Abstract
Background
Achaete-Scute Complex-Like 1 (ASCL1) is a transcription factor important in the malignant development of Medullary Thyroid Cancer (MTC). Activation of Raf-1 signaling is associated with ASCL1 suppression and growth inhibition. Xanthohumol, a natural compound, has recently been shown to have anti-cancer properties. We thus hypothesized that Xanthohumol that would suppress growth by activating Raf-1 signaling, thus altering the malignant phenotype of MTC.
Methods
Human MTC cells were treated with Xanthohumol (0–30 μM) for up to 6 days. Proliferation was measured by a MTT colorimetric assay. Western blot analysis was performed for ASCL1 and markers of Raf-1 pathway activation.
Results
Treatment of MTC cells with Xanthohumol resulted in a dose dependent inhibition of growth. Additionally, induction of phosphorylated ERK1/2 and a reduction of ASCL1 protein was noted.
Conclusions
Xanthohumol is a potent Raf-1 activator in MTC cells. This compound suppresses MTC growth, alters the malignant phenotype and warrants further pre-clinical study.
Keywords: Medullary Thyroid Cancer, Neuroendocrine Tumor, Achaete-Scute Complex-Like 1, Phosphorylated ERK1/2, Xanthohumol
Introduction
Medullary thyroid cancer (MTC) is a neuroendocrine tumor (NET) derived from the calcitonin producing thyroid C cells and accounts for 3–5% of all cases of thyroid cancer 1, 2. While early surgery is potentially curative, more than 50% of patients with MTC will have persistent disease, manifested by elevated post-operative calcitonin levels 3. While reoperation maybe potentially curative in patients with disease confined to local lymph nodes, there is no current therapy for widely metastatic disease4, 5. Additionally, the many debilitating symptoms associated with MTC, such as airway obstruction and diarrhea, are also difficult to treat.
Our group has had a long standing interest in the role of Achaete-Scute Complex-Like 1 (ASCL1) in neuroendocrine tumorigenesis. A basic helix-loop-helix transcription factor that plays an essential role in the development of the central and peripheral nervous systems, ASCL1 is critical in neuroendocrine tissue 6, 7. We have previously shown that that ASCL1 is not present in normal adult tissue but is highly expressed in a subset of NETs that include MTC8, 9. Knockout ASCL1 −/− mice have vastly reduced numbers of thyroid C-cells, suggesting a critical role for ASCL1 in C-cell development10. Given the important role of ASCL1 in MTC, pathways that control expression of this transcription factor are thus of particular interest.
Previous work by our group, using an artificial overexpression model, has shown that activation of the Raf-1/MEK 1/2 (Mitogen-activated protein kinase kinase)/ERK 1/2 (extracellular regulated kinase) pathway can alter the expression of ASCL1 in human MTC cells7. Briefly, active Raf-1 triggers a phosphorylation cascade that results in the phosphorylation of ERK1/2, an intracellular effector molecule11. In order to elucidate the importance of this pathway in MTC, our group established an estrogen inducible Raf-1 overexpression model using the human MTC cell line TT7. Induction of Raf-1 signaling with estrogen leads to a progressive induction of phosphorylated ERK1/2 and suppression of ASCL1 in vitro and in vivo7, 12. The identification of Raf-1 pathway activating drugs would therefore provide potential therapeutic options for patients with intractable MTC.
Xanthohumol (XN) is a prenylated chalcone derived from hops (Humulus lupus) and has recently shown promise as a potential chemotherapeutic agent in a variety of human malignancies 13–16. Anti-cancer action has been noted in prostate cancer, a variety of hematologic malignancies, melanoma and hepatocellular carcinoma13–16 As such, we hypothesized that Xanthohumol would be able to suppress the growth of human MTC and alter the expression of the key transcription factor ASCL1. We additionally hypothesized that these anti-cancer effects would be associated with activation of the Raf-1 pathway, a known tumor suppressing pathway in MTC.
Materials and Methods
Cell Culture and Cell Proliferation Assay
Human MTC cells (TT) were obtained from American Type Culture Collection (Manassas, VA) and maintained as previously described17. MTC cell proliferation was measured by the methylthiazolyldiphenyl-tetrazolium bromide (MTT) (Sigma-Aldrich, St. Louis, MO) rapid colorimetric assay as previously described 17. Briefly, cells were seeded on 24-well plates and incubated for 24 hours under standard conditions. The cells were then treated with Xanthohumol (Sigma-Aldrich), 0–30 μM, in quadruplicate and incubated for up to 6 days. The MTT assay was performed every 2 days by replacing the standard medium with 250 μL of serum-free medium containing MTT (0.5 mg/mL) and incubated at 37°C for 4 hours. After incubation, 750 μL of dimethyl sulfoxide (DMSO; Sigma-Aldrich) was added to each well and mixed thoroughly. The plates were then measured at 540 nm using a spectrophotometer (μQuant; Bio-Tek Instruments, Winooski, VT).
Western Blot Analysis
TT cells were treated with XN (0–30 μM) for 48 hours and whole cell lysates were prepared as previously described 17. Total protein concentrations were quantified with a bicinchoninic acid assay kit (Pierce Biotechnology, Rockford, IL). Denatured cellular extracts were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis, transferred onto nitrocellulose membranes (Bio-Rad, Hercules, CA), blocked in milk, and incubated with appropriate antibodies as previously described18. The following primary antibody dilutions were used: 1:1,000 for ASCL1 (BD Pharmingen, San Diego, CA), phospho-ERK1/2 and total ERK1/2 (Cell Signaling Technology, Beverly, MA) and 1:10,000 for Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Trevigen, Gaithersburg, MD). Horseradish peroxidase conjugated goat anti-rabbit or goat anti-mouse secondary antibodies (Cell Signaling) were used, depending on the source of the primary antibody. For visualization of the protein signal, Immun-star HRP substrate (Bio-Rad Laboratories) or SuperSignal West Femto (Pierce Biotechnology) kits were used, according to the manufacturer’s instructions, and then the blots were exposed to x-ray films.
Results
Xanthohumol inhibits MTC proliferation
Previous studies have suggested that XN is capable of suppressing the in vitro growth of a variety of other cancer lines13–16. Based upon this work, we hypothesized that XN would be capable of suppressing TT proliferation in vitro. To test this hypothesis we plated human TT cells as described above and treated them with increasing doses of XN. A statistically significant, dose dependent inhibition of growth was observed Figure 1. Significant growth inhibition was noted with 20 and 30 μM after 4 days of treatment and with all doses of XN after 6 days of treatment (p < 0.05). These data suggest that XN is capable of inhibiting the proliferation of MTC cells.
Figure 1.
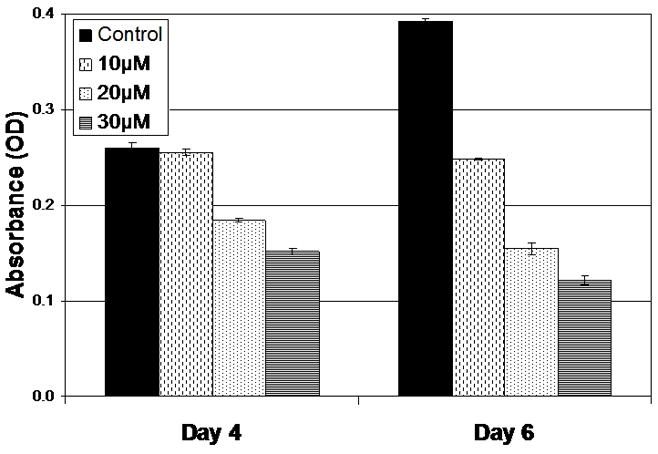
XN inhibits the proliferation of MTC cells. TT MTC cells were treated with the indicated concentrations for up to 6 days. Cell viability was determined by the MTT colorimetric assay. Experiments were performed in quadruplicate and data are plotted as mean ± SEM. Treatments were significantly different from control after 4 days of treatment with 20 μM and 30 μM and 6 days of treatment with all doses (p ≤ 0.001).
Xanthohumol alters the malignant phenotype
After showing that there was a significant inhibition of growth by the MTT assay, we were next interested in determining if XN could alter the malignant phenotype. As mentioned, ASCL1 plays an important role in the malignant development of MTC and can be viewed as an important marker of malignancy7, 19. Western blot analysis was performed for ASCL1 on TT cells treated for 4 days with increasing doses of XN. A modest inhibition of ASCL1 was noted after treatment with 10 μM XN, while treatment with either 20 or 30 μM resulted in almost no detectable ASCL1 Figure 2A. This suggests that XN treatment is able to alter the expression of this MTC transcription factor.
Figure 2.
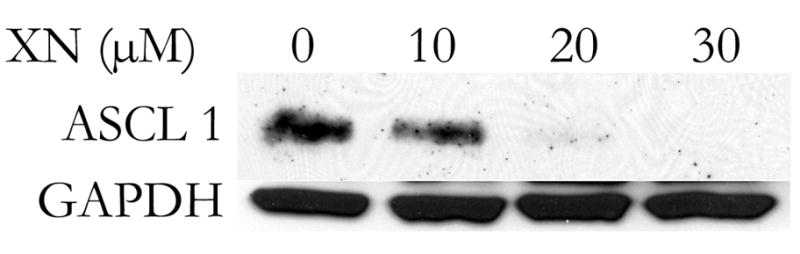
Figure 2A. XN suppresses the level of ASCL1 protein in MTC cells. Human MTC cells were treated for 4 days with the indicated doses of XN and western blot analysis for ASCL1 was performed. A dose dependent suppression of ASCL1 was observed. GAPDH is included as a loading control.
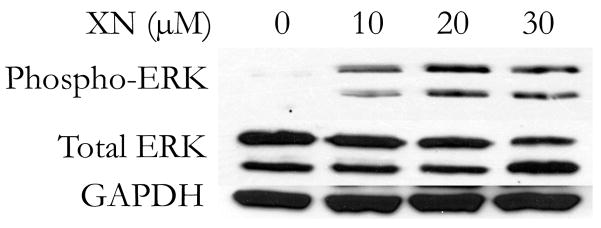
Figure 2B. XN induces phosphorylation of ERK1/2 in human MTC cells. Human MTC cells were treated for 4 days with the indicated doses of XN and western blot analysis performed for phosphorylated and total ERK1/2. A dose dependent increase in the proportion of phosphorylated ERK1/2 was observed, along with no change in total ERK 1/2. GAPDH is included as a loading control.
Xanthohumol induces the phosphorylation of ERK1/2
Activation of the Raf-1 pathway, as evidenced by phosphorylation of ERK1/2 has been shown to be a potent tumor suppressor in MTC both in vitro and in vivo7, 12. Western blot analysis for phosphorylated ERK1/2 was performed on TT cells treated with XN for 4 days, as a marker of Raf-1 pathway activation. A dose dependent induction in phosphorylated ERK1/2 was observed with no change in total ERK 1/2 Figure 2B. These finding suggest that XN induces the phosphorylation of ERK1/2, suggesting it is capable of activating the Raf-1 pathway, a known tumor suppressor in MTC.
Discussion
The significant morbidity associated with metastatic MTC as well as the lack of viable treatment options highlights the importance of novel therapeutic strategies 1, 20. While MTC accounts for only 3–5% of thyroid malignancies, it is responsible for approximately 14% of deaths1, 2, 20. Patients typically suffer from a variety of endocrinopathies and, though potentially curative, surgical resection may not be possible with metastatic disease1, 2, 20.
Important in the malignant development of MTC, ASCL1 can be modulated through overexpression of active Raf-17. This modulation can also be accomplished in a nude mouse xenograft model12. The potential to alter the phenotype and suppress the growth of MTC makes identification of compounds that can activate Raf-1 signaling an important strategy in the treatment of MTC.
We present here our data suggesting that XN, a natural compound derived from hops, is capable of inducing phosphorylated ERK1/2, and that this activation is associated with an alteration in the malignant phenotype and significant growth suppression. It appears that treatment with 10 μM XN efficiently induces Raf-1 pathway activation. This low level activation, however, is associated only with a minimal amount of growth and ASCL1 suppression after 4 days, though significant growth inhibition is observed at 6 days. Likely the low dose coupled with the slow proliferation rate of TT cells is the basis of this observation and these finding support the fact that XN can alter the growth and malignant phenotype of MTC in a dose dependent fashion.
In summary, XN is shown here to alter the malignant phenotype and suppress the growth of MTC. These changes are associated with induction of phosphorylated ERK1/2, a marker of a proven tumor-suppressing pathway. Given the relative non-toxic nature of XN, these data suggest that XN is an attractive target for additional pre-clinical investigation.
Acknowledgments
Financial Support
Howard Hughes Medical Research Institute (MRC)
NIH – R21 CA117117 (HC)
NIH – R01 CA109053 (HC)
NIH – RO1 CA121115 (HC)
American College of Surgeons: George H. A. Clowes Jr. Memorial Research Career Development Award (HC)
Carcinoid Cancer Foundation Research Award (HC)
Footnotes
Note: This work has been accepted and will be presented at the August, 2009 meeting of the Midwest Surgical Association. We certify that all authors give their approval for publication and that this work is unpublished.
References
- 1.Chen H, Roberts JR, Ball DW, et al. Effective long-term palliation of symptomatic, incurable metastatic medullary thyroid cancer by operative resection. Ann Surg. 1998 Jun;227(6):887–895. doi: 10.1097/00000658-199806000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sippel RS, Kunnimalaiyaan M, Chen H. Current management of medullary thyroid cancer. Oncologist. 2008 May;13(5):539–547. doi: 10.1634/theoncologist.2007-0239. [DOI] [PubMed] [Google Scholar]
- 3.Hahm JR, Lee MS, Min YK, et al. Routine measurement of serum calcitonin is useful for early detection of medullary thyroid carcinoma in patients with nodular thyroid diseases. Thyroid. 2001 Jan;11(1):73–80. doi: 10.1089/10507250150500694. [DOI] [PubMed] [Google Scholar]
- 4.Moley JF, Lairmore TC, Phay JE. Hereditary endocrinopathies. Curr Probl Surg. 1999 Sep;36(9):653–762. doi: 10.1016/s0011-3840(99)80001-x. [DOI] [PubMed] [Google Scholar]
- 5.Kebebew E, Kikuchi S, Duh QY, Clark OH. Long-term results of reoperation and localizing studies in patients with persistent or recurrent medullary thyroid cancer. Arch Surg. 2000 Aug;135(8):895–901. doi: 10.1001/archsurg.135.8.895. [DOI] [PubMed] [Google Scholar]
- 6.Chen H, Thiagalingam A, Chopra H, et al. Conservation of the Drosophila lateral inhibition pathway in human lung cancer: a hairy-related protein (HES-1) directly represses achaete-scute homolog-1 expression. Proc Natl Acad Sci U S A. 1997 May;94(10):5355–5360. doi: 10.1073/pnas.94.10.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sippel RS, Carpenter JE, Kunnimalaiyaan M, Chen H. The role of human achaete-scute homolog-1 in medullary thyroid cancer cells. Surgery. 2003 Dec;134(6):866–871. doi: 10.1016/s0039-6060(03)00418-5. discussion 871–863. [DOI] [PubMed] [Google Scholar]
- 8.Borges M, Linnoila RI, van de Velde HJ, et al. An achaete-scute homologue essential for neuroendocrine differentiation in the lung. Nature. 1997 Apr 24;386(6627):852–855. doi: 10.1038/386852a0. [DOI] [PubMed] [Google Scholar]
- 9.Chen H, Biel MA, Borges MW, et al. Tissue-specific expression of human achaete-scute homologue-1 in neuroendocrine tumors: transcriptional regulation by dual inhibitory regions. Cell Growth Differ. 1997 Jun;8(6):677–686. [PubMed] [Google Scholar]
- 10.Lanigan TM, DeRaad SK, Russo AF. Requirement of the MASH-1 transcription factor for neuroendocrine differentiation of thyroid C cells. J Neurobiol. 1998 Feb 5;34(2):126–134. [PubMed] [Google Scholar]
- 11.Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007 May 14;26(22):3279–3290. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- 12.Vaccaro A, Chen H, Kunnimalaiyaan M. In-vivo activation of Raf-1 inhibits tumor growth and development in a xenograft model of human medullary thyroid cancer. Anticancer Drugs. 2006 Aug;17(7):849–853. doi: 10.1097/01.cad.0000217424.36961.47. [DOI] [PubMed] [Google Scholar]
- 13.Dell’Eva R, Ambrosini C, Vannini N, Piaggio G, Albini A, Ferrari N. AKT/NF-kappaB inhibitor xanthohumol targets cell growth and angiogenesis in hematologic malignancies. Cancer. 2007 Nov 1;110(9):2007–2011. doi: 10.1002/cncr.23017. [DOI] [PubMed] [Google Scholar]
- 14.Delmulle L, Bellahcene A, Dhooge W, et al. Anti-proliferative properties of prenylated flavonoids from hops (Humulus lupulus L.) in human prostate cancer cell lines. Phytomedicine. 2006 Nov;13(9–10):732–734. doi: 10.1016/j.phymed.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Ho YC, Liu CH, Chen CN, Duan KJ, Lin MT. Inhibitory effects of xanthohumol from hops (Humulus lupulus L.) on human hepatocellular carcinoma cell lines. Phytother Res. 2008 Nov;22(11):1465–1468. doi: 10.1002/ptr.2481. [DOI] [PubMed] [Google Scholar]
- 16.Koo JH, Kim HT, Yoon HY, et al. Effect of xanthohumol on melanogenesis in B16 melanoma cells. Exp Mol Med. 2008 Jun 30;40(3):313–319. doi: 10.3858/emm.2008.40.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kunnimalaiyaan M, Vaccaro AM, Ndiaye MA, Chen H. Overexpression of the NOTCH1 intracellular domain inhibits cell proliferation and alters the neuroendocrine phenotype of medullary thyroid cancer cells. J Biol Chem. 2006 Dec 29;281(52):39819–39830. doi: 10.1074/jbc.M603578200. [DOI] [PubMed] [Google Scholar]
- 18.Greenblatt DY, Vaccaro AM, Jaskula-Sztul R, et al. Valproic acid activates notch-1 signaling and regulates the neuroendocrine phenotype in carcinoid cancer cells. Oncologist. 2007 Aug;12(8):942–951. doi: 10.1634/theoncologist.12-8-942. [DOI] [PubMed] [Google Scholar]
- 19.Chen H, Kunnimalaiyaan M, Van Gompel JJ. Medullary thyroid cancer: the functions of raf-1 and human achaete-scute homologue-1. Thyroid. 2005 Jun;15(6):511–521. doi: 10.1089/thy.2005.15.511. [DOI] [PubMed] [Google Scholar]
- 20.Udelsman R, Chen H. The current management of thyroid cancer. Adv Surg. 1999;33:1–27. [PubMed] [Google Scholar]