Summary
Positive social interactions and social support may protect against various forms of mental and physical illness, although the mechanisms for these effects are not well identified. The socially monogamous prairie vole, which – like humans – forms social bonds and displays high levels of parasympathetic activity, has provided a useful model for investigating neurobiological systems that mediate the consequences of sociality. In the present study, adult female prairie voles were exposed to social isolation or continued pairing with a female sibling (control conditions) for 4 weeks. During weeks 3 and 4 of this period, animals were administered oxytocin (20µg/50µl, SC) or saline vehicle (50µl, SC) daily for a total of 14 days. In Experiment 1 autonomic parameters were recorded during and following isolation or pairing. Isolation (vs. pairing) significantly increased basal heart rate (HR) and reduced HR variability and vagal regulation of the heart; these changes in isolated animals were prevented with oxytocin administration. In Experiment 2 behaviors relevant to depression [sucrose intake and swimming in the forced swim test (FST)] were measured as a function of isolation. Isolation reduced sucrose intake and increased immobility in the FST; these behaviors also were prevented by oxytocin. Administration of oxytocin did not significantly alter cardiac, autonomic or behavioral responses of paired animals. These findings support the hypothesis that oxytocinergic mechanisms can protect against behavioral and cardiac dysfunction in response to chronic social stressors, and can provide insight into social influences on behavior and autonomic function in humans.
Keywords: cardiac, cardiovascular disease, depression, mood, parasympathetic nervous system, peptide, prairie vole, social behavior
Introduction
The absence of positive social interactions and social support can contribute to alterations in behavior and physiology, including both negative mood states and cardiovascular dysfunction (Knox and Uvnäs-Moberg, 1998; Kiecolt-Glaser and Newton, 2001; Krantz and McCeney, 2002; Cacioppo et al., 2002). For instance, in humans perceived loneliness is associated with symptoms of depression and increased cardiovascular reactivity during exposure to a mental stressor (Steptoe et al., 2004). Maladaptive grief, affective disorders and autonomic dysfunction have been associated with the disruption of social bonds or perceived loneliness in humans (Prigerson et al., 1994; Cacioppo et al., 2002; Berkman et al., 2004). Social stressors also produce behavioral alterations and autonomic and cardiac dysfunction in non-human animals, using operational dependent measures that have been validated in rodents (Grippo et al., 2007b; 2007d; 2008). These data suggest that social stressors and the lack of positive social interactions significantly influence behavior and physiology in both humans and non-human animals. However, the mechanisms linking the absence of social interactions to mental and cardiovascular health remain to be described. Of particular interest in understanding the consequences of social stressors are neuroendocrine systems that regulate social bonding, mental health and autonomic function.
The mammalian neuropeptide, oxytocin, is highly sensitive to the social environment, and regulates behavior (including affective behaviors) and autonomic function (Uvnäs-Moberg, 1998; Carter, 2003; Michelini et al., 2003). However, the specific functions of oxytocin in the context of various social and environmental experiences are not well understood. Oxytocin has been shown to be released in specific central nervous system regions and in the circulation during positive social experiences, and has been suggested to facilitate positive social interactions including social memory, pair bonding, and parental behavior (Carter, 1998; Cho et al., 1999; Ferguson et al., 2000; 2001; Carter, 2003; Young and Wang, 2004). For instance, intracerebroventricular (ICV) administration of oxytocin (and also arginine vasopressin) led to increased positive social contact and an increased preference for a familiar social partner in both female and male prairie voles; these effects were blocked with pretreatment of either an oxytocin receptor antagonist or a vasopressin V1a receptor antagonist, suggesting that these related peptides may facilitate positive social behaviors if either the oxytocin or vasopressin receptor is available (Cho et al., 1999).
However, increased levels of oxytocin both in the central and peripheral nervous system also have been associated with stressful events in humans and rodents (Gibbs, 1984; Nishioka et al., 1998; Taylor et al., 2006; Grippo et al., 2007b). For example, higher plasma levels of oxytocin in women were correlated with “gaps in social relationships,” lower overall quality of social interactions, and absence of positive relations with partners (Taylor et al., 2006). Several animal studies have shown related increases in endogenous oxytocin to stressful events. For example, oxytocin (but not vasopressin) was shown to be released specifically in the hypothalamic paraventricular nucleus (shown via microdialysis) and in the plasma during an acute shaker stress paradigm (10 minutes) in male rats (Nishioka et al., 1998). Similarly, oxytocin levels were increased both in the hypothalamic paraventricular nucleus (shown via immunohistochemical staining) and in the plasma following prolonged (4 weeks) social isolation versus pairing with a sibling in female prairie voles (Grippo et al., 2007b). Also, in prairie voles intense acute stressors, including the resident-intruder paradigm (Grippo et al., 2007a; 2007b) and restraint (Pournajafi and Carter, unpublished observations), were associated with increases in plasma oxytocin. Finally, mice that are genetically deficient for oxytocin or its receptor have been shown to be highly reactive to psychogenic stressors such as shaker stress or restraint, although they continue to show normal reactions to physical stressors such as insulin-induced hypoglycemia (Mantella et al., 2004; Takayanagi et al., 2005).
These and other studies suggest that oxytocin is a component of an endogenous system with the capacity to buffer against both physical and emotional stressors (Uvnäs-Moberg, 1998; Carter, 1998; Neumann et al., 2000; Heinrichs et al., 2003). This system, regulated in part by oxytocin, appears to coordinate adaptive endocrine and autonomic reactions to stressors, possibly by upregulating parasympathetic and/or reducing sympathoadrenal responses (see for instance Porges, 1998; 2001; 2007).
Studies that focus on the consequences of social stressors and the role of peptides using valid and relevant animal models will promote a greater understanding of the social factors that contribute to behavioral and autonomic dysregulation. The socially monogamous prairie vole is a rodent species that exhibits social behaviors that parallel those observed in humans, including an active engagement in and reliance on the social environment, living in pairs or family groups, and displaying bi-parental care of offspring (Carter et al., 1995; Getz and Carter, 1996; Carter and Keverne, 2002). The behavioral, autonomic, and cardiac consequences of negative social stressors in this species are only recently being investigated. Prairie voles are highly sensitive to social isolation from either a same-sex sibling or an opposite-sex partner; following short- or long-term isolation prairie voles show alterations in behaviors that may be relevant to depression, including immobility in a forced swim test (FST) and reduced responsiveness to a rewarding stimulus (Grippo et al., 2008; Bosch et al., 2008). Research from our laboratories also demonstrates that long-term social isolation in prairie voles (versus pairing with a sibling of the same sex) produces several autonomic and cardiac disruptions indicative of potential cardiovascular pathophysiology, including increased resting heart rate (HR), reduced HR variability, increased cardiac responsiveness to acute stressors, disrupted sympathovagal balance, and increased heart weight (Grippo et al., 2007d).
Given the utility of the prairie vole model for experimental investigations of behavioral and physiological responses to social experiences, the present study was designed to investigate the effects of exogenously administered oxytocin in mediating behavioral, autonomic, and cardiac responses in female prairie voles exposed to long-term social isolation. The present study builds upon previous research showing that female prairie voles are especially sensitive to social isolation (Grippo et al., 2007b; 2007d; 2008), and that oxytocin may be of particular importance in regulating behavioral and physiological responses to stressors (Uvnäs-Moberg, 1998; Carter, 1998; Neumann et al., 2000; Heinrichs et al., 2003; Grippo et al., 2007b). We conducted two parallel experiments to investigate the hypothesis that negative autonomic (Experiment 1) and behavioral (Experiment 2) consequences, which typically follow 2–4 weeks of social isolation in adult, female prairie voles, would be prevented by long-term peripheral administration of exogenous oxytocin.
Methods
Animals
Female prairie voles (Microtus ochrogaster) – 60–90 days of age, 35–55 grams in weight, descendants of a wild stock caught near Champaign, Illinois – were maintained on a 14/10h light/dark cycle (lights on at 0630h), with a temperature of 25±1°C and relative humidity of 21±4g/m3. Food (Purina rabbit chow) and water were available ad libitum unless otherwise specified. Offspring were removed from breeding pairs at 21 days of age and housed in same-sex sibling pairs; only one sibling from each pair was used for the experimental procedures described here. All procedures were conducted according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the University of Illinois at Chicago Institutional Animal Care and Use Committee.
Females were chosen for these experiments for several reasons. First, female prairie voles may be especially sensitive to the effects of social stressors (see for instance Cushing and Carter, 2000; Grippo et al., 2007a). Second, the use of females allows for direct comparisons with previous experiments conducted by our laboratory that involved manipulation of the social environment (Grippo et al., 2007a; 2007d; 2008). Third, female prairie voles do not show a spontaneous puberty or estrous cycle; the ovaries remain inactive until the female has physical contact with a male, allowing for the use of reproductively intact animals without the need for artificially controlling the estrous cycle (Carter et al., 1987). Finally female rodents are an understudied group both in behavioral and physiological investigations relating to mood and cardiovascular disorders (see discussion in Konkle et al., 2003).
Experimental Design
The present study design consisted of two experiments. Experiment 1 (n=26) focused on cardiac and autonomic measures and Experiment 2 (n=31) focused on behavioral measures, in female prairie voles exposed to either social isolation or control conditions (continual pairing with a female sibling) for a total of 4 weeks, and administered either oxytocin or saline vehicle during weeks 3 and 4 of this period. The timeline of procedures is displayed in Table 1 and Table 2; specific experimental procedures are described in the following sections.
Table 1.
Timeline of procedures used in Experiment 1.
| Procedure | Schedule |
|---|---|
| Telemetric transmitter implantation | Days 1–3 |
| Recovery in divided cages | Days 2–38* |
| Recovery in home cages (with siblings) | Days 7–15* |
| Undisturbed baseline period; body weight measurements | Days 15–19* |
| • ECG measurements | |
| • Activity measurements | |
| Weeks 1 and 2 isolation or pairing | Days 20–33 |
| • ECG measurements | |
| • Activity measurements | |
| Body weight measurements | Day 34 |
| Weeks 3 and 4 isolation or pairing; oxytocin or vehicle administration | Days 34–47 |
| • ECG measurements | |
| • Activity measurements | |
| Body weight measurements | Day 48 |
| Pharmacological autonomic bockade | Days 48–53† |
| • ECG measurements | |
| • Activity measurements | |
| Sacrifice under anesthesia | Day 55 |
| • Body weight measurements | |
| • Heart weight measurements | |
Note: All acute procedures were conducted during the light period (3–5 hours following light onset); all ECG and activity measures were continuous throughout the periods listed.
Abbreviations: ECG, electrocardiogram.
Depending on date of transmitter implantation.
Drug administration was counterbalanced across groups, 48 hours between each drug injection.
Table 2.
Timeline of procedures used in Experiment 2.
| Procedure | Schedule |
|---|---|
| Adaptation to 2% sucrose | Days 1–7 |
| Baseline fluid intake tests; body weight measurements | Days 8–14* |
| Weeks 1 and 2 isolation or pairing | Days 15–28 |
| Week 2 fluid intake test; body weight measurements | Day 29 |
| Weeks 3 and 4 isolation or pairing; oxytocin or vehicle administration | Days 29–42 |
| Week 4 fluid intake test; body weight measurements | Day 43 |
| FST training period | Day 45 |
| FST test period | Day 46 |
Note: All acute procedures were conducted during the light period (3–5 hours following light onset).
Abbreviations: FST, forced swim test.
2 fluid intake tests were conducted, separated by 5 days.
Long-term isolation from a same-sex sibling was used here for the following reasons. First, these experiments are part of a series of studies investigating the effects of the disruption of established social bonds on a variety of behavioral, autonomic, and cardiac parameters. Thus it was possible to compare directly the current results with those from previous protocols (see for instance Grippo et al., 2007d). Second, the present design permits the investigation of the consequences of long-term social stressors, which may involve chronic alterations in behavior as well as autonomic and cardiovascular function.
EXPERIMENT 1, SPECIFIC METHODS
Telemetric Transmitter Implantation
Wireless radiofrequency transmitters [Data Sciences International (DSI), St. Paul, MN] were implanted for long-term electrocardiogram (ECG) recordings using procedures similar to those described previously (Sgoifo et al., 1996). Transmitters were implanted intraperitoneally under aseptic conditions in anesthetized (ketamine 67mg/kg, SC; xylazine 13.33mg/kg, SC; NLS Animal Health, Owings Mills, MD) animals. Animals were placed under a warming lamp, and the surgical area was shaved and cleaned. Small rostral-to-caudal skin and muscle incisions were made on the ventral surface of the body. The transmitter was inserted into the abdominal cavity, and sutured to the muscle while the muscle was sutured closed. The leads were directed rostrally using a trochar and sleeve, and anchored in place with permanent sutures (DII placement). Skin incisions were sutured closed, and subcutaneous fluids were administered as necessary. All animals were housed for 5 days in custom-designed divided cages (see Grippo et al., 2007c) to permit adequate healing of suture wounds in the instrumented animal. Animals were then returned to the standard home cages (with the sibling) for an additional 5–7 days before the onset of experimentation.
Radiotelemetric Recordings
ECG and activity signals were recorded with a radiotelemetry receiver (DSI; sampling rate 5 kHz for ECG and 256 Hz for activity, 12-bit precision digitizing). These parameters were recorded continuously during an undisturbed baseline period (3–5 days) and throughout all experimental procedures.
Social Isolation
Following surgical recovery and collection of undisturbed baseline data, animals were randomly divided into paired (control; n=13) or isolated (n=13) conditions. Isolated animals were separated from the sibling and housed individually (without sensory cues from the sibling) for 4 weeks; paired animals were continually housed with the siblings during this period. Handling, cage changing and measuring of body weight were matched between the groups.
Oxytocin Administration
During weeks 3 and 4 of the isolation/pairing period, animals received a daily subcutaneous injection of either oxytocin (20µ/50µl/vole; n=6 paired, n=6 isolated) or sterile saline vehicle (50µl/vole; n=7 paired, n=7 isolated). This dose was selected based on prior evidence that it would increase positive social behaviors in adult prairie voles (Cushing and Carter, 2000). Injections were given during the light period between 0900h and 1100h, in a counterbalanced fashion. Each animal received a total of 14 injections.
Pharmacological Autonomic Blockade
Following 4 weeks of isolation or pairing, HR was measured under the following conditions: β-adrenergic receptor blockade (atenolol, 8mg/kg, IP; Sigma-Aldrich, St. Louis, MO); cholinergic receptor blockade [atropine methyl nitrate (atropine), 4mg/kg, IP; Sigma-Aldrich]; and dual blockade (both drugs), using procedures described previously (Grippo et al., 2007d). These doses were chosen for their ability to completely block the respective autonomic inputs to the heart according to previously published results from voles (Ishii et al., 1996; Grippo et al., 2007c; 2007d) and preliminary analyses conducted by our laboratory. Drugs were administered in a counterbalanced fashion over a 6-day period (48 hours between each drug administration).
Heart and Body Weight
Body weight was recorded prior to the onset of experimentation (baseline) and following 2 and 4 weeks of social isolation/pairing. At the conclusion of the study, each animal was euthanized with an overdose of ketamine (67mg/kg, SC) and xylazine (13.33mg/kg, SC). The heart was removed and weighed.
Quantification of Radiotelemetric Recordings
Quantification of Telemetric Variables
Quantification of telemetric variables was conducted according to procedures described previously (Grippo et al., 2007c; 2007d). Multiple segments of 1–5 minutes of stable, continuous raw ECG and locomotor activity data were used to evaluate HR, HR variability and activity. Data segments were matched across subjects and time points, and were used to calculate all cardiac and activity variables.
Resting Cardiac Parameters
Because activity in prairie voles occurs in short bouts throughout the light and dark periods (Grippo et al., 2007c), resting cardiac parameters were derived from ECG data sampled during a period of at least 1 hour of minimal activity [5 counts/minute (cpm) or lower], and included the average of 2–5 minute intervals of continuous ECG data collected every 30–60 minutes (4–15 minutes of accumulated data from each animal).
HR was evaluated using vendor software (DSI, St. Paul, MN), and R-wave detections were verified with a custom-designed software package. The R-R intervals were analyzed for variations (HR variability) using a custom-designed software package, and included standard deviation of all R-R (normal-to-normal; N-N) intervals [SDNN index (Task Force of the European Society of Cardiology and North American Society of Pacing and Electrophysiology, 1996)] and amplitude of respiratory sinus arrhythmia [RSA (see Porges, 2007)].
RSA was assessed using a modification of procedures described elsewhere (Yongue et al., 1982; Grippo et al., 2007c), which have been validated in prairie voles and other species (Porges et al., 1982; Grippo et al., 2007c). The ECG signal was exported into a data file and examined using a custom-designed software package to ensure that all R waves were properly detected. The R-R intervals were resampled at 20 Hz and, to comply with the assumption of stationarity, detrended with a 21-point cubic moving polynomial to remove low frequency (trend) components below 0.5 Hz. The residuals of this procedure were free of aperiodic and slow periodic processes that may have violated the assumption of stationarity. A bandpass filter was applied to define RSA by extracting only the variance in the HR spectrum between the frequencies of 1–4 Hz. This frequency range has been associated with spontaneous breathing in the vole, and has been confirmed by preliminary analyses (see Grippo et al., 2007c).
Social Isolation and Oxytocin Administration
ECG and activity data were recorded throughout the period of social isolation or pairing and oxytocin or vehicle administration. Resting parameters of HR, SDNN index, and RSA were calculated using the same manner as described above in “Resting Cardiac Parameters.”
Autonomic Blockade
The peak HR response (beginning 30 minutes following each drug injection) was evaluated using continuous ECG data according to procedures described previously (Grippo et al., 2007c). Briefly, the data were manually examined to determine the peak HR response during a window of 1 hour that included a stable ECG recording that was not confounded by movement artifact (3–10 minutes of ECG data from each animal).
Data Analysis
All data are presented as means ± SEM for the indicated analyses, figures and tables. A probability value of P<.05 was considered to be statistically significant. Periods of ECG involving animal movement artifact were excluded from the analyses. For repeated measures, data were analyzed with 3-factor mixed-design ANOVAs, with group (paired/isolated) and peptide (oxytocin/vehicle) as independent variables and the following as repeated dependent variables: HR; SDNN index; RSA amplitude; autonomic blockade agent; or body weight. Description of the 3-factor ANOVA results includes significant main effects and 3-way interactions. Heart-body weight ratios were analyzed with a 2-way independent groups ANOVA, with group and peptide as independent variables. Multiple comparisons were conducted using Tukey’s Honestly Significant Difference (HSD) or Student’s t-test with a Bonferroni correction (hypothesis-driven, statistically justified comparisons).
EXPERIMENT 2, SPECIFIC METHODS
Social Isolation
As in Experiment 1, animals were randomly divided into paired (n=15) or isolated (n=16) conditions for a total of 4 weeks. Isolation, pairing and handling procedures were identical to those described in Experiment 1.
Oxytocin Administration
During weeks 3 and 4 of the isolation/pairing period, all animals received a daily injection of oxytocin (n=8 paired, n=8 isolated) or sterile saline (n=7 paired, n=8 isolated) for a total of 14 days, using the same dose, route, time schedule and procedures as described in Experiment 1.
Fluid Intake
Intake of water and 2% sucrose were measured as an operational index of anhedonia (reduced responsiveness to a previously-demonstrated pleasurable stimulus; the reduction of sucrose intake and preference vs. control and baseline values) (Willner et al., 1987). This operational index was chosen due to the clear and comprehensive demonstration of face, construct, and predictive validity of this test in rodents (Willner et al., 1987; 1992; Willner, 2005), and the prior application of this test as an index of behavioral changes as a result of negative social environmental manipulations in prairie voles (Grippo et al., 2007a; 2007b; 2007d; 2008).
Ad libitum 2% sucrose was available to all animals for 1 week prior to experimentation to allow for adaptation to its taste. Fluid intake tests were conducted during the light period prior to isolation/pairing (baseline) and following 4 weeks of this period, using procedures described elsewhere (Grippo et al., 2007d). Briefly, food and water were removed from the cage for 16 hours prior to the test; animals were monitored visually periodically throughout this period to ensure that no adverse effects were experienced. One hour prior to the test, all animals (paired and isolated) were moved into clean, individual cages to ensure accurate fluid intake measurements of paired animals; all animals were monitored visually during this period to ensure that no adverse effects were experienced. Water and 2% sucrose were placed on the cage in premeasured bottles, and fluid intake was monitored for 1 hour. Animals were returned to the home cages immediately following the test.
Forced Swim Test
Investigation of swimming behavior in the FST was used as an index of learned helplessness (e.g., “behavioral despair”). The FST was designed initially as a pharmacological model of depression with predictive validity (see Porsolt et al., 1977), however, more recently the face and construct validity of this test in rodents have been described (Willner, 1984; Cryan et al., 2005). Following isolation/pairing (48 hours following the last fluid intake test), all animals were exposed to the FST during the light period, over a 2-day period, using the modified FST procedures described previously (Cryan et al., 2005; Grippo et al., 2008). A clear, cylindrical Plexiglas tank (height 46 cm; diameter 20 cm) was filled to a depth of 18 cm with tap water (23–25° C). Animals were placed individually into the tank for a total of 15 minutes (training period; Day 1). Twenty-four hours later, animals were again placed into the tank for a total of 5 minutes (test period; Day 2). The tank was rinsed thoroughly and filled with clean water prior to testing each animal. Each animal was placed under a warming lamp immediately following the swim period, for a total of 15 minutes, and then was returned to the home cage.
Behaviors were recorded using a video camera, and defined as: swimming, movements of the forelimbs and hindlimbs without breaking the surface of the water; struggling, forelimbs breaking the surface of water; climbing, attempts to climb the walls of the tank; and immobility, no limb or body movements (floating) or using limbs to keep afloat without corresponding trunk movements. Struggling, climbing and swimming were summed to provide one index of active coping behaviors; immobility was used as the operational index of learned helplessness (Cryan et al., 2005).
Data Analysis
As in Experiment 1, data are presented as means ± SEM, and a probability value of P<.05 was considered to be statistically significant. Repeated measures were analyzed with 3-factor mixed-design ANOVAs, with group (paired/isolated) and peptide (oxytocin/vehicle) as independent variables and the following as repeated dependent variables: body weight; sucrose intake; water intake; or sucrose preference [%preference=(sucrose intake/total fluid intake)×100]. Swimming behaviors were scored by 3 independent, experimentally-blind raters, and the results averaged; these results were compared using a 2-way independent groups ANOVA with group and peptide as independent variables. Multiple comparisons were conducted as described in Experiment 1.
Results
EXPERIMENT 1
Resting Cardiac Parameters
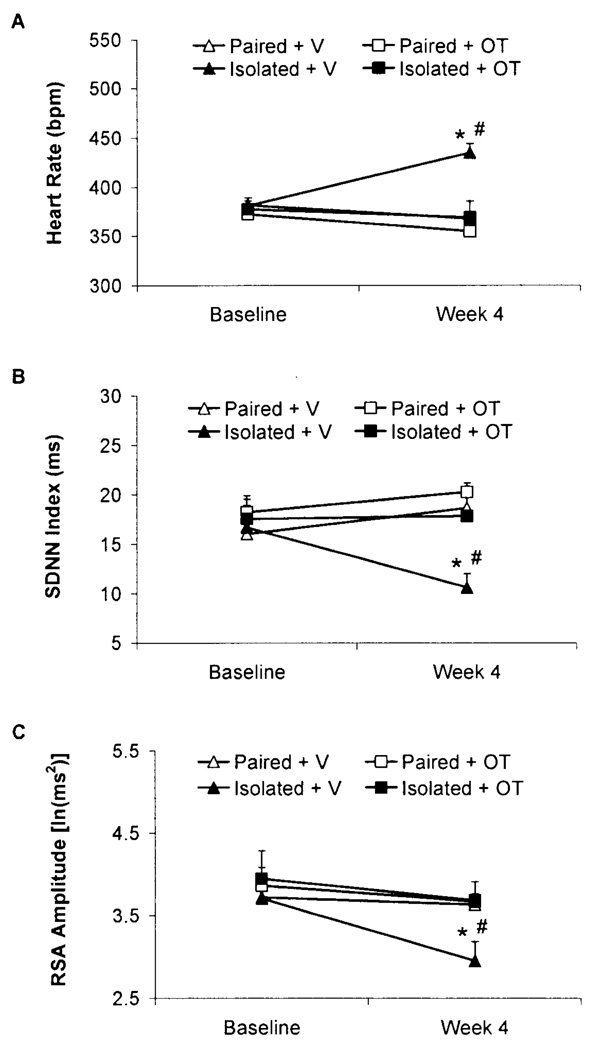
The following group names are used herein: Paired+Vehicle (V), Paired+Oxytocin (OT), Isolated+V, and Isolated+OT. Isolation significantly increased resting HR and reduced HR variability (both SDNN index and RSA amplitude) relative to control conditions; these changes were prevented by oxytocin administration (Fig. 1).
Fig. 1.
Mean (+SEM) HR (Panel A), SDNN index (Panel B), and RSA amplitude (Panel C) in paired or isolated prairie voles administered daily oxytocin (OT; 20µ/50µl/vole, SC) or vehicle (V; 50µl/vole, SC) at baseline and following 4 weeks of isolation/pairing. *P<.05 vs. respective group values; #P<.05 vs. respective baseline value. Note the scale differences among the 3 panels.
The ANOVA for resting HR yielded a 3-way interaction among time x group x peptide [F(1,22)=8.30, P<.05]; and main effects of group [F(1,22)=8.72, P<.05] and peptide [F(1,22)=9.90, P<.05]. No significant differences in HR were found among the 4 groups at baseline (P>.05 for all comparisons); following 4 weeks of social isolation/pairing, the Isolated+V group displayed a higher HR relative to its respective baseline value, the Isolated+OT group, and the two paired groups (P<.05 for all comparisons). In contrast to the increased HR in the Isolated+V group, no significant differences in HR were found among the Paired+V, Paired+OT, and Isolated+OT groups following 4 weeks of isolation/pairing (P>.05 for all comparisons).
The ANOVA for resting SDNN index yielded a 3-way interaction among time x group x drug [F(1,22)=8.99, P<.05]; and a main effect of peptide [F(1,22)=4.90, P<.05]. No significant differences in SDNN index were found among the 4 groups at baseline (P>.05 for all comparisons); following 4 weeks of social isolation/pairing, the Isolated+V group displayed a significantly lower SDNN index vs. its respective baseline value, the Isolated+OT group, and the two paired groups (P<.05 for all comparisons). In contrast to the reduced SDNN index in the Isolated+V group, no significant differences in SDNN index were found among the Paired+V, Paired+OT, and Isolated+OT groups following 4 weeks of isolation/pairing (P>.05 for all comparisons).
The ANOVA for resting RSA amplitude yielded a 3-way interaction among time x group x drug [F(1,22)=3.75, P<.05]; and main effects of group [F(1,22)=6.08, P<.05], peptide [F(1,22)=11.38, P<.05], and time [F(1,22)=8.11, P<.05]. No significant differences in RSA amplitude were observed among the 4 groups at baseline (P>.05 for all comparisons); following 4 weeks of social isolation/pairing, the Isolated+V group displayed a lower RSA amplitude vs. its respective baseline value, the Isolated+OT group, and the two paired groups (P<.05 for all comparisons). In contrast to the reduced RSA amplitude in the Isolated+V group, no significant differences in RSA amplitude were found among the Paired+V, Paired+OT, and Isolated+OT groups following 4 weeks of isolation/pairing (P>.05 for all comparisons).
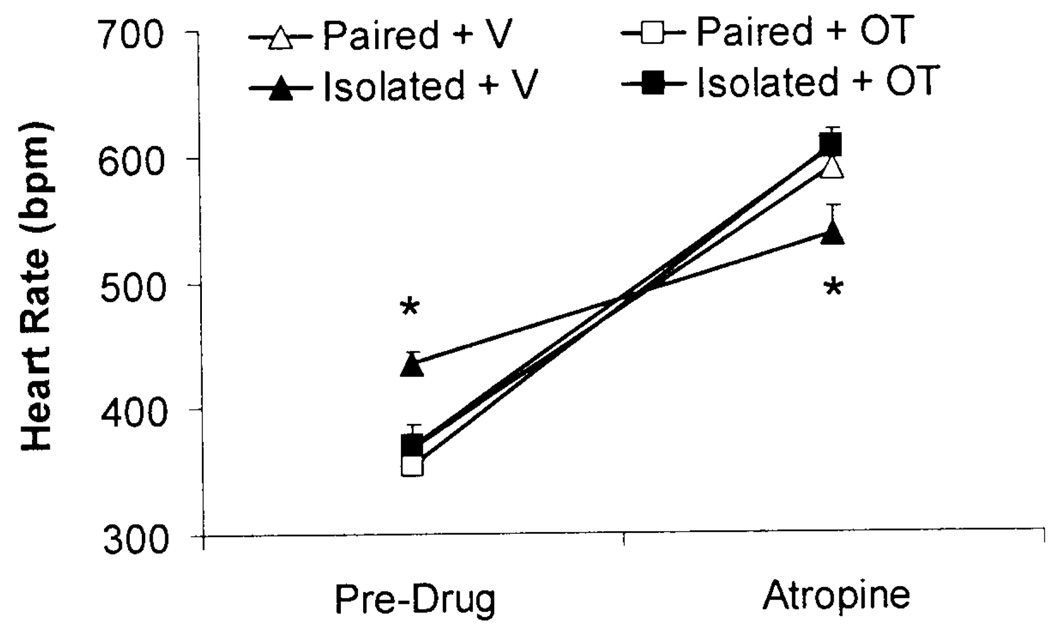
Selective Pharmacological Autonomic Blockade
Isolation significantly reduced vagal control of the heart relative to paired conditions, shown by an attenuated HR response following atropine administration. Oxytocin prevented this change (Fig. 2). Isolation was not associated with an alteration of sympathetic control of the heart nor was it associated with a change in HR following combined atropine and atenolol administration; oxytocin also had no effect on the HR responses to these pharmacological agents (data not shown).
Fig. 2.
Mean (+SEM) HR prior to and following atropine administration (4mg/kg, IP) in paired or isolated prairie voles administered daily oxytocin (OT; 20µ/50µ/vole, SC) or vehicle (V; 50µ/vole, SC). *P<.05 vs. Isolated+OT, Paired+OT, and Paired+V values at the same time point.
The ANOVA for autonomic blockade yielded a 3-way interaction among autonomic blockade agent x group x peptide [F(2,22)=5.45, P<.05]; and a main effect of autonomic blockade agent [F(2,22)=548.75, P<.05]. No significant differences in HR were observed among the 4 groups following atenolol administration or following a combination of atropine and atenolol (P>.05 for all comparisons). However, the HR of the Isolated+V group following atropine administration was significantly attenuated vs. the Isolated+OT group and the two paired groups (P<.05 for all comparisons), suggesting an alteration of vagal regulation of the heart in the Isolated+V group. In contrast to the attenuated HR response following atropine administration in the Isolated+V group, no significant differences in HR were found among the Paired+V, Paired+OT, and Isolated+OT group following atropine administration (P>.05 for all comparisons).
Body and Heart Weights
Neither isolation (vs. pairing) nor oxytocin (vs. vehicle) significantly affected body weight. Isolation was associated with an increase in heart-body weight ratio; however, oxytocin did not significantly affect this variable (Table 3).
Table 3.
Absolute body and heart weights, and heart-body weight ratios following 4 weeks of paired or isolated conditions plus either oxytocin (20µ/50µl/vole) or vehicle (50µl/vole) in Experiment 1.
| Body | Heart Weight | Heart-Body | |
|---|---|---|---|
| Weight (g) | (g) | Weight Ratio | |
| Paired+V | 42 ± 1 | 0.17 ± 0.017 | 0.004 ± 0.0003 |
| Paired+OT | 42 ± 2 | 0.19 ± 0.012 | 0.004 ± 0.0002 |
| Isolated+V | 43 ± 1 | 0.25 ± 0.016 | 0.005 ± 0.0003 |
| Isolated+OT | 43 ± 2 | 0.23 ± 0.005 | 0.005 ± 0.0002 |
Note: Values represent mean ± SEM. The weight of the radiotelemetry transmitter is excluded from the body weights listed here. Statistical significance is not noted given the lack of a significant interaction between group and peptide (see text for a further description of the results).
The ANOVA for body weight yielded a main effect of time [F(1,22)=11.23, P<.05]; the body weights (exclusive of the transmitter weights) of all groups were slightly higher at the end of the experiment vs. the respective baseline weights. The ANOVA yielded no other significant effects; no follow-up analyses were conducted.
The ANOVA for heart-body weight ratio yielded a main effect of group [F(1,22)=13.17, P<.05]; both isolated groups had slightly higher heart-body weight ratios than both paired groups. The ANOVA yielded no other significant effects; no follow-up analyses were conducted.
EXPERIMENT 2
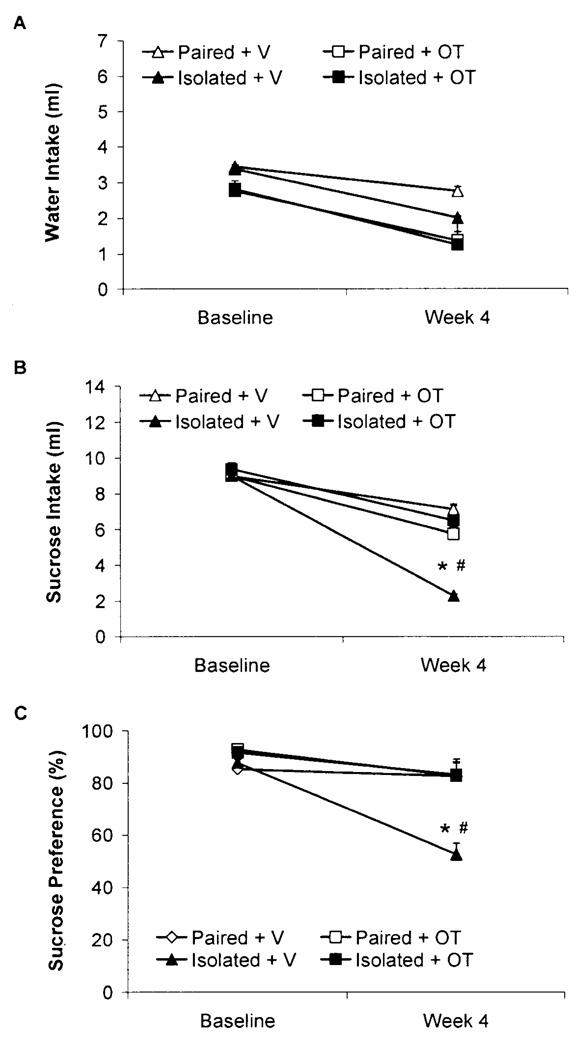
Fluid Intake and Body Weight
Isolation significantly reduced sucrose intake and sucrose preference; oxytocin administration prevented these changes (Fig. 3). Neither isolation (vs. pairing) nor oxytocin (vs. vehicle) affected body weight (Table 2).
Fig. 3.
Mean (+SEM) water intake (Panel A), sucrose intake (Panel B), and sucrose preference (Panel C) in paired or isolated prairie voles administered daily oxytocin (OT; 20µ/50µ/vole, SC) or vehicle (V; 50µ/vole, SC) at baseline and following 4 weeks of isolation/pairing. *P<.05 vs. respective group values; #P<.05 vs. respective baseline value. Note the scale differences among the 3 panels.
The ANOVA for water intake yielded a main effect of drug [F(1,27)=4.43, P<.05], due to a slightly higher intake of water in the two vehicle groups vs. the two oxytocin groups at both time points. Statistically justified follow-up analyses, however, did not yield a significant differences in water intake between the vehicle and oxytocin groups (P>.05). No other significant effects were found.
The ANOVA for sucrose intake yielded a 3-way interaction among time x group x drug [F(1,27)=7.68, P<.05]; and main effects of group [F(1,27)=4.38, P<.05] and time [F(1,27)=51.19, P<.05]. No significant differences in sucrose intake were observed among the 4 groups at baseline (P>.05 for all comparisons); following 4 weeks of social isolation/pairing, the Isolated+V group displayed a significant reduction in sucrose intake vs. its respective baseline intake, the Isolated+OT group, and the two paired groups (P<.05 for all comparisons). In contrast to the reduction in sucrose intake in the Isolated+V group, no significant differences in sucrose intake were found among the Paired+V, Paired+OT, and Isolated+OT groups following 4 weeks of isolation/pairing (P > .05 for all comparisons).
The ANOVA for sucrose preference yielded a 3-way interaction among time x group x drug [F(1,27)=4.11, P=.05]; and main effects of drug [F(1,27)=6.45, P<.05] and time [F(1,27)=11.40, P<.05]. No significant differences in sucrose preference were observed among the 4 groups at baseline (P>.05 for all comparisons); following 4 weeks of social isolation/pairing, the Isolated+V displayed a significant reduction in sucrose preference vs. its respective baseline preference, the Isolated+OT group, and the two paired groups (P<.05 for all comparisons). In contrast to the reduction in sucrose preference in the Isolated+V group, no significant differences in sucrose preference were found among the Paired+V, Paired+OT, and Isolated+OT groups following 4 weeks of isolation/pairing (P>.05 for all comparisons).
The ANOVA for body weight yielded no significant effects; no follow-up analyses were performed.
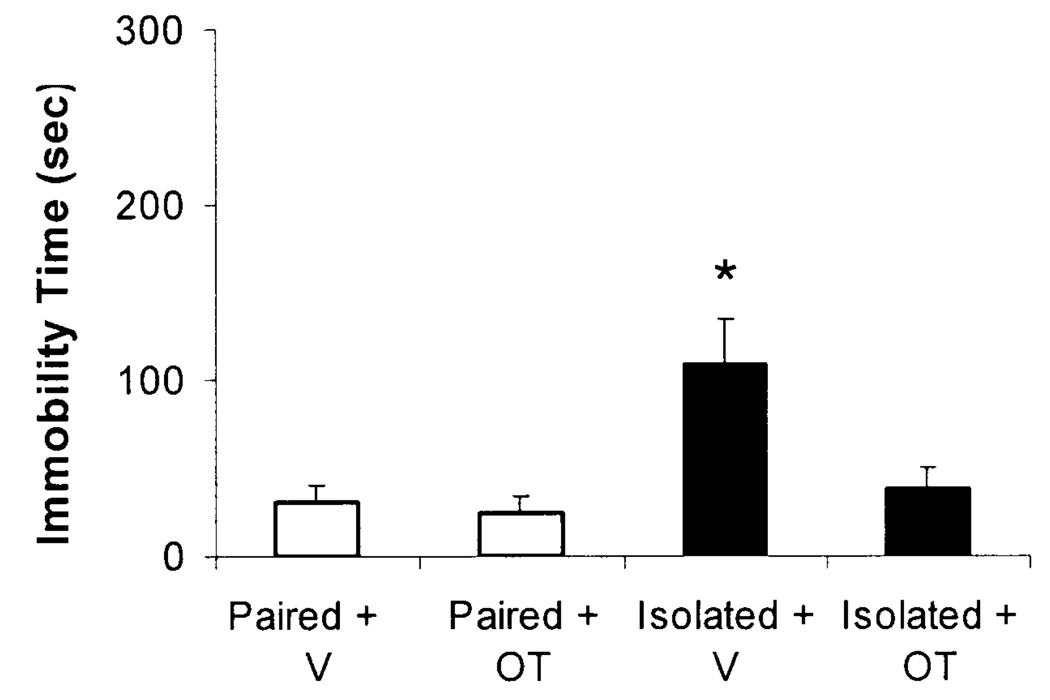
Forced Swim Test
Isolation did not significantly alter FST behaviors during the training period (Day 1; data not shown), but led to a significant increase in immobility and a decrease in active coping behaviors during the test period (Day 2). Oxytocin prevented these changes (Fig. 4).
Fig. 4.
Mean (+SEM) immobility time during a 5-min FST in paired or isolated prairie voles administered daily oxytocin (OT; 20ug/50ul/vole, SC) or vehicle (V; 50ul/vole, SC). *P<.05 vs. Isolated+OT, Paired+OT, and Paired+V values at the same time point.
The ANOVA for immobility during the test period yielded a group x peptide interaction [F(1,27)=4.16, P<.05]; and main effects of group [F(1,27)=8.09, P<.05] and peptide [F(1,27)=5.80, P<.05]. The Isolated+V group displayed increased immobility vs. the Isolated+OT group [t(13)=2.38, P<.05] and the two paired groups [t(13)=2.94, P<.05 and t(13)=2.70, P<.05 vs. Paired+OT and Paired+V groups, respectively]. In contrast to the increased immobility in the Isolated+V group, no significant differences in immobility were observed among the Paired+V, Paired +OT, and Isolated+OT groups during the FST (P>.05 for all comparisons).
The ANOVA for active coping behaviors during the test period yielded a group x peptide interaction [F(1,27)=4.17, P<.05]; and main effects of group [F(1,27)=8.10, P<.05] and peptide [F(1,27)=5.78, P<.05]. The Isolated+V group displayed decreased active coping behaviors vs. the Isolated+OT group [t(13)=2.37, P<.05] and the two paired groups [t(13)=2.93, P<.05 and t(13)=2.69, P<.05 vs. Paired+OT and Paired+V groups, respectively]. In contrast to the decrease in active coping behaviors in the Isolated+V group, no significant differences in active coping behaviors were observed among the Paired+V, Paired +OT, and Isolated+OT groups during the FST (P>.05 for all comparisons).
Discussion
Previous research in humans and prairie voles suggests that long-term social stressors, such as social isolation, produce behavioral changes, some of which may be relevant to depression, as well as autonomic dysfunction indicative of potential cardiac pathophysiology (Kiecolt-Glaser and Newton, 2001; Krantz and McCeney, 2002; Cacioppo et al., 2002; Grippo et al., 2007d; 2008). Further, the oxytocinergic system may be activated under certain stressful conditions (e.g., during long-term isolation), possibly in an attempt to compensate for altered neuroendocrine and autonomic regulation as a result of disrupted social bonds. This theory, along with previous data suggesting that oxytocin buffers behavioral and physiological responses to various stressors (Legros et al., 1987; Windle et al., 1997; Carter, 1998; Neumann, 2002; Heinrichs et al., 2003; Heinrichs and Domes, 2008), led us to investigate the effects of exogenously administered oxytocin on the behavioral and autonomic consequences of social isolation in the prairie vole model. The present findings indicate that long-term peripheral administration of oxytocin can prevent detrimental behavioral and physiological consequences of social isolation in female prairie voles, including behaviors that have been described as operational indices relevant to depression in rodents (reduced sucrose consumption, immobility in a FST), cardiac disturbances (increased HR, reduced HR variability) and autonomic imbalance (withdrawal of vagal regulation of the heart). The present findings extend previous research showing that oxytocin can have antidepressant properties (Arletti and Bertolini, 1987) and may modulate behavioral responses to short-term separation (Insel and Wintink, 1991).
Specifically, 4 weeks of isolation induced behavioral changes in prairie voles, consistent with previous studies that have employed short- or long-term separation paradigms in this species (Bosch et al., 2008; Grippo et al., 2008). Anhedonia, the reduced responsiveness to pleasurable stimuli that is often observed in human depression (Keller et al., 1995), was exhibited in isolated prairie voles evidenced by a significant reduction in sucrose intake and preference. This behavioral change represents a specific hedonic deficit, similar to previous reports from other rodent species (Willner et al., 1996; Grippo et al., 2002; 2006). Water intake was unchanged in isolated animals, indicating that social isolation does not produce a generalized deficit in ingestive behavior, and the reduction in sucrose intake was not secondary to a reduction in body weight. In addition to this behavioral index of anhedonia, isolation was associated with a behavioral index of learned helplessness, evidenced by a reduction in active coping behaviors and an increase in immobility on Day 2 of the FST (but not during the training period on Day 1). This behavioral change in the isolated group, indexed by an increase in putative “helpless” behavior in an operational test that has been described to possess face, construct, and predictive validity (Porsolt et al., 1977; Willner, 1984; Cryan et al., 2005), is similar to previously published data from isolated prairie voles (Grippo et al., 2008). In the present study, both behavioral changes were prevented in isolated animals by the administration of oxytocin, suggesting that administration of this peptide may protect against some of the negative behavioral consequences of social isolation.
In addition to behavioral changes during operational tests of anhedonia and learned helplessness, social isolation in prairie voles also produced cardiac and autonomic disruptions. Isolation led to increased resting HR and reduced HR variability (both SDNN index and RSA amplitude). Isolation also was associated with a specific withdrawal of vagal regulation of the heart, shown by an attenuated HR response following atropine administration and a specific reduction in RSA amplitude. Disruptions in autonomic function, manifest as increased HR or reduced HR variability, have been described in humans with affective disorders and in an animal model of depression (Pitzalis et al., 2001; Grippo et al., 2002). These disturbances also are common in heart disease, predicting mortality in myocardial infarction and heart failure (Ferrari et al., 2003; Guzzetti et al., 2005). Similar to the protective effect on behavioral changes, oxytocin had a protective effect on resting cardiac parameters and the vagal influence on cardiac function in isolated animals. However, while the functional physiological consequences of social isolation were prevented by oxytocin administration, the structural consequences were not. Isolation led to a significant increase in heart-body weight ratio (versus pairing; main effect of group, irrespective of peptide administration), consistent with previous findings from isolated prairie voles (Grippo et al., 2007d) and rodent models of heart disease (Francis et al., 2001). This increased ratio may represent pathological structural changes such as ventricular hypertrophy, or alternatively might be indicative of increased cardiac muscle mass for non-pathological reasons.
The precise mechanisms through which oxytocin is protective against behavioral, autonomic and cardiac responses to social isolation are yet to be elucidated. Importantly, oxytocin did not produce any significant alterations in paired animals, suggesting that its protective effects in the present paradigm were not a function of generalized down-regulation of behavioral or autonomic processes. Rather, the effects of oxytocin were specifically apparent under conditions of chronic stress (e.g., long-term social isolation). A role for oxytocin in the protective effects of social experiences has been suggested by studies in several species (Carter, 1998; Neumann et al., 2000; Heinrichs et al., 2003; DeVries and Panzica, 2006). Evidence from rats indicates that oxytocin receptor binding is increased in the hippocampus by various stressful experiences, including a single prolonged stressor as well as a series of repeated, unpredictable stressors (Liberzon and Young, 1997). Also, exogenous oxytocin down-regulates hypothalamic-pituitary-adrenal axis activity, shown by reductions in circulating cortisol in humans following a psychosocial stressor (Trier Social Stress Task) (Heinrichs et al., 2003). Some of the effects seen here may be secondary to these central or peripheral changes.
The behavioral and cardiovascular alterations shown here may reflect actions of oxytocin at several sites in the central or peripheral nervous system. Receptors for oxytocin have been identified in nuclei that regulate parasympathetic functions, including dorsal motor nucleus of the vagus, nucleus ambiguus and nucleus tractus solitarius (NTS) (see Higa et al., 2002). Oxytocin receptors also have been identified in cardiac tissue (Jankowski et al., 2004); this is a possible site of action for oxytocin’s beneficial effects on cardiac function in the present study, as this peptide was administered peripherally. However, oxytocin likely produces central changes that affect downstream processes such as autonomic outflow and behavioral responses to isolation. While circulating oxytocin is purported to cross the blood-brain barrier in small quantities (approximately 0.2% of subcutaneously administered oxytocin crosses the blood-brain barrier in adult rodents) (see Jones and Robinson, 1982; Ermisch et al., 1985), chronic peripheral administration of this peptide produces many of the same responses that occur following central release of oxytocin, including reductions in blood pressure and regulation of motivated behaviors (Arletti et al., 1992; Petersson et al., 1996; Caldwell et al., 1996; Liberzon et al., 1997). Similarly, peripheral administration of oxytocin in rats has been shown to alter receptor function in central regions associated with stressor responsiveness and cardiovascular regulation, including hypothalamus, amygdala, NTS, and locus coeruleus (Petersson et al., 1998; 2005). It is also possible that biologically active fragments of the oxytocin molecule cross the blood-brain barrier (see De Wied et al., 1993), producing central effects.
The present findings suggest that social isolation produces altered neural regulation of the heart, which can, in turn, influence both behavioral dysregulation and cardiovascular pathophysiology. As detailed in the Polyvagal Theory (Porges, 2007), reductions in the influence of the myelinated vagal pathways originating in the nucleus ambiguus – which produce RSA – are associated with an increase in biobehavioral defense strategies. Dampened RSA facilitates the sympathetic excitation necessary for fight-flight mobilization behaviors. Social isolation in turn produced significant reductions in RSA, an associated increase in HR and reduction in SDNN index (as detailed in Figure 1), and increased cardiac and neuroendocrine reactivity to acute social stressors (Grippo et al., 2007b; 2007d). Moreover, the dampened levels of RSA also may be related to a lowered threshold to trigger a vestigial vagal circuit originating in the dorsal motor nucleus of the vagus that may be related to syncope, clinical bradycardia, apnea, and cardiac arrhythmias. Since the dorsal motor nucleus of the vagus contains high levels of oxytocin receptors, the actions of oxytocin in this pathway could protect the mammalian nervous system from behavioral and autonomic shut-down during periods of prolonged stress or immobility (see Porges, 1998; 2003). This hypothesis is supported by the fact that the isolation-enhanced immobility in the FST was reduced in oxytocin-treated females (Figure 4).
Specific limitations of the current study design may have had an impact on the findings described here. A limitation of the present study (and also inherent in many studies of social interactions) is that the experimental design may have introduced uncontrolled variables associated with temporary separation of control animals from their respective siblings. However, if this were a significant confounding factor in the current results, the differences between paired and isolated groups may have been less pronounced or absent; whereas several robust differences in both behavior and cardiac function were observed between paired and isolated animals. A second limitation of the present experimental design involves the type of isolation employed. Although the disruption of established sibling bonds was the focus of the current investigation for the reasons cited above (see Methods), it may also be relevant to investigate the disruption of established opposite-sex bonds [such as the design described by Bosch and colleagues (2008)]. A third limitation of this study is that the behavioral variables studied here were focused very specifically on those that have been shown to be relevant to depression in rodents. Because other behaviors (such as those relevant to anxiety) may be very important in the context of social behavior, peptides, and autonomic function, future studies will benefit from focusing on additional behavioral alterations in prairie voles exposed to long-term social isolation. A final limitation of the present study was the lack of a specific investigation of central versus peripheral administration of oxytocin. While this may limit the conclusions that can be drawn from the current results with respect to the precise site(s) of action of oxytocin, the present findings provide a first step in increasing our understanding of potential peptide involvement in mediating behavioral and physiological responses to long-term social isolation.
In summary, the present findings demonstrate that administration of exogenous oxytocin mediates behavioral and physiological consequences of long-term social isolation in female prairie voles. Understanding the neural, autonomic, and behavioral pathways of oxytocin’s beneficial effects will offer an important perspective on the protective role of peptides and social support in the context of both physical and emotional disorders.
Table 4.
Body weights in paired and isolated groups treated with either oxytocin (20µ/50µ/vole) or vehicle (50µ/vole) in Experiment 2.
| Baseline | Week 4 | |
|---|---|---|
| (g) | Isolation/Pairing (g) | |
| Paired+V | 35 ± 2 | 36 ± 4 |
| Paired+OT | 38 ± 1 | 39 ± 2 |
| Isolated+V | 38 ± 3 | 40 ± 4 |
| Isolated+OT | 39 ± 3 | 37 ± 3 |
Note: Values represent mean ± SEM. The body weights of the groups in Experiment 2 were slightly lower than those in Experiment 1.
Acknowledgements
This research was funded by National Institutes of Health grants MH67446 (SWP), MH72935 (CSC), MH73233 (AJG), and MH77581 (AJG). The investigators thank Iman Hassan, Damon Lamb, Gregory Lewis, Lisa Sanzenbacher, Eric Schmidt, Maulin Shah, and Patrin Suppatkul for valuable assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arletti R, Benelli A, Bertolini A. Oxytocin involvement in male and female sexual behavior. Ann. NY Acad. Sci. 1992;652:180–193. doi: 10.1111/j.1749-6632.1992.tb34354.x. [DOI] [PubMed] [Google Scholar]
- Arletti R, Bertolini A. Oxytocin acts as an antidepressant in two animal models of depression. Life Sci. 1987;41:1725–1730. doi: 10.1016/0024-3205(87)90600-x. [DOI] [PubMed] [Google Scholar]
- Berkman LF, Melchior M, Chastang JF, Niedhammer I, Leclerc A, Goldberg M. Social integration and mortality: a prospective study of French employees of Electricity of France-Gas of France: the GAZEL Cohort. Am. J. Epidemiol. 2004;159:167–174. doi: 10.1093/aje/kwh020. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Nair HP, Ahern TH, Neumann ID, Young LJ. The CRF system mediates increased passive stress-coping behavior following the loss of a bonded partner in a monogamous rodent. Neuropsychopharmacology. 2008;34:1406–1415. doi: 10.1038/npp.2008.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Hawkley LC, Crawford LE, Ernst JM, Burleson MH, Kowalewski RB, Malarkey WB, Van Cauter E, Berntson GG. Loneliness and health: potential mechanisms. Psychosom. Med. 2002;64:407–417. doi: 10.1097/00006842-200205000-00005. [DOI] [PubMed] [Google Scholar]
- Caldwell JD, Walker CH, O’Rourke ST, Faggin BM, Morris M, Mason GA. Analogies between oxytocin systems of the uterus and brain. Horm. Metabol. Res. 1996;28:65–74. doi: 10.1055/s-2007-979131. [DOI] [PubMed] [Google Scholar]
- Carter CS. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology. 1998;23:779–818. doi: 10.1016/s0306-4530(98)00055-9. [DOI] [PubMed] [Google Scholar]
- Carter CS. Developmental consequences of oxytocin. Physiol. Behav. 2003;79:383–397. doi: 10.1016/s0031-9384(03)00151-3. [DOI] [PubMed] [Google Scholar]
- Carter CS, DeVries AC, Getz LL. Physiological substrates of mammalian monogamy: the prairie vole model. Neurosci. Biobehav. Rev. 1995;19:303–314. doi: 10.1016/0149-7634(94)00070-h. [DOI] [PubMed] [Google Scholar]
- Carter CS, Keverne EB. The neurobiology of social affiliation and pair bonding. Horm. Brain Behav. 2002;1:299–337. [Google Scholar]
- Carter CS, Witt DM, Schneider J, Harris ZL, Volkening D. Male stimuli are necessary for female sexual behavior and uterine growth in prairie voles (Microtus ochrogaster) Horm. Behav. 1987;21:74–82. doi: 10.1016/0018-506x(87)90032-8. [DOI] [PubMed] [Google Scholar]
- Cho MM, DeVries AC, Williams JR, Carter CS. The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster) Behav. Neurosci. 1999;113:1071–1080. doi: 10.1037//0735-7044.113.5.1071. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci. Biobehav. Rev. 2005;29:547–569. doi: 10.1016/j.neubiorev.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Cushing BS, Carter CS. Peripheral pulses of oxytocin increase partner preferences in female, but not male, prairie voles. Horm. Behav. 2000;37:49–56. doi: 10.1006/hbeh.1999.1558. [DOI] [PubMed] [Google Scholar]
- De Wied D, Diamant M, Fodor M. Central nervous system effects of the neurohypophyseal hormones and related peptides. Front. Neuroendocrinol. 1993;14:251–302. doi: 10.1006/frne.1993.1009. [DOI] [PubMed] [Google Scholar]
- DeVries GJ, Panzica GC. Sexual differentiation of central vasopressin and vasotocin systems in vertebrates: different mechanisms, similar endpoints. Neuroscience. 2006;138:947–955. doi: 10.1016/j.neuroscience.2005.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermisch A, Ruhle HJ, Landgraf R, Hess J. Blood-brain barrier and peptides. J. Cerebral Blood Flow Metab. 1985;5:350–357. doi: 10.1038/jcbfm.1985.49. [DOI] [PubMed] [Google Scholar]
- Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J. Neurosci. 2001;21:8278–8285. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT. Social amnesia in mice lacking the oxytocin gene. Nature Genetics. 2000;25:284–288. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- Ferrari R, Censi S, Mastrorilli F, Boraso A. Prognostic benefits of heart rate reduction in cardiovascular disease. Eur. Heart. J. 2003;Suppl. 5 Suppl. G:G10–G14. [Google Scholar]
- Francis J, Weiss RM, Wei SG, Johnson AK, Felder RB. Progression of heart failure after myocardial infarction in the rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;281:R1734–R1745. doi: 10.1152/ajpregu.2001.281.5.R1734. [DOI] [PubMed] [Google Scholar]
- Getz LL, Carter CS. Prairie-vole partnerships. Am. Scientist. 1996;84:56–62. [Google Scholar]
- Gibbs DM. Dissociation of oxytocin, vasopressin and corticotropin secretion during different types of stress. Life Sci. 1984;35:487–491. doi: 10.1016/0024-3205(84)90241-8. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Beltz TG, Weiss RM, Johnson AK. The effects of chronic fluoxetine treatment on chronic mild stress-induced cardiovascular changes and anhedonia. Biol. Psychiatry. 2006;59:309–316. doi: 10.1016/j.biopsych.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Cushing BS, Carter CS. Depression-like behavior and stressorinduced neuroendocrine activation in female prairie voles exposed to chronic social isolation. Psychosom. Med. 2007a;69:149–157. doi: 10.1097/PSY.0b013e31802f054b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Gerena D, Huang J, Kumar N, Shah M, Ughreja R, Carter CS. Social isolation induces behavioral and neuroendocrine disturbances relevant to depression in female and male prairie voles. Psychoneuroendocrinology. 2007b;32:966–980. doi: 10.1016/j.psyneuen.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Lamb DG, Carter CS, Porges SW. Cardiac regulation in the socially monogamous prairie vole. Physiol. Behav. 2007c;90:386–393. doi: 10.1016/j.physbeh.2006.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Lamb DG, Carter CS, Porges SW. Social isolation disrupts autonomic regulation of the heart and influences negative affective behaviors. Biol. Psychiatry. 2007d;62:1162–1170. doi: 10.1016/j.biopsych.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Moffitt JA, Johnson AK. Cardiovascular alterations and autonomic imbalance in an experimental model of depression. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002;282:R1333–R1341. doi: 10.1152/ajpregu.00614.2001. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Wu KD, Hassan I, Carter CS. Social isolation in prairie voles induces behaviors relevant to negative affect: toward the development of a rodent model focused on co-occurring depression and anxiety. Depress. Anxiety. 2008;25:E17–E26. doi: 10.1002/da.20375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzzetti S, La Rovere MT, Pinna GD, Maestri R, Borroni E, Porta A, Mortara A, Malliani A. Different spectral components of 24 h heart rate variability are related to different modes of death in chronic heart failure. Eur. Heart J. 2005;26:357–362. doi: 10.1093/eurheartj/ehi067. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol. Psychiatry. 2003;54:1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Domes G. Neuropeptides and social behavior: effects of oxytocin and vasopressin in humans. Prog. Brain Res. 2008;170:337–350. doi: 10.1016/S0079-6123(08)00428-7. [DOI] [PubMed] [Google Scholar]
- Higa KT, Mori E, Viani FF, Morris M, Michelini LC. Baroreflex control of heart rate by oxytocin in the solitary-vagal complex. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002;282:R537–R545. doi: 10.1152/ajpregu.00806.2000. [DOI] [PubMed] [Google Scholar]
- Insel TR, Wintink AJ. Central administration of oxytocin modulates the infant rat's response to social isolation. Eur. J. Pharmacol. 1991;203:149–152. doi: 10.1016/0014-2999(91)90806-2. [DOI] [PubMed] [Google Scholar]
- Ishii K, Kuwahara M, Tsubone H, Sugano S. Autonomic nervous function in mice and voles (Microtus arvalis): investigation by power spectral analysis of heart rate variability. Lab. Animals. 1996;30:359–364. doi: 10.1258/002367796780739880. [DOI] [PubMed] [Google Scholar]
- Jankowski M, Danalache B, Wang D, Bhat P, Hajjar F, Marcinkiewicz M, Paquin J, McCann SM, Gutkowska J. Oxytocin in cardiac ontogeny. Proc. Natl. Acad. Sci. 2004;31:13074–13079. doi: 10.1073/pnas.0405324101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PM, Robinson IC. Differential clearance of neurophysin and neurohypophyseal peptides from cerebrospinal fluid in conscious guinea pigs. Neuroendocrinology. 1982;34:297–302. doi: 10.1159/000123316. [DOI] [PubMed] [Google Scholar]
- Keller MB, Klein DN, Hirschfeld RM, Kocsis JH, McCullough JP, Miller I, First MB, Holzer CP, Keitner GI, Marin DB. Results of the DSM-IV mood disorder field trial. Am. J. Psychiatry. 1995;152:843–849. doi: 10.1176/ajp.152.6.843. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Newton TL. Marriage and health: his and hers. Psychol. Bull. 2001;127:472–503. doi: 10.1037/0033-2909.127.4.472. [DOI] [PubMed] [Google Scholar]
- Knox SS, Uvnäs-Moberg K. Social isolation and cardiovascular disease: an atherosclerotic pathway? Psychoneuroendocrinology. 1998;23:877–890. doi: 10.1016/s0306-4530(98)00061-4. [DOI] [PubMed] [Google Scholar]
- Konkle AT, Baker SL, Kentner AC, Barbagallo LS, Merali Z, Bielajew C. Evaluation of the effects of chronic mild stressors on hedonic and physiological responses: sex and strain compared. Brain Res. 2003;992:227–238. doi: 10.1016/j.brainres.2003.08.047. [DOI] [PubMed] [Google Scholar]
- Krantz DS, McCeney MK. Effects of psychological and social factors on organic disease: a critical assessment of research on coronary heart disease. Annu. Rev. Psychol. 2002;53:341–369. doi: 10.1146/annurev.psych.53.100901.135208. [DOI] [PubMed] [Google Scholar]
- Legros JJ, Chiodera P, Geenen V, von Frenckell R. Confirmation of the inhibitory influence of exogenous oxytocin in cortisol and ACTH in man: evidence of reproducibility. Acta Endocrinol. 1987;114:345–349. doi: 10.1530/acta.0.1140345. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Trujillo KA, Akil H, Young EA. Motivational properties of oxytocin in the conditioned place preference paradigm. Neuropsychopharmacology. 1997;17:353–359. doi: 10.1016/S0893-133X(97)00070-5. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Young EA. Effects of stress and glucocorticoids on CNS oxytocin receptor binding. Psychoneuroendocrinology. 1997;22:411–422. doi: 10.1016/s0306-4530(97)00045-0. [DOI] [PubMed] [Google Scholar]
- Mantella RC, Vollmer RR, Rinaman L, Li X, Amico JA. Enhanced corticosterone concentrations and attenuated Fos expression in the medial amygdala of female oxytocin knockout mice exposed to psychogenic stress. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;287:1495–1504. doi: 10.1152/ajpregu.00387.2004. [DOI] [PubMed] [Google Scholar]
- Michelini LC, Marcelo MC, Amico J, Morris M. Oxytocinergic regulation of cardiovascular function: studies in oxytocin-deficient mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;284:H2269–H2276. doi: 10.1152/ajpheart.00774.2002. [DOI] [PubMed] [Google Scholar]
- Neumann ID. Involvement of the brain oxytocin system in stress coping: interactions with the hypothamo-pituitary-adrenal axis. Prog. Brain Res. 2002;139:147–162. doi: 10.1016/s0079-6123(02)39014-9. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Krömer SA, Toschi N, Ebner K. Brain oxytocin inhibits the (re)activity of the hypothalamo-pituitary-adrenal axis in male rats: involvement of hypothalamic and limbic brain regions. Regul Pept. 2000;96:31–38. doi: 10.1016/s0167-0115(00)00197-x. [DOI] [PubMed] [Google Scholar]
- Nishioka T, Anselmo-Franci JA, Li P, Callahan MF, Morris M. Stress increases oxytocin release within the hypothalamic paraventricular nucleus. Brain Res. 1998;781:57–61. doi: 10.1016/s0006-8993(97)01159-1. [DOI] [PubMed] [Google Scholar]
- Petersson M, Alster P, Lundeberg T, Uvnäs-Moberg K. Oxytocin causes a long-term decrease of blood pressure in female and male rats. Physiol. Behav. 1996;60:1311–1315. doi: 10.1016/s0031-9384(96)00261-2. [DOI] [PubMed] [Google Scholar]
- Petersson M, Diaz-Cabiale Z, Narváez JA, Fuxe K, Uvnäs-Moberg K. Oxytocin increases the density of high affinity alpha2-adrenoceptors within the hypothalamus, the amygdala and the nucleus of the solitary tract in ovariectomized rats. Brain Res. 2005;1049:234–239. doi: 10.1016/j.brainres.2005.05.034. [DOI] [PubMed] [Google Scholar]
- Petersson M, Uvnäs-Moberg K, Erhardt S, Engberg G. Oxytocin increases locus coeruleus alpha 2-adrenoreceptor responsiveness in rats. Neurosci. Lett. 1998;255:115–118. doi: 10.1016/s0304-3940(98)00729-0. [DOI] [PubMed] [Google Scholar]
- Pitzalis MV, Iacoviello M, Todarello O, Fioretti A, Guida P, Massari F, Mastropasqua F, Russo GD, Rizzon P. Depression but not anxiety influences the autonomic control of heart rate after myocardial infarction. Am. Heart J. 2001;141:765–771. doi: 10.1067/mhj.2001.114806. [DOI] [PubMed] [Google Scholar]
- Porges SW. Love: an emergent property of the mammalian autonomic nervous system. Psychoneuroendocrinology. 1998;23:837–861. doi: 10.1016/s0306-4530(98)00057-2. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal theory: phylogenetic substrates of a social nervous system. Int. J. Psychophysiol. 2001;42:123–146. doi: 10.1016/s0167-8760(01)00162-3. [DOI] [PubMed] [Google Scholar]
- Porges SW. Social engagement and attachment: a phylogenetic perspective. Ann. NY Acad. Sci. 2003;1008:31–47. doi: 10.1196/annals.1301.004. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal perspective. Biol. Psychol. 2007;74:116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW, McCabe PM, Yongue BG. Respiratory-heart rate interactions: psychophysiological implications for pathophysiology and behavior. In: Cacioppo J, Petty R, editors. Perspectives in cardiovascular psychophysiology. New York: Guilford Publications, Inc; 1982. pp. 223–264. [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Prigerson HG, Reynolds CF, III, Frank E, Kupfer DJ, George CJ, Houck PR. Stressful life events, social rhythms, and depressive symptoms among the elderly: an examination of hypothesized causal linkages. Psychiatry Res. 1994;51:33–49. doi: 10.1016/0165-1781(94)90045-0. [DOI] [PubMed] [Google Scholar]
- Sgoifo A, Stilli D, Medici D, Gallo P, Aimi B, Musso E. Electrode positioning for reliable telemetry ECG recordings during social stress in unrestrained rats. Physiol. Behav. 1996;60:1397–1401. doi: 10.1016/s0031-9384(96)00228-4. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Owen N, Kunz-Ebrecht SR, Brydon L. Loneliness and neuroendocrine, cardiovascular, and inflammatory stress responses in middle-aged men and women. Psychoneuroendocrinology. 2004;29:593–611. doi: 10.1016/S0306-4530(03)00086-6. [DOI] [PubMed] [Google Scholar]
- Takayanagi Y, Yoshida M, Bielsky IF, Ross HE, Kawamata M, Onaka T, Yanagisawa T, Kimura T, Matzuk MM, Young LJ, Nishimori K. Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. Proc. Natl. Acad. Sci. 2005;102:16096–16101. doi: 10.1073/pnas.0505312102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Task Force of the European Society of Cardiology & North American Society of Pacing Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- Taylor SE, Gonzaga GC, Klein LC, Hu P, Greendale GA, Seeman TE. Relation of oxytocin to psychological stress responses and hypothalamic-pituitary-adrenocortical axis activity in older women. Psychosom. Med. 2006;68:238–245. doi: 10.1097/01.psy.0000203242.95990.74. [DOI] [PubMed] [Google Scholar]
- Uvnäs-Moberg K. Oxytocin may mediate the benefits of positive social interaction and emotions. Psychoneuroendocrinology. 1998;23:819–835. doi: 10.1016/s0306-4530(98)00056-0. [DOI] [PubMed] [Google Scholar]
- Willner P. The validity of animal models of depression. Psychopharmacology. 1984;83:1–16. doi: 10.1007/BF00427414. [DOI] [PubMed] [Google Scholar]
- Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52:90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- Willner P, Moreau J-L, Nielsen CK, Papp M, Sluzewska A. Decreased hedonic responsiveness following chronic mild stress is not secondary to loss of body weight. Physiol. Behav. 1996;60:129–134. doi: 10.1016/0031-9384(95)02256-2. [DOI] [PubMed] [Google Scholar]
- Willner P, Muscat R, Papp M. Chronic mild stress-induced anhedonia: a realistic animal model of depression. Neurosci. Biobehav. Rev. 1992;16:525–534. doi: 10.1016/s0149-7634(05)80194-0. [DOI] [PubMed] [Google Scholar]
- Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology. 1987;93:358–364. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- Windle RJ, Shanks N, Lightman SL, Ingram CD. Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats. Endocrinology. 1997;138:2829–2834. doi: 10.1210/endo.138.7.5255. [DOI] [PubMed] [Google Scholar]
- Yongue BG, McCabe PM, Porges SW, Rivera M, Kelley SL, Ackles PK. The effects of pharmacological manipulations that influence vagal control of the heart on heart period, heart-period variability and respiration in rats. Psychophysiology. 1982;19:426–432. doi: 10.1111/j.1469-8986.1982.tb02499.x. [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang Z. The neurobiology of pair bonding. Nature Neurosci. 2004;7:1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]