending Children’s Hospital (n = 307). Families could return a postcard indicating that they did not want to be contacted by phone about the study. Twenty families returned these postcards, refusing contact about the study without us being able to determine eligibility. We reached 261 of the remaining 287 families by phone and determined that 90 were not eligible. Of the 171 eligible families, 39 refused and 132 agreed. Thus, our effective response rate was 77%.
We interviewed adolescents with diabetes immediately before or after their clinic visit in the General Clinical Research Center, which is separate from and not associated with the diabetes clinic. Parent consent and child assent were obtained at the initial interview. Interviews were conducted aloud, with the exception of the depression scale which was completed by the child in private due to its sensitive nature. Children were provided with response cards (i.e., 1=not at all; 2 = a little; 3 = a lot) for standardized instruments and paid for their participation.
One year later (Time 2 [T2]), we interviewed 127 of the 132 (96%) children. Two years later (Time 3 [T3]), we interviewed 126 (95%) of the children; three years later (Time 4 [T4]), we interviewed 127 (96%) of the children; and four years later (Time 5 [T5]), we interviewed 126 (95%) of the children with diabetes. Annual interviews were conducted as close as possible to the yearly anniversary from the baseline interview. If a participant cancelled an appointment or scheduled an appointment more than a month after this anniversary, we scheduled the annual interview in the home. On average, the time between followup interviews was 380 days T1–T2; 362 days T2–T3; 366 days T3–T4; and 363 days T4–T5. The majority (88%) of interviews were conducted within 45 days of the anniversary. Missing data were due to scheduling difficulty, inability to reach the child to set up the interview, or lack of interest on the part of the child.
Instruments
Stressful life events
We based our stressful life events checklist on Yeaworth, York, Hussey, Ingle, and Goodwin’s (1980) Adolescent Life Change Event Scale. We used 23 of those items (omitted items more relevant to older adolescents, such as started a job), added 3 other items from Newcomb et al.’s (1981) measure, and added 8 items that we used in a previous study of adolescents with diabetes (automobile accident, close friend move away, argument with a friend, end a friendship, lose something valuable, have difficulties with homework, insulted or laughed at, peer pressure; Helgeson & Fritz, 1996). We added these 8 items in the previous study based on pilot interviews with adolescents because the original instrument was dated, and we wanted to include more common, contemporary items. In that longitudinal study, the modified stressful life event index predicted concurrent psychological distress, concurrent metabolic control, and declines in metabolic control over 4 months. All items reflect general domains of stress relevant to adolescents, none of which are related to diabetes specifically.
Psychological distress
We administered three measures of psychological distress. First, we administered the abbreviated form of the Children’s Depression Inventory (CDI; Kovacs, 1985; 2001). The CDI is a self-report measure designed for children and adolescents. The abbreviated form consists of 10 items that are comprehensible at a first-grade reading level. Alphas were .76, .59, .78, .80, and .74. We measured anxiety with the 7-item scale that Stark and Laurent (2001) developed in response to concerns about the inability to distinguish depressive symptoms and anxiety in children. These were the 7 items from the Revised Children’s Manifest Anxiety Scale that were found to reflect anxiety uniquely. The authors provided convergent and discriminate validity for their measure. To increase variability in the scale and make the response format consistent with other items, we changed the true/false format to 3-point scales (not at all true, sort of true, very true of me). The internal consistencies in the present study were .68, .72, .73, .76 and .72, which are comparable to the alphas reported by Stark and Laurent (2001). Consistent with the authors’ intent, we found much lower correlations between our measures of anxiety and depressive symptoms (r’s ranged from .27 to .59) than is commonly found in the literature. We used the 3-item anger subscale of the Differential Emotions Scale (Izard, Libero, Putman & Haynes, 1993). Test-retest reliability is high, and validity with comparable scales has been reported. To be consistent with other scales, we changed the response format to a 3-point scale. The internal consistencies were .76 at T1, T2 and T3, .79 at T4, and .73 at T5, which are slightly lower than the .85 the authors reported. Because the three scales were only modestly related (r’s ranged .22 to .43 at T1), we examined them separately.
Self-care behavior
We employed two measures of self-care behavior. First, we administered a modification of the widely used 14-item Self-Care Inventory to adolescents (La Greca, Swales, Klemp, & Madigan, 1988). This instrument asks respondents to indicate how well they followed their physician’s recommendations for glucose testing, insulin administration, diet, exercise, and other diabetes-related behaviors. Each item is rated on a 1 (never do it) to 5 (always do this as recommended) scale. This scale reflects domains of self-care that have been regarded as important by the American Diabetes Association, and it has been associated with metabolic control among adolescents in a number of studies (e.g., Delamater, Applegate, Eidson, & Nemery, 1998; La Greca, Follansbee, & Skyler, 1990; Korbel, Wiebe, Berg, & Palmer, 2007). We updated this scale by adding 8 more contemporary items: 3 negative behaviors from Weissberg-Benchell et al. (1995: made up blood tests results because numbers were too high, made up blood test results because did not really test, took extra insulin because ate inappropriate food); 3 negative behaviors of our own (skipping meals, skipping injections, eating foods that should be avoided); and 2 positive behaviors (rotating injection sites; measuring food). The positive behaviors used the above-mentioned scale; the negative items were scored on a similar scale ranging from 1 (never do it) to 5 (very often). We reverse scored negative items, summed across items, and took the average. Our revised measure was correlated .94 with La Greca’s original 14-item scale at T1. The internal consistency was adequate at all times (alpha = .78, .82, .80, .82, and .81, respectively).
Second, we downloaded data from adolescents’ blood glucose meters which they brought to the clinic. When we confirmed our interview appointment by phone, we asked adolescents if they used more than one blood glucose meter and, if so, to bring both of them. When two meters were brought to the clinic, we downloaded the data from both of them. However, it is certainly possible that a few adolescents brought only one meter to the clinic and left the other meter at home. On average, meters contained about 2 months of data (mean number of days ranged from 59 to 67 across the five waves; SD’s ranged from 23 to 50). We calculated the average number of meter readings taken per day. The average number taken per day over the course of the study was about 4 (means ranged from 3.71 to 3.88; SD’s ranged from 1.38 to 1.57).
Metabolic control
Metabolic control was measured with hemoglobin A1C (HbA1C) obtained at the clinic appointment measured by HPLC (Tosoh Instruments) with normal range of 4.6–6.1%. HbA1C values indicate the average blood glucose level over the past 2–3 months. The average HbA1C at T1 for our sample was 8.04 (SD = 1.31) and at T5 was 8.90 (SD = 1.83). Higher numbers indicate worse metabolic control. Current HbA1C recommendations for 13 to 19-year-old adolescents are below 7.5% (American Diabetes Association, 2008).
Overview of Statistical Analyses
We used longitudinal growth curve modeling or multi-level modeling (Singer & Willett, 2003) with HLM-6 (Raudenbush, Bryk, Cheong, & Congdon, 2004) to examine the extent to which stressful life events predicted changes in outcomes over time. We had three mental health outcomes (depressive symptoms, anxiety, anger), two measures of self-care behavior (self-care index, average number of meter readings per day), and one indicator of metabolic control (hemoglobin A1c). Because this is an emerging technique in the field, we briefly outline its most important strengths for the current study.
Multi-level modeling has numerous advantages over ordinary least squares (OLS) regression. First, with multi-level modeling, one is able to take advantage of all available data, including data from participants who missed an assessment. Thus, if a participant missed one or two assessments, his or her data from the other assessments were included in the analyses. Second, multi-level modeling can be used when one expects variables to be correlated across time, a substantial improvement over OLS, which assumes that this autocorrelation is zero. Finally, and most importantly, multi-level modeling allows one to examine individual variability in rates of change. The rate of change is calculated for each individual, then aggregated across individuals. One can examine the relation of individual characteristics that change over time (i.e., time-varying predictors) as well as individual characteristics that do not change over time (i.e., time invariant predictors) to outcomes that change over time. In this paper, both stressful life events and age were considered to be time-varying predictors, and sex was considered to be a time invariant predictor. We examined the relation of stressful life events at each wave of assessment to the outcome measured at the same time. We also examined how the age and sex of the adolescent at each wave of assessment were associated with the outcome at the same time, and determined whether adolescent age and sex influenced the relation of stressful life events to outcomes. That is, we were able to examine whether the relation of stressful life events to outcomes was moderated by adolescent sex or age.
We first used multi-level modeling to examine whether stressful life events predicted each outcome cross-sectionally and whether this effect was moderated by age or sex. Consistent with Singer and Willett’s (2003) recommendations, we deleted nonsignificant parameters from the final model. Stressful life events were uncentered in the analyses, as 0 was a meaningful number. Age was centered at the youngest age of the participant, so that 0 represented the youngest participant in the study. Sex was scored such that 0 represented female and 1 represented male. Significant interactions were decomposed using the HLM-6 software (Raudenbush et al., 2004) which plots the outcome for participants who score at the 25th and 75th percentile of the independent variables. For the lagged analyses, we examined the relation of stressful life events at Tn to outcomes measured at Tn+1 controlling for outcomes measured at Tn. The lagged analyses take into consideration four lags: T1 to T2; T2 to T3; T3 to T4; T4 to T5.
Results
Background Analyses
Before predicting our primary outcomes, we thought it useful to determine whether either of our potential moderator variables (age, sex) was related to stressful life events. Using multilevel modeling, age significantly predicted life events (.224, SE = .065, p < .001) and sex marginally predicted life events (−.775, SE = .46, p = .09), such that older children and females reported more stressful events.
Cross-Sectional Analyses
Mental health
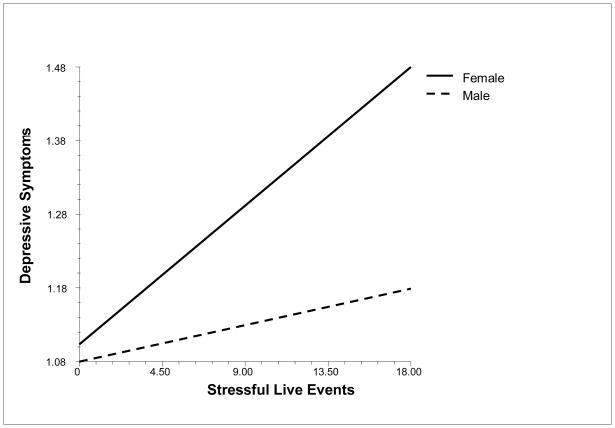
Stressful life events predicted more depressive symptoms (.015, SE = .003, p < .001). Stressful life events did not interact with age but did interact with sex (−.015, SE = .006, p < .01), such that stressful life events were more strongly related to depressive symptoms for females than males (see Figure 1). Stressful life events interacted with age to predict anxiety (.005, SE = .002, p < .05) and anger (.06, SE = .003, p = .05). The relations of stressful life events to anxiety and anger were stronger for older than younger adolescents.
Figure 1.
Relation of Stressful Life Events to Depressive Symptoms for Males and Females
Self-care behavior
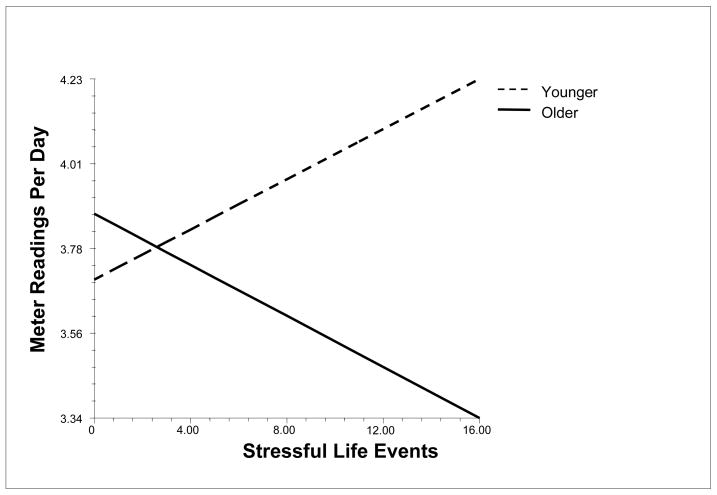
Stressful life events predicted poorer self-care behavior (−.04, SE = .007, p < .001). Stressful life events interacted with age to predict number of meter readings (−.03, SE = .012, p < .05), such that life events were related to less frequent meter readings for older teens and more frequent meter readings for younger teens (see Figure 2). Stressful life events did not interact with sex to predict each measure of self-care.
Figure 2.
Relation of Stressful Life Events to Meter Readings for Older (75th percentile) and Younger (25th percentile) Adolescents
Metabolic control
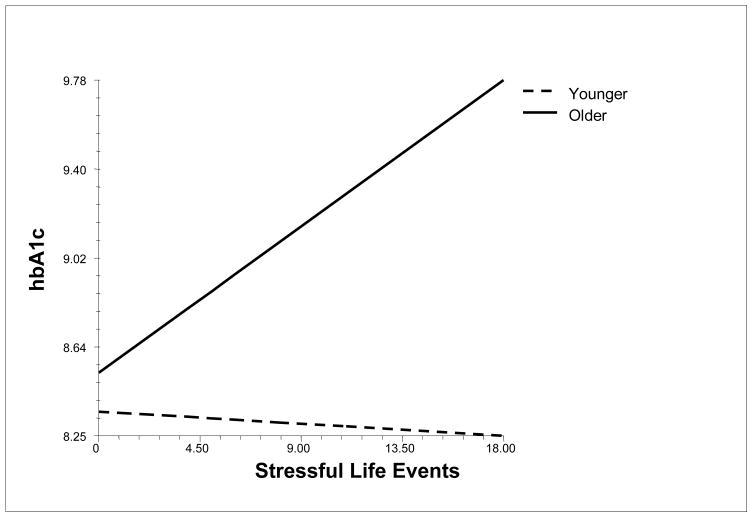
Stressful life events interacted with age to predict metabolic control (.03, SE = .011, p < .01), such that more life events were associated with poor metabolic control for older but not younger adolescents (see Figure 3). Stressful life events did not interact with sex to predict metabolic control
Figure 3.
Relation of Stressful Life Events to Metabolic Control (hbA1c) for Older (75th percentile) and Younger (25th percentile) Adolescents
Longitudinal Analyses
Mental health
Stressful life events predicted changes in depressive symptoms (.006, SE = .002, p < .05), changes in anxiety (.017, SE = .005, p < .005), and changes in anger (.036, SE = .008, p < .001)—all in the direction of more life events predicting increases in psychological distress over time. There were no interactions with age or sex.
Self-care behavior
Stressful life events predicted changes in self-care behavior and meter readings (−.015, SE = .005, p < .005; −.044, SE = .023, p = .05), in the direction of more events predicting declines in self-care. There were no interactions with age or sex.
Metabolic control
Stressful life events predicted changes in metabolic control over time (.042, SE = .019, p < .05), such that more life events were associated with declines in metabolic control over time. There were no interactions with age or sex.
Mediation
Our final goal was to examine whether psychological distress or self-care behavior mediated the relation between stressful life events and metabolic control. In the cross-sectional analyses, stressful life events interacted with age to predict anxiety, anger, meter readings, and metabolic control. Of these three potential mediators, anger and meter readings were associated with poor metabolic control (.22, SE = .09, p < .05; −.42, SE = .05, p < .001, respectively) and could be examined as potential mediators of the life events by age interaction on metabolic control. We examined mediation in two ways. First, we entered both anger and meter readings in the equation along with the life events by age interaction to predict metabolic control. In the final equation, anger was not significant, meter readings was significant (−.29, SE = .046, p < .001), and the stressful life events by age interaction was no longer significant (.007, SE = .010, p = .48). We also used the calculation of random indirect and direct effects developed by Bauer, Preacher, and Gil (2006) to examine mediation for multi-level models. Using this method, we found that the indirect effect for anger was not significant but the indirect effect for meter readings was significant (.006, SE = .002, z = 2.36, p < .05) and accounted for 25% of the effect of life events on metabolic control.
In the lagged analyses, stressful life events predicted the three distress indices, the two self-care indices, and metabolic control. Of the predictor variables, only depressive symptoms and meter readings were associated with metabolic control (.744, SE = .335, p < .05; −.092, SE = .037, p < .05; respectively) and could be examined as potential mediators of the relation of life events to metabolic control. When depressive symptoms and meter readings were entered into the equation with life events, the effects for depressive symptoms and meter readings were both significant (.73, SE = .336, p < .05; −.091, SE = .039, p < .05) and the effect for life events was no longer significant (.014, SE = .017, p = .42). However, the Bauer et al. (2006) method for testing the significance of indirect effects showed that neither mediator was significant.
Discussion
The primary goal of the study was to determine whether stressful life events were associated with metabolic control for adolescents with diabetes. Using growth curve modeling with a fairly large sample of adolescents, we found that stressful life events were associated with poor metabolic control concurrently and predicted declines in metabolic control over time. In the cross-sectional analyses, this relation only appeared among older teens. Because we enrolled 10 to 14-year-olds in the study and followed them for five years, we were able to examine the relation of stressful life events to metabolic control for a larger age range—10 through 18 years of age. Thus, one reason that a relation between stress and metabolic control has not been found consistently in previous research is that younger adolescents’ metabolic control may not be affected by stressful life events. There are several reasons that stressful life events may play a larger role in the health of older than younger adolescents. First, older adolescents have greater knowledge, increased complexity of thought, and are able to understand the consequences of events more fully (Holmbeck et al., 2006). Second, younger adolescents may be less affected by stressful life events because parents intervene on their behalf. Parents may exert a stronger effect on the behavior of younger teens, buffering any adverse effect of life stressors. Our cross-sectional finding for meter readings supports this possibility. For older children, more stressful life events were associated with fewer meter readings. But for younger children, more stressful life events were associated with more frequent meter readings. It may be that parents of younger children are sensitive to the stress in their child’s environment, aware of its potential impact on metabolic control, and intervene by ensuring that the behavior—testing blood sugar—is enacted. Because previous studies have often employed wide age ranges of adolescents with small sample sizes, they have not been able to test whether the relation of stressful life events to metabolic control held for only a portion of the sample. It also is the case that age was related to more stressful life events; thus, stronger relations may have appeared among older adolescents because the frequency of stressful life events increased in this age group.
A second goal of the study was to determine whether we could explain the relation of stressful life events to poor metabolic control. We noted that there are two pathways by which stress is thought to influence metabolic control: (1) the neuroendocrine system and (2) behavior. Although we did not have direct measures of neuroendocrine function, we did assess psychological distress which is thought to be influenced by neuroendocrine hormones. Stressful events predicted all three indicators of psychological distress in cross-sectional and longitudinal analyses, but there was no evidence that psychological distress mediated the relation of stressful life events to metabolic control. Because we did not have direct measures of neuroendocrine function, we cannot rule out that pathway in the present study. However, there was some evidence that behavior mediated this relation. Stressful life events predicted both the self-care behavior index and the average number of meter readings taken per day in both cross-sectional and longitudinal analyses. The life events by age interaction was reduced to nonsignificance when meter readings was statistically controlled while the effect for meter readings remained significant. These findings are consistent with the idea that stressful life events detract from self-care behavior, including blood glucose testing, which then has adverse effects on metabolic control. This model appears to hold only for older adolescents. In addition, this mediational pathway was not significant in the longitudinal analyses. Thus, more work is certainly needed to elucidate the precise mechanism by which stress may adversely affect metabolic control among adolescents.
Although the focus of the study was on metabolic control, it also is important to note that stressful life events were associated with indicators of psychological distress, confirming previous research that has demonstrated such a relation (e.g., Byrne et al., 2007). We anticipated that these relations would be stronger for girls than boys. There was only modest evidence that girls were more responsive to stress than boys. In cross-sectional analyses, stressful life events were more strongly associated with depressive symptoms for girls than boys. Importantly, there was no interaction between stressful life events and sex on either measure of self care or metabolic control. Thus, although stressful life events might pose a more significant source of distress for girls, stressful life events did not seem to have any stronger effects on girls’ behavior or metabolic control in this study.
These results have implications for clinicians’ treatment of children and adolescents with type 1 diabetes. In discussions with adolescents, clinicians should probe for information about potential stressors. Alternatively, clinician offices could easily administer a brief life events checklist prior to the appointment and use that instrument as a screen or vehicle for discussion. When there has been an increase in stressful life events, it would be helpful for clinicians, family, and the adolescent to understand the potential impact on behavior.
Before concluding, we must acknowledge several limitations of this study. First, the sample of children was quite homogeneous with respect to race, limiting the generalizabililty of the findings. Future research would benefit by examining stressful life events in a more diverse group both racially and economically. Second, our assessments were spaced quite far apart—once each year. Although a strength of the study is its longitudinal design, future research would benefit from studies that capture the more proximal effects of stress on blood glucose. Daily diary or ecological momentary methods are designs that could help to discern the relation of stress to blood glucose. Finally, we focused on a global checklist of stressful life events. Future research would benefit from examining specific dimensions or categories of stressors.
In sum, following a fairly large sample of adolescents for five years and spanning a large age range, we were able to demonstrate that stressful life events are associated with poor metabolic control, that this relation holds for the older end of the adolescent spectrum, and that stressful life events predicted a deterioration in metabolic control over time. We also showed that self-care behavior may be a primary contributor to this relation.
Acknowledgments
Portions of this article were presented at the 2008 Meeting of the Society of Behavioral Medicine. The authors acknowledge the support of grant R01 DK60586 from the National Institutes of Health to conduct this work and the support of the Pediatric Clinical and Translational Research Center at Children’s Hospital (GCRC grant, 5MO1 RR00084). The authors extend their appreciation to Pamela Snyder for her management of the day-to-day aspects of the project as well as for the statistical analyses. The authors are grateful to Laura Kiley, Abigail Kunz Vaughn, Michelle Merriman, Elizabeth Muia, and Laura Viccaro for conducting the majority of the adolescent interviews. We also acknowledge the support of the clinic staff of Children’s Hospital of Pittsburgh.
Footnotes
We did not rule out participants who had conditions that can be comorbid with diabetes, such as celiac disease or hypothyroidism. Two patients had celiac disease and three patients had hypothyroidism at study start.
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/journals/hea
Contributor Information
Vicki S. Helgeson, Carnegie Mellon University
Oscar Escobar, Children’s Hospital of Pittsburgh.
Linda Siminerio, University of Pittsburgh Medical Center.
Dorothy Becker, Children’s Hospital of Pittsburgh.
References
- Allison KR, Adlaf EM, Ialomiteanu A, Rehm J. Predictors of health risk behaviours among young adults: Analysis of the national population health survey. Canadian Journal of Public Health. 1999;90:85–89. doi: 10.1007/BF03404107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association. Standards of medical care in diabetes-2008. Diabetes Care. 2008;31(Supplement 1):S12–S54. doi: 10.2337/dc08-S012. [DOI] [PubMed] [Google Scholar]
- Bauer DJ, Preacher KJ, Gil KM. Conceptualizing and testing random indirect effects and moderated mediation in multilevel models: New procedures and recommendations. Psychological Methods. 2006;11:142–163. doi: 10.1037/1082-989X.11.2.142. [DOI] [PubMed] [Google Scholar]
- Balfour L, White DR, Schiffrin A, Dougherty G, Dufrense J. Dietary disinhibition, perceived stress, and glucose control in young, Type 1 diabetic women. Health Psychology. 1993;12:33–38. doi: 10.1037//0278-6133.12.1.33. [DOI] [PubMed] [Google Scholar]
- Brooks-Gunn J. How stressful is the transition to adolescence for girls? In: Colten ME, Gore S, editors. Adolescent stress: Causes and consequences. Hawthorne, New York: Aldine De Gruyter; 1991. pp. 131–151. [Google Scholar]
- Byrne DG, Davenport SC, Mazanov J. Profiles of adolescent stress: The development of the Adolescent Stress Questionnaire (ASQ) Journal of Adolescence. 2007;30:393–416. doi: 10.1016/j.adolescence.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Byrne DG, Mazanov J. Adolescent stress and future smoking behavior: A prospective investigation. Journal of Psychosomatic Research. 2003;54:313–321. doi: 10.1016/s0022-3999(02)00411-7. [DOI] [PubMed] [Google Scholar]
- Delamater AM. Stress, coping, and metabolic control among youngsters with diabetes. In: La Greca AM, Siegel LJ, Wallander JL, Walker CE, editors. stress and coping in child health. New York: The Guilford Press; 1992. pp. 191–211. [Google Scholar]
- Delamater AM, Applegate B, Edison M, Nemery R. Increased risks for poor metabolic control in minority youths with Type 1 diabetes. Diabetes. 1998;47:A326. [Google Scholar]
- Delamater AM, Bubb J, Kurtz SM, Kuntze J, Smith JA, White NH, Santiago JV. Physiologic responses to acute psychological stress in adolescents with type 1 diabetes mellitus. Journal of Pediatric Psychology. 1988;13:69–86. doi: 10.1093/jpepsy/13.1.69. [DOI] [PubMed] [Google Scholar]
- Ge X, Lorenz FO, Conger RD, Elder GH, Jr, Simons RL. Trajectory of stressful life events and depressive symptoms during adolescence. Developmental Psychology. 1994;30:467–483. [Google Scholar]
- Goetsch VL, Wiebe DJ. Diabetes mellitus: considerations of the influence of stress. In: Goreczny AJ, editor. Handbook of health and rehabilitation psychology. New York, NY: Plenum Press; 1995. pp. 513–533. [Google Scholar]
- Goldston DB, Kovacs M, Obrosky DS, Iyengar S. A longitudinal study of life events and metabolic control among youths with insulin-dependent diabetes mellitus. Health Psychology. 1995;14:409–414. doi: 10.1037//0278-6133.14.5.409. [DOI] [PubMed] [Google Scholar]
- Graber JA. Internalizing problems during adolescence. In: Lerner RM, Steinberg L, editors. The handbook of adolescent psychology. 2. Hoboken, NJ: John Wiley and Sons, Inc; 2004. [Google Scholar]
- Greening L, Stoppelbein L, Konishi C, Jordan SS, Moll G. Child routines and youths’ adherence to treatment for Type 1 diabetes. Journal of Pediatric Psychology. 2007;32:437–447. doi: 10.1093/jpepsy/jsl029. [DOI] [PubMed] [Google Scholar]
- Hanson CL, Henggeler SW, Burghen GA. Social competence and parental support as mediators of the link between stress and metabolic control in adolescents with insulin-dependent diabetes mellitus. Journal of Consulting and Clinical Psychology. 1987;55:529–533. doi: 10.1037/0022-006X.55.4.529. [DOI] [PubMed] [Google Scholar]
- Hanson SL, Pichert JW. Perceived stress in diabetes control in adolescents. Health Psychology. 1986;5:439–452. doi: 10.1037//0278-6133.5.5.439. [DOI] [PubMed] [Google Scholar]
- Helgeson VS, Fritz HL. Implications of unmitigated communion and communion for adolescent adjustment to Type 1 diabetes. Women’s Health: Research on Gender, Behavior, and Policy. 1996;2:163–188. [Google Scholar]
- Helgeson VS, Lopez LC, Kamarck T. Peer relationships and diabetes: Retrospective and ecological momentary assessment approaches. Health Psychology. 2009;28:273–282. doi: 10.1037/a0013784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status. New Haven, CT: Yale University; 1975. Unpublished manuscript. [Google Scholar]
- Holmbeck GN, Friedman D, Abad M, Jandasek B. Development and psychopathology in adolescence. In: Wolfe DA, Mash EJ, editors. Behavioral and emotional disorders in adolescents. New York: The Guilford Press; 2006. pp. 21–55. [Google Scholar]
- Izard CE, Libero DZ, Putman P, Haynes OM. Stability of emotion experiences and their relations to traits of personality. Journal of Personality and Social Psychology. 1993;64:847–860. doi: 10.1037//0022-3514.64.5.847. [DOI] [PubMed] [Google Scholar]
- Johnson J, Cohen P, Gould M, Kasen S, Brown J, Brook J. Childhood adversities, interpersonal difficulties, and risk for suicide attempts during late adolescence and early adulthood. Archives of General Psychiatry. 2002;59:741–749. doi: 10.1001/archpsyc.59.8.741. [DOI] [PubMed] [Google Scholar]
- Kager VA, Holden EW. Preliminary investigation of the direct and moderating effects of family and individual variables on the adjustment of children and adolescents with diabetes. Journal of Pediatric Psychology. 1992;17:491–502. doi: 10.1093/jpepsy/17.4.491. [DOI] [PubMed] [Google Scholar]
- Kiviniemi MT, Voss-Humke AM, Seifert AL. How do I feel about the behavior? The interplay of affective associations with behaviors and cognitive beliefs as influence on physical activity behavior. Health Psychology. 2007;26:152–158. doi: 10.1037/0278-6133.26.2.152. [DOI] [PubMed] [Google Scholar]
- Korbel CD, Wiebe DJ, Berg CA, Palmer DL. Gender differences in adherence to type 1 diabetes management across adolescence: The mediating role of depression. Children’s Health Care. 2007;36:83–98. [Google Scholar]
- Kovacs M. The Children’s Depression Inventory (CDI) Psychopharmacology Bulletin. 1985;21:995–998. [PubMed] [Google Scholar]
- Kovacs M. Children’s Depression Inventory: Technical manual. North Tonawanda, NY: Multi-Health Systems, Inc; 2001. [Google Scholar]
- Kramer JR, Ledolter J, Manos GN, Bayless ML. Stress and metabolic control in diabetes mellitus: Methodological issues and an illustrative analysis. Annals of Behavioral Medicine. 2000;22:17–28. doi: 10.1007/BF02895164. [DOI] [PubMed] [Google Scholar]
- La Greca AM, Follansbee D, Skyler JS. Developmental and behavioral aspects of diabetes management in youngsters. Children’s Health Care. 1990;19:132–139. [Google Scholar]
- La Greca AM, Swales T, Klemp S, Madigan S. Self-care behaviors among adolescents with diabetes. Paper presented at the Ninth Annual Convention of the Society for Behavioral Medicine.1988. [Google Scholar]
- Larson R, Ham M. Stress and “storm and stress” in early adolescence: The relationship of negative events with dysphoric affect. Developmental Psychology. 1993;29:130–140. [Google Scholar]
- Leonard BJ, Jang YP, Savik K, Plumbo MA. Adolescents with type 1 diabetes: Family functioning and metabolic control. Journal of Family Nursing. 2005;11:102–121. doi: 10.1177/1074840705275152. [DOI] [PubMed] [Google Scholar]
- Newcomb MD, Huba GJ, Bentler PM. A multidimensional assessment of stressful life events among adolescents: Derivation and correlates. Journal of Health and Social Behavior. 1981;22:400–415. [Google Scholar]
- Raudenbush S, Bryk A, Cheong YF, Congdon R. HLM 6: Hierarchical linear and nonlinear modeling. Lincolnwood, IL: Scientific Software International, Inc; 2004. [Google Scholar]
- Shih JH, Eberhart NK, Hammen CL, Brennan PA. Differential exposure and reactivity to interpersonal stress predict sex differences in adolescent depression. Journal of Clinical Child and Adolescent Psychology. 2006;35:103–115. doi: 10.1207/s15374424jccp3501_9. [DOI] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis. New York: Oxford University Press; 2003. [Google Scholar]
- Smith MS, Mauseth R, Palmer JP, Pecoraro R, Wenet G. Glycosylated hemoglobin and psychological adjustment in adolescents with diabetes. Adolescence. 1991;26:31–40. [PubMed] [Google Scholar]
- Stark KD, Laurent J. Joint factor analysis of the Children’s Depression Inventory and the Revised Children’s Manifest Anxiety Scale. Journal of Clinical Child Psychology. 2001;30:552–567. doi: 10.1207/S15374424JCCP3004_11. [DOI] [PubMed] [Google Scholar]
- Stevens SJ, Murphy BS, McKnight K. Traumatic stress and gender differences in relationship to substance abuse, mental health, physical health, and HIV risk behavior in a sample of adolescents enrolled in drug treatment. Child Maltreatment. 2003;8:46–57. doi: 10.1177/1077559502239611. [DOI] [PubMed] [Google Scholar]
- Weissberg-Benchell J, Wirtz P, Glasgow AM, Turek J, Tynan WD, Ward J. Adolescent diabetes management and mismanagement. Diabetes Care. 1995;18:77–82. doi: 10.2337/diacare.18.1.77. [DOI] [PubMed] [Google Scholar]
- Yeaworth RC, York J, Hussey MA, Ingle ME, Goodwin T. The development of an Adolescent Life Change Event Scale. Adolescence. 1980;15:91–98. [PubMed] [Google Scholar]