Abstract
Inflammation following tissue damage promotes lymphocyte recruitment, tissue remodelling, and wound healing while maintaining self tolerance. Endogenous signals associated with tissue damage and cell death have been proposed to initiate and instruct immune responses following injury. Here we have examined the effects of elevated levels of a candidate endogenous danger signal, heat shock cognate protein 70 (Hsc70), on stimulation of inflammation and autoimmunity following cell damage. We find that damage to pancreatic β-cells expressing additional cytosolic Hsc70 leads to an increased incidence of diabetes in a transgenic mouse model. Steady-state levels of activated APC and T cell populations in the draining lymph node were enhanced, which further increased following streptozotocin-induced β-cell death. In addition, pro-inflammatory serum cytokines, and lymphocyte recruitment were increased in Hsc70 transgenic mice. Islet-antigen-specific T cells underwent a greater extent of proliferation in the lymph nodes of mice expressing Hsc70 following β-cell damage, suggesting elevated antigen presentation following release of antigen in the presence of Hsc70. These findings suggest that an elevated content of Hsc70 in cells undergoing necrotic or apoptotic cell death can increase the extent of sterile inflammation and increase the susceptibility to autoimmunity.
Introduction
Sterile wounding produces inflammation that is able to recruit and activate phagocytes, promote scavenging and processing of cellular debris, and activate lymphocytes to effect tissue remodelling and wound healing. Sterile inflammatory pathways initiated following cell death or tissue injury share features of pathogen-triggered immune responses, and are now recognized to be initiated by endogenous signals which sound the alarm to indicate damage and altered tissue homeostasis requiring immune attention and repair (1). A growing list of endogenous danger signals, termed damage associated molecular patterns (DAMPs)6 and “alarmins” have been identified which promote immune responses. These include heat shock proteins (HSPs), monosodium urate, high mobility group box-1, low molecular weight hyaluronic acid, and ATP (2, 3). Innate immune cells, including macrophage and dendritic cells (DCs), are thought to detect these extracellular DAMPs and initiate inflammatory responses through a variety of receptors which may include toll-like receptors (TLRs) and other “foreign” pattern recognition receptors (4).
It appears to be critical, therefore, to limit the exposure of danger signals following injury, as well as during physiological cell death, in order to avoid excessive inflammation and inappropriate immune activation and to maintain tolerance to released self antigens. Apoptotic cell death is thought to sequester pro-inflammatory cellular contents, preventing extracellular release of DAMPs, as well as triggering anti-inflammatory clearance by phagocytes (5). Rapid clearance of apoptotic cells, however, may be crucial for preventing autoimmune activation following cell death (6). Overload of clearance receptors or accumulation of dead cell debris may trigger pro-inflammatory responses during prolonged generation of apoptotic cells (5). Also, apoptotic cells can progress to secondary necrosis and expose their sequestered intracellular contents and DAMPs to the innate immune system. For example, β-cells that die by apoptosis and progress to secondary necrosis were found to stimulate efficient self antigen presentation and to activate self-reactive T cells in a TLR2-dependent manner (7). Furthermore, “stressful” apoptosis may increase the expression of additional danger or clearance factors, including Hsp70, Hsp90, or calreticulin, which promote uptake and antigen cross presentation with concomitant APC maturation, thus enhancing responses against cell-associated antigens. This form of cell death has been suggested to critically influence the immunogenicity of tumor cells (8).
Immunity against self antigens might be more likely to result during conditions of cell death which release sufficient DAMPs or which display a signature of a stress-response. Cell damage models of autoimmune diabetes have been described which could reflect an excess of DAMP-induced inflammation or dead cell overload. These include streptozotocin (STZ), alloxan (9), cyclophosphamide (10), and Coxsackie-B virus (11), all of which have direct β-cell-specific cytotoxic effects followed by induced immune responses. STZ is a reactive glucose analogue which is taken up specifically by pancreatic β-cells and causes DNA damage and nitric oxide-generation (9). Caspase-mediated β-cell apoptosis following multiple low dose (MLD) treatments with STZ leads to an immune-mediated diabetes (12). T cells and macrophages have been implicated in mediating complete β-cell destruction following STZ insult (13, 14), and inflammatory cytokines including IL-1, IL-15, IL-18, and IL-23 have been found to play a role in the onset of diabetes, suggesting an innate response instructing subsequent lymphocyte-dependent β-cell destruction (15-18).
In this study we have addressed the question of whether the extent of inflammation following cell damage could be tipped to further progress to autoimmune activation if cells expressed increased levels of endogenous danger signals. Heat shock proteins have been suggested to provide pro-inflammatory and immunity enhancing signals following their release from necrotic cells (19, 20). Hsc70 is a constitutively expressed member of the Hsp70 family, which performs housekeeping functions of protein folding/unfolding, clathrin uncoating, and chaperoning of lysosome-targeting proteins (21). While recombinant human Hsp70 and mixtures of highly purified mouse Hsp70/Hsc70 were previously shown to enhance autoantigen-specific CD8+ T cell activation and promote autoimmune diabetes (22), others have attributed the pro-inflammatory activities of HSPs to contaminating endotoxin (23, 24). To directly assess the effects of increased release of pre-existing cellular Hsc70, in situ, we used the STZ-damage induced diabetes model in mice transgenically engineered to express additional cytosolic Hsc70 in pancreatic β-cells, and combined this with the RIP-LCMV-GP antigen model to examine auto-antigen-specific CD8+ T cell responses. We find that while resting mice displayed only modestly enhanced activation of immune cell populations, β-cell death induced by STZ, whether necrotic or apoptotic, produced a higher incidence of diabetes associated with immune cell recruitment and activation in Hsc70-expressing animals. These results suggest that endogenous danger signal protein levels can determine the susceptibility to autoimmune activation following cell death.
Materials and Methods
Transgenic mice production and maintenance
The full length coding region of bovine Hsc70 (kindly provided by Prof. David McKay, UCSF) was cloned into the BamH1 site of the plasmid pKool, downstream of the rat insulin promoter, replacing the LCMV-GP cDNA (25, 26), and used for pro-nuclear injection of F1 (C57Bl/6J × DBA/2J) mouse fertilized oocytes at the Rockefeller University Transgenic Service Laboratory. Four RIP-Hsc70 founder lines were identified with germline transmission of the transgene. Mice were maintained at the Ontario Cancer Institute, and at the University of Manchester. All experimental protocols were in accordance with Canadian Government guidelines and the Animals (Scientific Procedures) Act 1986, and were performed under an approved UK Home Office Project License. Mice were bred with previously described RIP-GP and RIP-GP / P14 TCR strains (22, 25) on the C57Bl/6J background for more than 10 generations. RIP-Hsc70 / RIP-GP mice were backcrossed to generate homozygous RIP-GP+/+ / RIP-Hsc70+/+ mice.
Chemicals and Reagents
Streptozotocin was purchased from Sigma (Poole, UK). Antibodies specific for Hsp/Hsc70 (clone 5A5), or for Hsc70 only (clone 1B5) were purchased from Stressgen (Victoria, Canada). All other antibodies were from BD Biosciences except where noted. LPS (E. coli serotype 026:B6), and CFSE were from Sigma. Primers used for Hsc70 genotyping and RT-PCR were H1 (3′-tggcttagacaaaaaggttgg), and H2 (3′-cttggggatacgggttgag) and control β-actin primers were Act1 (3′-atggatgacgatatcgctgc) and Act2 (3′-ctagaagcacttgcggtgcac). Peptide GP33-41 (KAVYNFATM) was synthesized by Sigma Genosys (Haverhill, UK). LCMV (Armstrong strain) was administered i.v. at a dose of 2000 pfu. Blood glucose concentration was monitored using an Accu-Chek Advantage glucose meter with Accu-Chek Advantage II test strips (Roche, UK).
RT-PCR
Total pancreatic RNA was prepared using Trizol (Invitrogen, Paisley, UK) and cDNA was prepared with Superscript II reverse transcriptase (Invitrogen), according to the manufacturer’s instructions, using oligo-dT primers. Serial dilutions were then used for PCR with the primers above for 35 cycles.
Pancreas Histology
Freshly removed pancreas tissue was snap frozen in OCT compound (RA Lamb, Eastbourne, UK) in liquid nitrogen, and stored at −80 C. 10 μm section were cut with a Leica cryostat microtome and mounted on gelatin coated glass slides. Tissue was fixed in 4% paraformaldehyde or 50:50 acetone:methanol, endogenous peroxidase was quenched with glucose and glucose oxidase (Sigma) followed by blocking with heterologous serum and avidin (Vector Labs, Peterborough, UK), and processed for antibody and/or Hematoxylin and Eosin staining. Tissue images were acquired on a Nikon E400 microscope using Eclipse software. Pancreatic β-cells and infiltrates were identified using anti-insulin, anti-CD4, anti-CD8, anti-CD44, or anti-MHC class II antibodies coupled to biotin, and detected with Streptavidin-HRP and developed using DAB solution (Vector Labs). Double staining was performed using HRP and alkaline phosphatase detection using an ABC kit (Vector Labs) according to the manufacturer’s instructions
Lymph node cell isolation and FACS analysis
PDLN and ILN were removed into FACS buffer (PBS + 2% FBS), ground between frosted glass slides, and strained through a 100 μm filter. Cells were recovered by centrifugation, treated with ACK buffer (0.15 M NH4Cl, 10 mM KHCO3, 0.1 mM EDTA) to lyse red blood cells, and washed in Hank’s Balanced Salt Solution (HBSS, Invitrogen). Following recovery by centrifugation, cells were resuspended in FACS buffer and stained for activation markers using fluorescently-labeled antibodies. Stained populations were analyzed on a FACScalibur (BD Biosciences) using Cell Quest or Flowjo (Treestar, USA) software.
Multiple low dose (MLD-) STZ and single intermediate dose (SID-) STZ treatments
STZ was dissolved in 0.1 M citrate buffer pH 4.5 and injected i.p. within 5 minutes. A dose of 50 mg/kg was injected on five consecutive days (MLD) or a single dose of 125, 150, or 200 mg/kg was administered i.p., and blood glucose monitored over several weeks to identify incidence of diabetes. Mice were considered diabetic when their blood glucose was greater than 15 mM for at least 3 readings measured every 2-4 days.
Cytokine measurement
Blood was collected via the lateral tail vein, or following terminal cardiac puncture, allowed to clot for 30 minutes at room temperature, the serum recovered following centrifugation, and stored at −20 C. Simultaneous determination of multiple cytokines was performed using cytometric bead array (CBA) Flex-sets (BD Biosciences) for IFNγ, IL-12p70, IL-2, and IL-10. Fluorescence data were acquired on a FACSarray (BD Biosciences), and compared with standards to determine cytokine concentrations, using FCAParray software. For sandwich enzyme-linked immunosorbent assay (ELISA), samples were diluted in ELISA blocking buffer (PBS containing 2% BSA), and added to coated plates (IL-1β capture antibody clone B122 (eBioscience)), washed with PBS plus 0.05% Tween-20, and detected using biotinylated anti-IL1β detection antibody (polyclonal, eBioscience), and visualized using Avidin-HRP and TMB reagent measured at 630nm.
Lymphocyte recruitment and in vivo proliferation
P14 TCR Tg mouse splenocytes were prepared and labeled with carboxyfluorescein-succinimidyl-ester (CFSE, Sigma, UK). For some experiments, CD8+ T cells were purified using MACS CD8a-bead selection on an AutoMACS (Miltenyi, Bergisch Gladbach, Germany) according to the manufacturer’s instructions. Transfer of 1×107 cells into STZ-treated mice was by i.p. injection in HBSS.
Statistics
Significant differences between experimental groups with normal distribution were determined using Student’s t-test and ANOVA. Non-parametric data were analyzed for significant difference between experiment groups using the Mann-Whitney test. Differences between Kaplan-Meier incidence curves were determined by logrank test using GraphPad Prism software.
Results
Characterization of RIP-Hsc70 Tg mice
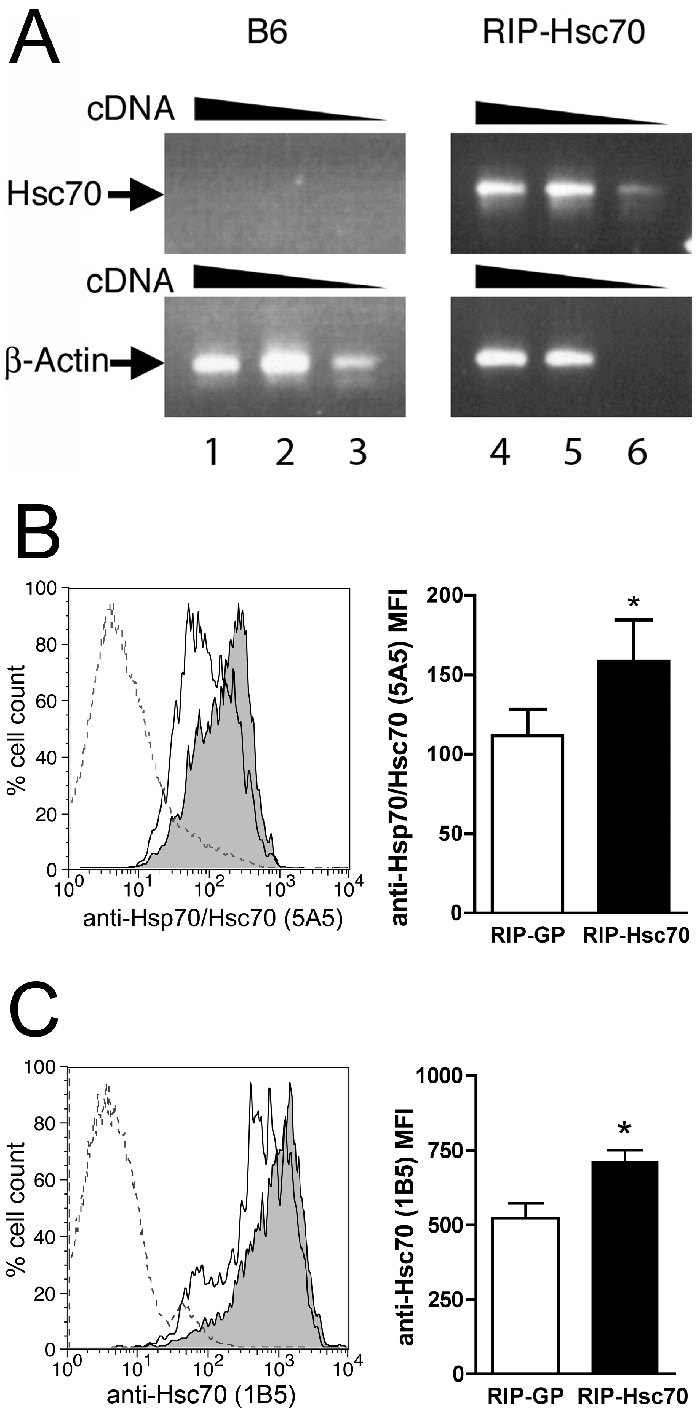
We used the full-length bovine Hsc70 coding region cloned downstream of the rat insulin promoter (RIP-Hsc70) to generate transgenic (Tg) mice. Four founder lines were identified with germline transmission of the transgene. RIP-Hsc70 Tg mice expressed mRNA specific for bovine Hsc70 in the pancreas (Fig. 1A), and over-expressed Hsc70 protein, as detected by intracellular flow-cytometric staining (Fig. 1B,C), and immunofluorescence staining of freshly isolated pancreatic islets (data not shown). RIP-Hsc70 mice were otherwise indistinguishable from wildtype littermates and no abnormalities or pathology were observed during their lifespan, nor were significant difference seen between founder lines.
Figure 1.
Expression of Hsc70 in pancreas of RIP-Hsc70 Tg mice. (A) RT-PCR analysis of Hsc70 cDNA levels from C57Bl/6J wildtype littermates (B6, lanes 1-3) versus RIP-Hsc70 Tg mice (lanes 4-6), compared with β-actin controls. (B) Total pancreas cells were stained for intracellular content of Hsp/Hsc70 using anti-Hsp70 clone 5A5, gating on the high granularity β-cell population. In the left panel, the outline trace shows staining of RIP-GP controls, filled trace shows fluorescence of RIP-Hsc70+/+ Tg mice, dotted line shows staining with secondary detecting antibody alone. The right panel shows mean fluorescence intensity (MFI) average of Hsp/Hsc70 stains from RIP-GP and RIP-Hsc70+/+ mice. (C) Total pancreas cells were stained for intracellular content of Hsc70 using anti-Hsc70-specific clone 1B5, gating on the high granularity β-cell population. In the left panel, the outline trace shows staining of RIP-GP controls, filled trace shows fluorescence of RIP-Hsc70+/+ Tg mice, dotted line shows staining with secondary detecting antibody alone. The right panel shows mean fluorescence intensity (MFI) average of Hsc70 stains from RIP-GP and RIP-Hsc70+/+ mice. n=6 mice, * indicates p<0.05. Representative results of at least 3 independent experiments are shown.
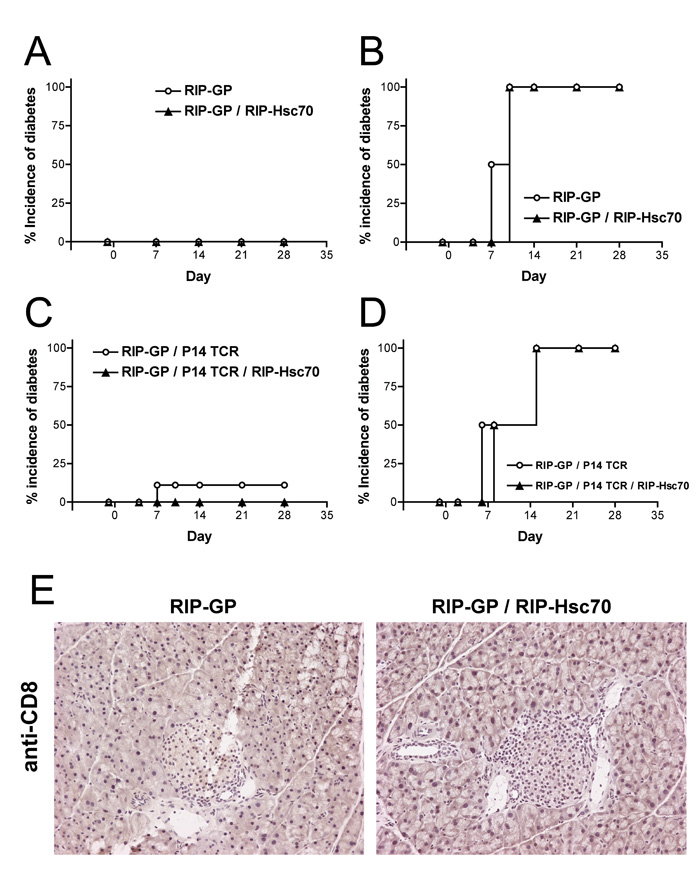
To examine the influence of β-cell Hsc70 over-expression on induced diabetic responses, the RIP-Hsc70 Tg mice were crossed with the RIP-LCMV-glycoprotein (RIP-GP) antigen model (25). RIP-GP mice can be stimulated to acquire diabetes by infection with lymphocytic choriomeningitis virus (LCMV), which carries the GP auto-antigen. Immunity raised against the viral GP results in concomitant immunity against GP expressed in pancreatic β-cells, resulting in CTL-mediate destruction of β-cells, loss of insulin production, elevation of blood glucose, and diabetes (25). To determine if Hsc70 expression altered the immune response against GP, adult RIP-GP and RIP-GP / RIP-Hsc70 Tg mice were monitored for blood glucose levels over several weeks. No spontaneous diabetes was observed in any RIP-GP / RIP-Hsc70 mice (Fig. 2A). To assess if elevated Hsc70 expression promoted enhanced peripheral tolerance against GP, we infected single RIP-GP and double RIP-GP / RIP-Hsc70 Tg mice with LCMV and measured the ability to stimulate a diabetogenic anti-LCMV-GP response. Both groups of mice displayed rapid 100% incidence of diabetes in response to LCMV infection (Fig. 2B), indicating that Hsc70 expression does not promote tolerance against the co-localized GP antigen in double Tg mice.
Figure 2.
Expression of Hsc70 in islet β-cells does not alter immunological ignorance or responsiveness in the RIP-GP diabetes model. (A) Incidence of spontaneous diabetes in untreated adult RIP-GP and RIP-GP / RIP-Hsc70 mice observed by blood glucose measurement for 4 weeks. n=10 mice. (B) Incidence of diabetes induced by infection with LCMV on day 0. n=6 mice. (C) Incidence of spontaneous diabetes in untreated adult RIP-GP / P14 TCR and RIP-GP / RIP-Hsc70 / P14 TCR mice. n=10 mice. (D) Incidence of diabetes in Tg mice stimulated with 100 μg GP33-41 peptide plus 100 μg LPS, administered i.v. on day 0. n=4 mice. Experiments were performed at least 3 times. (E) Pancreatic sections from untreated adult RIP-GP or RIP-GP / RIP-Hsc70 mice were stained for CD8-positive T cells. Sections show an absence of detectable cells infiltrating pancreatic tissue or islets. Representative islets from each strain are shown. Histological analysis was performed on 6-8 mice of each strain, examining 5-10 islets per mouse, in 3 independent experiments.
CD8+ T cell responses have been demonstrated to mediate destruction of the RIP-GP β-cells (25). “P14” TCR mice are transgenic for a T cell receptor recognizing the immuno-dominant H-2Db-restricted epitope of LCMV-GP (27). In the RIP-GP / P14 TCR model, P14 CD8+ T cells develop normally and circulate in a naive state, in ignorance of the tissue-restricted GP antigen (25, 28). To examine CD8+ T cell responses against islet GP-antigen in the RIP-Hsc70 background, we crossed RIP-Hsc70 mice with RIP-GP / P14 TCR mice, to produce triple Tg mice. These mice did not display enhanced spontaneous diabetes (Fig. 2C), and isolated P14 T cells responded normally to peptide stimulation (data not shown). RIP-GP / P14 TCR mice can be stimulated to acquire diabetes using antigenic peptide plus a strong innate activation signal, such as LPS, anti-CD40, CpG ODN, or Hsp70 (22, 28, 29). To ensure P14 T cells were not tolerant to GP in the RIP-Hsc70 background, we administered peptide GP33-41 plus LPS to triple Tg mice and assessed the ability to stimulate APC and T cell activation. The presence of Hsc70 in β-cells had no effect on the ability of peptide immunization to activate diabetogenic P14 T cell function, leading to destruction of β-cells and hyperglycemia (Fig. 2D). Resting RIP-GP / RIP-Hsc70 mice were found to have no spontaneous pancreatic infiltrates of CD8+ T cells (Fig. 2E), nor were elevated numbers of MHC class II+ or CD4+ cells found within islets of naïve mice (data not shown). Large, healthy islets found in RIP-GP / RIP-Hsc70 double transgenic mice showed no indication of increased spontaneous apoptosis or necrosis due to transgene expression (Fig 2E and data not shown). Together these results demonstrate that elevation of intracellular Hsc70 levels in β-cells does not cause spontaneous inflammation, nor does it affect the development and function of islet antigen-specific CD8+ T cells.
Increased lymphocyte activation in draining lymph nodes of RIP-Hsc70 Tg mice
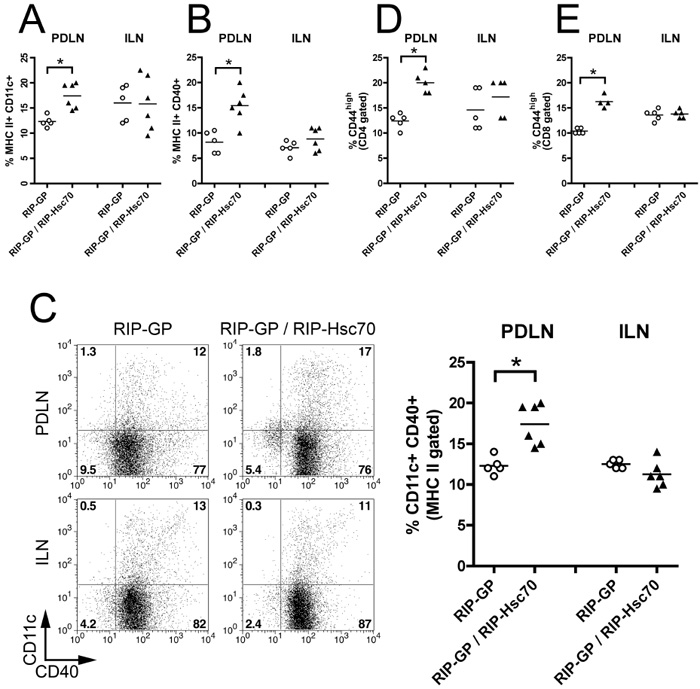
We then characterized the status of immune cells in the draining lymph nodes (LN) of RIP-GP / RIP-Hsc70 homozygous double Tg mice, compared to single Tg RIP-GP littermates. Double Tg mice had increased populations of partially activated APCs and T cells in the pancreatic draining lymph node (PDLN) but not the inguinal LN (ILN). The proportion of MHC class II+ CD11c+ DCs was significantly increased in the PDLN of double Tg mice compared with single RIP-GP Tg mice (Fig. 3A). The frequency of activated MHC class II+ CD40+ APCs and CD11c+ CD40+ DCs were also increased in PDLN of mice expressing RIP-Hsc70 (Fig. 3B,C). Furthermore, a significantly increased proportion of CD44-expressing CD4 and CD8 T cells were seen in the PDLN of Hsc70-expressing mice compared to non-Hsc70 Tg littermates in the PDLN but not the ILN (Fig. 3D,E). Total lymph node cell numbers were similar in both mouse strains (RIP-GP PDLN = 1.5 +/− 0.8 ×106 cells; RIP-GP / RIP-Hsc70 PDLN = 1.8 +/− 0.6 × 106 cells; RIP-GP ILN = 5.1 +/− 1.2 × 106 cells; RIP-GP / RIP-Hsc70 ILN = 5.5 +/− 0.8 × 106 cells). These findings indicate a mild but significantly activated immune status in the PDLN of RIP-Hsc70 Tg mice, albeit one that does not result in any detectable resting pathology in the mice.
Figure 3.
Increase in activated APCs and T cells in PDLN of RIP-Hsc70 mice. PDLN and ILN cells from RIP-GP or RIP-GP / RIP-Hsc70 were isolated and stained for (A) MHC class II and CD11c double positive cells (gated on total LN cells); (B) MHC class II and CD40 double positive cells (gated on total LN cells); (C) CD11c and CD40 double positive cells (gated on MHC class II-positive cells); (D) CD4 and CD44; (E) CD8 and CD44. Individual stained cell populations from each mouse strain and the mean is shown in each graph and a representative FACS plot illustrating CD11c and CD40 double positive cells (gated on MHC class II-positive cells) is shown (C, left panel). n=4-6 mice,* indicates p<0.05. Experiments were performed at least 3 times with similar results.
Elevated expression of Hsc70 increases diabetes following islet β-cell damage
Next, we challenged single and double Tg mice with the β-cell-specific drug, STZ, to produce partial β-cell necrosis in order to release intracellular contents, including transgenically expressed Hsc70. A single high dose (SHD) of STZ (80-200 mg/kg) is thought to produce a combination of apoptosis and necrotic damage of β-cells (30, 31). Interestingly, a role for an immune response in the SHD-STZ model is suggested from studies using TLR4-deficient C3H mice (32) and those demonstrating activation of islet-antigen-specific T cells in PDLN after a single STZ dosing (11, 31, 33, 34).
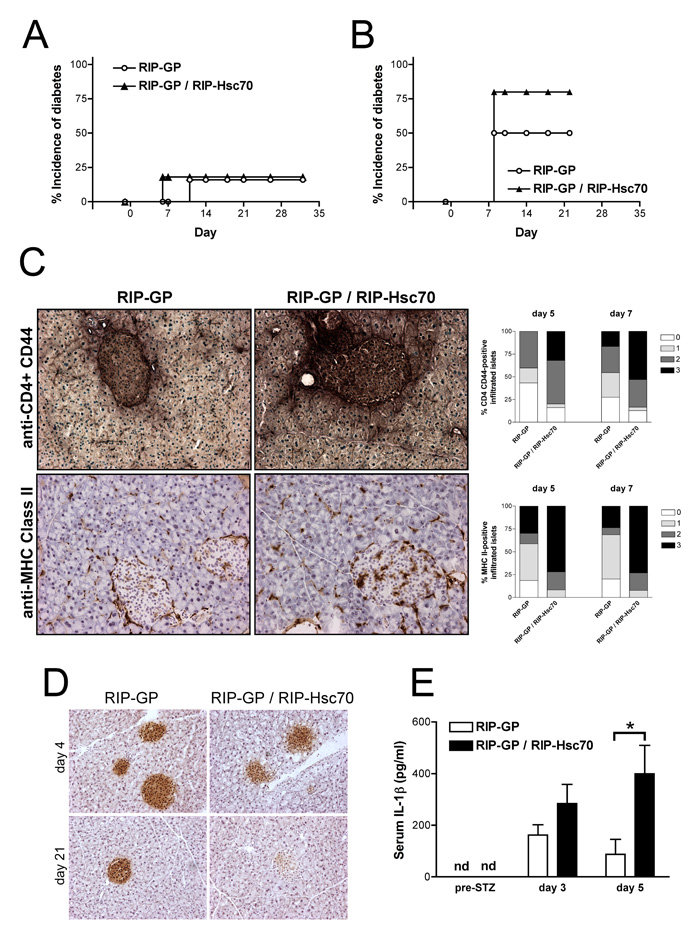
To assess the effects of damage to Hsc70-expressing β-cells on autoimmunity, we first tested a single intermediate dose (SID) of STZ (125 mg/kg), to produce limited necrotic damage to β-cells without causing complete cytotoxic ablation of β-cells and insulin production. Treatment of RIP-GP or RIP-GP / RIP-Hsc70 mice with SID-STZ resulted in only a low incidence of diabetes (20%) in both strains (Fig. 4A). Intriguingly, RIP-Hsc70 mice displayed durable, long lasting hyperglycemia starting earlier than single Tg mice. This dose of STZ was not overly cytotoxic to β-cells, as assessed by normal blood glucose concentrations between 2-7 days following treatment and normal, although infiltrated, islet morphology a few days after dosing (data not shown).
Figure 4.
Increased incidence of diabetes and inflammation in RIP-GP / RIP-Hsc70 mice following STZ-induced necrotic β-cell damage. Incidence of diabetes in RIP-GP and RIP-GP / RIP-Hsc70 following administration of a single i.p. dose of (A) 125 mg/kg or (B) 150 mg/kg STZ on day 0. n=7-12 mice. p < 0.05 between RIP-GP and RIP-GP / RIP-Hsc70 incidence using 150mg/kg STZ. (C) Pancreatic tissue was frozen on days 5 and 7 following administration of 125 mg/kg STZ to RIP-GP and RIP-GP / RIP-Hsc70 mice. Sections were double or single stained for CD4-positive and CD44-positive cells (upper panels), and MHC class II-positive cells (lower panels). Representative sections from day 7 pancreas containing infiltrated islets are shown. Islets were scored as 0 = no cell staining; 1 = peri-islet staining only, 2 = intra-islet staining, 3 = extensive peri-islet and intra-islet staining, shown in the graphs to the right. A total of 30-60 islets from multiple sections of 4 treated mice of each strain on each day were examined. p<0.05 for CD4/CD44 and p=0.006 for MHC II scores between RIP-GP and RIP-GP / RIP-Hsc70 scoring 2 or 3 on both days. (D) Mice were given a single injection of 150 mg/kg STZ, and the pancreas analyzed 4 or 21 days later. Sections were stained for insulin-positive β-cells remaining in the islets. (E) Serum samples were obtained before and on days 3 and 5 following administration of 125 mg/kg STZ and levels of IL-1β were determined by ELISA. * indicates p<0.05 for n=2-4 mice. Experiments were performed at least 3 times with similar results.
Since we observed a gradual increase in diabetes (from 0% to 100% incidence) at STZ doses between 100 and 200 mg/kg (data not shown), we assessed susceptibility to STZ-induced diabetes in each strain by increasing the dose to 150 mg/kg. We observed 50% incidence of diabetes in RIP-GP mice starting over 7 days after the drug administration, while the incidence of diabetes in RIP-GP / RIP-Hsc70 mice was increased to greater than 75% (Fig. 4B). Also, double Tg mice showed an increased infiltrate of CD4+ CD44+ cells in the islets, and had increased numbers of MHC class II+ APC in the pancreas and infiltrating the islet, both at 125 and 150 mg/kg doses (Fig. 4C and data not shown). The presence of cell infiltrates correlated with high blood glucose. The dose of 150 mg/kg did not cause complete cytotoxic damage to all β-cells, as revealed by insulin-positive islet staining at early time points after administration, in both strains (Fig. 4D), However, insulin-positive β-cells disappeared over 7-21 days as mice became diabetic, and this was more pronounced in RIP-Hsc70 mice (Fig. 4D). An early systemic inflammatory response, as assessed by IL-1β levels, was detected in the serum at days 3 and 5 post STZ-administration, which was significantly elevated in RIP-Hsc70 mice (Fig. 4E). At the time points examined, however, no significant increases in LN cell activation was detected following SID-STZ-treatment including proportions of CD11c+ DCs, MHC class II+ CD40+, CD44+ CD4+, and CD44+ CD8+ cells (data not shown). Overall, an enhanced inflammatory response was observed in the pancreas and serum of RIP-Hsc70 mice following limited necrosis of islet β-cells, which was followed by a higher incidence of diabetes.
STZ-induced diabetes in RIP-GP / RIP-Hsc70 / P14 TCR mice
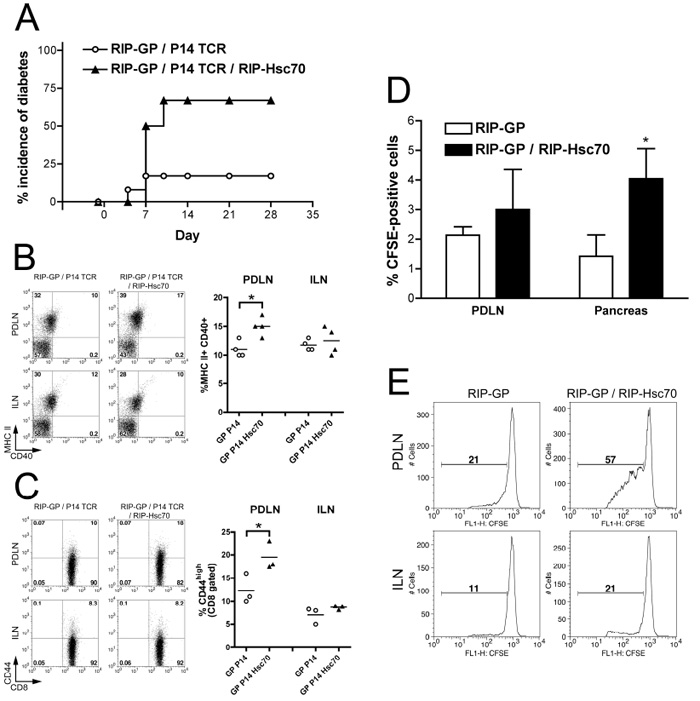
Next, we sought to assess the role of GP-specific T cells in diabetes induced by STZ-treatment, by using Tg mice additionally expressing the P14 TCR. A single high dose of 200 mg/kg was found to induce a high incidence of diabetes in RIP-GP / RIP-Hsc70 / P14 mice, while producing a low incidence of diabetes in controls containing only RIP-GP and P14 TCR transgenes (Fig. 5A). An increased proportion of high CD40-expressing and MHC Class II+ APCs were seen in the PDLN of mice expressing additional RIP-Hsc70, following STZ administration, compared to the control group (Fig. 5B, left and right panels). Also, the proportion of activated GP-specific CD8+ T cells was increased in the PDLN of STZ-treated RIP-Hsc70 mice (Fig. 5C). To focus on the responses of the GP-antigen specific T cells, we transferred CFSE-labeled P14 splenocytes into single Tg or RIP-GP / RIP-Hsc70 double Tg mice, and gave a subsequent damage-inducing dose of STZ. Twenty-four hours later, total pancreatic and PDLN cells were prepared and the presence of CFSE+ P14 cells was examined. Mice harboring additional β-cell Hsc70 contained more P14 cells recruited to both the PDLN and pancreas (Fig. 5D). Further examination at later time points demonstrated that the transferred CD8+ P14 cells in the PDLN underwent a greater extent of cell division in mice containing RIP-Hsc70, compared to the control strain (Fig. 5E). Together, these data suggest that release of islet antigen in the presence of elevated Hsc70 leads to enhanced tissue and LN recruitment of lymphocytes, including islet antigen-specific T cells and APCs, and increased antigen presentation stimulating CD8+ T cell proliferation in the draining lymph nodes.
Figure 5.
Increased incidence of diabetes and activation of GP-specific T cells in RIP-GP / P14 TCR / RIP-Hsc70 mice following STZ-induce damage. (A) Incidence of diabetes in RIP-GP / P14 TCR and RIP-GP / P14 TCR / RIP-Hsc70 mice following administration of a single i.p. dose of 200 mg/kg STZ on day 0. n=10 mice. PDLN and ILN cells were prepared on day 5 following STZ administration and stained for (B) MHC class II and CD40 (gated on total LN cells); and (C) CD8 and CD44 (gating on CD8+ cells). FACS plots indicating cellular fluorescence levels are shown in the left panels and percentage of cells in the double positive quadrant of individual mice are shown in the right panels. n=3-4 mice, * indicates p<0.05. Experiments were performed at least 3 times with similar results. (D) Splenocytes from P14 TCR mice were prepared and stained with CFSE, and transferred into RIP-GP or RIP-GP / RIP-Hsc70 mice. The next day, mice were given a single i.p. injection of 150 mg/kg STZ. 24 hours later, the pancreas and PDLN were removed and examined for recruitment of P14 cells by flow cytometry, gated on CD8-positive cells. n=3-6, * indicates p<0.05. (E) On day 6 following STZ injection, lymph nodes were isolated from mice which had received CFSE-labeled P14 splenocytes, stained for CD8+ T cells, and analyzed by flow cytometry. Plots show CFSE intensity gated on CD8-positive cells, with a marker showing CFSE dilutions below initial staining level, indicating the proportion of dividing cells. Results in panels (D) & (E) are representative of 4 mice in 2 separate experiments.
Elevated expression of Hsc70 during apoptosis increases diabetes
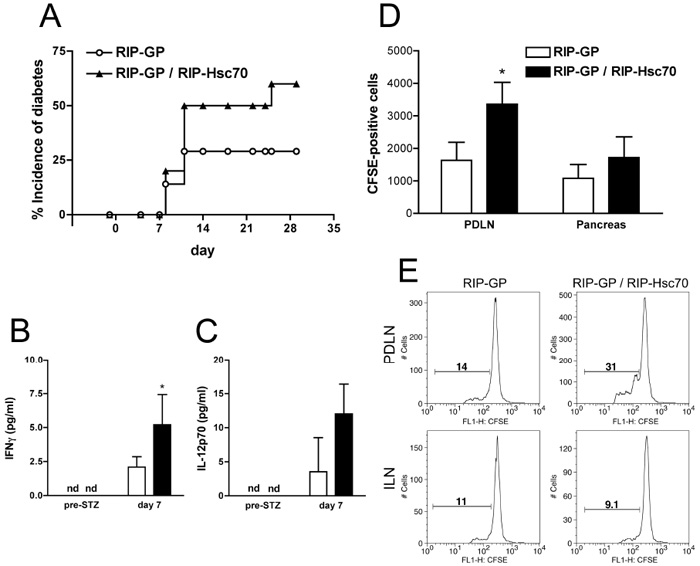
We then assessed the MLD-STZ model of diabetes, to determine if there was a difference between release of expressed Hsc70 following cellular apoptosis versus necrosis. The control group of RIP-GP mice given 5 doses of 50 mg/kg STZ showed 30% incidence of diabetes (Fig. 6A). However RIP-Hsc70 mice had a greater incidence of diabetes (60%) in response to this treatment. The MLD-STZ treatment resulted in detectable serum cytokines, including IFNγ and IL-12p70, and these levels were significantly increased in RIP-Hsc70 mice (Fig. 6B,C).
Figure 6.
Increased incidence of diabetes and inflammatory responses in RIP-GP / RIP-Hsc70 mice following MLD-STZ-induced apoptotic β-cell damage. (A) Incidence of diabetes in RIP-GP and RIP-GP / RIP-Hsc70 Tg mice following administration of 5 daily i.p. injections of 50 mg/kg STZ. n=7-10 mice. Two days after the last STZ injection, serum was collected and analyzed for cytokines using a mouse CBA flex set. Levels of IFNγ (B) and IL-12p70 (C) above the threshold for standard detection are shown. Cytokine levels in naïve animals were not detectable (nd). Experiments were performed at least 3 times with similar results. (D) Mice were given MLD-STZ treatment for 5 days. CD8+ T cells from spleen of P14 TCR mice were prepared by MACS positive selection, stained with CFSE, and transferred into RIP-GP or RIP-GP / RIP-Hsc70 mice one day before the last STZ injection. Two days later the pancreas and PDLN were removed and examined for recruitment of P14 cells by flow cytometry. n=3, * indicates p<0.05. (E) On day 7 after the first STZ injection, PDLN and ILN were isolated from mice which had received CFSE-labeled P14 splenocytes, stained for CD8+ T cells, and analyzed by flow cytometry. Plots show CFSE intensity gated on CD8-positive cells, with a marker showing CFSE dilutions below initial staining level, indicating the proportion of dividing cells. Results in panels (D) & (E) are representative of 4 mice in 2 separate experiments.
To track the islet antigen-specific T cell response during MLD-STZ-induced diabetes, CFSE-labeled P14 splenocytes were transferred into mice one day before the last dose of STZ, then the PDLN and pancreas were collected on day 5 and 7. CD8+ P14 T cells were found in the PDLN in greater numbers in RIP-Hsc70 mice on day 5 (Fig. 6D). The pancreas had no significant increase in transferred Ag-specific cells at this time point. When the PDLN cells were examined at a later time point, i.e. day 7, an increased extent of CD8+ T cell proliferation was seen in RIP-Hsc70 mice (Fig. 6E). Together, these results demonstrate that release of transgenically expressed Hsc70 from islets during either necrosis or apoptosis results in increased inflammation, infiltration, and islet-antigen-specific T cell activation, which can promote autoimmune destruction of β-cells and diabetes.
Discussion
Extracellular appearance of HSPs is thought to represent a danger signal to the immune system. We have previously shown that Hsp70 administered with self peptide can interfere with tolerance induction and cause diabetes by increasing DC activation and CTL responses, similar to PAMP or CD40-mediated stimulation (22, 28). Release of endogenous HSPs was therefore speculated to stimulate APC function and activate autoreactive T cells during their release from damaged cells. Here we used Tg over-expression of Hsc70 in β-cells to test whether elevated levels of this HSP increased the occurrence of autoimmune disease in β-cell damage-induced models of diabetes. Hsc70 was chosen as the most abundant HSP in unstressed cells which could be exposed upon sudden necrotic cell death. Tg over-expression avoids the introduction of exogenous contaminants (for example, associated proteins and endotoxin) and was chosen in preference to administration of commercial Hsc70 preparations, which could not be guaranteed endotoxin-free. A potential risk, however, is the possibility that high over-expression of a transgene using the rat insulin promoter could cause translational stress and induce non-specific β-cell death or increase sensitivity to STZ-induced death. While we could find no evidence of spontaneous β-cell death in resting RIP-Hsc70 mice, we cannot rule out contribution of such an effect. Nevertheless, we have presented results which suggest that additional endogenous Hsc70 provides an immune stimulus, detectable in PDLN of resting mice, and that its release upon necrotic or apoptotic cell death increases the recruitment of APC and T cell to the damaged tissue and draining LN and further stimulates lymphocyte activation resulting in autoimmune disease.
To our knowledge, this is the first study aimed at increasing the cellular levels of the constitutively expressed Hsc70 protein in pancreatic β-cells. This protein is already present in most cells to perform housekeeping functions including protein folding, clathrin-uncoating, nuclear protein transfer, and chaperone mediated autophagy (21). Since bovine Hsc70 is 99% identical to mouse Hsc70, we think it likely that the levels of endogenous mouse Hsc70 may play a similar and critical role in sensitivity to autoimmunity following cell damage. However, since Hsc70 is only 85% identical to inducible Hsp70 (iHsp70), there may be distinct properties of these two proteins which could differently affect their functional roles during β-cell damage and diabetes (see below).
Previously, it was reported that Tg mice expressing iHsp70 ubiquitously exhibited enhanced resistance to cerebral infarction and myocardial disfunction following ischemic injury (35). Protection against these pathologies may be due to the function of iHsp70 to promote protein re-folding, enhance recovery from cellular stress, and increase cell survival. Alternatively iHsp70 might protect cells from death by its reported ability to inhibit apoptosis (36). A recent report of Tg expression of iHsp70 causing growth defects and early death from lung and lymph node tumors suggests that suppression of apoptosis by iHsp70 can have dramatic pathological consequences including cellular transformation (37). In another Tg model, iHsp70 gene expression under the control of the human insulin promoter was reported to produce spontaneous diabetes mellitus in one transgenic line (38). The nature of the diabetes was not characterized further, however, since in several other founder lines, additional expression of iHsp70 in the thymus and in T cells was observed which was accompanied by lymphoma. Again this suggests pro-survival activities of over-expressed iHsp70 can promote transformation. Any pro-inflammatory activity of iHsp70 in these models may therefore have been overshadowed by its pro-survival function, reduction of cell death, and intracellular retention.
Extracellular Hsp70 has been implicated in a variety of inflammatory and autoimmune conditions (reviewed in Refs. (20, 39)). More recently, extracellular iHsp70 has been reported to increase activation of hepatocytes following ischemia-reperfusion injury (40) and to induce airway inflammatory responses (41). Importantly, extracellular Hsc70 was shown to play a critical role in myocardial inflammation following injury (42). In addition to release during cellular necrosis, there are now recognized to be active, stress-induced secretion mechanisms for HSPs, including Hsc70 (43, 44). The enhanced immune responses we observed following MLD-STZ suggest that Hsc70 may become exposed on apoptotic β-cells or that secondary necrosis occurs in situ.
A potential mechanism by which endogenous Hsc70 might be promoting islet-directed autoimmune responses is by acting as an innate danger signal, DAMP, or alarmin (3). Signaling receptors for Hsp70-family members have been demonstrated on APCs, including CD91, CD40, TLR2/TLR4/CD14, CCR5 and the scavenger receptors SR-A, CD36, SREC, FEEL, and Lox-1 (reviewed in Ref. (45)). Several reports, however, have suggested that low endotoxin contamination of recombinant and commercial HSP preparations may be responsible for immuno-stimulatory activity, as some responses are lost following endotoxin removal (23, 24). Currently there is support for both an intrinsic danger activity of HSPs, as wells as an adjuvant function for enhancing the response to bacterial products (46).
However, another property of Hsc70 that may be involved in its immunostimulatory function is antigen chaperoning and enhancement of cross presentation. Cross presentation of extracellular antigens sampled by APCs is required for stimulation of CD8+ T cells, and in the steady state this promotes cross-tolerance to self-antigen acquired in a non-co-stimulatory environment (47). HSPs of the 70 and 90 kDa families have been reported to chaperone antigenic peptides efficiently into the cross presentation pathway (48). Antigens associated with Hsc70 have been shown to stimulate CTL responses (49) and immunization with Hsc70-epitope fusions efficiently generates anti-tumour immunity via enhanced T cell activation (50). In our Tg model, if islet GP antigen associates with the expressed Hsc70, then this complex might enhance cross-presentation and stimulation of diabetogenic CTLs following its release during β-cell death. However, Hsc70-chaperoned GP antigen could also be released during peri-natal or juvenile waves of β-cell apoptosis (34) and may be cross-presented to alter GP-specific T cell priming in the PDLN. Enhancing juvenile apoptosis in β-cells was shown to tolerize diabetogenic T cells, and decrease subsequent immune activation and diabetes in the NOD and LCMV-NP models (31). However, the effects of altered presentation of islet antigen-Hsc70 complexes during steady state cross-tolerization on later exposure to islet antigen or β-cell damage is not known. Further studies are necessary to determine whether differences in cross-priming during peri-natal or juvenile β-cell apoptosis in the RIP-Hsc70 background could be responsible for enhancing adult autoimmune responses to β-cell damage.
Acknowledgements
The authors would like to thank Jonathan Miller for comments on the manuscript.
Footnotes
This work was supported by an MRC (UK) New Investigator Award (to D.G.M.) and a BBSRC (UK) research grant (to D.G.M.). P.S.O. is supported by the Canadian Cancer Society, grant #17506. P.S.O. holds a Canada Research Chair in Infection and Immunity.
- DC
- dendritic cells
- DAMP
- damage associated molecular pattern
- ILN
- inguinal lymph node
- PDLN
- pancreatic draining lymph node
- STZ
- streptozotocin
- MLD
- multiple low dose
- SHD
- single high dose
- SID
- single intermediate dose
- LCMV-GP
- lymphocytic choriomeningitis virus glycoprotein
- RIP
- rat insulin promoter
- Tg
- transgenic
Disclosures The authors declare that they have no competing financial interest.
Publisher's Disclaimer: “This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. This manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the United States National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org.”
References
- 1.Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8:279–289. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rock KL, Hearn A, Chen CJ, Shi Y. Natural endogenous adjuvants. Springer Semin Immunopathol. 2005;26:231–246. doi: 10.1007/s00281-004-0173-3. [DOI] [PubMed] [Google Scholar]
- 3.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 4.Ishii KJ, Koyama S, Nakagawa A, Coban C, Akira S. Host innate immune receptors and beyond: making sense of microbial infections. Cell Host Microbe. 2008;3:352–363. doi: 10.1016/j.chom.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–975. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 6.Roos A, Xu W, Castellano G, Nauta AJ, Garred P, Daha MR, van Kooten C. Mini-review: A pivotal role for innate immunity in the clearance of apoptotic cells. Eur J Immunol. 2004;34:921–929. doi: 10.1002/eji.200424904. [DOI] [PubMed] [Google Scholar]
- 7.Kim HS, Han MS, Chung KW, Kim S, Kim E, Kim MJ, Jang E, Lee HA, Youn J, Akira S, Lee MS. Toll-like receptor 2 senses beta-cell death and contributes to the initiation of autoimmune diabetes. Immunity. 2007;27:321–333. doi: 10.1016/j.immuni.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 8.Kepp O, Tesniere A, Schlemmer F, Michaud M, Senovilla L, Zitvogel L, Kroemer G. Immunogenic cell death modalities and their impact on cancer treatment. Apoptosis. 2009;14:364–375. doi: 10.1007/s10495-008-0303-9. [DOI] [PubMed] [Google Scholar]
- 9.Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res. 2001;50:537–546. [PubMed] [Google Scholar]
- 10.O’Brien BA, Harmon BV, Cameron DP, Allan DJ. Nicotinamide prevents the development of diabetes in the cyclophosphamide-induced NOD mouse model by reducing beta-cell apoptosis. J Pathol. 2000;191:86–92. doi: 10.1002/(SICI)1096-9896(200005)191:1<86::AID-PATH573>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 11.Horwitz MS, Ilic A, Fine C, Rodriguez E, Sarvetnick N. Presented antigen from damaged pancreatic beta cells activates autoreactive T cells in virus-mediated autoimmune diabetes. J Clin Invest. 2002;109:79–87. doi: 10.1172/JCI11198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liadis N, Murakami K, Eweida M, Elford AR, Sheu L, Gaisano HY, Hakem R, Ohashi PS, Woo M. Caspase-3-dependent beta-cell apoptosis in the initiation of autoimmune diabetes mellitus. Mol Cell Biol. 2005;25:3620–3629. doi: 10.1128/MCB.25.9.3620-3629.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paik SG, Fleischer N, Shin SI. Insulin-dependent diabetes mellitus induced by subdiabetogenic doses of streptozotocin: obligatory role of cell-mediated autoimmune processes. Proc Natl Acad Sci U S A. 1980;77:6129–6133. doi: 10.1073/pnas.77.10.6129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ihm SH, Lee KU, Rhee BD, Min HK. Initial role of macrophage in the development of anti-beta-cell cellular autoimmunity in multiple low-dose streptozotocin-induced diabetes in mice. Diabetes Res Clin Pract. 1990;10:123–126. doi: 10.1016/0168-8227(90)90033-p. [DOI] [PubMed] [Google Scholar]
- 15.Sandberg JO, Andersson A, Eizirik DL, Sandler S. Interleukin-1 receptor antagonist prevents low dose streptozotocin induced diabetes in mice. Biochem Biophys Res Commun. 1994;202:543–548. doi: 10.1006/bbrc.1994.1962. [DOI] [PubMed] [Google Scholar]
- 16.Lukic ML, Stosic-Grujicic S, Shahin A. Effector mechanisms in low-dose streptozotocin-induced diabetes. Dev Immunol. 1998;6:119–128. doi: 10.1155/1998/92198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lukic ML, Mensah-Brown E, Wei X, Shahin A, Liew FY. Lack of the mediators of innate immunity attenuate the development of autoimmune diabetes in mice. J Autoimmun. 2003;21:239–246. doi: 10.1016/s0896-8411(03)00115-x. [DOI] [PubMed] [Google Scholar]
- 18.Mensah-Brown EP, Shahin A, Al-Shamisi M, Wei X, Lukic ML. IL-23 leads to diabetes induction after subdiabetogenic treatment with multiple low doses of streptozotocin. Eur J Immunol. 2006;36:216–223. doi: 10.1002/eji.200535325. [DOI] [PubMed] [Google Scholar]
- 19.Srivastava P. Roles of heat-shock proteins in innate and adaptive immunity. Nat Rev Immunol. 2002;2:185–194. doi: 10.1038/nri749. [DOI] [PubMed] [Google Scholar]
- 20.Millar DG, Ohashi PS. Hsp70 family members, danger signals and autoimmunity. In: Asea AA, De Maio A, editors. Heat Shock Proteins: Potent Mediators of Inflammation and Immunity. Springer Netherlands; 2007. pp. 189–211. [Google Scholar]
- 21.Dice JF. Chaperone-mediated autophagy. Autophagy. 2007;3:295–299. doi: 10.4161/auto.4144. [DOI] [PubMed] [Google Scholar]
- 22.Millar DG, Garza KM, Odermatt B, Elford AR, Ono N, Li Z, Ohashi PS. Hsp70 promotes antigen-presenting cell function and converts T-cell tolerance to autoimmunity in vivo. Nat Med. 2003;9:1469–1476. doi: 10.1038/nm962. [DOI] [PubMed] [Google Scholar]
- 23.Tsan MF, Gao B. Endogenous ligands of Toll-like receptors. J Leukoc Biol. 2004;76:514–519. doi: 10.1189/jlb.0304127. [DOI] [PubMed] [Google Scholar]
- 24.Marincek BC, Kuhnle MC, Srokowski C, Schild H, Hammerling G, Momburg F. Heat shock protein-antigen fusions lose their enhanced immunostimulatory capacity after endotoxin depletion. Mol Immunol. 2008;46:181–191. doi: 10.1016/j.molimm.2008.07.039. [DOI] [PubMed] [Google Scholar]
- 25.Ohashi PS, Oehen S, Buerki K, Pircher H, Ohashi CT, Odermatt B, Malissen B, Zinkernagel RM, Hengartner H. Ablation of “tolerance” and induction of diabetes by virus infection in viral antigen transgenic mice. Cell. 1991;65:305–317. doi: 10.1016/0092-8674(91)90164-t. [DOI] [PubMed] [Google Scholar]
- 26.Ehl S, Hombach J, Aichele P, Rulicke T, Odermatt B, Hengartner H, Zinkernagel R, Pircher H. Viral and bacterial infections interfere with peripheral tolerance induction and activate CD8+ T cells to cause immunopathology. J Exp Med. 1998;187:763–774. doi: 10.1084/jem.187.5.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pircher H, Burki K, Lang R, Hengartner H, Zinkernagel RM. Tolerance induction in double specific T-cell receptor transgenic mice varies with antigen. Nature. 1989;342:559–561. doi: 10.1038/342559a0. [DOI] [PubMed] [Google Scholar]
- 28.Garza KM, Chan SM, Suri R, Nguyen LT, Odermatt B, Schoenberger SP, Ohashi PS. Role of antigen-presenting cells in mediating tolerance and autoimmunity. J Exp Med. 2000;191:2021–2027. doi: 10.1084/jem.191.11.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lang KS, Recher M, Junt T, Navarini AA, Harris NL, Freigang S, Odermatt B, Conrad C, Ittner LM, Bauer S, Luther SA, Uematsu S, Akira S, Hengartner H, Zinkernagel RM. Toll-like receptor engagement converts T-cell autoreactivity into overt autoimmune disease. Nat Med. 2005;11:138–145. doi: 10.1038/nm1176. [DOI] [PubMed] [Google Scholar]
- 30.Saini KS, Thompson C, Winterford CM, Walker NI, Cameron DP. Streptozotocin at low doses induces apoptosis and at high doses causes necrosis in a murine pancreatic beta cell line, INS-1. Biochem Mol Biol Int. 1996;39:1229–1236. doi: 10.1080/15216549600201422. [DOI] [PubMed] [Google Scholar]
- 31.Hugues S, Mougneau E, Ferlin W, Jeske D, Hofman P, Homann D, Beaudoin L, Schrike C, Von Herrath M, Lehuen A, Glaichenhaus N. Tolerance to islet antigens and prevention from diabetes induced by limited apoptosis of pancreatic beta cells. Immunity. 2002;16:169–181. doi: 10.1016/s1074-7613(02)00273-x. [DOI] [PubMed] [Google Scholar]
- 32.Kaku K, McGill J, Province M, Permutt MA. A single major gene controls most of the difference in susceptibility to streptozotocin-induced diabetes between C57BL/6J and C3H/HeJ mice. Diabetologia. 1989;32:716–723. doi: 10.1007/BF00274530. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, O’Brien B, Trudeau J, Tan R, Santamaria P, Dutz JP. In situ beta cell death promotes priming of diabetogenic CD8 T lymphocytes. J Immunol. 2002;168:1466–1472. doi: 10.4049/jimmunol.168.3.1466. [DOI] [PubMed] [Google Scholar]
- 34.Turley S, Poirot L, Hattori M, Benoist C, Mathis D. Physiological beta cell death triggers priming of self-reactive T cells by dendritic cells in a type-1 diabetes model. J Exp Med. 2003;198:1527–1537. doi: 10.1084/jem.20030966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mestril R. The use of transgenic mice to study cytoprotection by the stress proteins. Methods. 2005;35:165–169. doi: 10.1016/j.ymeth.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 36.Beere HM. “The stress of dying”: the role of heat shock proteins in the regulation of apoptosis. J Cell Sci. 2004;117:2641–2651. doi: 10.1242/jcs.01284. [DOI] [PubMed] [Google Scholar]
- 37.Vanhooren V, Liu XE, Desmyter L, Fan YD, Vanwalleghem L, Van Molle W, Dewaele S, Praet M, Contreras R, Libert C, Chen C. Over-expression of heat shock protein 70 in mice is associated with growth retardation, tumor formation, and early death. Rejuvenation Res. 2008;11:1013–1020. doi: 10.1089/rej.2008.0783. [DOI] [PubMed] [Google Scholar]
- 38.Seo JS, Park YM, Kim JI, Shim EH, Kim CW, Jang JJ, Kim SH, Lee WH. T cell lymphoma in transgenic mice expressing the human Hsp70 gene. Biochem Biophys Res Commun. 1996;218:582–587. doi: 10.1006/bbrc.1996.0103. [DOI] [PubMed] [Google Scholar]
- 39.Johnson JD, Fleshner M. Releasing signals, secretory pathways, and immune function of endogenous extracellular heat shock protein 72. J Leukoc Biol. 2006;79:425–434. doi: 10.1189/jlb.0905523. [DOI] [PubMed] [Google Scholar]
- 40.Galloway E, Shin T, Huber N, Eismann T, Kuboki S, Schuster R, Blanchard J, Wong HR, Lentsch AB. Activation of hepatocytes by extracellular heat shock protein 72. Am J Physiol Cell Physiol. 2008;295:C514–520. doi: 10.1152/ajpcell.00032.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chase MA, Wheeler DS, Lierl KM, Hughes VS, Wong HR, Page K. Hsp72 induces inflammation and regulates cytokine production in airway epithelium through a TLR4- and NF-kappaB-dependent mechanism. J Immunol. 2007;179:6318–6324. doi: 10.4049/jimmunol.179.9.6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zou N, Ao L, Cleveland JC, Jr., Yang X, Su X, Cai GY, Banerjee A, Fullerton DA, Meng X. Critical role of extracellular heat shock cognate protein 70 in the myocardial inflammatory response and cardiac dysfunction after global ischemia-reperfusion. Am J Physiol Heart Circ Physiol. 2008;294:H2805–2813. doi: 10.1152/ajpheart.00299.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barreto A, Gonzalez JM, Kabingu E, Asea A, Fiorentino S. Stress-induced release of HSC70 from human tumors. Cell Immunol. 2003;222:97–104. doi: 10.1016/s0008-8749(03)00115-1. [DOI] [PubMed] [Google Scholar]
- 44.Ireland HE, Leoni F, Altaie O, Birch CS, Coleman RC, Hunter-Lavin C, Williams JH. Measuring the secretion of heat shock proteins from cells. Methods. 2007;43:176–183. doi: 10.1016/j.ymeth.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 45.Calderwood SK, Mambula SS, Gray PJ, Jr., Theriault JR. Extracellular heat shock proteins in cell signaling. FEBS Lett. 2007;581:3689–3694. doi: 10.1016/j.febslet.2007.04.044. [DOI] [PubMed] [Google Scholar]
- 46.Osterloh A, Breloer M. Heat shock proteins: linking danger and pathogen recognition. Med Microbiol Immunol. 2008;197:1–8. doi: 10.1007/s00430-007-0055-0. [DOI] [PubMed] [Google Scholar]
- 47.Luckashenak N, Schroeder S, Endt K, Schmidt D, Mahnke K, Bachmann MF, Marconi P, Deeg CA, Brocker T. Constitutive crosspresentation of tissue antigens by dendritic cells controls CD8+ T cell tolerance in vivo. Immunity. 2008;28:521–532. doi: 10.1016/j.immuni.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 48.Binder RJ, Srivastava PK. Peptides chaperoned by heat-shock proteins are a necessary and sufficient source of antigen in the cross-priming of CD8+ T cells. Nat Immunol. 2005;6:593–599. doi: 10.1038/ni1201. [DOI] [PubMed] [Google Scholar]
- 49.Wieland A, Denzel M, Schmidt E, Kochanek S, Kreppel F, Reimann J, Schirmbeck R. Recombinant complexes of antigen with stress proteins are potent CD8 T-cell-stimulating immunogens. J Mol Med. 2008;86:1067–1079. doi: 10.1007/s00109-008-0371-x. [DOI] [PubMed] [Google Scholar]
- 50.Mizukami S, Kajiwara C, Ishikawa H, Katayama I, Yui K, Udono H. Both CD4+ and CD8+ T cell epitopes fused to heat shock cognate protein 70 (hsc70) can function to eradicate tumors. Cancer Sci. 2008;99:1008–1015. doi: 10.1111/j.1349-7006.2008.00788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]