Abstract
The main purpose of this study was to determine if differences in life spans of two different strains of mice are associated with the thiol redox state of their tissues and mitochondria. A comparison, based on amounts of reduced and oxidized glutathione (GSH, GSSG) and reactive protein thiols, was made between short-lived SAM (P8) mice and the longer-lived C57BL/6 mice at 13 months of age. The average life span of the latter mouse strain is approximately 48% longer than the former strain. Analyses of plasma, tissue homogenates and mitochondria of liver, kidney, heart, brain and skeletal muscle indicated that, in general, amounts of GSH and reactive protein sulfhydryls and GSH:GSSG ratios were lower and concentrations of GSSG were higher in the SAM than the C57BL/6 mice. Differences in the redox state between the two strains were more consistent and pronounced in skeletal muscle than in other tissues, and in mitochondria than in their respective tissue homogenates. Overall, the results support the view that the shorter-lived SAM mice exhibit a relatively higher level of oxidative stress than the longer-lived C57BL/6 mice, which is consistent with the predictions of the oxidative stress hypothesis of aging. Intra-species comparisons may be useful for the identification of biochemical characteristics associated with the variations in life spans.
Keywords: Senescence-accelerated mouse (SAM), Glutathione, Mitochondria, Aging, Oxidative stress, Reactive protein sulfhydryls
1. Introduction
Although the nature of the biochemical mechanisms underlying the aging process is presently poorly understood, free radicals and hydroperoxides, collectively termed ‘reactive oxygen species’ (ROS), have been widely postulated to play a causal role in the induction of senescent alterations (Harman, 1956). There are presently two different, but compatible, notions about the mechanisms by which ROS may cause age-related attenuations in cellular functions (Weindruch and Sohal, 1997). The classical view is that age-associated losses in the efficiency of cellular functions are primarily due to the accumulation of ROS inflicted oxidative damage to macromolecules, such as proteins, nucleic acids and lipids (reviewed in Beckman and Ames, 1998; Sohal et al., 2002). Indeed, a large body of correlative data demonstrates the link between aging and the increase of reaction products of ROS and cellular macromolecules in a variety of different species (Levine and Stadtman, 2001). Such ROS-mediated damage, particularly that to proteins, has been shown to deleteriously affect cellular functions (Stadtman and Levine, 2000; Sohal, 2002). The other, more recent, view is that besides the infliction of molecular damage, ROS also cause a gradual prooxidizing shift in the redox state of cells, which results in progressive alterations in cellular signaling and gene expression, thereby propelling the cells into increasingly advanced states of cellular senescence (Droge, 2002; Finkel, 2003).
The mechanism by which ROS alter overall cellular redox state is thought to initially involve oxidation of free and protein-bound accessible thiols (Reed, 1986; Schafer and Buettner, 2001; Giles et al., 2003). Although there are several redox couples, which collectively establish the cellular redox state, reduced:oxidized glutathione (GSH:GSSG) is the most abundant pair. Thus, the GSH:GSSG ratio is considered to be a sensitive indicator of the cellular redox state (Jones, 2002). Furthermore, loss of protein sulfhydryl groups and glutathionylation or thiolation can potentially regulate the functions of certain proteins (Cotgreave and Gerdes, 1998; Klatt and Lamas, 2000; Thomas and Mallis, 2001).
Results of previous studies in mice have indicated that during aging the glutathione redox state (GSH:GSSG ratio) in tissues and mitochondria shifts progressively towards oxidation (Rebrin et al., 2003, 2004). Concomitantly, there is an elevation in mitochondrial superoxide anion radical and hydrogen peroxide (H2O2) generation and an increase in the amounts of molecular damage, indicated by products such as protein carbonyls and 8-hydroxydeoxy-guanosine (reviewed in Sohal and Weindruch, 1996; Beckman and Ames, 1998). Reduction of caloric intake, which extends the life span of mice, retards such age-associated increases in rates of mitochondrial generation and in the amounts of oxidatively modified macromolecules, and attenuates the severity of the prooxidizing shift in the glutathione redox state (Sohal et al., 1994; Rebrin et al., 2003). Thus, it seems that the prooxidizing shift in the glutathione redox state of tissues in the mouse is associated with aging and life expectancy.
Various strategies have been employed to identify age-associated biochemical alterations that may play a causal role in the aging process. One approach, to identify potential longevity determinants, is to ascertain whether or not intra-species differences in life span are indeed correlating with suspected causal factors. The rationale is that if differences in life spans reflect an underlying variation in the rate of aging, it may be reasoned that at similar chronological ages, the biochemical alterations associated with the aging process should be at a more advanced stage in the shorter-lived than in the longer-lived strain. The present study examines whether the thiol redox state is a correlate of life span of two different strains of mice, namely C57BL/6 and SAM (senescence accelerated mice): the former has 48% longer average life span than the latter.
Specifically, the present study reports a comparison of GSH and GSSG content, GSH:GSSG ratios and amounts of reactive protein sulfhydryls in homogenates and mitochondria of liver, brain, heart, skeletal muscle and kidney, between the longer-lived C57BL/6 mouse and the shorter-lived SAM (P8) mouse.
2. Materials and methods
2.1. Reagents
Calibration standards (GSH, GSSG and cysteine) were obtained from Sigma Chemical Co. (St Louis, MO). Acetonitrile, meta-phosphoric acid (MPA) and 1-octane sulfonic acid were acquired from EM Science (Gibbstown, NJ). Deionized water was prepared using a Millipore Milli-Q System. All other chemicals were HPLC grade or of the highest purity available.
2.2. Animals
Male SAM and C57BL/6Nnia mice (13 months of age) were obtained from the National Institute on Aging, National Institutes of Health. SAM (P8) (senescence accelerated-prone mice) mice were originally received from the University of California, Berkley. Mice were fed ad libitum and maintained under standard conditions.
2.3. Isolation of mitochondria
At 13 months of age, mice were killed by cervical dislocation and their tissues (liver, kidney, brain, heart and skeletal muscle) were quickly removed and placed in ice-cold anti-oxidant buffer (50 mM potassium phosphate buffer, pH 7.4, containing 2 mM EDTA and 0.1 mM butylated hydroxytoluene). Mice (two animals in each age group) were usually dissected within 1 h, and all subsequent procedures were performed at 4 °C. EDTA-containing blood (50 µl of 100 mM EDTA solution for each ml of blood) was centrifuged at 2000×g for 3 min to separate plasma. Tissue homogenization procedures and buffers used for isolation of mitochondria were identical to those described previously (Rebrin et al., 2003). Briefly, pieces of minced tissues were homogenized in 10 vol (w/v) of the indicated isolation buffer: for liver tissue, 0.25 M sucrose, 3 mM EDTA, 10 mM Tris buffer, pH 7.4; for kidney, 0.22 M mannitol, 70 mM sucrose, 10 mM EGTA, 2 mM Hepes, pH 7.4; for heart, 0.3 M sucrose, 0.03 M nicotinamide, 0.02 M EDTA, pH 7.4; for brain, 0.32 M sucrose, 1 mM EDTA, 10 mM Tris, pH 7.4; for skeletal muscle, buffer 1, containing 0.12 M KCl, 2 mM MgCl2, 1 mM EGTA, 0.5 mg/ml BSA, 20 mM Hepes, pH 7.4 and buffer 2, containing 0.3 M sucrose, 0.1 mM EGTA, 2 mM Hepes, pH 7.4. Low and high speed differential centrifugations for mitochondrial isolation were, respectively, 1000 g for 10 min and 17,500 g for 10 min for liver; 600 g for 10 min and 15,000 g for 10 min for kidney; 700 g for 10 min and 10,000 g for 10 min for heart; 1300 g for 3 min and 21,200 g for 10 min for brain; 600 g for 12 min and 17,000 g for 12 min in buffer 1 and 1200 g for 12 min and 12,000 g for 12 min in buffer 2 for skeletal muscle. Mitochondria from all tissues were isolated within 1–2 h after tissue dissection, except those from brain, which required a longer isolation time (up to 3 h) due to additional steps involving Percoll gradient centrifugation (Sims, 1993).
2.4. Sample preparation for glutathione analysis
Immediately after homogenization of the tissues, 200 µl aliquots of the crude homogenates were mixed with 200 µl of ice-cold 10% (w/v) MPA, incubated for 30 min on ice and centrifuged for 20 min at 14,000×g at 4 °C. Supernatants were transferred to autosample vials and either injected immediately or stored at −80 °C until analysis. Storage of deproteinized tissue samples in 5% (w/v) MPA at −80 °C for up to 6 months was found to have no effect on the concentrations of GSH and GSSG.
For measurements of mitochondrial glutathione content, aliquots (150–200 µl) of fresh mitochondrial preparations were washed once with a 100-fold excess of buffer and pelleted by centrifugation. This washing step was found to be critical in order to remove excess GSH and GSSG from the cytosolic fraction. Mitochondria were then resuspended in 200 µl of 5% (w/v) MPA. After 20 min of incubation on ice, samples were centrifuged for 20 min at 18,000 g at 4 °C. Supernatants were transferred into autosample vials and used for injections immediately or stored at −80 °C until analysis.
For the measurements of reactive protein sulfhydryls, 200 µl of homogenates were mixed with 50 µl of solution containing 50 mM GSSG. After incubation of this mixture for 2 h at 37 °C, 250 µl of ice-cold 10% MPA was added. After 20 min of incubation on ice, samples were centrifuged for 20 min at 18,000 g at 4 °C. Protein pellets from acid precipitation were washed three times in 5% MPA to remove the free (non-protein bound) glutathione. Protein-bound glutathione was subsequently released by incubation of washed protein pellets with 0.5 M sodium borohydride for 30 min at 37 °C.
The protein content of homogenates and mitochondria was determined by the BCA protein assay, according to the manufacturer’s instructions (Pierce, Rockford, IL).
2.5. HPLC-Coulometric EC detection
Samples were separated by HPLC, equipped with a Shimadzu Class VP solvent delivery system, using a reverse phase C18 Luna (II) column (3 μ; 4.6× 150 mm), obtained from Phenomenex (Torrance, CA, USA). The mobile phase for isocratic elution consisted of 25 mM monobasic sodium phosphate, 0.5 mM of the ion-pairing agent 1-octane sulfonic acid, 2% (v/v) acetonitrile, pH 2.7, adjusted with 85% phosphoric acid. The flow rate was 0.7 ml/min. Under these conditions, the separation of aminothiols was completed in 35 min; cysteine was the first and GSSG was the last eluting peak, with retention times of 5 and 30 min, respectively. Deproteinated samples were injected directly onto the column using a Shimadzu autosampler. Calibration standards were prepared by dilution of 2 mM stock solutions of cysteine, GSH and GSSG in 5% (w/v) meta-phosphoric acid, and were injected at regular intervals to ensure uniform standardization. Each sample was injected twice, and the average of the peak areas was used for calculations of the aminothiol concentrations. Following HPLC separation, aminothiols were detected with a model 5600 CoulArray® electrochemical detector (ESA, Inc., Chelmsford, MA, USA), equipped with a four-channel analytical cell. Increasing potentials of + 400, + 600, + 750 and + 875 mV were applied on channels 1–4, respectively. Cysteine and GSH were detected on channel 3 (+ 750 mV), whereas GSSG was detected on channel 4 (+ 875 mV). The low potentials on channels 1 (+ 400 mV) and 2 (+ 600 mV) were used as an oxidative screen to eliminate interfering compounds that oxidize at a lower voltage than the GSH and GSSG.
2.6. Statistics
Statistical significance was determined by unpaired Student’s t-tests. P < 0.05 was considered to be significant.
3. Results
3.1. Life spans
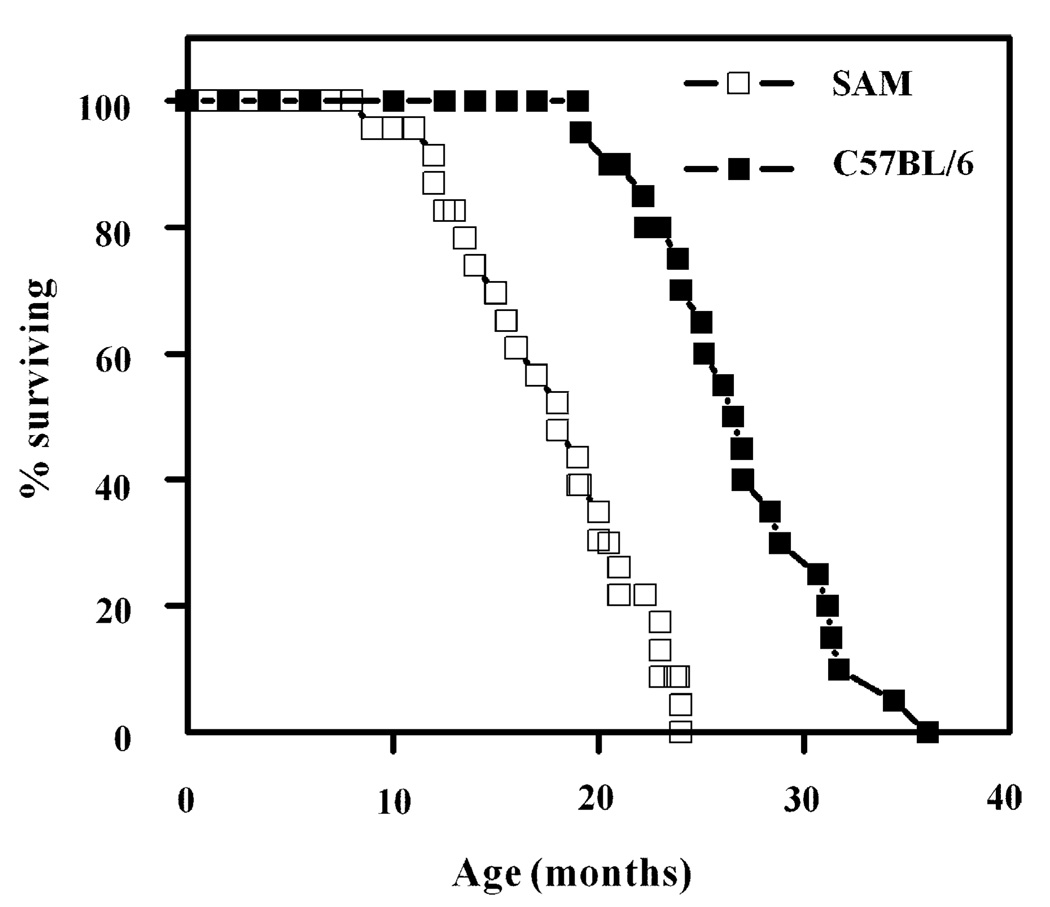
Survivorship curves of male C57BL/6 and SAM (P8) mice are presented in Fig. 1. The average life span of the SAM mice was 17.8 ± 4.3 months and the age at which 90% mortality had occurred was 23 months (n = 18), whereas the average life span and age of 90% mortality of male C57BL/6 mice were 26.4 ± 4.0 and 31.3 months (n = 22), respectively, as reported previously (Forster et al., 2003). Biochemical comparisons were made at 13 months of age, which precedes the onset of dying phase. This should avoid the complications associated with pathologies that occur during this period.
Fig. 1.
Survivorship of C57BL/6 (n = 22) and SAM (P8) (n = 18) mice as a function of age. Mortality data for C57BL/6 mice were taken from a previous study (Forster et al., 2003).
3.2. Comparison of GSH, GSSG and cysteine content in blood serum between the two strains of mice
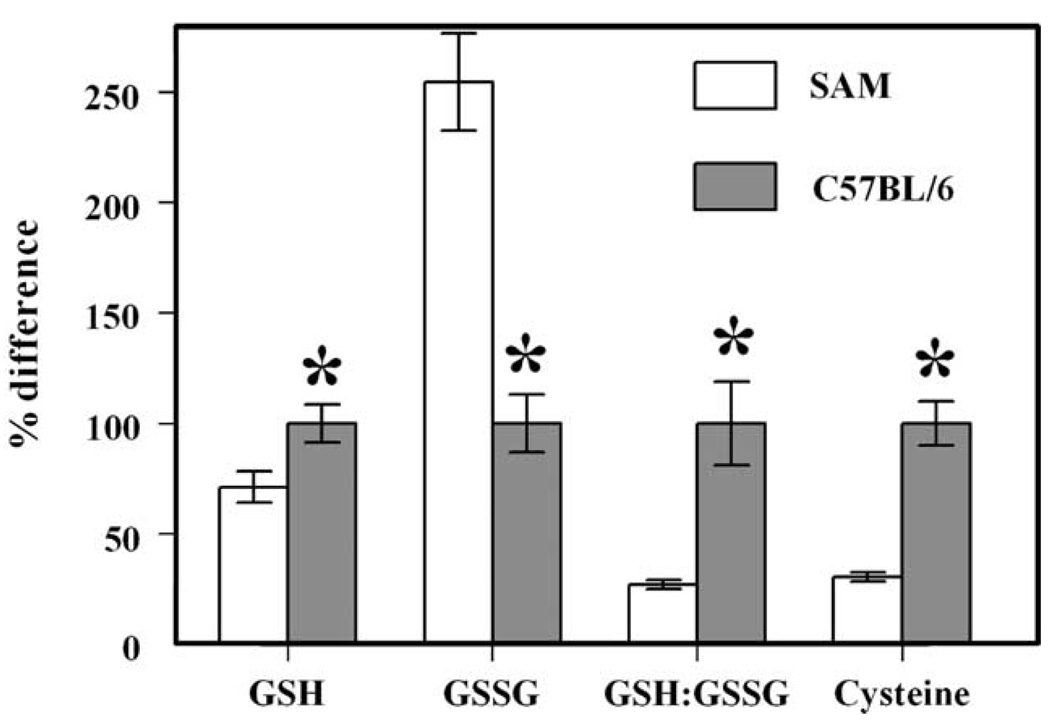
At 13 months of age, the concentrations of GSH and cysteine were, respectively, 29 and 69% lower, whereas GSSG content was 2.5-fold greater in the SAM mice, as compared to the C57BL/6 mice (Fig. 2). Consequently, the GSH:GSSG ratio was 70% lower in SAM than in C57BL/6 mice.
Fig. 2.
Percent differences in concentrations of GSH, GSSG and cysteine and GSH:GSSG ratio in blood serum between 13-month-old SAM (n = 5) and C57BL/6 (n = 6) mice. The absolute concentrations of GSH, GSSG and cysteine in blood serum of the C57BL/6 mouse were 1.76 ± 0.15, 0.038 ± 0.008 and 6.58 ± 0.67 µM, respectively, and the GSH:GSSG ratio was 47 ± 9. All values are mean ± s.d.; the *indicates a significant difference (P<0.005) based on unpaired t-test.
3.3. Comparison of GSH, GSSG and reactive protein sulfhydryl content in tissue homogenates
There were considerable variations in GSH content (up to 17-fold) among homogenates of different tissues (Table 1). In both strains of mice, the rank order of GSH concentration was liver > brain > heart > skeletal muscle > kidney. In C57BL/6 mice, in comparison to the liver, the relative amounts of GSH were 10% in kidney, 39% in heart, 51% in brain and 11% in the hindlimb skeletal muscle. GSSG concentration also varied several fold in different tissues. Compared to the level in the liver, the GSSG concentration was 83% in brain, 30% in heart, 25% in skeletal muscle and 133% in the kidney.
Table 1.
Concentrations of reduced glutathione (GSH) and glutathione disulfide (GSSG) in homogenates and mitochondria of different tissues of SAM (n = 5), and C57BL/6 (n = 6) mice at 13 months of age
| Tissues | Homogenate | Mitochondria | ||
|---|---|---|---|---|
| GSH (nmol/mgprotein) | GSSG (nmol/mgprotein) | GSH (nmol/mgprotein) | GSSG (nmol/mgprotein) | |
| Liver | ||||
| SAM | 25.2± ±2.1 | 0.18±0.06 | 7.17±0.48# | 0.18±0.02# |
| C57BL | 26.3±1.9 | 0.12±0.02 | 9.41±1.06 | 0.11±0.01 |
| Brain | ||||
| SAM | 12.6±2.2 | 0.10±0.02 | 4.22±0.25# | 0.030±0.005 |
| C57BL | 13.5±0.9 | 0.10±0.01 | 6.31±0.43 | 0.025±0.002 |
| Heart | ||||
| SAM | 6.3±0.4# | 0.28±0.09 | 4.54±0.33 | 0.30±0.04 |
| C57BL | 10.3±0.5 | 0.36±0.06 | 5.10±0.37 | 0.21±0.02 |
| Skeletal muscle | ||||
| SAM | 2.6±0.2# | 0.08±0.01# | 1.12±0.09# | 0.102±0.012# |
| C57BL | 3.0±0.2 | 0.03±0.01 | 1.37±0.11 | 0.048±0.004 |
| Kidney | ||||
| SAM | 1.4±0.2# | 0.18±0.02 | 1.79±0.15 | 0.0080±0002# |
| C57BL | 2.8±0.2 | 0.16±0.01 | 1.99±0.26 | 0.0101±0.0008 |
Denotes significant statistical difference (P<0.05) compared to C57BL/6 mice.
The GSH content of homogenates was similar in liver and brain, but was significantly lower in kidney, heart and skeletal muscle of SAM than C57BL/6 mice. For instance, compared to C57BL/6 mice, the GSH content of SAM mice was 52% in kidney, 60% in heart and 87% in skeletal muscle. The GSSG content of homogenates was similar in the two strains in all the tissues except skeletal muscle where it was relatively higher in the SAM mice.
The GSH:GSSG ratios varied in homogenates from 17:1 in the kidney to 128:1 in the brain of C57BL/6 mice (Fig. 3). Ratios of GSH:GSSG were significantly higher in homogenates of kidney and skeletal muscle of C57BL/6 than SAM mice, but were similar in liver, heart and brain.
Fig. 3.
GSH:GSSG ratios in homogenates (top panel) and mitochondria (bottom panel) of liver (L), kidney (K), heart (H), brain (B) and skeletal muscle (SM) of 13-month-old SAM (n = 5) and C57BL/6 (n = 6) mice. All values are nmol/mg protein ± s.d.; *indicates a significant difference (P<0.005) based on unpaired t-test.
The amounts of reactive protein sulfhydryls were higher in C57BL/6 mice than in SAM mice in all the tissues examined, except the brain, where there was no significant difference (Fig. 4).
Fig. 4.
Amounts of reactive protein sulfhydryls in homogenates of liver (L), kidney (K), heart (H), brain (B) and skeletal muscle (SM) of SAM (n = 5) and C57BL/6 (n = 6) mice at 13 months of age. All values are nmol/mg protein ± s.d.; *indicates a significant difference (P<0.005) based on unpaired t-test.
3.4. Comparison of mitochondrial GSH and GSSG content
In both strains of mice, the GSH content of the mitochondrial fraction was considerably lower than in the corresponding tissue homogenate, except in the kidney of SAM mice, where it was similar (Table 1). In C57BL/6 mice, compared to the homogenates, mitochondrial GSH concentration was 35% in liver, 71% in kidney, 50% in heart, 47% in brain and 45% in skeletal muscle. In general, mitochondrial GSSG concentration was lower than in the respective homogenate.
Mitochondrial GSH content also varied greatly among different tissues. In C57BL/6 mice, compared to the level in the liver mitochondria, GSH concentration was 67% in brain, 54% in heart, 15% in skeletal muscle and 21% in kidney. Similarly, mitochondrial GSSG concentration varied up to 21-fold among different tissues.
Compared to the SAM mice, amounts of GSH in mitochondria from liver, brain and skeletal muscle were, respectively, 32, 50 and 21% higher in the C57BL/6 mice, whereas there were no significant differences in the kidney or heart. In contrast, the GSSG content in mitochondria from the liver and skeletal muscle was significantly higher in SAM than in C57BL/6 mice, although in kidney it was significantly lower (Table 1). The mitochondrial GSH:GSSG ratios were also higher in C57BL/6 mice than in SAM mice in all tissues, except the kidney (Fig. 3). The difference in mitochondrial GSH:GSSG ratio between the two strains was most notable in the brain.
4. Discussion
The main findings of this study are that, in both the SAM and C57BL/6 strains of mice, the redox state differs among different tissues and between mitochondria and their corresponding tissue homogenates. Most of the tissues of the shorter-lived SAM mouse exhibit a lower ratio of GSH:GSSG as well as lower amounts of reactive protein thiols than corresponding tissues of the longer-lived C57BL/6 mouse.
The redox status of tissue is primarily dependent upon the amount of GSH and the ratio between GSH and GSSG (Schafer and Buettner, 2001). Present results indicate the existence of large differences in amounts of GSH and GSSG and GSH:GSSG ratios among homogenates and mitochondria from different tissues of the same mouse. Significant differences in these parameters also exist between tissue homogenates and their respective mitochondria. For instance, up to 17- and 10-fold variations in the amounts of GSH were encountered in different tissue homogenates of SAM and C57BL/6 mice, respectively. The relative concentrations of GSH present in mitochondria as compared to their respective tissue homogenates varied from 35 to 71%. Similarly, the GSH:GSSG ratios in tissue homogenates ranged from 17:1 in the kidney to 128:1 in the brain of C57BL/6 mice. Mitochondrial GSH:GSSG ratios varied not only among different tissues, but also in comparison with their respective tissue homogenates. In contrast, there was considerably less variation in reactive protein sulfhydryl content among different tissues. Altogether, these data suggest that different redox environments exist in different cellular compartments and tissues. The rank order of reducing conditions was liver > brain > heart > skeletal muscle > kidney. The inter-tissue variability also emphasizes that studies on oxidative stress and aging should be conducted on several different tissues and not restricted to a single organ.
The GSH and GSSG contents of tissues are dependent upon a number of factors related to the biosynthesis of GSH and its catabolism by oxidation and degradation (Reed, 1986; Griffith, 1999; Bharath et al., 2002). Concentrations of GSH precursor molecules, such as cysteine, and activities of enzymes such as glutamate cysteine ligase, the rate limiting enzyme in GSH biosynthesis, and GSSG reductase, are thought to be critical factors in the augmentation of GSH. Under aerobic conditions, GSH is catabolised in a variety of reductive reactions, including the reduction of H2O2 by glutathione peroxidase, elimination of xenobiotics by glutathione-S-transferases, reduction of oxidized forms of thioredoxin, dethiolation of proteins, and direct reactions with H2O2 and various other ROS. Thus, lower oxidant load and higher biosynthetic activity would be associated with relatively high level of GSH. Indeed, caloric restriction in mice and rats, a regimen that attenuates rates of mitochondrial generation (Sohal et al., 1994; Barja, 2002) and elevates amounts of coenzyme Q and vitamin E (Kamzalov and Sohal, 2004), as well as catalase in some tissues (Yu, 1996), results in an increase in GSH levels, particularly in mitochondria (Rebrin et al., 2003). A comparison of GSH concentrations in homogenates of different tissues and mitochondria between the SAM and C57BL/6 mice indicates that in three out of five tissues examined the GSH concentration is higher in the latter strain than in the former, which suggests that reductive capacity tends to be greater in the longer-lived strain.
Steady-state concentrations of GSSG and ratios of GSH to GSSG are widely recognized to be sensitive indicators of oxidative stress in tissues (Schafer and Buettner, 2001; Jones, 2002; Droge, 2002). While GSSG concentrations reflect the gap between the rates of GSSG formation and its reconversion to GSH or extrusion from cells, the GSH:GSSG ratio is indicative of the overall redox state. Comparisons of GSSG content together with GSH:GSSG ratios between the two strains of mice, studied here, indicate that except kidney mitochondria, all the tissue homogenates and mitochondria in SAM mice exhibit either a higher amount of GSSG content and/or a lower ratio of GSH:GSSG than the C57BL/6 mice, thereby strongly suggesting that tissues of the former mouse strain exhibit a relatively more oxidizing state than those of the latter strain. This inference is consistent with previous findings made in the SAM mice. As summarized by Hosokava (2002), at comparable ages SAM mice contain relatively higher concentrations of lipid peroxides, protein carbonyls and 8-hydroxy-deoxyguanosine than the longer-lived control mice.
The main question that arises from this study is whether the more oxidizing redox state of SAM mice, compared to the C57BL/6 mice, could be at least partially responsible for the differences in their life spans. Obviously, the correlative nature of the present study cannot establish a cause and effect relationship. Nevertheless, a pro-oxidizing shift in redox state and particularly the thiolation of proteins can induce a multiplicity of alterations in cellular functions. Amounts of reactive protein sulfhydryls, which were lower in SAM than in C57BL/6 mice, have been found previously to decrease in mouse tissues as function of age (Dubey et al., 1996). Loss of protein sulfhydryls is often associated with reversible thiolation of proteins. Protein thiolation is apparently induced by different mechanisms that involve thiol/disulfide exchange or one or two electron oxidations of cysteinyl residues (Costa et al., 2003; Giles et al., 2003). Protein thiolation is potentially reversible by disulfide/thiol exchange or via the mediation of glutaredoxin and thioredoxin. Protein thiolation/dethiolation is considered to be physiologically relevant as proteins falling within diverse functional categories such as signal transduction, transcription factors, and metabolic enzymes have been shown to be regulated by this mechanism (Droge, 2002).
In conclusion, the main contribution of the present study is the demonstration that, at the same chronological age, the shorter-lived SAM mouse strain is at a relatively more advanced state of oxidative stress, as reflected by the thiol redox state, than the longer-lived C57BL/6 mouse strain. This finding, along with the previously reported data that amounts of macromolecular oxidative damage are also relatively higher in the shorter-lived SAM mouse, supports the main prediction of the oxidative stress hypothesis of aging, namely, that the rate of aging is dependent upon the level of oxidative stress. Furthermore, it seems that intra-species comparisons may be useful in the identification of biochemical characteristics associated with variations in the rate of aging.
Acknowledgements
This research was supported by the National Institute on Aging-National Institutes of Health (Grant RO1 AG 13563).
Abbreviations
- GSH
reduced glutathione
- GSSG
oxidized glutathione or glutathione disulfide
- ROS
reactive oxygen species
- SAM
senescence-accelerated mouse.
References
- Barja G. Rate of generation of oxidative stress-related damage on animal longevity. Free Radic. Biol. Med. 2002;33:1167–1172. doi: 10.1016/s0891-5849(02)00910-3. [DOI] [PubMed] [Google Scholar]
- Beckman KB, Ames BN. The free radical theory of aging matures. Physiol. Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- Bharath S, Hsu M, Kaur D, Rajagopalan S, Andersen JK. Glutathione, iron and Parkinson’s desease. Biochem. Pharmacol. 2002;64:1037–1048. doi: 10.1016/s0006-2952(02)01174-7. [DOI] [PubMed] [Google Scholar]
- Costa NJ, Dahm CC, Hurrel F, Taylor ER, Murphy MP. Interaction of mitochondrial thiols with nitric oxide. Antioxid. Redox Signal. 2003;5:291–305. doi: 10.1089/152308603322110878. [DOI] [PubMed] [Google Scholar]
- Cotgreave IA, Gerdes RG. Recent trends in glutathione biochemistry. Glutathione–protein interactions: a molecular link between oxidative stress and cell proliferation? Biochem. Biophys. Res. Commun. 1998;242:1–9. doi: 10.1006/bbrc.1997.7812. [DOI] [PubMed] [Google Scholar]
- Droge W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- Dubey A, Forster MJ, Lal H, Sohal RS. Effect of age and caloric intake on protein oxidation in different brain regions and on behavioral functions of the mouse. Arch. Biochem. Biochem. Biophys. 1996;333:189–197. doi: 10.1006/abbi.1996.0380. [DOI] [PubMed] [Google Scholar]
- Finkel T. Oxidant signals and oxidative stress. Curr. Opin. Cell Biol. 2003;15:247–254. doi: 10.1016/s0955-0674(03)00002-4. [DOI] [PubMed] [Google Scholar]
- Forster MJ, Morris P, Sohal RS. Genotype and age influence the effect of caloric intake on mortality in mice. FASEB J. 2003;17:690–692. doi: 10.1096/fj.02-0533fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles NM, Watts AB, Giles GI, Fry FH, Littlechild JA, Jacob C. Metal and redox modulation of cysteine protein function. Chem. Biol. 2003;10:677–693. doi: 10.1016/s1074-5521(03)00174-1. [DOI] [PubMed] [Google Scholar]
- Griffith OW. Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Radic. Biol. Med. 1999;27:922–935. doi: 10.1016/s0891-5849(99)00176-8. [DOI] [PubMed] [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Hosokava M. A higher oxidative status accelerates senescence and aggravates age-dependent disorders in SAMP strains of mice. Mech. Ageing Dev. 2002;123:1553–1561. doi: 10.1016/s0047-6374(02)00091-x. [DOI] [PubMed] [Google Scholar]
- Jones DP. Redox potential of GSH/GSSG couple: assay and biological significance. Meth. Enzymol. 2002;348:93–112. doi: 10.1016/s0076-6879(02)48630-2. [DOI] [PubMed] [Google Scholar]
- Kamzalov S, Sohal RS. Effect of age and caloric restriction on coenzyme Q and α-tocopherol levels in the rat. Exp. Gerontol. 2004;39:1199–1205. doi: 10.1016/j.exger.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Klatt P, Lamas S. Regulation of protein function by S-glutathiolation in response to oxidative and nitrosative stress. Eur. J. Biochem. 2000;267:4928–4944. doi: 10.1046/j.1432-1327.2000.01601.x. [DOI] [PubMed] [Google Scholar]
- Levine RL, Stadtman ER. Oxidative modification of proteins during aging. Exp. Gerontol. 2001;36:1495–1502. doi: 10.1016/s0531-5565(01)00135-8. [DOI] [PubMed] [Google Scholar]
- Rebrin I, Kamzalov S, Sohal RS. Effects of age and caloric restriction on glutathione redox state in mice. Free Radic. Biol. Med. 2003;35:626–635. doi: 10.1016/s0891-5849(03)00388-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebrin I, Bayne A-CV, Mockett RJ, Sohal RS. Free aminothiols, glutathione redox state and protein mixed disulfides in aging drosophila melanogaster. Biochem. J. 2004;382:131–136. doi: 10.1042/BJ20040506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed DJ. Regulation of reductive processes by glutathione. Biochem. Pharmacol. 1986;35:7–13. doi: 10.1016/0006-2952(86)90545-9. [DOI] [PubMed] [Google Scholar]
- Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- Sims NR. Mitochondrial isolation from brain: strategy, techniques, and criteria of purity. In: Lash LH, Jones DP, editors. Mitochondrial Dysfunction. San Diego, CA: Academic Press; 1993. pp. 29–41. [Google Scholar]
- Sohal RS. Role of oxidative stress and protein oxidation in the aging process. Free Radic. Biol. Med. 2002;33:37–44. doi: 10.1016/s0891-5849(02)00856-0. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Weindruch R. Oxidative stress, caloric restriction and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal RS, Ku H-H, Agarwal S, Forster MJ, Lal H. Oxidative damage, mitochondrial oxidant generation, and antioxidant defenses during aging and in response to food restriction in the mouse. Mech. Ageing Dev. 1994;74:121–133. doi: 10.1016/0047-6374(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Mockett RJ, Orr WC. Mechanisms of aging: an appraisal of the oxidative stress hypothesis. Free Radic. Biol. Med. 2002;33:575–586. doi: 10.1016/s0891-5849(02)00886-9. [DOI] [PubMed] [Google Scholar]
- Stadtman ER, Levine RL. Protein oxidation. Ann. NY Acad. Sci. 2000;899:191–208. doi: 10.1111/j.1749-6632.2000.tb06187.x. [DOI] [PubMed] [Google Scholar]
- Thomas JA, Mallis RJ. Aging and oxidation of reactive protein sulfhydryls. Exp. Gerontol. 2001;36:1519–1526. doi: 10.1016/s0531-5565(01)00137-1. [DOI] [PubMed] [Google Scholar]
- Weindruch R, Sohal RS. Caloric intake and aging. N. Engl. J. Med. 1997;337:986–994. doi: 10.1056/NEJM199710023371407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu BP. Aging and oxidative stress: Modulation by dietary restriction. Free Radic. Biol. Med. 1996;21:651–668. doi: 10.1016/0891-5849(96)00162-1. [DOI] [PubMed] [Google Scholar]