SUMMARY
The NF-κB activating kinase IKKβ suppresses early chemically-induced liver tumorigenesis by inhibiting hepatocyte death and compensatory proliferation. To study IKKβ’s role in late tumor promotion and progression, we developed a transplant system that allows initiated mouse hepatocytes to form hepatocellular carcinomas (HCC) in host liver after a long latency. Deletion of IKKβ long after initiation accelerated HCC development and enhanced proliferation of tumor initiating cells. These effects of IKKβ/NF-κB were cell autonomous and correlated with increased accumulation of reactive oxygen species (ROS) that led to JNK and STAT3 activation. Hepatocyte-specific STAT3 ablation prevented HCC development. The negative crosstalk between NF-κB and STAT3, which is also evident in human HCC, is a critical regulator of liver cancer development and progression.
INTRODUCTION
Carcinogenesis, consisting of initiation, promotion and progression, is a multistage process governed by cumulative genetic and epigenetic alterations (Nowell, 1976). Tumor initiators induce genetic changes that cause proto-oncogene activation and/or loss of tumor suppressors (Hennings et al., 1993). Initiation alone, however, is insufficient for cancer development and tumor promotion is needed for expansion of initiated cells into pre-malignant lesions that progress into malignant tumor masses. While initiation is brief and irreversible, tumor promotion and progression are long-lasting processes that up to a point may be reversed, thereby providing a rationale for chemo-intervention. Tumor promotion is thought to depend on an interaction between initiated cells and their microenvironment (Albini and Sporn, 2007) and inflammation is a frequent tumor promoter (Karin, 2006). Through production of proinflammatory cytokines, chemokines and ROS, the inflammatory microenvironment exerts a constant evolutionary pressure on initiated cells while supporting their proliferation and expansion (Coussens and Werb, 2002). Relative to early tumor promotion, the mechanisms that control tumor progression and malignant conversion are poorly understood. An improved understanding of these late steps in the tumorigenic process is of great importance as it has been estimated that most individuals harbor pre-malignant lesions that never or rarely progress to full blown neoplasms (Boucher and Yakovlev, 1997).
One of the slowest cancers to appear and grow is HCC, the third leading cause of cancer-related death worldwide (Parkin et al., 2001). HCC usually appears after exposure to liver carcinogens or infection with one of two hepatitis viruses, HBV or HCV, but its evolution and growth may take more than 30 years (Bosch et al., 2004; Tong et al., 1995). HCC frequently develops in the context of hepatosteatosis and liver cirrhosis following chronic liver damage due to oxidative and endoplasmic reticulum (ER) stress, accompanied by inflammation that drives the compensatory proliferation of surviving hepatocytes (El-Serag and Rudolph, 2007; Parekh and Anania, 2007; Wang and Weinman, 2006). Cirrhotic livers contain pre-malignant lesions ranging from dysplastic foci to dysplastic hepatocyte nodules (Wanless, 1995). These lesions are more proliferative than the surrounding parenchyma and resemble early HCC (Hytiroglou et al., 2007). A small number of these lesions undergo malignant conversion, whose rate may be accelerated by environmental factors (Seki et al., 2000; Takayama et al., 1990). Pre-malignant lesions are also found in chemically-induced rodent models of HCC (Pitot, 1990), but their conversion into frank HCC is controversial (Sell and Leffert, 2008). Understanding the molecular mechanisms underlying the progression and malignant conversion of pre-malignant lesions is critical for any effort to slow down or prevent HCC development. However, animal models for studying HCC progression are scarce.
By contrast, early steps in the molecular etiology of HCC have been extensively studied using transgenic or chemically-induced mouse HCC models (Calvisi and Thorgeirsson, 2005; Fausto, 1999). Using the chemical procarcinogen diethylnitrosamine (DEN) to induce HCC in mice, we made the initially surprising discovery that hepatocyte-specific ablation of the IKKβ subunit of the IκB kinase (IKK) complex dramatically enhances HCC induction (Maeda et al., 2005). These findings stand in marked contrast to the outcome of IKKβ deletion in enterocytes, which prevents development of colitis associated cancer (CAC) (Greten et al., 2004). Yet, in both HCC and CAC, deletion of IKKβ in myeloid cells (Kupffer cells in the liver and lamina propria macrophages and dendritic cells in the colon) attenuates tumor development due to reduced expression of tumor promoting cytokines (Greten et al., 2004; Grivennikov et al., 2009; Maeda et al., 2005). The anti-tumorigenic activity of hepatocyte IKKβ was suggested to be due to induction of NF-κB-dependent pro-survival and anti-oxidant genes. Indeed, DEN administration to hepatocyte IKKβ-deficient (IkkβΔhep) mice results in elevated ROS accumulation, hepatocyte death and compensatory proliferation, all of which are prevented by the anti-oxidant butylated hydroxyanisole (BHA) (Kamata et al., 2005; Maeda et al., 2005). Similar findings were obtained in mice lacking the NEMO/IKKγ regulatory subunit (Nemo/IkkγΔhep mice), whose hepatocyte specific ablation results in spontaneous liver damage, hepatosteatosis, fibrosis and HCC development, which are all preventable by BHA administration (Luedde et al., 2007). By contrast, in other mouse models of HCC that depend on chronic inflammation and not on liver damage and death-driven compensatory proliferation, hepatocyte IKKβ and NF-κB were found to promote tumor development (Haybaeck et al., 2009; Pikarsky et al., 2004). Under such conditions, NF-κB activation in hepatocytes is needed for expression of chemokines and cytokines that organize and maintain an inflammatory microenvironment.
Although DEN administration activates IKK transiently and does not lead to chronic hepatitis (Maeda et al., 2005), DEN-induced HCC depends on production of the NF-κB-regulated cytokine IL-6 by resident Kupffer cells (Naugler et al., 2007; Sakurai et al., 2008). In this case, Kupffer cells are activated by IL-1α released by dying hepatocytes and the absence of parenchymal IKKβ enhances IL-6 production. Interestingly, male mice produce more IL-6 upon DEN administration than females and this accounts for the marked male bias in HCC induction (Naugler et al., 2007). Although it remains to be established whether differential IL-6 production accounts for the gender bias in human HCC, a recent epidemiological study identified elevated serum IL-6 as a critical and reliable predictor of progression from HBV-induced hepatitis to HCC (Wong et al., 2009). Elevated IL-6 also correlates with accelerated progression from HCV-induced hepatitis to HCC especially in women (Nakagawa et al., 2009), which produce more IL-6 after menopause (Cioffi et al., 2002). The post-menopausal increase in IL-6 production may explain the delayed onset of HCC in women relative to men (Chen et al., 2006). IL-6 transduces its signals via a heterodimeric receptor composed of the cytokine binding IL-6R subunit and the gp130 signaling subunit (Kamimura et al., 2003). Recently, activating gp130 mutations were identified as the causal event in non-malignant hepatic adenomas (Rebouissou et al., 2009). These mutations activate the Ras-MAPK pathway and STAT3, an oncogenic transcription factor that is critical for CAC development (Bromberg and Wang, 2009; Grivennikov et al., 2009).
To further dissect the molecular mechanisms that govern liver carcinogenesis, here we examine the role of IKKβ-driven NF-κB in HCC progression as well as its relationships with STAT3 in both DEN-induced HCC in mice and human clinical specimens.
RESULTS
A transplant system for studying progression/malignant conversion of initiated hepatocytes
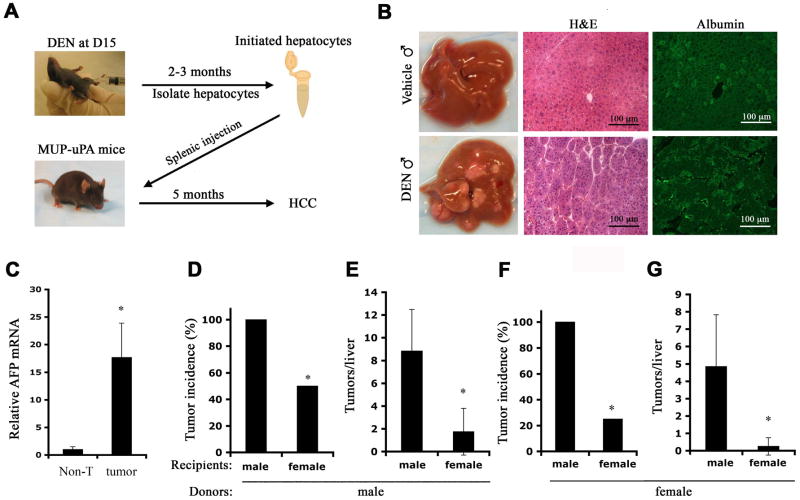
To dissociate initiation and early tumor promotion from HCC progression and malignant conversion, we established an experimental system for studying late events that affect hepatocarcinogenesis. We treated C57BL/6 mice with DEN at 2 weeks of age and waited for 3 months to allow hepatocyte initiation and clonal expansion. At that point, hepatocytes were isolated and transplanted via splenic injection into livers of MUP-uPA transgenic mice (Figure 1A). The latter over-express urokinase-type plasminogen activator (uPA) in their hepatocytes and are therefore subjected to low grade but continuous liver damage and regeneration, making them ideal recipients for exogenous hepatocytes (Weglarz et al., 2000). Furthermore, MUP-uPA mice develop mild liver fibrosis but no HCC by 8 months of age (Figure S1A). Their livers also exhibit elevated expression of IL-6 mRNA (Figure S1B) and enhanced ROS accumulation (Figure S1C). All of these changes resemble the microenvironment within which human liver cancer forms. Within a month, transplanted hepatocytes marked with green fluorescent protein (GFP) formed small islands in the recipient’s liver but otherwise were barely distinguishable from host hepatocytes (Figure S1D). By 5 months after transplantation, male MUP-uPA mice receiving hepatocytes from DEN-initiated males developed multiple tumor nodules that were absent in mice receiving hepatocytes from vehicle-injected mice (Figure 1B) or in untransplanted MUP-uPA mice (data not shown). The tumors, which exhibited a typical trabecular HCC structure and expressed albumin and elevated amounts of the HCC marker α-fetoprotein (AFP; Figure 1B,C), are likely to be derived from the transplanted DEN-initiated cells. The latter failed to grow in normal C57BL/6 mice (data not shown), suggesting that the MUP-uPA liver microenvironment is conducive and essential for conversion of initiated hepatocytes into HCC.
Figure 1. A transplant system for studying HCC progression.
(A) A diagram of experimental protocol. C57BL/6 pups were given a single i.p. injection of DEN (25 mg/kg) when 15 days old. Hepatocytes were isolated 2–3 months later and transplanted into 3 weeks old MUP-uPA mice via intra-splenic injection. Recipients were sacrificed 5 months later for liver tumor analysis.
(B) Representative livers of male MUP-uPA mice 5 months after transplantation with hepatocytes from vehicle- or DEN-treated male mice. Liver sections were stained with H&E and albumin antibody. Magnification bar = 100 μm.
(C) Relative expression of α-fetoprotein (AFP) mRNA was determined by real-time PCR in liver tumors (tumor) and surrounding non-tumor liver (Non-T). n = 4; *: p < 0.01 by t test.
(D, E) DEN-treated male or (F, G) female mice were used as hepatocyte donors to MUP-uPA recipients of the indicated gender. Tumor incidence (D, F) and multiplicity (E, G) were determined at 5 months post-transplantation. n = 8–10 for each group; *: p < 0.01 by t test (E, G) or *: p < 0.01 by chi-square test (D, F).
Please also see Figure S1.
To further characterize this system and determine its physiological relevance, we examined whether the host gender affects HCC formation by transplanted hepatocytes. We injected male and female mice with DEN and transplanted their hepatocytes into MUP-uPA hosts of either gender. Five months later, male recipients of male hepatocytes exhibited at least one HCC per liver, whereas less than 50% of female recipients of male hepatocytes bore tumors (Figure 1D). Furthermore, tumor multiplicity was 5-times higher in male hosts (Figure 1E). Even more striking results were obtained with transplanted female hepatocytes (Figure 1F, G). Although fewer tumors were seen in this case, consistent with the gender bias in HCC induction (Naugler et al., 2007), male hosts of initiated female hepatocytes exhibited 4-fold higher tumor incidence and nearly 20-times higher tumor multiplicity than female hosts of female hepatocytes (Figure 1F, G). Since MUP-uPA expression is similar between males and females [(Weglarz et al., 2000) and data not shown], these data demonstrate that gender plays a critical role not only in HCC initiation and early promotion but also in tumor progression. Removing initiated hepatocytes from the female microenvironment where they hardly progress into HCC to a male microenvironment results in a substantial enhancement of HCC development. These findings support the physiological relevance of the transplant system.
Deletion of Ikkβ in initiated hepatocytes enhances tumorigenic potential
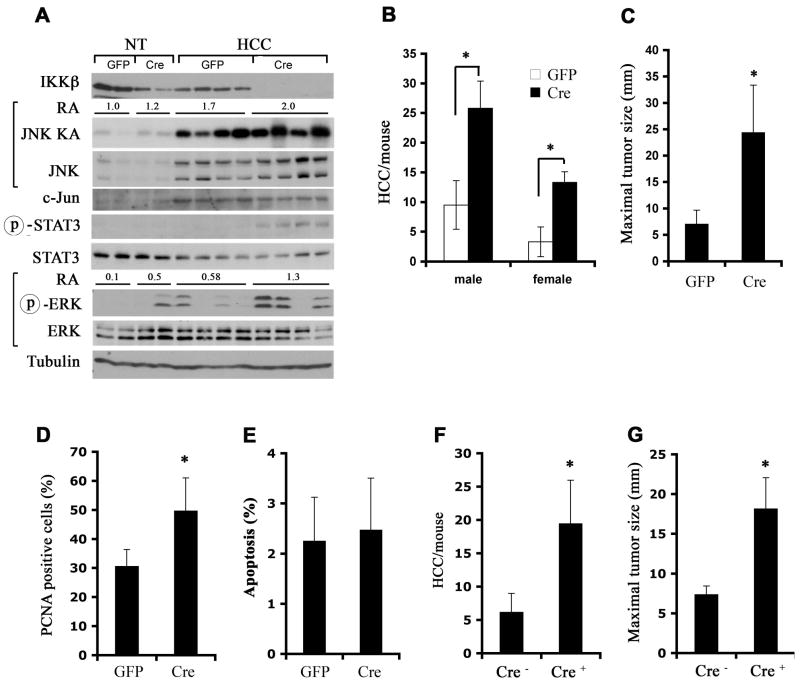
Next we examined whether the IKKβ-NF-κB pathway, which inhibits DEN-induced (Maeda et al., 2005) and spontaneous (Luedde et al., 2007) HCC development, most likely by suppressing death-driven compensatory proliferation that occurs during early tumor promotion (Karin, 2006), also affects progression. To this end, we gave Ikkβf/f male mice, which express normal amounts of IKKβ, DEN and transferred their initiated hepatocytes into MUP-uPA mice. After one month, male and female hosts were injected with GFP- or Cre-expressing adenoviruses (Adv) to delete Ikkβ in transplanted hepatocytes. Adv infection caused mild liver injury, indicated by a small elevation in circulating ALT (Figure S2). Administration of Adv-Cre induced efficient IKKβ deletion (Figure 2A) and resulted in a 3–4-fold increase in tumor multiplicity and size in both male and female recipients relative to Adv-GFP infection (Figure 2B, C). These results are consistent with the previous observation that inactivation of IKKβ in cultured hepatocytes enhances their proliferation (Koch et al., 2009).
Figure 2. IKKβ deletion after initiation enhances HCC formation and growth.
(A–C) Ikkβf/f male pups were DEN initiated and used as hepatocyte donors into MUP-uPA mice. IKKβ in transplanted hepatocytes was deleted 1 month later by injection of Adv-Cre. Adv-GFP was used as a control. Mice were sacrificed 4 months later and whole cell lysates were prepared from dissected HCCs and surrounding non-tumor livers (NT).
(A) Lysates were gel separated and immunoblotted with the indicated antibodies or used for measurement of JNK kinase activity by immunecomplex kinase assay. Relative JNK kinase activity (KA) and ERK phosphorylation in the different samples were determined by densitometry and the average relative activities (RA) for each group of samples are indicated.
(B) HCCs per liver were counted and (C) maximal tumor size was measured. n = 7~10 for each group; *: p < 0.05.
(D, E) Tumor cell proliferation and apoptosis were determined by PCNA (D) and TUNEL (E) staining, respectively, of paraffin embedded liver sections (n = 10 each group; *: p < 0.05).
(F, G) Ikkβf/f (Cre−) and Ikkβf/f/Mx1-Cre (Cre+) DEN-initiated males were used as hepatocyte donors to male MUP-uPA recipients. IKKβ deletion was accomplished by poly(IC) injection 1 month after transplantation. HCC multiplicity (F) and maximal sizes (G) were determined 4 months later (n = 10 each group; *: p < 0.01).
Please also see Figure S2.
In addition to complete IKKβ deletion, Adv-Cre administration increased STAT3 and ERK phosphorylation in HCCs relative to Adv-GFP administration (Figure 2A). However, JNK and c-Jun expression and JNK kinase activity, which were elevated in HCCs relative to non-tumor liver tissue, did not show large differences between IKKβ-expressing and non-expressing HCCs. Ikkβ-deleted HCCs contained more proliferating (PCNA-positive) cells than IKKβ-expressing tumors (Figure 2D), but the rate of HCC apoptosis was not affected by the IKKβ status (Figure 2E).
As an alternative approach to delete Ikkβ after tumor initiation, we used DEN-initiated Ikkβf/f/Mx1-Cre mice as hepatocyte donors. These mice express Cre recombinase from the interferon (IFN)-inducible Mx1 promoter (Kuhn et al., 1995), such that administration of the IFN-inducer poly(IC) results in efficient Ikkβ deletion in liver (Maeda et al., 2005). Using this experimental set-up, we deleted Ikkβ one month after transplantation. This resulted in a large increase in HCC multiplicity and size in hosts receiving initiated hepatocytes from Ikkβf/f/Mx1-Cre donors relative to hosts transplanted with Ikkβf/f hepatocytes (Figure 2F,G). These results clearly demonstrate that in addition to enhancing tumor initiation and/or early promotion, deletion of Ikkβ in initiated hepatocytes augments and/or accelerates HCC progression.
Ikkβ deletion enhances hepatosphere formation and tumorigenic potential
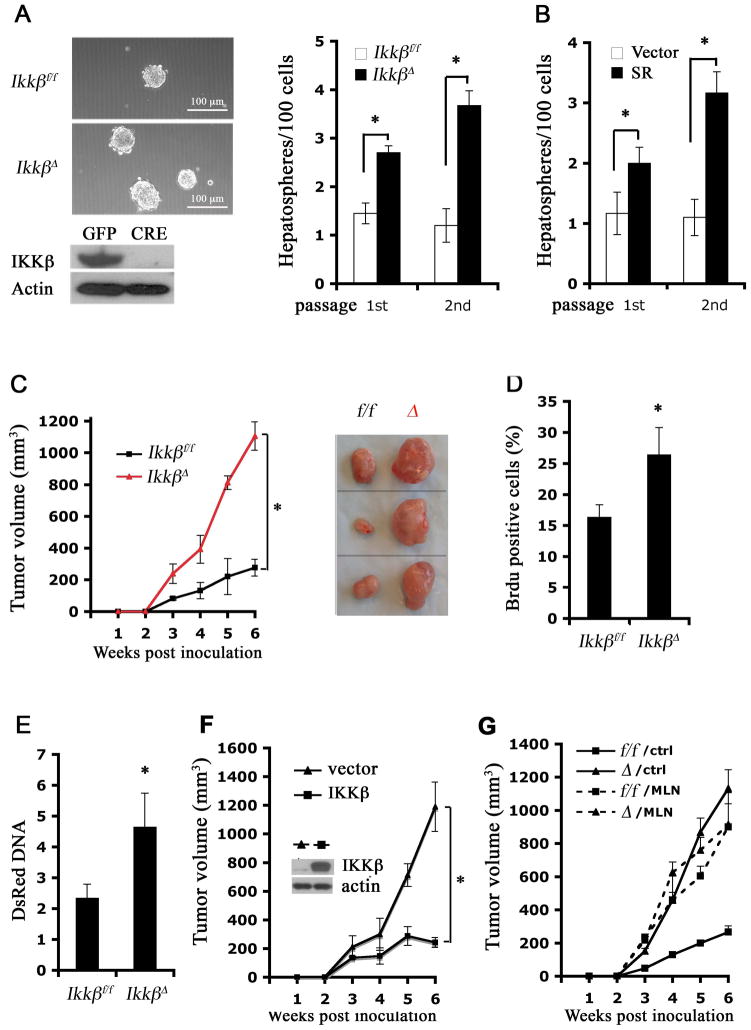
To further examine cell autonomous effects of IKKβ in malignant hepatocytes, we cultured DEN-induced HCCs from Ikkβf/f mice. Initially, HCC cells failed to proliferate and gradually died in standard hepatocyte culture medium (data not shown). Addition of phenobarbital, a liver tumor promoter (Kaufmann et al., 1988), and EGF overcame this problem and allowed the derivation of several cell strains from DEN-induced HCCs (dih). Three of the strains (dih10-12) expressed both albumin and AFP, consistent with being derived from AFP-expressing HCCs (Figure S3A). All dih cells were albumin positive, suggesting little contamination, if any, with non-parenchymal cells (Figure S3B). These cells showed increased PCNA expression and enhanced STAT3 phosphorylation relative to primary hepatocytes, but did not exhibit an obvious increase in gp130 or β-catenin phosphorylation under standard culture conditions (Figure S3C). Infection of dih cells with Adv-Cre resulted in nearly complete Ikkβ deletion (IkkβΔ) (Figure 3A). IkkβΔ dih cells grew in multi-layers and formed spheroids even under non-confluent conditions, while Ikkβf/f dih cells mainly grew as monolayers (Figure S3D). When plated onto Petri dishes without serum, Ikkβf/f dih cells formed a few floating spheroids (hepatospheres) that could be passaged in culture to yield secondary hepatospheres (Figure 3A). Interestingly, IkkβΔ dih cells formed twice as many primary hepatospheres and 3-fold more secondary hepatospheres than Ikkβf/f dih cells. Transduction of Ikkβf/f dih cells with an IκB super-repressor lentivirus (Boehm et al., 2007) also enhanced hepatosphere formation (Figure 3B), suggesting that IKKβ inhibits hepatosphere formation via NF-κB.
Figure 3. IKKβ deletion in initiated cells enhances proliferation and self-renewal of HCC progenitors.
(A) DEN-induced HCC (dih) cell strains from Ikkβf/f mice were cultured and infected with Adv-GFP or Adv-Cre. Whole cell lysates of infected dih10 cells were immunoblotted for IKKβ (bottom panel). The cells were further cultured without serum on Petri dishes to form hepatospheres (upper panels). Numbers of 10 and 20 hepatospheres formed per 100 plated cells were determined (n = 3; *: p < 0.05). Magnification bar = 100 μm.
(B) Ikkβf/f dih10 cells were infected with an empty retrovirus (vector) or a retrovirus expressing IκBα super-repressor (SR). Stably transfected cells were selected and cultured as above and hepatosphere formation was analyzed (n = 3; *: p < 0.05).
(C, D) IKKβ expressing (Ikkβf/f) and deficient (IkkβΔ) dih10 cells (2.5 × 106 each)were s.c. injected into 8 weeks old C57BL/6 mice. (C) Allograft volume was measured weekly (n = 5; *: p < 0.01). Dissected tumors are shown on the right. (D) BrdU incorporation into tumors was determined (n = 5; *: p < 0.05).
(E) Ikkβf/f and IkkβΔ dih12 cells were labeled with dsRed and 4 × 105 cells were seeded into MUP-uPA mouse livers. Relative amounts of dsRed DNA in transplanted MUP-uPA livers were determined by real-time PCR 3 weeks post-inoculation and normalized to actin DNA as a measurement of cell growth (n = 5; *: p < 0.05).
(F) IkkβΔ dih10 cells were reconstituted with a control vector or WT IKKβ expression vector and were s.c. injected into 8 weeks old C57BL/6 mice. Allograft volume was measured weekly (n = 5; *: p < 0.01).
(G) Ikkβf/f and IkkβΔ dih10 cells were s.c. injected as above. Starting from day 2, mice were treated daily with MLN120B or vehicle by oral gavage. Tumor volume was measured weekly (n = 5; p < 0.01, f/f/control vs. f/f/MLN by one-way ANOVA).
Please also see Figure S3.
In mammary cancer, spheroid-forming cells were suggested to be tumor progenitors (Al-Hajj and Clarke, 2004). To test whether Ikkβ deletion in dih cells enhances tumorigenic potential, we subcutaneously implanted Ikkβf/f and IkkβΔ dih10 cells into C57BL/6 mice. IkkβΔ cells grew faster than Ikkβf/f cells and after 6 weeks formed tumors that were 4-times larger than those formed by Ikkβf/f cells (Figure 3C) and had higher proliferative index (Figure 3D). A similar difference in proliferative potential between Ikkβf/f and IkkβΔ dih cells was seen when the cells were grown in the liver microenvironment: dsRed-labeled IkkβΔdih12 cells formed faster growing HCCs in MUP-uPA livers than Ikkβf/f dih12 cells (Figure 3E). Conversely, retroviral-mediated reconstitution of IKKβ in IkkβΔ dih10 cells suppressed tumorigenic growth (Figure 3F). Thus, the effect of Ikkβ deletion on hepatoma growth is reversible. Enhanced tumorigenic growth of subcutaneously inoculated dih10 cells was also seen upon treatment of tumor-bearing mice with the specific IKKβ inhibitor MLN120B (Wen et al., 2006). MLN120B enhanced tumorigenic growth of Ikkβf/f dih cells and not IkkβDelta; cells and its effect was equivalent to that of Ikkβ deletion (Figure 3G). These data demonstrate that IKKβ is an inhibitor of HCC growth and progression and suggest that its effect is direct and not due to irreversible genetic alterations.
STAT3 activity is elevated in the absence of IKKβ due to ROS-mediated SHP1/2 inactivation
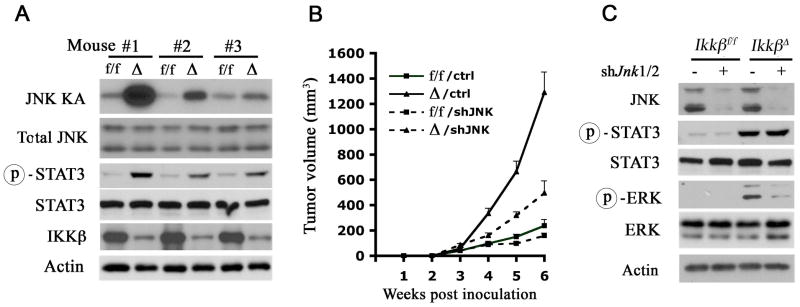
To determine how loss of IKKβ accelerates tumor growth and progression, we examined its effect on signaling pathways that affect hepatocyte proliferation. Subcutaneous tumors formed by IkkβΔ dih cells exhibited a tendency towards higher JNK activity, but the effect was variable (Figure 4A). A more consistent change was increased STAT3 phosphorylation in IkkβΔ dih tumors. Despite the variable effect of IKKβ on JNK activity in HCCs and subcutaneous tumors, silencing of JNK1/2 expression in dih10 cells suppressed their tumorigenic growth (Figure 4B) and the inhibitory effect was greater in IkkβΔ cells. Curiously, silencing of JNK1/2 expression decreased ERK phosphorylation in Ikkβ tumors, but had no effect on STAT3 phosphorylation (Figure 4C).
Figure 4. STAT3 is activated in the absence of IKKβ independently of JNK.
(A) Tumors derived from Ikkβf/f (f/f) and IkkβΔ (Δ) dih10 cells were collected and lysed. Tumor lysates were examined for JNK kinase activity (KA) and STAT3 phosphorylation.
(B, C) Ikkβf/f and Ikkβ Δ dih10 cells were infected with lentiviruses expressing either a control shRNA (control) or shRNAs against mouse Jnk1/2 (shJnk1/2) and implanted s.c. (B) Tumor growth was measured (n = 5; p < 0.05, Δ/control vs. Δ/shJnk1/2 by one-way ANOVA). (C) Tumors were collected and lysed. Lysates were examined for expression and phosphorylation of the indicated proteins.
Please also see Figure S4.
We examined the cause of elevated STAT3 activity in IkkβΔ dih cells. In vitro, both IL-6 and IL-22, which are major STAT3 activators in liver (Naugler et al., 2007; Zenewicz et al., 2007), led to higher STAT3 activity in IkkβΔ dih cells than in Ikkβf/f dih cells (Figure S4A, B). Conversely, expression of constitutively active IKKβ (IKKβEE) in IkkβΔ dih cells inhibited IL-6 induced STAT3 activation (Figure S4C). The IKKβ inhibitor MLN120B also enhanced IL-6 induced STAT3 activation in dih cells and a human liver cancer cell line (Figure S4D). Enhancement of STAT3 activation required a 24–48 hr pre-incubation with MLN120B, suggesting that IKKβ regulates STAT3 indirectly. No obvious differences in IL-6 or IL-22 expression were detected between IKKβ-expressing and non-expressing HCCs (Figure S4E).
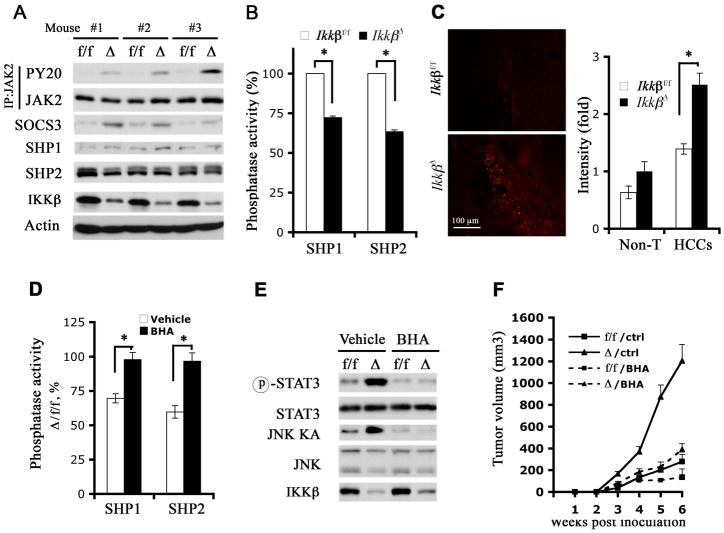
Given these results, we considered that increased STAT3 activity in IkkβΔ dih cells is due to a cell intrinsic effect on the STAT3 signaling pathway. In support of this, phosphorylation of JAK2, a Janus kinase involved in IL-6-mediated STAT3 activation (Kamimura et al., 2003), was enhanced in tumors formed by IkkβΔ dih cells relative to Ikkβf/f dih tumors (Figure 5A), suggesting that elevated STAT3 activation in IkkβΔ dih cells is the consequence of enhanced JAK2 activity. Indeed, inhibition of JAK2 expression by shRNA reduced IL-6 induced STAT3 activation in both Ikkβf/f and IkkβΔ dih cells (Figure S5A). We also examined other regulators of the JAK2-STAT3 pathway. Expression of SOCS3, a critical feedback inhibitor of cytokine signaling and a STAT3 target gene (Auernhammer et al., 1999; Brender et al., 2001), whose ablation enhances HCC development (Ogata et al., 2006), was elevated in IKKβ-deficient tumors (Figure 5A). SOCS3 upregulation is likely to be transcriptional because SOCS3 mRNA was also higher in the absence of IKKβ (Figure S5B). Thus, enhanced JAK2 activation cannot be due to diminished SOCS3 expression as previously found in the hypothalamus (Zhang et al., 2008).
Figure 5. ROS-mediated SHP1/2 inhibition in IKKβ-deficient HCCs correlates with STAT3 activation and accelerated tumor growth.
(A) Tumors derived from Ikkβf/f (f/f) and Ikkβ Δ (Δ) dih10 cells were collected and lysed. Tumor lysates were immunoprecipitated with an anti-JAK2 antibody and examined for JAK2 tyrosine phosphorylation with PY20 antibody. Tumor lysates were also used for immunoblot analyses with the indicated antibodies.
(B) SHP1 and SHP2 were immunoprecipitated from above tumor lysates and their phosphatase activities were measured (n = 4; *: p < 0.05).
(C) Fresh frozen sections of HCCs from transplanted MUP-uPA mice were analyzed for superoxide accumulation by DHE staining. Fluorescence intensity in several fields was quantitated by ImageJ and relative average increases in fluorescent intensity are shown (n = 3; *: p < 0.01). Magnification bar = 100 μm.
(D-F) Ikkβf/f (f/f) and IkkβΔ (Δ) dih10 cells 2.5 × 106 each were s.c. implanted into 8 weeks old C57BL/6 mice. Starting on day 2, the mice were switched to a diet containing vehicle or BHA (0.7%) for 6 weeks.
(D) Tumors were collected from BHA treated and untreated mice, lysed and SHP1/2 phosphatase activities were measured. The data were plotted as SHP1/2 phosphatase activities in IkkβΔ tumors relative to activities in Ikkβf/f tumors (n = 4; *: p < 0.05). (E) Tumor lysates were used to determine JNK kinase activity and STAT3 phosphorylation. (F) Tumor volume was measured weekly (n = 5; p < 0.01,/ctrl vs./BHA by one way ANOVA).
Please also see Figure S5.
Other negative regulators of JAK2-STAT3 signaling include the SH2-containing phosphatases, SHP1 and SHP2 (Valentino and Pierre, 2006). Tumors derived from dih cells expressed higher amounts of SHP2 than SHP1, but these were not significantly affected by IKKβ (Figure 5A). However, both SHP1 and SHP2 phosphatase activities were significantly lower in tumors formed by IkkβΔ dih cells relative to Ikkβf/f dih tumors (Figure 5B). SHP1 and SHP2 activities in IkkβΔ tumors were restored upon reconstitution with IKKβ (Figure S5C). To validate a role for SHP1/2 in regulation of STAT3, we overexpressed SHP2, the more abundant of the two phosphatases in HCC cells, and this resulted in a strong inhibition of IL-6 induced STAT3 phosphorylation in both Ikkβf/f and IkkβΔ dih cells (Figure S5D). These results strongly suggest that reduced SHP1/2 activities in IKKβ deficient HCCs are responsible for the elevated STAT3 activation.
SHP1/2 are members of the protein tyrosine phosphatase (PTP) family (Figure S5E), whose catalytic cysteine is highly susceptible to oxidation (Meng et al., 2002; Salmeen et al., 2003). Several PTPs are subject to reversible inactivation in response to growth factor (Meng et al., 2002) or cytokine induced ROS and this inactivation is potentiated in the absence of NF-κB (Kamata et al., 2005). We therefore examined ROS accumulation in HCCs and in dih cells. Staining with the ROS (superoxide) indicator dihydroethydine (DHE) revealed more ROS accumulation in HCCs relative to surrounding tissue and IKKβ-deficient HCCs stained stronger than IKKβ-expressing HCCs (Figure 5C). Cultured IkkβΔ dih cells also accumulated more ROS both under basal culture conditions and in response to IL-6 or EGF than Ikkβf/f dih cells and this was reversed by expression of constitutively active IKKβEE (Figure S5F,G). To examine the role of ROS in reduced SHP1/2 activities and STAT3 upregulation in IKKβ-deficient HCCs, we fed tumor-bearing mice with BHA, a potent anti-oxidant (Maeda et al., 2005). BHA feeding completely restored SHP1/2 phosphatase activities and reduced JNK activity and STAT3 phosphorylation in tumors formed by IkkβΔ dih cells to levels comparable to those in Ikkβf/f tumors (Figure 5D, E). Importantly, BHA consumption reduced IkkβΔ tumor growth to a level that was similar to that of Ikkβf/f tumors in untreated mice (Figure 5F). Collectively, these data suggest that ROS accumulation in IKKβ-deficient HCCs is responsible for reduced PTP activity, JNK and STAT3 activation, as well as accelerated tumor growth.
STAT3 activity is required for HCC formation and growth
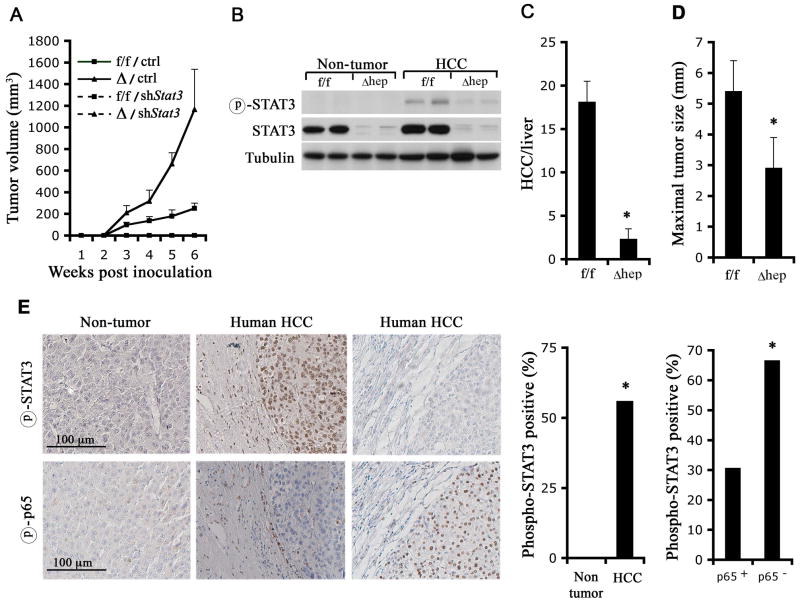
To examine the contribution of activated STAT3 to the enhanced tumorigenic potential of Ikkβ Δ dih cells, we treated tumor-bearing mice with AG490, an inhibitor of STAT3 phosphorylation (Eriksen et al., 2001). AG490 inhibited the growth of IkkβΔ subcutaneous tumors and had a more modest effect on Ikkβf/f tumorigenic growth (Figure S6A). Immunoblot analysis verified that AG490 inhibited STAT3 phosphorylation regardless of IKKβ status (Figure S6B). A similar effect on tumor growth was observed with another STAT3 inhibitor, S3I-201 (Figure S6C), which inhibits STAT3 activation through binding to its SH2 domain (Siddiquee et al., 2007). S3I-201 also inhibited STAT3 phosphorylation (Figure S6D). To more specifically address the role of STAT3 we silenced its expression in dih cells via lentiviral expression of a STAT3-specific shRNA (Figure S6E). In sharp contrast to cells transduced with a control lentivirus encoding scrambled shRNA which formed subcutaneous tumors, dih cells transduced with the STAT3 shRNA failed to grow into subcutaneous tumors regardless of their IKKβ status (Figure 6A).
Figure 6. STAT3 is required for HCC formation and growth.
(A) Ikkβf/f (f/f) and IkkβΔ (Δ) dih10 cells were infected with lentiviruses expressing either scrambled shRNA (ctrl) or an shRNA against mouse Stat3 (shStat3). Stably transfected cells were selected and 2.5x106 cells were s.c. implanted. Tumor growth was measured (n = 5; p < 0.01, f/f/ctrl vs. f/f/shSTAT3; p < 0.01, Δ/ctrl vs. Δ/shSTAT3 by one-way ANOVA).
(B–D) Stat3f/f and Stat3Δhep male mice were injected with 25 mg/kg DEN when 15 days old. Mice were sacrificed 8 months later and HCC induction was evaluated. (B) HCCs and surrounding non-tumor tissues were collected, lysed and STAT3 expression and phosphorylation were examined. (C) Tumor multiplicity (n = 10; *: p < 0.01) and (D) maximal tumor sizes (n = 10; *: p <0.05) were determined.
(E) STAT3 and NF-κB activation in human HCC. Upper panels: representative samples of non-tumor liver tissue and liver tissue containing HCC were stained with a phospho-STAT3 antibody. Lower panels: adjacent parallel sections of the same samples shown in the upper panels were stained with a phospho-p65 antibody. The bar graphs present the frequency of phospho-STAT3 positive HCC specimens amongst all HCCs or amongst p65-positive and p65-negative HCCs (* p<0.05 by chi-square analysis).
To further examine the role of STAT3 in HCC development, we administered DEN to 2 weeks old hepatocyte-specific STAT3-deficient mice (Stat3f/f/Alb-Cre mice = Stat3Δhep) (Figure 6B). Stat3Δhep mice were markedly resistant to DEN-induced HCC development with more than 6-fold reduction in HCC multiplicity relative to Stat3f/f mice (Figure 6C). Tumors in Stat3Δhep mice, which retained their STAT3 deficiency (Figure 6B), were also significantly smaller than HCCs in Stat3f/f mice (Figure 6D). We derived Stat3f/f dih cells from DEN-induced HCCs of Stat3f/f mice, but deletion of STAT3 from these cells by Ad-Cre infection resulted in inhibition of cell growth and induction of cell death (Figure S6F,G). These data strongly suggest that STAT3 is required for mouse HCC development, growth and survival.
We also examined the status of STAT3 activation in more than 50 different human HCC specimens and found that nearly 60% of them exhibited activated nuclear STAT3, which could not be detected in non-tumor liver tissue from the same patient (Figure 6E). As observed previously (Calvisi et al., 2006), STAT3 activation was more pronounced in the more aggressive tumors (Table S1). We also found that 25% (13/52) of human HCCs were positive for phospho-p65/RelA, an indicator of NF-κB activation. However, phospho-p65 positive HCCs were often negative for activated STAT3. Only 30.8% of phospho-p65 positive HCCs were positive for activated STAT3, whereas 66.7% of phospho-p65 negative HCCs exhibited activated STAT3 (Figure 6E). These data show that a major sub-fraction of human HCCs exhibit an inverse relationship between NF-κB and STAT3.
DISCUSSION
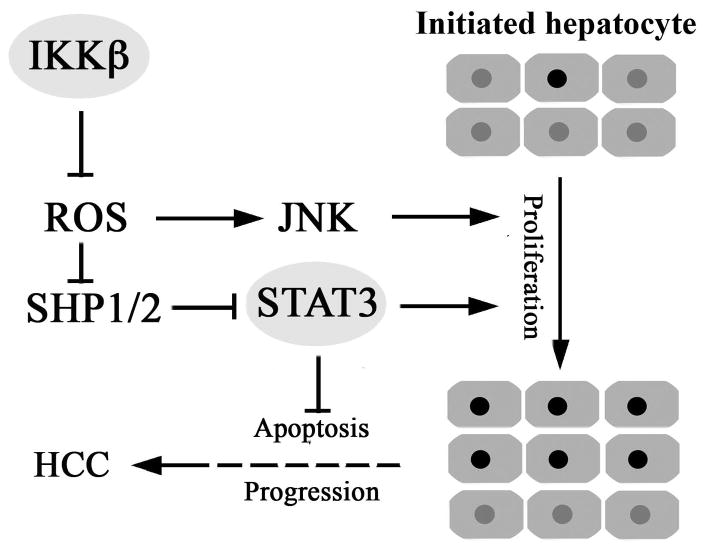
We have developed an experimental system based on transplantation of DEN-initiated hepatocytes into MUP-uPA mouse liver that allows one to examine factors and mechanisms that affect the progression of initiated, pre-neoplastic, hepatocytes into full-blown HCC. Using this system, we found that loss of IKKβ in initiated hepatocytes greatly enhances HCC development even when IKKβ is deleted or inhibited many months after tumor initiation. Previously, IKKβ and its regulatory subunit NEMO/IKKγ were found to negatively control HCC development in models that depend on compensatory proliferation triggered by chemically-induced or spontaneous hepatocyte death and liver injury (Luedde et al., 2007; Maeda et al., 2005). This inhibitory effect on HCC development was proposed to be exerted during early tumor promotion and be due to loss of NF-κB pro-survival activity, which is more severe in Nemo/IkkγΔhep than in IkkβΔhep mice (Karin, 2006). The current results, however, show that IKKβ-driven NF-κB also inhibits late tumor promotion and progression of initiated hepatoma cells through effects on ROS metabolism that exert a negative control over the STAT3 signaling pathway (Figure 7). STAT3 itself is frequently activated in human HCCs, especially in aggressive tumors with poor prognosis (Calvisi et al., 2006; Table S1) and we now show that STAT3 activation is subject to negative regulation by NF-κB and is essential for HCC induction. The inverse relationship between NF-κB and STAT3 also applies to a major sub-fraction of human HCCs.
Figure 7. A central role for IKKβ and ROS-controlled STAT3 signaling in HCC development.
Inactivation of IKKβ or other anti-oxidant defenses in hepatocytes favors ROS accumulation and leads to oxidative inhibition of PTPs, including SHP1 and SHP2. This results in activation of JNK and STAT3 which stimulate the proliferation of initiated pre-neoplastic hepatocytes. This contributes both to early tumor promotion and HCC progression. In addition, STAT3 activation suppresses apoptosis in progressing HCCs.
The ability to temporally and physically separate tumor initiation, which in our case occurs upon DEN-induced mutagenesis, from late tumor promotion and malignant progression, has been instrumental to the success of this study. Interestingly, transplantation of DEN-initiated hepatocytes from either a male or female donor to normal C57BL/6 recipients, as opposed to MUP-uPA mice, has never given rise to detectable HCCs. These findings underscore the importance of the microenvironment and circulatory system in tumor progression and malignant conversion. Although the exact factors responsible for the permissive nature of the MUP-uPA liver microenvironment remain to be determined, it should be noted that MUP-uPA mice experience chronic low grade liver injury accompanied by a small, but significant, elevation in IL-6 expression and ROS accumulation and eventually develop low grade liver fibrosis. These are the same kind of changes that accompany the development of human HCC.
Using the transplant system, as well as a culture system that allowed the derivation of the dih cell strains described above, we found that deletion or inhibition of IKKβ in initiated hepatocytes or HCC-derived cells increases their proliferative and tumorigenic potential. The effect is due to loss of NF-κB activity, because specific NF-κB inhibition through expression of IκB super-repressor results in a similar effect. Similar findings were made in squamous cell carcinoma (SCC), where NF-κB was shown to inhibit keratinocyte proliferation and Ras-induced tumorigenesis through negative regulation of JNK activity, whose exact mechanism was not identified (Dajee et al., 2003; Zhang et al., 2004). We now show that another way through which NF-κB inhibits proliferation and tumorigenesis is negative regulation of STAT3 activation. As shown previously for JNK in TNF-α-treated NF-κB-deficient cells (Kamata et al., 2005), enhanced STAT3 activation in IkkβΔ cells or tumors is due to oxidative inhibition of PTPs, whose catalytic cysteine is extremely susceptible to oxidation (Meng et al., 2002; Salmeen et al., 2003). While previous work has shown that PTPs are oxidatively inactivated under rather harsh conditions which favor ROS accumulation, such as TNF-α-induced cell death (Kamata et al., 2005), the present work shows that significant PTP inhibition and subsequent kinase activation occur under relatively normal conditions, as long as NF-κB-dependent anti-oxidant defenses (Kamata et al., 2005; Pham et al., 2004) are dismantled. The anti-oxidant function of NF-κB, which is exerted in part through expression of ferritin heavy chain and superoxide dismutase 2 (Kamata et al., 2005; Pham et al., 2004), is particularly important in the liver, an organ that is heavily engaged in oxidative metabolism. Indeed, the deletion of hepatocyte NEMO/IKKγ results in spontaneous liver damage, hepatosteatosis, fibrosis and HCC formation, all of which can be prevented by administration of an anti-oxidant (Luedde et al., 2007).
While our work demonstrates an essential and critical role for STAT3 in HCC development and progression, STAT3 has been known to be critically involved in several other malignancies, including SCC (Chan et al., 2004) and CAC (Bollrath et al., 2009; Grivennikov et al., 2009) and JAK2 or STAT3 inhibitors were found to inhibit the growth of several human cancers (Hedvat et al., 2009). Notably, we detected phosphorylated (i.e. activated) STAT3 in approximately 60% of human HCCs, with STAT3-positive tumors being more aggressive. These findings are consistent with those of other studies in which STAT3 was found to be activated in the majority of HCCs with poor prognosis and not in surrounding non-tumor tissue or normal liver (Calvisi et al., 2006; Lin et al., 2009). Expression of the STAT3 activating cytokine IL-6 is elevated in both liver cirrhosis and HCC (Tilg et al., 1992; Trikha et al., 2003) and was recently found to correlate with rapid progression from viral hepatitis to HCC (Nakagawa et al., 2009; Wong et al., 2009). In addition, activating mutations in the gene encoding the gp130 signaling subunit for IL-6 and other cytokine receptors were found to account for benign hepatic adenomas (Rebouissou et al., 2009). When combined with a β catenin activating mutation, these mutations which result in STAT3 activation lead to HCC development. Our findings suggest that NF-κB may also be engaged in negative regulation of STAT3 in a sub-fraction of human HCCs as the frequency of STAT3-positive HCCs is two-fold lower in NF-κB-positive HCCs than in NF-κB-negative HCCs. Altogether, there is little doubt that STAT3 is a key regulator of liver tumorigenesis in mice and men. Our results suggest that in addition to its role in early tumor development, where it suppresses apoptosis and enhances proliferation of pre-neoplastic cells (Bromberg and Wang, 2009), STAT3 plays a critical role during tumor progression when it is activated many months after the HCC initiating event caused by DEN exposure. We identified the IKK-NF-κB axis as a negative regulator of STAT3 activation, but it needs to be determined how NF-κB activity is downregulated during human hepatocarcinogenesis and evaluate its relative contribution to STAT3 activation vis a vis the effects of cytokines and growth factors. We also show that STAT3 can be activated in response to ROS accumulation and it should be noted that several of the most prominent HCC risk factors, including HCV and hepatosteatosis, result in oxidative stress (El-Serag and Rudolph, 2007; Parekh and Anania, 2007; Wang and Weinman, 2006).
The present work as well as previous work with IkkβΔhep and Nemo/IkkγΔhep mice (Luedde et al., 2007; Maeda et al., 2005; Sakurai et al., 2006) demonstrate an anti-tumorigenic role for NF-κB in hepatocytes. However, it was also described that NF-κB activation promotes hepatocarcinogenesis in Mdr2−/− mice, which develop cholestatic hepatitis and eventually liver cancer (Pikarsky et al., 2004), and in transgenic mice that overexpress lymphotoxin (LT)αβ in their liver (Haybaeck et al., 2009). Notably in both Mdr2−/− and LTαβ -transgenic mice, HCC development depends on chronic low grade inflammation and no liver injury has been observed either prior to or subsequent to NF-κB inhibition (Haybaeck et al., 2009; Pikarsky et al., 2004). Most likely in these models, in contrast to the injury-driven IkkβΔhep+DEN and Nemo/IkkγΔhep models, the main function of NF-κB in hepatocytes is to upregulate the expression of chemokines needed for recruitment of inflammatory cells that contribute to the microenvironment in which these tumors develop. In the Nemo/IkkγΔhep model, however, the absence of hepatocyte NF-κB results in ROS accumulation and its sequela: chronic liver damage, hepatosteatosis, fibrosis and eventual HCC development, whereas in the transplantation model described above IKKβ-deficient and -proficient initiated HCC cells are placed into a permissive microenvironment created by chronic proteolytic damage to the liver. In human hepatocarcinogenesis, which is mainly caused and promoted by chronic HCV or HBV infections or by hepatosteatosis, both inflammatory and injury/repair responses are likely to be involved (Berasain et al., 2009). In addition, some viruses inhibit NF-κB activation to facilitate cell killing and avoid immune detection (Roulston et al., 1999). Therefore, all of the above model systems bear some relevance to human HCC development and given the complex effects of IKK/NF-κB inhibition, a more attractive and likely candidate for therapeutic targeting is the STAT3 signaling pathway.
EXPERIMENTAL PROCEDURES
Mice and HCC induction
Ikkβf/f mice (Park et al., 2002) were backcrossed into the C57BL/6 background for at least 6 generations. Ikkβf/f/Mx1-Cre mice were described (Maeda et al., 2005). MUP-uPA transgenic mice were kindly provided by Eric P. Sandgren, University of Wisconsin-Madison, Madison, Wisconsin (Weglarz et al., 2000). Stat3Δhep mice were from David E. Levy at New York University (Lee et al., 2002). To induce HCC, 15 days old littermates were injected with 25 mg/kg DEN (Sigma, St Louis, Mo). DEN-injected mice were sacrificed either 3 months after DEN injection to be used as hepatocyte donors or maintained for 8 months to monitor HCC development. All mouse experimental protocols were approved by the UCSD Animal Care Program, following National Institutes of Health guidelines. Histology, gene expression and cell signaling were analyzed as described (Maeda et al., 2005; Sakurai et al., 2008).
Isolation and transplantation of primary hepatocytes
Primary hepatocytes were isolated from DEN-treated mice as described (Leffert et al., 1979). For transplantation, cell preparations whose viability was greater than 80% were used. Three weeks old MUP-uPA transgenic mice received 1.2 × 105 viable hepatocytes in 30 μl PBS via intra-splenic injection with a 30 G needle (Weglarz et al., 2000). Transplanted mice were sacrificed 5 months later to monitor HCC development. To delete Ikkβ in transplanted hepatocytes, mice were given 1 × 109 pfu of Adv-GFP or Adv-Cre via the tail vein one month post-transplantation. Alternatively, Ikkβf/f/Mx1-Cre hepatocytes were transplanted as above and the recipients given 3 injections (250 μg each) of poly(IC) every other day one month after hepatocyte transplantation.
Human HCC specimens and their analysis
Human HCC specimens were from Department of Internal Medicine, Division of Gastroenterology/Hepatology Medical University, Vienna. HCC samples were obtained during liver transplantation from a total of 52 patients (49 males, 3 females) who had no prior therapy before surgery (Sieghart et al., 2006). None of the patients was diagnosed with regional lymph node metastasis and only one patient had distal metastasis at the time of liver transplantation. Sections prepared from paraffin-embedded blocks were stained with either phospho-STAT3 antibody (Cell Signaling) or phospho-p65 antibody (Cell Signaling) at a dilution of 1:50. Conditions for use of all antibodies have been posted to http://biorating.com. Positive nuclear staining was scored. (***) = large area staining; (**) = staining of multiple smaller areas; (*) = staining of scattered few positive cells. Immunohistochemical staining of HCC specimens as well as retrospective clinical data collection and analysis were approved by the local ethics committee of the Medical University of Vienna, Austria.
Statistical analysis
Data are presented as means ± s.d.. Differences in means were analyzed by Student’s t test and one-way ANOVA. Tumor incidence (%) was analyzed by chi-square analysis. p values < 0.05 were considered significant.
Supplementary Material
Acknowledgments
We thank David Levy for providing Stat3Δhep mice. G.H. was supported by a postdoctoral fellowship from Damon Runyon Cancer Research Foundation and G.Y.Y was supported by American Diabetes Association Postdoctoral research grant to M.K.. V.T., H.O., and C.K. were supported by Arthritis Foundation Research Fellowship, Kanzawa Medical Research Foundation and American Institute for Cancer Research Fellowship, respectively. Research was supported by grants from the National Institute of Health (ES006376, CA113602 and CA118165) and the Superfund Basic Research Program (P42ES010337). M.K. is an American Cancer Society Research Professor.
Footnotes
Additional experimental procedures are available in Supplementary Information.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Hajj M, Clarke MF. Self-renewal and solid tumor stem cells. Oncogene. 2004;23:7274–7282. doi: 10.1038/sj.onc.1207947. [DOI] [PubMed] [Google Scholar]
- Albini A, Sporn MB. The tumour microenvironment as a target for chemoprevention. Nat Rev Cancer. 2007;7:139–147. doi: 10.1038/nrc2067. [DOI] [PubMed] [Google Scholar]
- Auernhammer CJ, Bousquet C, Melmed S. Autoregulation of pituitary corticotroph SOCS-3 expression: characterization of the murine SOCS-3 promoter. Proc Natl Acad Sci U S A. 1999;96:6964–6969. doi: 10.1073/pnas.96.12.6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berasain C, Castillo J, Perugorria MJ, Latasa MU, Prieto J, Avila MA. Inflammation and liver cancer. Ann N Y Acad Sci. 2009;1155:206–221. doi: 10.1111/j.1749-6632.2009.03704.x. [DOI] [PubMed] [Google Scholar]
- Boehm JS, Zhao JJ, Yao J, Kim SY, Firestein R, Dunn IF, Sjostrom SK, Garraway LA, Weremowicz S, Richardson AL, et al. Integrative genomic approaches identify IKBKE as a breast cancer oncogene. Cell. 2007;129:1065–1079. doi: 10.1016/j.cell.2007.03.052. [DOI] [PubMed] [Google Scholar]
- Bollrath J, Phesse TJ, von Burstin VA, Putoczki T, Bennecke M, Bateman T, Nebelsiek T, Lundgren-May T, Canli O, Schwitalla S, et al. gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell. 2009;15:91–102. doi: 10.1016/j.ccr.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Bosch FX, Ribes J, Diaz M, Cleries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127:S5–S16. doi: 10.1053/j.gastro.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Boucher KM, Yakovlev AY. Estimating the probability of initiated cell death before tumor induction. Proc Natl Acad Sci U S A. 1997;94:12776–12779. doi: 10.1073/pnas.94.24.12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brender C, Nielsen M, Kaltoft K, Mikkelsen G, Zhang Q, Wasik M, Billestrup N, Odum N. STAT3-mediated constitutive expression of SOCS-3 in cutaneous T-cell lymphoma. Blood. 2001;97:1056–1062. doi: 10.1182/blood.v97.4.1056. [DOI] [PubMed] [Google Scholar]
- Bromberg J, Wang TC. Inflammation and cancer: IL-6 and STAT3 complete the link. Cancer Cell. 2009;15:79–80. doi: 10.1016/j.ccr.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvisi DF, Ladu S, Gorden A, Farina M, Conner EA, Lee JS, Factor VM, Thorgeirsson SS. Ubiquitous activation of Ras and Jak/Stat pathways in human HCC. Gastroenterology. 2006;130:1117–1128. doi: 10.1053/j.gastro.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Calvisi DF, Thorgeirsson SS. Molecular mechanisms of hepatocarcinogenesis in transgenic mouse models of liver cancer. Toxicol Pathol. 2005;33:181–184. doi: 10.1080/01926230590522095. [DOI] [PubMed] [Google Scholar]
- Chan KS, Sano S, Kiguchi K, Anders J, Komazawa N, Takeda J, DiGiovanni J. Disruption of Stat3 reveals a critical role in both the initiation and the promotion stages of epithelial carcinogenesis. J Clin Invest. 2004;114:720–728. doi: 10.1172/JCI21032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Huang GT, Yang PM, Chen PJ, Lai MY, Chen DS, Wang JD, Sheu JC. Hepatitis B- and C-related hepatocellular carcinomas yield different clinical features and prognosis. Eur J Cancer. 2006;42:2524–2529. doi: 10.1016/j.ejca.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Cioffi M, Esposito K, Vietri MT, Gazzerro P, D’Auria A, Ardovino I, Puca GA, Molinari AM. Cytokine pattern in postmenopause. Maturitas. 2002;41:187–192. doi: 10.1016/s0378-5122(01)00286-9. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dajee M, Lazarov M, Zhang JY, Cai T, Green CL, Russell AJ, Marinkovich MP, Tao S, Lin Q, Kubo Y, Khavari PA. NF-kappaB blockade and oncogenic Ras trigger invasive human epidermal neoplasia. Nature. 2003;421:639–643. doi: 10.1038/nature01283. [DOI] [PubMed] [Google Scholar]
- El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- Eriksen KW, Kaltoft K, Mikkelsen G, Nielsen M, Zhang Q, Geisler C, Nissen MH, Ropke C, Wasik MA, Odum N. Constitutive STAT3-activation in Sezary syndrome: tyrphostin AG490 inhibits STAT3-activation, interleukin-2 receptor expression and growth of leukemic Sezary cells. Leukemia. 2001;15:787–793. doi: 10.1038/sj.leu.2402093. [DOI] [PubMed] [Google Scholar]
- Fausto N. Mouse liver tumorigenesis: models, mechanisms, and relevance to human disease. Semin Liver Dis. 1999;19:243–252. doi: 10.1055/s-2007-1007114. [DOI] [PubMed] [Google Scholar]
- Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L, Karin M. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haybaeck J, Zeller N, Wolf MJ, Weber A, Wagner U, Kurrer MO, Bremer J, Iezzi G, Graf R, Clavien PA, et al. A lymphotoxin-driven pathway to hepatocellular carcinoma. Cancer Cell. 2009;16:295–308. doi: 10.1016/j.ccr.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedvat M, Huszar D, Herrmann A, Gozgit JM, Schroeder A, Sheehy A, Buettner R, Proia D, Kowolik CM, Xin H, et al. The JAK2 inhibitor AZD1480 potently blocks Stat3 signaling and oncogenesis in solid tumors. Cancer Cell. 2009;16:487–497. doi: 10.1016/j.ccr.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennings H, Glick AB, Greenhalgh DA, Morgan DL, Strickland JE, Tennenbaum T, Yuspa SH. Critical aspects of initiation, promotion, and progression in multistage epidermal carcinogenesis. Proc Soc Exp Biol Med. 1993;202:1–8. doi: 10.3181/00379727-202-43511a. [DOI] [PubMed] [Google Scholar]
- Hytiroglou P, Park YN, Krinsky G, Theise ND. Hepatic precancerous lesions and small hepatocellular carcinoma. Gastroenterol Clin North Am. 2007;36:867–887. vii. doi: 10.1016/j.gtc.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- Kamimura D, Ishihara K, Hirano T. IL-6 signal transduction and its physiological roles: the signal orchestration model. Rev Physiol Biochem Pharmacol. 2003;149:1–38. doi: 10.1007/s10254-003-0012-2. [DOI] [PubMed] [Google Scholar]
- Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- Kaufmann WK, Ririe DG, Kaufman DG. Phenobarbital-dependent proliferation of putative initiated rat hepatocytes. Carcinogenesis. 1988;9:779–782. doi: 10.1093/carcin/9.5.779. [DOI] [PubMed] [Google Scholar]
- Koch KS, Maeda S, He G, Karin M, Leffert HL. Targeted deletion of hepatocyte Ikkbeta confers growth advantages. Biochem Biophys Res Commun. 2009;380:349–354. doi: 10.1016/j.bbrc.2009.01.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn R, Schwenk F, Aguet M, Rajewsky K. Inducible gene targeting in mice. Science. 1995;269:1427–1429. doi: 10.1126/science.7660125. [DOI] [PubMed] [Google Scholar]
- Lee CK, Raz R, Gimeno R, Gertner R, Wistinghausen B, Takeshita K, DePinho RA, Levy DE. STAT3 is a negative regulator of granulopoiesis but is not required for G-CSF-dependent differentiation. Immunity. 2002;17:63–72. doi: 10.1016/s1074-7613(02)00336-9. [DOI] [PubMed] [Google Scholar]
- Leffert HL, Koch KS, Moran T, Williams M. Liver cells. Methods Enzymol. 1979;58:536–544. doi: 10.1016/s0076-6879(79)58168-3. [DOI] [PubMed] [Google Scholar]
- Lin L, Amin R, Gallicano GI, Glasgow E, Jogunoori W, Jessup JM, Zasloff M, Marshall JL, Shetty K, Johnson L, et al. The STAT3 inhibitor NSC 74859 is effective in hepatocellular cancers with disrupted TGF-beta signaling. Oncogene. 2009;28:961–972. doi: 10.1038/onc.2008.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luedde T, Beraza N, Kotsikoris V, van Loo G, Nenci A, De Vos R, Roskams T, Trautwein C, Pasparakis M. Deletion of NEMO/IKKgamma in liver parenchymal cells causes steatohepatitis and hepatocellular carcinoma. Cancer Cell. 2007;11:119–132. doi: 10.1016/j.ccr.2006.12.016. [DOI] [PubMed] [Google Scholar]
- Maeda S, Kamata H, Luo JL, Leffert H, Karin M. IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005;121:977–990. doi: 10.1016/j.cell.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Meng TC, Fukada T, Tonks NK. Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol Cell. 2002;9:387–399. doi: 10.1016/s1097-2765(02)00445-8. [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Maeda S, Yoshida H, Tateishi R, Masuzaki R, Ohki T, Hayakawa Y, Kinoshita H, Yamakado M, Kato N, et al. Serum IL-6 levels and the risk for hepatocarcinogenesis in chronic hepatitis C patients; An analysis based on gender differences. Int J Cancer. 2009;125:2264–2269. doi: 10.1002/ijc.24720. [DOI] [PubMed] [Google Scholar]
- Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, Karin M. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–124. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- Nowell PC. The clonal evolution of tumor cell populations. Science. 1976;194:23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- Ogata H, Kobayashi T, Chinen T, Takaki H, Sanada T, Minoda Y, Koga K, Takaesu G, Maehara Y, Iida M, Yoshimura A. Deletion of the SOCS3 gene in liver parenchymal cells promotes hepatitis-induced hepatocarcinogenesis. Gastroenterology. 2006;131:179–193. doi: 10.1053/j.gastro.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Parekh S, Anania FA. Abnormal lipid and glucose metabolism in obesity: implications for nonalcoholic fatty liver disease. Gastroenterology. 2007;132:2191–2207. doi: 10.1053/j.gastro.2007.03.055. [DOI] [PubMed] [Google Scholar]
- Park JM, Greten FR, Li ZW, Karin M. Macrophage apoptosis by anthrax lethal factor through p38 MAP kinase inhibition. Science. 2002;297:2048–2051. doi: 10.1126/science.1073163. [DOI] [PubMed] [Google Scholar]
- Parkin DM, Bray FI, Devesa SS. Cancer burden in the year 2000. The global picture. Eur J Cancer . 2001;37(Suppl 8):S4–66. doi: 10.1016/s0959-8049(01)00267-2. [DOI] [PubMed] [Google Scholar]
- Pham CG, Bubici C, Zazzeroni F, Papa S, Jones J, Alvarez K, Jayawardena S, De Smaele E, Cong R, Beaumont C, et al. Ferritin heavy chain upregulation by NF-kappaB inhibits TNFalpha-induced apoptosis by suppressing reactive oxygen species. Cell. 2004;119:529–542. doi: 10.1016/j.cell.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E, Ben-Neriah Y. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–466. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- Pitot HC. Altered hepatic foci: their role in murine hepatocarcinogenesis. Annu Rev Pharmacol Toxicol. 1990;30:465–500. doi: 10.1146/annurev.pa.30.040190.002341. [DOI] [PubMed] [Google Scholar]
- Rebouissou S, Amessou M, Couchy G, Poussin K, Imbeaud S, Pilati C, Izard T, Balabaud C, Bioulac-Sage P, Zucman-Rossi J. Frequent in-frame somatic deletions activate gp130 in inflammatory hepatocellular tumours. Nature. 2009;457:200–204. doi: 10.1038/nature07475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roulston A, Marcellus RC, Branton PE. Viruses and apoptosis. Annu Rev Microbiol. 1999;53:577–628. doi: 10.1146/annurev.micro.53.1.577. [DOI] [PubMed] [Google Scholar]
- Sakurai T, He G, Matsuzawa A, Yu GY, Maeda S, Hardiman G, Karin M. Hepatocyte necrosis induced by oxidative stress and IL-1 alpha release mediate carcinogen-induced compensatory proliferation and liver tumorigenesis. Cancer Cell. 2008;14:156–165. doi: 10.1016/j.ccr.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T, Maeda S, Chang L, Karin M. Loss of hepatic NF-kappa B activity enhances chemical hepatocarcinogenesis through sustained c-Jun N-terminal kinase 1 activation. Proc Natl Acad Sci U S A. 2006;103:10544–10551. doi: 10.1073/pnas.0603499103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmeen A, Andersen JN, Myers MP, Meng TC, Hinks JA, Tonks NK, Barford D. Redox regulation of protein tyrosine phosphatase 1B involves a sulphenyl-amide intermediate. Nature. 2003;423:769–773. doi: 10.1038/nature01680. [DOI] [PubMed] [Google Scholar]
- Seki S, Sakaguchi H, Kitada T, Tamori A, Takeda T, Kawada N, Habu D, Nakatani K, Nishiguchi S, Shiomi S. Outcomes of dysplastic nodules in human cirrhotic liver: a clinicopathological study. Clin Cancer Res. 2000;6:3469–3473. [PubMed] [Google Scholar]
- Sell S, Leffert HL. Liver cancer stem cells. J Clin Oncol. 2008;26:2800–2805. doi: 10.1200/JCO.2007.15.5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiquee K, Zhang S, Guida WC, Blaskovich MA, Greedy B, Lawrence HR, Yip ML, Jove R, McLaughlin MM, Lawrence NJ, et al. Selective chemical probe inhibitor of Stat3, identified through structure-based virtual screening, induces antitumor activity. Proc Natl Acad Sci U S A. 2007;104:7391–7396. doi: 10.1073/pnas.0609757104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieghart W, Losert D, Strommer S, Cejka D, Schmid K, Rasoul-Rockenschaub S, Bodingbauer M, Crevenna R, Monia BP, Peck-Radosavljevic M, Wacheck V. Mcl-1 overexpression in hepatocellular carcinoma: a potential target for antisense therapy. J Hepatol. 2006;44:151–157. doi: 10.1016/j.jhep.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Takayama T, Makuuchi M, Hirohashi S, Sakamoto M, Okazaki N, Takayasu K, Kosuge T, Motoo Y, Yamazaki S, Hasegawa H. Malignant transformation of adenomatous hyperplasia to hepatocellular carcinoma. Lancet. 1990;336:1150–1153. doi: 10.1016/0140-6736(90)92768-d. [DOI] [PubMed] [Google Scholar]
- Tilg H, Wilmer A, Vogel W, Herold M, Nolchen B, Judmaier G, Huber C. Serum levels of cytokines in chronic liver diseases. Gastroenterology. 1992;103:264–274. doi: 10.1016/0016-5085(92)91122-k. [DOI] [PubMed] [Google Scholar]
- Tong MJ, el-Farra NS, Reikes AR, Co RL. Clinical outcomes after transfusion-associated hepatitis C. N Engl J Med. 1995;332:1463–1466. doi: 10.1056/NEJM199506013322202. [DOI] [PubMed] [Google Scholar]
- Trikha M, Corringham R, Klein B, Rossi JF. Targeted anti-interleukin-6 monoclonal antibody therapy for cancer: a review of the rationale and clinical evidence. Clin Cancer Res. 2003;9:4653–4665. [PMC free article] [PubMed] [Google Scholar]
- Valentino L, Pierre J. JAK/STAT signal transduction: regulators and implication in hematological malignancies. Biochem Pharmacol. 2006;71:713–721. doi: 10.1016/j.bcp.2005.12.017. [DOI] [PubMed] [Google Scholar]
- Wang T, Weinman SA. Causes and consequences of mitochondrial reactive oxygen species generation in hepatitis C. J Gastroenterol Hepatol . 2006;21(Suppl 3):S34–37. doi: 10.1111/j.1440-1746.2006.04591.x. [DOI] [PubMed] [Google Scholar]
- Wanless IR. Terminology of nodular hepatocellular lesions. International Working Party. Hepatology. 1995;22:983–993. doi: 10.1016/0270-9139(95)90324-0. [DOI] [PubMed] [Google Scholar]
- Weglarz TC, Degen JL, Sandgren EP. Hepatocyte transplantation into diseased mouse liver. Kinetics of parenchymal repopulation and identification of the proliferative capacity of tetraploid and octaploid hepatocytes. Am J Pathol. 2000;157:1963–1974. doi: 10.1016/S0002-9440(10)64835-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen D, Nong Y, Morgan JG, Gangurde P, Bielecki A, Dasilva J, Keaveney M, Cheng H, Fraser C, Schopf L, et al. A selective small molecule IkappaB Kinase beta inhibitor blocks nuclear factor kappaB-mediated inflammatory responses in human fibroblast-like synoviocytes, chondrocytes, and mast cells. J Pharmacol Exp Ther. 2006;317:989–1001. doi: 10.1124/jpet.105.097584. [DOI] [PubMed] [Google Scholar]
- Wong VW, Yu J, Cheng AS, Wong GL, Chan HY, Chu ES, Ng EK, Chan FK, Sung JJ, Chan HL. High serum interleukin-6 level predicts future hepatocellular carcinoma development in patients with chronic hepatitis B. Int J Cancer. 2009;124:2766–2770. doi: 10.1002/ijc.24281. [DOI] [PubMed] [Google Scholar]
- Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Karow M, Flavell RA. Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity. 2007;27:647–659. doi: 10.1016/j.immuni.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JY, Green CL, Tao S, Khavari PA. NF-kappaB RelA opposes epidermal proliferation driven by TNFR1 and JNK. Genes Dev. 2004;18:17–22. doi: 10.1101/gad.1160904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135:61–73. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.