Abstract
Objective
To identify obstetrical factors associated with development of levator ani injury after vaginal birth.
Methods
Magnetic resonance images were taken of the pelvic floor of 160 women 9 to 12 months postpartum after first term vaginal delivery. Half the women had de novo stress incontinence and half were continent controls. Abnormalities of the pubovisceral portion were identified on magnetic resonance as present or absent. Defect severity was further scored in each muscle from 0 (no defect) to 3 (complete muscle loss). A summed score for the two sides (0 to 6) was assigned and grouped as minor (0 – 3) or major (4 – 6). Obstetric details were collected. The association between obstetric variables and muscle injury were analyzed using Fisher exact test and t-tests.
Results
The following increased odds ratios for levator defect were found: forceps use 14.7 (95% confidence interval [CI] 4.9–44.3), anal sphincter rupture 8.1 (95% CI 3.3–19.5) and episiotomy 3.1 (95% CI 1.4–7.2) but not vacuum delivery 0.9 (95% CI 0.19–4.3), epidural use 0.9 (95% CI 0.4–2.0), or oxytocin use 0.8 (95% CI 0.3–1.8). Women with levator injury were 3.5 years older and had a 78-minute longer second stage of labor. Differences in gestational age, birth weight, and head circumference were not statistically significant. A major defect in the pubovisceral muscle was seen in 22 women and a minor defect in 7 women.
Conclusion
Injuries to the levator ani muscles in women after their first vaginal delivery are associated with several obstetric factors indicating difficult vaginal birth and with older age.
INTRODUCTION
Vaginal birth increases the chance that a woman will develop pelvic organ prolapse by 4 to 11 times1 and stress urinary incontinence by 2.7 times2. The way in which vaginal birth increases the risk for these impairments remains to be elucidated. Abnormalities of the levator ani muscle are seen on magnetic resonance (MR) images of women with pelvic floor dysfunction3–5. While conducting research into the relationship between vaginal birth and stress urinary incontinence, we discovered that levator ani muscle injury was found in parous women after vaginal delivery but not in nulliparasand that there was a 2-fold increase in muscle injury in stress incontinent women compared with primiparous continent controls6. This suggests a causal link between an identifiable birth injury and pelvic floor dysfunction.
Although obstetric factors responsible for anal sphincter rupture and fecal incontinence have been studied and include instrumental delivery and long second stage, 7–10 the factors associated with levator ani injury have not been defined. The purpose of this study was to test the hypothesis that levator ani muscle abnormalities in post-partum MR images are associated factors indicating difficult delivery, such as forceps, prolonged second stage of labor, and extensive perineal trauma, and to begin to explore whether different degrees of muscle defect were related to these obstetrical factors.
MATERIALS AND METHODS
Proton density MR images (echo time 15 ms, repetition time 4,000 ms) of the levator ani muscles were obtained in 160 women after their first vaginal delivery and 80 nulliparous controls with a 1.5-T superconducting magnet as part of an University of Michigan Institutional Review Board- approved study on urinary incontinence 9 to 12 months after vaginal delivery at term11. This study included 80 nulliparous continent women, 80 women who reported being continent before pregnancy but now had de novo stress urinary incontinence that persisted and was demonstrable on physical examination at least 6months after delivery, and an equal number of 80continent women recruited so that the mean age of the 3 cohorts were not different and the racial distribution in each group was similar. Potential subjects were identified based on their responses to a questionnaire mailed to primiparous women delivering on the University of Michigan Obstetrical Unit concerning continence status and their willingness to be contacted about research project participation and a subsequent telephone interview. Nulliparous continent women were recruited from the Ann Arbor area through newspaper advertisements and recruitment posters. The women selected as continent controls had proven continence on full bladder stress test and did not report symptoms of stress incontinence. The sample size of the parent study was chosen based on power calculations performed to detect differences in urethral closure pressure and urethral support. The study demographics, morphology, and number of levator ani defects seen have previously been reported.6 The study population of primiparous women included 143 white women, 6 African-American women, and 11 women from other racial groups. Enrollment took place between 1997 and 2001 with 87% of observations collected between the years 1998 to 2000. This secondary analysis compares the obstetric factors seen in 29 women who had a visible defect in the pubovisceral portion of the levator ani with the131 women who had normal muscle.
For this study, the severity of the defect in the pubovisceral portion of the levator ani muscle was determined on each side, independently, by two examiners, (R.K. and J.D.), based on our experience with normal levator ani muscle morphology and blinded to the patient’s clinical status.12,13 A scale of 0–3 was used: a score of 0 was assigned when no visible muscle damage was seen, a score of 1 when a mild abnormality of the muscle was visible, a score of 2 when a moderate defect was present, and a score of 3 was assigned for complete loss of visible muscle on that side. Findings noted in axial sections were correlated with findings seen in coronal images to confirm the nature and extent of muscle damage and to avoid identifying asymmetric muscle appearance that was due, not to muscle loss on one side, but to asymmetry in patient placement in the scanner. The scores from each side were added to achieve a summed score for each woman. This summed score for normal pubovisceral muscles bilaterally therefore resulted in a score of 0, whereas the absence of pubovisceral muscles bilaterally resulted in a summed score of 6 (Figure 1). After this grading, abnormalities were grouped into major defects, if the total score was 4–6, and minor defects, where the summed score was 1–3, with the exception that a summed score of 3 occurring from unilateral score of 3 (that is a 3 on one side and 0 on the other) was classified in the major group due to the closer similarity of its obstetric risk factors to major injury. Obstetric data were obtained from a chart review of all delivery records.
Figure 1.
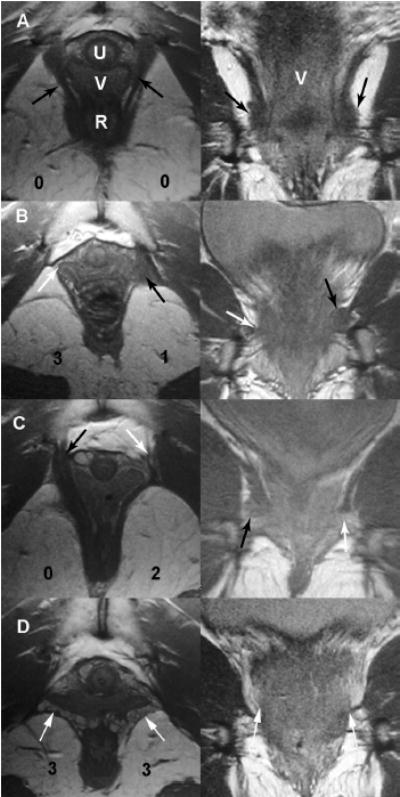
Examples of the appearance of different grades of levator ani pubovisceral muscle defects in axial and coronal magnetic resonance images. Example A represents a woman with normal muscles; B and D represent women with major defects, and C, a woman with a minor defect. Defect scores in the left panels represent the scores for each side. Black arrows identify normal muscle and white arrows represent areas where muscle is defective or should be present. U, urethra; V, vagina; R, rectum.
Statistical analysis was performed using Fisher exact test and the 2-tailed, 2-sample t test. We used a Bonferroni correction for the multiple comparisons such that significance was tested at alpha =.05/11 tests performed; P < .005 was considered significant.
RESULTS
Table 1 presents the clinical and demographic characteristics of the study sample, shown by defect status. Forceps delivery was the intervention most associated with a pubovisceral muscle injury. Sixty-six percent of women who had a forceps delivery sustained a levator ani injury compared with 25% of women with a vacuum delivery and 10.7% of women with a spontaneous vaginal delivery. Overall, 54.5% percent of women with a major defect were delivered by forceps, 9.1% by vacuum and 36.4% had a spontaneous delivery.
Table 1.
Comparison of Women with a Levator Ani Defect to Those Without a Defect in Terms of Discrete and Continuous Birth Variables
| Cases without a Defect N=131 Mean +/− SD | Cases with a Defect N=29 Mean +/−SD | Significance Level | ||
|---|---|---|---|---|
| Continuous Variables | ||||
| Age (years) | 29.3±4.7 | 32.8±5.9 | 0.0011 | |
| Gestational Age (Weeks) | 39.1±1.5 | 39.4±1.2 | 0.3798 | |
| Second Stage (minutes) | 92.5±67.5 | 170.5±117.5 | 0.0001 | |
| Birth Weight (kg) | 3.4±0.5 | 3.6±0.5 | 0.1016 | |
| Body mass index | 24.9±5.0 | 23.8±4.8 | 0.30 | |
| Head Circumference (cm) | 34.9±1.4 | 35.5±1.5 | 0.0764 | |
| Discrete Variables | N (%) | N (%) | Odds Ratio (confidence intervals) | Significance Level |
| Race | ||||
| White | 83.3 | 16.7 | 2.06 (0.66–6.4) | .21 |
| Nonwhite | 70.6 | 29.4 | ||
| Forceps Delivery | 6 (4.6%) | 12 (41.4%) | 14.7 (4.9–44.3) | 0.0001 |
| Vacuum Delivery | 10 (7.6%) | 2 (6.9%) | 0.9 (.19–4.3) | 0.6255 |
| Episiotomy | 45 (34.4%) | 18 (62.1%) | 3.1 (1.3–7.2) | 0.006 |
| Anal Sphincter Rupture | 22 (16.8%) | 18 (62.1%) | 8.1 (3.3–19.5) | 0.0001 |
| Epidural | 90 (68.7%) | 19 (65.5%) | 0.9 (0.4–2.0) | .4481 |
| Oxytocin | 71 (54.2%) | 14 (48.3%) | 0.8 (0.3–1.7) | .3541 |
The defect group was significantly older, had more anal sphincter ruptures, and more episiotomies than the group without defects (although with the Bonferroni correction for multiple t tests, the P value of this last difference fell slightly below threshold for statistical significance). Anal sphincter ruptures include partial and complete third-degree and fourth- degree lacerations. Because of the highly interrelated nature of forceps delivery, long second stage, sphincter laceration and episiotomy, we felt the sample was not large enough to render logistic regression reliable.
One quarter of all delivered women sustained some degree of anal sphincter rupture; 52.5% of these women with a sphincter rupture had a spontaneous delivery, 12.5% a vacuum delivery, and 35% a forceps delivery. Of women with a pubovisceral injury, 62.1% also had an injury to the anal sphincter. Birth weight and head circumference were both slightly greater for the defect group, but these differences were not statistically significant at this sample size. There were no differences by gestational age, epidural, or oxytocin use.
Table 2 presents the obstetric variables associated with major and minor levator defects for those measures where differences were found between women with levator defects and those with normal muscles. It reveals that for the variables of age, second stage, and forceps, women with minor defects were closer to those with normal muscle, whereas for episiotomy and anal sphincter rupture, minor defects more closely resembled major defects. Twenty of the 29 women with levator ani defects had de novo stress incontinence since delivery (P= .037); this is twice the expected rate from the parent study, in which women with stress incontinence and continent women were enrolled in equal numbers. Details regarding symptoms have been previously reported. 6
Table 2.
Comparison of Cases With Normal Muscle to Those With Major and Minor Defects in Terms of Discrete and Continuous Birth Variables
| Variable | Normal N = 131 | Minor Defect Cases N=7 Mean +/− SD | Major Defect Cases N=22 Mean +/− SD |
|---|---|---|---|
| Continuous Variables | |||
| Age (years) | 29.3±4.7 | 28.9±6.4 | 34.0±5.1** |
| Second Stage (minutes) | 92.5±67.5 | 79.4±62.0 | 194.9±118.1** |
| Discrete Variables | N (%) | N (%) | |
| Forceps Delivery | 6 (4.6%) | 0 (0.0%) | 12 (54.5%)** |
| Anal Sphincter Laceration | 45 (34.4%) | 4 (57.1%)* | 14 (63.6%) |
| Episiotomy | 22 (16.8%) | 5 (71.4%)* | 13 (59.1%) |
Minor differs from none at p< 0.05
Major differs from Minor at p< 0.05
DISCUSSION
The present study demonstrates that several obstetric and maternal factors are associated with an increased likelihood of levator ani muscle injury during vaginal delivery. Not unexpectedly, these are the signs of a difficult birth and include long second stage, forceps delivery, and anal sphincter laceration. Further analysis in larger groups will be needed to identify which of these factors are causal and which are associative. Because forceps, anal sphincter lacerations, and episiotomy are obstetrically interrelated factors, a sample size sufficient for a reliable multivariate analysis that includes all of them will be needed to determine independent effects after accounting for length of second stage.
The role that forceps delivery might play in levator ani injury requires careful scrutiny. Is it the need for forceps or the action of the forceps that is associated with injury? In the vast majority of these births, forceps were needed because of some degree of soft tissue dystocia and prolongation of the second stage. Levator damage may have occurred if the delivery was allowed to occur without forceps. The need for forceps may, therefore, be at work here as much as the effect of the forceps. The need for forceps may, therefore, be at work here as much as the effect of the forceps. There are, however, logical mechanisms whereby instrumented vaginal delivery could increase damage. During forceps delivery as much as 45 lbs of force are placed on the forceps14, so the tissues must stretch to accommodate the increasing diameter of the fetal head much more rapidly than they would during spontaneous delivery. Forceps have been implicated as a major cause of anal sphincter damage, which exhibited a strong association with levator ani injury in our study.7,8,15 Further work has demonstrated that the pubococcygeal muscle seen to be injured is the part of the levator ani muscles that undergoes the greatest degree of lengthening during vaginal delivery, suggesting that this injury may be due to rupture of the muscle from overstretching.16 Previously, however, Gainey17,18 showed a 3-fold reduction in levator damage detected on physical examination in women delivered slowly with forceps in conjunction with early episiotomy, and Ranney19 reported a reduction in the need for prolapse repair in women delivered slowly with forceps. The fact that liberal use of forceps has also been shown to decrease pelvic floor damage indicates that it may not be the forceps themselves, but the need for forceps or how they are used that causes the increase in damage.
The issue of interventions such as forceps or episiotomy and levator ani muscle injury raises interesting questions of cause and association. In our unit, where episiotomy is not routine, episiotomy may have been more common in women with levator ani defects because it was performed when a difficult delivery was anticipated; episiotomy may therefore have been an epiphenomenon. The association with anal sphincter rupture seems logical because a birth that is sufficiently traumatic to injure one muscle maybe expected to be more likely to damage another muscle. We are not suggesting that reducing episiotomy would decrease injury. In fact, Gainey17, 18 and Ranney,19 using early generous mediolateral episiotomy, have documented a decrease in injury to the levator ani muscle and occurrence of prolapse. Conversely, avoiding episiotomy could possibly lead to increased muscle stretching and therefore increased injury. Although there are strongly held beliefs for and against episiotomy, only appropriately designed scientific studies can answer these questions. Now that it is possible to objectively document the occurrence of levator ani and anal sphincter injury, carefully conducted trials can be carried out to determine how interventions affect both of these factors.
Several other studies also document prolonged second stage length as a risk factor for pelvic floor injury.8,20,21 The American College of Obstetricians and Gynecologists defines prolonged second stage of labor as being longer than 3 hours in a nulliparous woman in the presence of regional anesthesia and 2 hours without it.22 Prolonged second stage may be due to a dystocia of labor as a result of pelvic floor resistance. Because it is often overcome by instrumental delivery, it is important to separate the effect of the delivery method from the preceding second stage length.
We have introduced a new grading system to assess the degree of levator ani muscle injury. This simple system provides a graded evaluation of the degree of the visible damage to the muscle. After examining the specific score (0–6) for each woman, it seemed that grouping scores into normal (0), minor (1–3) and major (4–6 or unilateral 3) was a simple way to look at the broad categories of injury. This system of assessing disruption of normal structure was chosen specifically to assess which women sustained birth injury. It must be emphasized that this system quantifies the amount of visible damage, but not the amount of muscle present, which is a separate issue. A woman with robust muscles may lose half of her pubovisceral muscle and still have more muscle than a woman born with small muscles who has not sustained an injury. Measuring muscle volume would not, therefore, reliably indicate which women suffered muscle damage. Also, we are unable to determine the role of denervation injury in the pathogenesis of muscle damage, and future studies availing of neurophysiological testing may provide information complimentary to that obtained with MR. Whether muscle defect or remaining muscle volume is more strongly associated with pelvic organ prolapse will require further research.
This study sample was part of a project that recruited equal numbers of primiparous women who developed de novo stress incontinence after first term vaginal birth. This oversampling of stress incontinent women means that the frequency with which levator defects are seen is not representative of the normal population. We observed 29 pubovisceral defects in 160 women (18%). Future studies that seek to define the proportion of women who develop injuries will require sampling a large representative population of delivering women, a study that is probably prohibitively expensive at the present time because of the cost of MR imaging scans. For that reason, more efficient case– control studies will probably be needed to answer specific questions, one at a time, until a less expensive alternative to MR imaging can be developed and properly validated. The present research provides important insights into what the next questions should be and sufficient early data to use in answering additional questions.
Pelvic organ prolapse is more strongly associated with birth than either urinary or fecal incontinence. Therefore, data concerning obstetric factors and “pelvic floor dysfunction” must be interpreted based on which of these issues is to be addressed. Recommendations for obstetric practice should not be made on an isolated outcome, for example, stress incontinence. Proving that an intervention fails to prevent stress incontinence does not mean that it might not influence pelvic organ prolapse. Research into the role of vaginal birth and pelvic organ prolapse has been hampered by the long lag between the delivery and appearance of the prolapse and also because of the lack of a consistently accepted way of assessing the status of the pelvic organs. If, as ongoing research in our unit is showing, levator injury is proven to be a link between vaginal birth and increased risk of pelvic organ prolapse, then this marker can be used to study women soon after birth to gain further insight. The availability of a standardized clinical measurement (pelvic organ prolapse quantification system) 23 also facilitates such a study. With these new tools, we should be able to clarify the role of vaginal birth and each type of pelvic floor dysfunction and then make recommendations concerning preventive strategies that would benefit the greatest number of women at the lowest risk to those not destined to develop these problems.
Acknowledgments
We gratefully acknowledge support from NIH grants R01 DK51405.
References
- 1.Mant J, Painter R, Vessey M. Epidemiology of genital prolapse: observations from the Oxford Family Planning Association Study. Br J Obstet Gynaecol. 1997;104:579–85. doi: 10.1111/j.1471-0528.1997.tb11536.x. [DOI] [PubMed] [Google Scholar]
- 2.Rortveit G, Hannestad YS, Daltveit AK, Hunskaar S. Age- and type-dependent effects of parity on urinary incontinence: the Norwegian EPINCONT study. Obstet Gynecol. 2001;98:1004–10. doi: 10.1016/s0029-7844(01)01566-6. [DOI] [PubMed] [Google Scholar]
- 3.Kirschner-Hermanns R, Wein B, Niehaus S, Schaefer W, Jakse G. The contribution of magnetic resonance imaging of the pelvic floor to the understanding of urinary incontinence. Br J Urol. 1993;72:715–8. doi: 10.1111/j.1464-410x.1993.tb16254.x. [DOI] [PubMed] [Google Scholar]
- 4.Tunn R, Paris S, Fischer W, Hamm B, Kuchinke J. Static magnetic resonance imaging of the pelvic floor muscle morphology in women with stress urinary incontinence and pelvic prolapse. Neurourol Urodyn. 1998;17:579–89. doi: 10.1002/(sici)1520-6777(1998)17:6<579::aid-nau2>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 5.Hoyte L, Schierlitz L, Zou K, Flesh G, Fielding J. Two- and 3-dimensional MRI comparison of levator ani structure, volume, and integrity in women with stress incontinence and prolapse. Am J Obstet Gynecol. 2001;185:11–9. doi: 10.1067/mob.2001.116365. [DOI] [PubMed] [Google Scholar]
- 6.DeLancey JO, Kearney R, Chou Q, Speights S, Binno S. The appearance of levator ani muscle abnormalities in magnetic resonance images after vaginal delivery. Obstet Gynecol. 2003;101(1):46–53. doi: 10.1016/s0029-7844(02)02465-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sultan AH, Kamm MA, Hudson CN, Bartram CI. Analsphincter disruption during vaginal delivery. N Engl J Med. 1993;329:1905–11. doi: 10.1056/NEJM199312233292601. [DOI] [PubMed] [Google Scholar]
- 8.Donnelly V, Fynes M, Campbell D, Johnson H, O’Connell PR, O’Herlihy C. Obstetric events leading to anal sphincter damage. Obstet Gynecol. 1998;92:955–61. doi: 10.1016/s0029-7844(98)00255-5. [DOI] [PubMed] [Google Scholar]
- 9.Meyer S, Hohlfeld P, Achtari C, Russolo A, De Grandi P. Birth trauma: short and long term effects of forceps delivery compared with spontaneous delivery on various pelvic floor parameters. BJOG. 2000;107:1360–5. doi: 10.1111/j.1471-0528.2000.tb11648.x. [DOI] [PubMed] [Google Scholar]
- 10.Christianson LM, Bovbjerg VE, McDavitt EC, Hullfish KL. Risk factors for perineal injury during delivery. Am J Obstet Gynecol. 2003;189:255–60. doi: 10.1067/mob.2003.547. [DOI] [PubMed] [Google Scholar]
- 11.Chou Q, DeLancey J. A structured system to evaluate urethral support anatomy in magnetic resonance images. Am J Obstet Gynecol. 2001;185:44–50. doi: 10.1067/mob.2001.116368. [DOI] [PubMed] [Google Scholar]
- 12.Strohbehn K, Ellis JH, Strohbehn JA, DeLancey JO. Magnetic resonance imaging of the levator ani with anatomic correlation. Obstet Gynecol. 1996;87:277–85. doi: 10.1016/0029-7844(95)00410-6. [DOI] [PubMed] [Google Scholar]
- 13.Tunn R, Delancey JO, Howard D, Ashton-Miller JA, Quint LE. Anatomic variations in the levator ani muscle, endopelvic fascia, and urethra in nulliparas evaluated by magnetic resonance imaging. Am J Obstet Gynecol. 2003;188:116–21. doi: 10.1067/mob.2003.58. [DOI] [PubMed] [Google Scholar]
- 14.Pearse WH. Electronic recording of forceps delivery. Am J Obstet Gynecol. 1963;86:43–51. doi: 10.1016/0002-9378(63)90075-9. [DOI] [PubMed] [Google Scholar]
- 15.Sultan AH, Kamm MA, Hudson CN, Bartram CI. Third degree obstetric anal sphincter tears: risk factors and outcome of primary repair. BMJ. 1994;308:887–91. doi: 10.1136/bmj.308.6933.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lien KC, Mooney B, DeLancey JO, Ashton-Miller JA. Levator ani muscle stretch induced by simulated vaginal birth. Obstet Gynecol. 2004;103:31–40. doi: 10.1097/01.AOG.0000109207.22354.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gainey HL. Postpartum observation of pelvic tissue damage. Am J Obstet Gynecol. 1943;45:457–66. doi: 10.1016/s0002-9378(16)37836-x. [DOI] [PubMed] [Google Scholar]
- 18.Gainey HL. Postpartum observation of pelvic tissue damage: further studies. Am J Obstet Gynecol. 1955;70:800–7. doi: 10.1016/s0002-9378(16)37836-x. [DOI] [PubMed] [Google Scholar]
- 19.Ranney B. Decreasing numbers of patients for vaginal hysterectomy and plasty. S D J Med. 1990;43:7–12. [PubMed] [Google Scholar]
- 20.Allen RE, Hosker GL, Smith AR, Warrell DW. Pelvic floor damage and childbirth: a neurophysiological study. Br J Obstet Gynaecol. 1990;97:770–9. doi: 10.1111/j.1471-0528.1990.tb02570.x. [DOI] [PubMed] [Google Scholar]
- 21.Cheng YW, Hopkins LM, Caughey AB. How long is too long: Does a prolonged second stage of labor in nulliparous women affect maternal and neonatal outcome? Am J Obstet Gynecol. 2004;191:933–8. doi: 10.1016/j.ajog.2004.05.044. [DOI] [PubMed] [Google Scholar]
- 22.American College of Obstetricians and Gynecologists. ACOG practice bulletin 17. Washington, DC: ACOG; 2000. Operative vaginal delivery. [Google Scholar]
- 23.Bump RC, Mattiasson A, Bo K, Brubaker LP, DeLancey JO, Klarskov P, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996;175:10–7. doi: 10.1016/s0002-9378(96)70243-0. [DOI] [PubMed] [Google Scholar]


