Summary
In attempts to understand the social determinants of health, strong associations have been found between measures of loneliness, physiological stress processes, and physical and mental health outcomes. Feelings of loneliness are hypothesized to have implications for physiological stress processes, including activity of the hypothalamic-pituitary-adrenal (HPA) axis. In a community sample of young adults, multilevel modeling was used to examine whether trait and state feelings of loneliness were related to changes in levels of the stress-sensitive hormone cortisol, and whether the associations between loneliness and cortisol were mediated or moderated by the presence of concurrent depression or high levels of chronic life stress. Results indicated that trait loneliness was associated with a flattening of the diurnal cortisol rhythm. In addition, both daily and momentary state variations in loneliness were related to cortisol. Prior-day feelings of loneliness were associated with an increased cortisol awakening response the next morning and momentary experiences of loneliness during the day were associated with momentary increases in cortisol among youth who also had high chronic interpersonal stress. Results were significant after covarying current depression, both chronic and momentary reports of stress, and medical and lifestyle covariates. This study expanded on prior work by investigating and revealing three different time-courses of association between loneliness and HPA axis activity in young adults: trait, daily and momentary.
Keywords: loneliness, cortisol diurnal rhythms, HPA axis, young adults, momentary emotion, CAR
Introduction
Loneliness is a feeling that emerges when social relationships are thought to be deficient, and may arise from a perceived lack of intimacy or lack of companionship (Peplau and Perlman, 1982). Experiencing loneliness can include notions of isolation, disconnection, and “not fitting in”. Loneliness has emerged as an important contributor to physiological stress processes and physical and mental health outcomes (Cacioppo et al., 2003; Steptoe et al., 2004).
Scholars have examined the impact of loneliness on neuroendocrine, immune and cardiovascular responses (Uchino et al., 1996). Loneliness is associated with higher blood pressure (Cacioppo et al., 2002; Steptoe et al., 2004; Hawkley et al., 2006), and impaired or underactive immune function (Pressman et al., 2005). Studies have found possible links between loneliness and cancer (Fox et al., 1994) and a large epidemiological study found that loneliness was associated with both morbidity and mortality (Herlitz et al., 1998; Seeman, 2000). Feelings of loneliness have been significantly associated with mental and physical health outcomes independent of the size of one’s social network or social connections (e.g. see Pressman et al., 2005; Nausheen et al., 2007).
Loneliness and cortisol
More recently, scholars have started to look at the effect of loneliness on the hypothalamic pituitary adrenal axis (HPA axis) as indexed by cortisol levels (Cacioppo et al., 2000b; Steptoe, et al., 2004; Adam et al., 2006). Much of this research has been on middle-age and older adults, and yet late adolescents and young adults spend the most time alone, and feel lonelier, than any other age group under the age of 60 (Larson, 1990; Rokach, 2001). To our knowledge only three studies to date have examined associations between cortisol and loneliness in the college or young adult years. Their results represented different time courses of loneliness experience and varying measures of cortisol (Cacioppo et al., 2002; Pressman et al., 2005; Adam, 2006). For example, Cacioppo et al. (2002) found elevated mean cortisol levels (based on measures aggregated separately for morning, afternoon and evening) in chronically lonely college students but did not find changes in the shape of diurnal cortisol rhythms across the day between lonely and non-lonely students. They did not examine momentary or daily experiences of loneliness. In contrast, Pressman et al. (2005) found that high levels of momentary or daily experiences of loneliness were associated with elevated morning cortisol and elevated evening cortisol. Adam (2006) found that being alone was associated with higher momentary cortisol in an adolescent population.
While prior studies have examined both trait and momentary influences of loneliness on cortisol, the importance of day-to-day variations in cortisol have only recently been brought to light. A study by Adam et al. (2006) took the novel approach of examining how day-to-day changes in emotional experience (including loneliness) related to day-to-day changes in cortisol diurnal rhythms in older adults. Prior research has generally conceptualized this type of daily variation in diurnal cortisol rhythms as error variation. However, Adam and her colleagues found that emotional experiences and diurnal cortisol patterns covaried in systematic ways. For example, experiences of sadness and loneliness on a particular day predicted a higher cortisol awakening response the following morning.
Given that loneliness and cortisol have been related across multiple time scales in prior research, a goal of the current study was to examine whether momentary state loneliness, day-to-day variation in loneliness, or trait levels of loneliness were the strongest predictors of cortisol activity. Momentary and daily state loneliness were measured using diary reports over the relevant periods of time (moments, days) and trait loneliness was measured using a well-validated trait loneliness questionnaire.
Psychopathology and chronic life stress: potential mediators or moderators?
Researchers have commonly acknowledged loneliness as a strong correlate of depression (Shaver and Brennan, 1991; Segrin, 1998; Nolen-Hoeksema and Ahrens, 2002; Cacioppo et al., 2006) and that there is great overlap in the constructs of loneliness and depression (Hagerty and Williams, 1999). Studies have also found associations between chronic stress and cortisol, finding stress to be associated with increased cortisol awakening responses (Pruessner et al., 1997; Wust et al., 2000), and a flattening of the diurnal rhythm (Gunnar and Vazquez, 2001). In this paper, we included major depression, momentary stress, daily stress and chronic interpersonal stress in our models, to examine whether they mediated or accounted for any loneliness-cortisol associations. It can also be hypothesized that having multiple vulnerabilities, such as both loneliness and depression, or high loneliness and multiple life events, might have multiplicative impacts on cortisol activity. As a result, we also examined whether major depression or chronic life stress might have moderating (amplifying) effects on any loneliness-cortisol associations.
In summary, this study sought to understand whether momentary or day-to-day changes in loneliness or chronic, ongoing feelings of isolation and loneliness were more powerfully associated with HPA axis activity. Multi-level linear regressions (HLM) were used to examine these questions, as this approach allowed us to simultaneously examine momentary, daily and between-person differences in cortisol levels in relation to loneliness experienced across each of these three time frames. Several hypotheses were tested in this paper.
First, we hypothesized that youth with heightened levels of trait loneliness would have altered diurnal cortisol rhythms, in particular flatter cortisol slopes across the waking day. Secondly, we hypothesized that there would be day-to-day covariation between loneliness and cortisol such that high levels of loneliness or sadness would be associated with an altered diurnal rhythm the following day, specifically in the form of a higher cortisol awakening response. Finally, we hypothesized that higher momentary lonely/sad (as well as higher momentary stress) would be associated with higher levels of momentary cortisol.
Method
Participants
Participants were recruited from two large public high schools—one in a Chicago suburb and one in the greater Los Angeles area. Students participated in this study as part of their involvement in a larger, longitudinal study on the development of mood and anxiety disorders. Students were selected to participate in the longitudinal study based on their scores on the Neuroticism scale of the EPQ-R (EPQ-R, Eysenck et al., 1975). In order to increase the number of students in the sample at high risk for the subsequent development of mood and anxiety disorders, students who scored in the upper third on this measure were oversampled, such that two thirds of the participants in the study scored in the upper third on the EPQ-R. Although the larger study was based at two sites, this paper only used data from a subset of participants at the Chicago suburban site who participated in several subsequent waves of cortisol data collection. Due to budgetary constraints, the youth from the Los Angeles suburban site were not invited to provide multiple waves of cortisol data collection. There were 835 students screened for the study at the Chicago suburban site. Of these, 588 were invited to participate in the longitudinal study, 337 (57%) consented and 306 (91% of consented sample) completed the baseline assessment. Of the study participants, a random subsample of 243 (79%) participants was invited to participate in the cortisol task. Of those invited, 173 (71%) agreed to participate and successfully completed the first wave of data collection. This study uses data from the second wave of cortisol data collection (occurring approximately two years after initial screening), when measures of loneliness and compliance monitoring were introduced into data collection.
Youth in this analysis include a subsample of 121 participants (81%) who were in the first wave and agreed to participate in the longitudinal cortisol data collection. While there was significant attrition, there were no race differences (Black: t = .84, p = .40; Hispanic: t = −.17, p = .86; Multiple/Other: t = 1.47, p = .14; Caucasian: t = −1.06, p = .29) or gender differences (t = .81, p = .42) between the sub-sample (N=121) and the original sample (N=173) who completed the first wave of cortisol data collection. Exclusion criteria for the current analyses were use of corticosteroid based medications (N = 9), pregnancy (N =1), or provision of insufficient data (N= 2) which led to an analytic sample of 108 (27 male) with an average age of 19.022 (SD = .437). Descriptive statistics on all measures are in Table 1.
Table 1.
Descriptive Statistics for Dependent and Independent Variables (N=108)
| Variable | Mean | SD | Minimum | Maximum |
|---|---|---|---|---|
| Average Waketime Cortisol nmol/L | 8.256 | 6.307 | 0.100 | 49.350 |
| Average Wake + 30 min. Cortisol nmol/L | 13.404 | 7.850 | 0.100 | 49.730 |
| Average Wake + 3 hr. Cortisol nmol/L | 7.523 | 5.739 | 0.100 | 48.760 |
| Average Wake + 8 hr. Cortisol nmol/L | 5.067 | 5.144 | 0.330 | 49.610 |
| Average Wake + 12 hr. Cortisol nmol/L | 3.488 | 3.942 | 0.170 | 28.480 |
| Average Bedtime Cortisol nmol/L | 3.425 | 5.549 | 0.040 | 49.870 |
| Cortisol Awakening Response | 5.407 | 7.302 | −18.210 | 22.760 |
| Time Since Waking | 6.420 | 6.210 | 0.000 | 23.080 |
| Sad Lonely Factor | 0.383 | 0.603 | 0.000 | 3.000 |
| Nervous Stress Factor | 0.760 | 0.661 | 0.000 | 3.000 |
| Loneliness Scale | 2.020 | 1.691 | 0.000 | 6.000 |
| Chronic Interpersonal Stress | 2.264 | 0.342 | 1.750 | 3.690 |
| Caffeine | 0.070 | 0.132 | 0.000 | 0.800 |
| Smoking | 0.030 | 0.104 | 0.000 | 0.690 |
| Age | 19.022 | 0.437 | 17.940 | 20.110 |
| Percentage | SD | N | ||
| Clinical Major Depression | 4.1% | 0.207 | 5 | |
| Oral Contraceptive Use | 23.1% | 0.435 | 28 | |
| Follicular Stage | 19.0% | 0.205 | 23 | |
| Male | 22.3% | 0.241 | 27 | |
| African American | 18.2% | 0.196 | 22 | |
| Hispanic | 5.8% | 0.063 | 7 | |
| Multiple/Other Race | 17.4% | 0.188 | 21 | |
Note: Raw cortisol values (nmol/L) are presented for descriptive purposes but log transformed values are used in all analyses
Procedure
Participants who agreed to take part in the longitudinal study were interviewed once a year using the Structured Clinical Interview for DSM–IV (SCID; First et al., 2002) to determine current and past diagnoses of mood and anxiety disorders. They also completed a life stress interview about chronic and episodic stress (Hammen et al., 1987, Hammen 1991). Participants in the longitudinal cortisol part of the study provided salivary cortisol and filled out diary entries six times a day for three consecutive weekdays. We asked participants to avoid atypical days such as days when important exams were taking place, birthdays, or vacations. Participants were informed that, in addition to completing diaries and cortisol samples, they would also be asked to wear a watch-like device that signaled them for sampling times. The watches were in fact actigraphs (Actiwatch Score), which capture an ongoing record of movement across the day, from which objective measures of waketimes, bedtimes and sleep quality can be determined (see DeSantis et al., in press; Sadeh and Acebo, 2002). Participants were instructed to put on the watch the night before starting the study and to leave it on until the morning after the last day of the study. Participants were paid $30 for the completion of the second wave of data collection and received an analysis of their sleep and activity patterns over their three days of sampling.
Diary reports and saliva sampling
Participants were sent a study packet that included the watch, three diary booklets, straws, a mechanical kitchen timer (to assist with the accurate timing of the 30 minute post-awakening sample), vials and labels for the saliva sampling, and a health/medical and sleep questionnaire. The study procedures were explained in detail by phone. Participants also received a reminder call the night before they were to start the procedure, during which procedures were reviewed and any remaining questions were answered.
This study used a momentary diary method (Ecological Momentary Assessment (EMA); Stone and Shiffman, 1994), which involved participants completing diary reports of where they were, who they were with, and what they were thinking and feeling at selected moments in the course of their everyday lives. The diary reports also asked youth specific questions regarding the intake of caffeine, alcohol, food, medication or nicotine in the last hour and whether they had taken a nap or had experienced pain in the last hour. Participants were asked to fill out a diary form immediately as they woke up, thirty minutes afterwards, at bed time, and three other times throughout the day when signaled by the watch. Participants also provided samples of saliva (to be assayed for cortisol) in conjunction with each diary report.
Participants wore the watches that were preprogrammed to beep to signal the three midday diary entries. These beeps occurred at approximately 3 hours, 8 hours, and 12 hours after participants’ typical wakeup times. This timing was chosen to best model the shape of the cortisol diurnal rhythm while avoiding the post-prandial surges associated with meal times. The mid-day beeps were set to vary from day-to-day around the above-mentioned times (within plus or minus ½ hour) so that the exact timing of the signal would be unanticipated by participant.
Monitoring compliance
Compliance with the timing of the morning cortisol sampling in relation to objective waketimes were assessed, as noncompliance as been shown to influence estimates of cortisol parameters (Kudielka et al., 2003). In particular, recent work has shown the improper timing of samples relative to actual waketimes influences estimates of the cortisol awakening response (Dockray et al., 2008). Compliance was determined by comparing actigraph estimates of participant wake times with when they reported completing their morning saliva samples. Participants were considered “compliant” if they provided their wakeup samples within −5 to 5 minutes of the actigraph wake times and the wakeup + 30 min sample 25–35 minutes after the actigraph wake time (for additional details see DeSantis et al., in press).
Measures
Cortisol
Cortisol was collected by passive drool; participants expelled saliva through straws into a sterile 2 mL cryogenic vial. They wrote the exact date and time of the sample on a label and affixed the label to the vial. Completed samples were returned to the laboratory by courier. Once returned to the laboratory, samples were refrigerated at −20 degrees Celsius until they were sent by courier to the Biochemisches Labor at the University of Trier, Germany to be assayed. Cortisol values are not significantly affected by transport over a period of several days without refrigeration (Clements and Parker, 1998). Samples were assayed in duplicate, using a competitive solid phase time-resolved fluorescence immunoassay with fluorometric endpoint detection (DELFIA). Fluorescence was detected using a DELFIA-Fluorometer (Wallac, Turku, Finland).The intra-assay coefficient of variation was between 4.0% and 6.7%, and the inter-assay coefficients of variation were between 7.1% and 9.0%. Cortisol values were natural logarithmically transformed prior to analysis to correct for a strong positive skew in the cortisol distribution.
Trait Loneliness
Youth completed a shortened version of the Revised UCLA Loneliness Scale (R-UCLA; Russell et al., 1980) for use in large scale surveys which has been validated in several studies (Hughes et al., 2004). The three item scale asked “how often do you feel that you lack companionship”, “how often do you feel left out” and “how often do you feel isolated from others”. Participants could circle “hardly ever”, “some of the time”, or “often”. An average score was created by summing all of the items with higher scores indicating greater loneliness and then standardized. In this sample, the alpha of the scale was .84.
State Loneliness and State Stress
Participants were asked to indicate in their diary reports how much they felt each of the following mood states when they were beeped: happy, tired, friendly, cooperative, nervous, lonely, sleepy, active, frustrated, hardworking, alert, caring, worried, relaxed, irritable, stressed, determined, sad, cheerful, and productive. Each mood state was rated on a scale of 0 (not at all) to 3 (very much). The items were subjected to a principal axis factoring, using an oblimin rotation. Five factors emerged and we used two unit-weighted factors in our analysis as they were our mood states of interest: lonely/sad (lonely, sad; α = .70) and nervous/stress (nervous, frustrated, worried, irritable, stressed; α= .75). Both factors were positively skewed so were natural log transformed for all analyses. The factor lonely/sad was our primary construct of interest however nervous/stress was included to ensure that we were detecting cortisol associations that were specific to loneliness rather than a general negative affective state. Momentary and daily averages of these variables were used in analyses.
Chronic Interpersonal Stress
Participants completed an interview about chronic and episodic stress during their diagnostic assessment. Chronic interpersonal stress was assessed using a semi-structured interview developed by Hammen (Hammen et al., 1987; Hammen 1991) The interview assessed participants’ level of functioning over the last year in ten life domains. We focused on the interpersonal relationship domains of close friendships, social group relations, romantic relationships, and relations with family members because functioning in interpersonal relationships has been related to cortisol functioning in past research (Adam and Gunnar, 2001). Each domain was rated by the interviewer on a five point scale that indicates the severity of chronic stress in that domain, with 1 indicating exceptionally good functioning and no stress in the domain, and 5 indicating extreme adversity and impairment. The average level of chronic interpersonal stress across the two years prior to cortisol assessment was used. Inter-rater reliability was assessed by having trained interviewers listen to recordings of a subset of life stress interviews. Across the four domains of chronic interpersonal stress, intra-class correlation coefficients ranged from .65 to .80 (n = 38). Inter-rater reliability for a composite of the four domains of interpersonal stress was .78 across all pairs of coders.
Clinical, Demographic and Health Covariates
The Structured Clinical Interview for DSM–IV (SCID; First et al., 1995) was used to determine current and lifetime psychiatric diagnoses of anxiety, mood, psychotic, alcohol and substance use, somatoform, and eating disorders. The SCID has demonstrated adequate reliability (Skre et al., 1991; Williams et al., 1992) and is widely accepted and used in clinical research. In this analytic sample, there were five participants who had been diagnosed with clinical MDD during the days of cortisol testing.
Several demographic characteristics and medical controls were included in the model. Dichotomous race dummy variables (Caucasian as the excluded group) were included as previous literature has demonstrated that African Americans have a flatter diurnal rhythm (flatter slopes from wake-up to bedtime) (DeSantis et al., 2007; Cohen et al., 2006). Other known covariates of cortisol are gender, birth control (e.g., Meulenberg et al., 1987), stage of the menstrual cycle (follicular: days 1–13 of the menstrual cycle), alcohol use, caffeine use (Lovallo et al., 2005), nicotine use (Kirschbaum et al., 1992), physical exercise (Jacks et al., 2002), sleeping or napping, wake time, and pain. Of these, only gender, birth control, follicular menstrual stage, caffeine use, nicotine use and wake time were significantly related to cortisol in preliminary analyses; these were retained as covariates in our analyses. An intercorrelation matrix of all dependent and independent variables and covariates is provided in Table 2.
Table 2.
Intercorrelation Table of Main Independent Variables, Covariates and Dependent Variable (N=108)
| 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Waketime Cortisol µg/dl | −0.151 | −0.793** | −.017 | −.038 | .007 | −.105 | −.119 | −.144 | .093 | .239* | .018 | .088 | .099 | −.073 | −.152 | .069 | −.102 |
| 2. CARa | 0.127 | .023 | .081 | .135 | −.081 | .071 | −.037 | −.111 | −.188* | .171 | −.002 | −.018 | −.206* | −.237* | −.041 | .143 | |
| 3 Wake to Bed Cortisol Slope | −.067 | .077 | .022 | .130 | .177* | .135 | −.099 | −.076 | .053 | −.147 | −.095 | .108 | .093 | .019 | .044 | ||
| 4. Time Since Waking | .149 | .198* | −.040 | .006 | .203* | .121 | −.053 | .050 | .153 | −.043 | −.165 | .094 | −.141 | −.115 | |||
| 5. Sad Lonely Factor | .609** | .269** | .110 | −.038 | .009 | −.041 | .022 | .002 | .030 | −.026 | .017 | −.068 | −.150 | ||||
| 6. Nervous Stress Factor | .187* | .024 | .056 | .066 | −.027 | .015 | .071 | −.009 | .000 | .023 | −.051 | −.092 | |||||
| 7. Trait Loneliness Scale | .130 | .206* | −.018 | −027 | −0.19 | −.148 | .009 | .161 | −.012 | .042 | .013 | ||||||
| 8. Major Depressive Disorder | .088 | −.054 | .022 | −.003 | −.025 | .060 | −.021 | .220* | .123 | −.104 | |||||||
| 9. Chronic Interpersonal Life Stress | −.004 | −.087 | −.022 | .073 | −.081 | .089 | .153 | −.050 | .031 | ||||||||
| 10. Caffeine | .336** | −.061 | −.002 | .099 | .124 | −.138 | .023 | .117 | |||||||||
| 11. Smoking | .016 | −.034 | .158 | .148 | −.113 | −.045 | .041 | ||||||||||
| 12. Follicular Stage | .319** | −.112 | −.287** | −.029 | .051 | .039 | |||||||||||
| 13. Oral Contraceptive Use | −.025 | −.325** | −.026 | .021 | .092 | ||||||||||||
| 14. Age | .288** | −.087 | .156 | .076 | |||||||||||||
| 15. Gender (Male=1) | −0.068 | −0.059 | −0.003 | ||||||||||||||
| 16. African American | −.128 | −.238 | |||||||||||||||
| 17. Hispanic | −.124 | ||||||||||||||||
| 18. Multiple/Other Race | |||||||||||||||||
Notes: p <.05
p <.01
Cortisol Awakening Response (Cortisol Wake + 30 value − Cortisol Waking Value)
Analytic Plan
After presenting basic descriptive statistics and intercorrelations among our key variables, associations among state and trait loneliness and cortisol were tested using three-level hierarchical growth curve models to account for the nesting of moments within days and days nested within persons (Raudenbush and Bryk, 2002; Singer and Willett, 2003; Adam et al., 2006). In these models, which are described in greater detail below, levels of cortisol for each person at each moment was the outcome variable, and was predicted by both moment-level predictors (Level 1), day-varying predictors (Level 2), and stable individual differences (Level 3). Lag models were introduced at the day level following previous work by Adam et al. (2006). Mood states measured the day before (prior day) as well as those measured the day of (same day) each day of cortisol sampling were included at Level 2, predicting changes in diurnal cortisol measures from day-to-day. It should be noted that while we used three days of emotion data, only two days of cortisol data (maximum of 12 data points) could be included per participant as we did not have mood data available for the day before the first day of testing.
Model
First, an unconditional model was created looking at the distribution of variation of log cortisol across moments, days and persons. Next, an equation was created to model the shape of each person’s diurnal rhythm and the size of their cortisol awakening response. A time variable indicating how long since waking the sample was given was included. This variable was constructed by subtracting the wake-up time from the exact time of each sample such that time could be interpreted as 0=wake-up, .5=wake up + 30, 2.5=2.5 hours after waking, etc. A time since waking squared parameter was also included to better model the acceleration and curvilinearity rate of decline and shape of the cortisol diurnal curve from waking to bedtime. A dummy variable representing the CAR sample (1=sample taken 30 minutes after waking) was included at Level 1 to model the positive deviation of the CAR cortisol value from the waking-to-bedtime diurnal curve, a reflection of the size of the cortisol awakening response. In this model, π0ij, the intercept, represented each person’s average cortisol level at wake-up, π1ij reflected each person’s CAR, and π2ij and π3ij together reflect each person’s average slope across the day from wake up to bedtime (excluding the CAR). The full models including all independent variables and covariates are shown below.
| Level 1 |
| Level 2 |
| Level 3 |
These analytic techniques allowed us to observe within-person changes in cortisol as predicted by moment-to-moment changes in feeling lonely/sad, day-to-day changes in cortisol diurnal rhythms as predicted by day-to-day changes in feeling lonely/sad, and whether trait loneliness was related to the average or typical diurnal cortisol rhythm across the days of testing. It also allowed us to test the extent to which the strength and direction of these associations was modified by characteristics of the individual, including presence of MDD, chronic stress, gender, race, and age. Furthermore, we captured the three time courses simultaneously in one model thus illustrating the independent contributions of each time course of loneliness.
Results
Descriptive statistics for our primary independent and dependent variables and covariates are presented first (Table 1) and followed by simple correlations among these variables (Table 2). Next, the multilevel models that are our primary analysis are presented and summarized in Table 3.
Table 3.
Loneliness and Cortisol: Three Level Model (N = 108)
| Fixed Effects | Coefficient | SE | Interpretation |
|---|---|---|---|
| Wakeup Cortisol Level, Π0 | |||
| Average wakeup cortisol level,β00 | |||
| Level 1 Intercept, γ000 | 1.913** | .0642 | awakening level = 7.038 nmol/L |
| Level 2 Same Day Nervous/Stress, β01 | −.074 | .066 | n.s. |
| Level 2 Prior Day Nervous/Stress, β02 | −.205 | .107 | 2% decrease per 10 % increase in Nervous/Stress |
| Level 2 Same Day Lonely/Sad, β03 | −.172 | .142 | n.s. |
| Level 2 Prior Day Lonely/Sad, β04 | −.200 | .134 | n.s. |
| Trait Loneliness, γ001 | −.039 | .044 | n.s. |
| Cortisol Awakening Response, Π1 | |||
| Average size of CAR, β10 | |||
| Level 1 Intercept, γ100 | 509** | .062 | 66.3% increase in cortisol at CAR sample |
| Level 2 Same Day Nervous/Stress, β11 | −.149 | .115 | n.s. |
| Level 2 Prior Day Nervous/Stress, β12 | .197 | .215 | n.s. |
| Level 2 Same Day Lonely/Sad, β13 | .142 | .269 | n.s. |
| Level 2 Prior Day Lonely/Sad, β14 | .477 | .203 | 4.77% increase per 10 % increase in Lonely/Sad |
| Trait Loneliness, γ101 | .020 | .039 | n.s. |
| Time Since Waking, Π2 | |||
| Average slope at wake at wakeup, β20 | |||
| Level 1 Intercept, γ200 | −.091** | .014 | 9.5% decrease per hour at awakening |
| Trait Loneliness, γ201 | .020* | .010 | 2% flatter |
| Major Depressive Disorder, γ202 | .209* | .089 | 23.2% flatter |
| TimeSinceWaking2, Π3 | |||
| Average asymptotic slope, β3 | |||
| Level 1 Intercept, γ300 | .001 | .001 | n.s. |
| Trait Loneliness, γ301 | −.001* | .000 | .01% less |
| Momentary Lonely/Sad, Π4 | |||
| Average momentary sad-lonely factor, β40 | |||
| Level 1 Intercept, γ400 | .062 | .087 | n.s. |
| Trait Loneliness, γ401 | −0.99* | .049 | 10.4% decrease per 1 SD increase |
| Chronic Interpersonal Stress, γ402 | .161+ | .094 | 17.5% increase per 1 SD increase |
| Momentary Nervous/Stress, Π5 | |||
| Average momentary nervous-stress, β50 | |||
| Level 1 Intercept of Momentary Stress, γ500 | .031 | .057 | n.s. |
| Trait Loneliness, γ501 | .006 | .039 | n.s. |
Note: p<.05
p<.01
All l Level 1 predictors are uncentered. Chronic Interpersonal Stress and Major Depressive Disorder are included as covariates for each parameter but only shown when significant. Also controlling for nicotine use, caffeine use, oral contraceptive use, follicular stage, race/ethnicity, waketime, compliance at wake up and 30 minutes post wake up. All cortisol levels and mood factors have been log transformed.
Descriptives (Table 1)
Demographic and Health Controls
Our sample consisted of 58 Caucasian youth (53.7%), 22 African American youth (18.2%), 7 Hispanic youth (5.8%) and 21 multiple race/other race youth (17.4%) with a mean age of 19.02 (SD = .44). Twenty-five females in our sample were using oral contraceptives (23% of total sample). Twenty-three females (25% of total sample) were in their follicular stage of their menstrual cycle. Over the three days of testing, 17% of our sample smoked cigarettes prior to at least one of their cortisol samples and 48.2% of the sample had caffeine prior to at least one of their cortisol samples. The mean level of chronic interpersonal stress in the sample was 2.3 (SD = .34) on a scale of 1–5, with 1 indicating the most optimal relationship and 5 indicating the most stressful. There were no significant differences by gender or race.
Trait Loneliness and Momentary Lonely/Sad Factor
The mean level of trait loneliness was 2.07 (SD = 1.7) on a scale of 1–6 with 1 indicating no feelings of loneliness and 6 indicating high levels of loneliness. There were no differences by race, but males reported higher levels of loneliness than females (t = 2.80, p < .10) with males reporting average levels of 2.50 (SD = 1.3) and females reporting average levels of 1.86 (SD = 1.8). The mean level of diary reported momentary lonely/sad across all moments and youth was .38 (SD = .6) on a scale of 0–3. There were no differences in momentary loneliness by race, gender or day of testing.
Intercorrelations among Covariates, Independent Variables and Cortisol (Table 2)
Several of the health and demographic controls were significantly associated with the size of the cortisol awakening response. There were statistically significant differences in the CAR by gender (r = −.21, p <. 05) indicating that males had smaller awakening responses than females. Nicotine consumption was also negatively correlated with the CAR (r = −.19, p <. 05), such that a lower awakening response was associated with higher levels of nicotine consumption. There were also racial differences; African American youth (n = 21) had smaller cortisol awakening responses than adolescents in other race categories (r = −2.37, p < .05). Trait loneliness was positively associated with momentary lonely/sad (r = .27, p < .05), momentary nervous/stress (r = .19, p < .05) and chronic interpersonal life stress (r = .21, p < .05).
Multilevel Models of Cortisol, Loneliness and Covariates (see Table 3)
All results are presented in Table 3. The outcome of interest, cortisol level, was log transformed, thus the effect sizes for all of the variables with the exception of the momentary mood factors can be interpreted as a percent change per one unit change in the independent variable after utilizing the following formula: β%change = ((e^β)−1). The momentary mood factors are log transformed therefore the effect sizes can be interpreted as % change when there is a 1% change in the mood factor.
Participants showed the expected diurnal pattern of high morning levels (γ000 = 1.913, p<.001; equivalent to 7.038 nmol/l), and a decline in cortisol across the day (γ200 = −.091, p <.01) at a rate of 9.5% per hour at waking. They also showed an approximately 66.3% (γ100 = .509, p <.01) rise a half hour after waking (the CAR awakening response). The quadratic term was not significant (γ300 = .001, p = n.s.), but we kept this in the model because it has been found to be important in prior research (Adam and Gunnar, 2001).
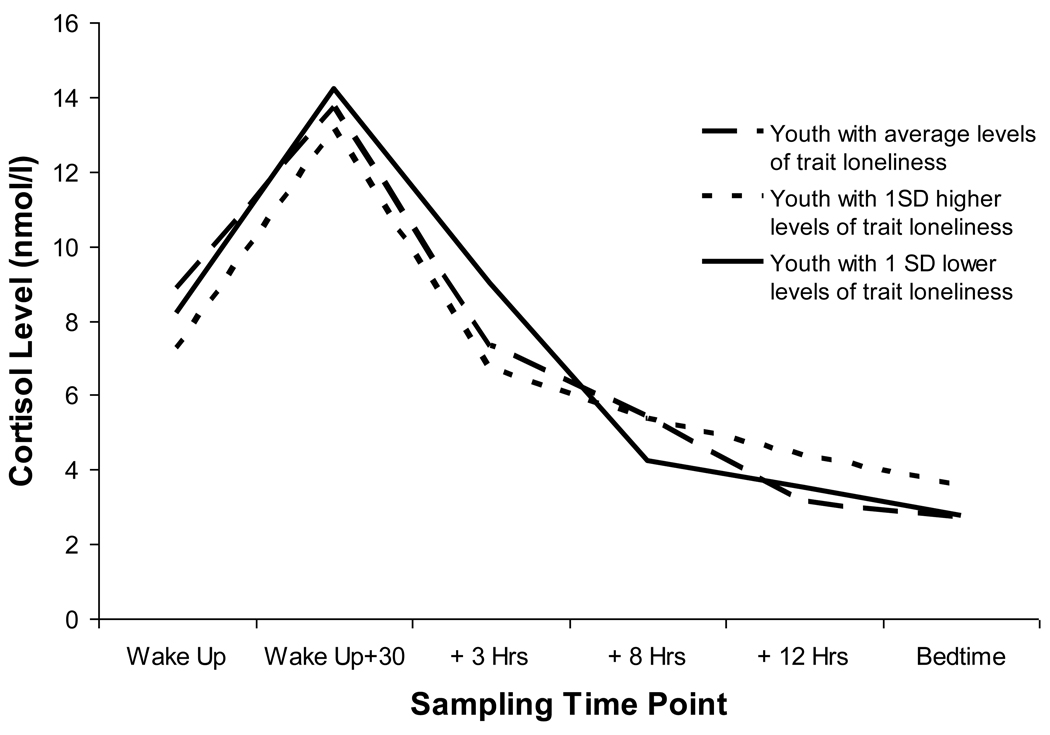
Trait loneliness (see Figure 1)
Figure 1.
Average cortisol rhythms across the waking day by trait loneliness
Trait levels of loneliness were associated with several parameters defining the shape of the diurnal cortisol curve. Higher levels of trait loneliness were associated with flatter diurnal cortisol slopes (γ201 = .020, p < .05) and less curvilinearity of the rhythm across the waking day (γ309 = −.001, p < .05). These effects were significant even when controlling for momentary, prior day and concurrent day levels of lonely/sad and nervous/stress.
Current Day and Prior Day Lonely/sad
Higher prior day feeling lonely/sad was significantly associated with a greater CAR the following morning (β14 = .477, p < .05; 4.77% increase per 10% increase in lonely/sad) whereas levels of lonely/sad on the same day that cortisol was measured, and prior day levels of nervous/stress were not. In addition, prior day feeling nervous/stress were associated with lower average wake up levels of cortisol (β02 = −.205, p < 10), while measures of lonely/sad were not significantly associated with waking levels of cortisol. This indicates that it is prior day, not concurrent, feelings of loneliness or stress that appeared to have an impact on next morning cortisol. These results were significant even when time of waking each day, and compliance with the timing of the wakeup and CAR samples, relative to objective waketimes, were included in the model, such that the differences cannot be attributed to later wake times or noncompliance with cortisol procedures. While not shown here, average levels of feeling lonely/sad or feeling nervous/stress across all three days were not significantly associated with average cortisol slopes. In short, it was the dynamic changes in these variables from day-to-day that predicted alterations in cortisol.
Momentary Lonely/sad
Neither momentary levels of lonely/sad nor momentary levels of nervous/stress, on average for all individuals, were significantly associated with momentary elevations of cortisol (deviations in cortisol above each person’s expected level for that time of day) (Π4 = .062, p = n.s.; Π5 = .031, p = n.s.). However, the association between momentary lonely/sad and cortisol was modified by levels of chronic interpersonal life stress. Youth who experienced one standard deviation higher levels of chronic interpersonal life stress had higher levels of cortisol above their own expected levels for that time of day (Π4 = .062, p = n.s.) when they were experiencing higher momentary loneliness/sadness (γ402 = .161, p < .10). A surprising result was that trait levels of loneliness also moderated the state lonely/sad-cortisol association (γ401 = −.099, p < .05) indicating that while on average most youth experienced higher levels of cortisol when feeling acutely lonely/sad, youth with higher trait levels (experience feelings of loneliness more frequently) actually experienced lower levels of cortisol when they were reporting momentary lonely/sad feelings.
Covariates
Including of the covariates did not change the results substantially, but revealed some additional associations. Experiencing a major depressive episode (MDD) during the days of testing appeared to be associated with significantly flatter slopes across the waking day (γ202 = .209, p < .05). Depressed youth had about 23.2% flatter slopes than youth without the presence of the disorder, however it should be noted that there were only 5 youth in this category. Additional analyses conducted on the subsample of youth (N=103) that did not have MDD produced similar results as those presented here. Momentary levels of cortisol were elevated in youth who were 1 SD higher in chronic interpersonal life stress when experiencing moments of lonely/sad (γ403 = .132, p < .10). Prior day feelings of lonely/sad predicted similar increases in the CAR the next day (β14 = .518, p < .05; 5.18% increase per 10% increase in lonely/sad). Trait loneliness predicted flatter cortisol rhythms (γ201 = .020, p < .05).
Average levels of chronic interpersonal stress did not significantly predict any of cortisol parameters beyond it’s interactions with state loneliness noted above. Males, on average, had a smaller CAR than their female counterparts (γ107 = −.207, p =.10) which has been demonstrated in previous literature. Hispanics also had a smaller CAR than their non-Hispanic white counterparts (γ108 = −.361, p <.10). Nicotine use predicted a greater deceleration of cortisol across the day (γ308 = −.309, p < .05). Lastly, being multi-racial or identifying as “other race” was associated with lower levels of cortisol during moments when feeling lonely/sad (γ405 = −.795, p < .05) and lower cortisol when feeling higher levels of stress (γ505 = −.385, p < .05) as compared to their Caucasian counterparts.
Discussion
This study found three different dynamic pathways through which loneliness is associated with cortisol activity. It is particularly notable that loneliness was associated with altered cortisol activity over three different time courses. We found clear support for our first hypothesis that youth with high levels of trait or chronic loneliness would have flatter slopes across the waking day. This association was independent of the presence of MDD or high levels of chronic interpersonal stress, both of which have previously been related to flatter diurnal cortisol slopes (Schildkraut et al., 1989; Adam and Gunnar, 2001; Peeters et al., 2004). Flat slopes have been interpreted by some to be an indication of chronic stress (Miller et al., 2007), and as such, it makes sense that trait loneliness would be related to this particular aspect of cortisol functioning, as trait loneliness reflects a chronic interpersonal stressor (Cacioppo et al., 2003).
Similarly, we found support for our second hypothesis that high daily levels of lonely/sad are associated with an altered diurnal rhythm the following day. Even when controlling for concurrent day feelings of lonely/sad, prior day levels were associated with a 30% increase in the cortisol awakening response the next morning. Furthermore, these findings were independent of the effects of day-to-day changes in feeling nervous or stressed -- another type of negative affect typically thought to be associated with cortisol. This prior-day loneliness predicting next-day CAR finding replicates Adam et al.’s (2006) findings in older adults that prior day increases in loneliness and sadness predicted a greater cortisol awakening response the following morning. Our findings thus add further evidence in support of the “boost” hypothesis that Adam et al. (2006) proposed. This hypothesis suggests that an increase in the CAR, in response to adverse prior-day social experience, such as loneliness, may provide an extra energetic “boost” to help the individual meet the demands of the next day. Another possible interpretation is that an increased CAR the next day is evidence of a prolonged stress response and may signify a maladaptive rather than adaptive reaction. Studies have shown associations between increased CAR and chronic stress (Schulz et al., 1998; Pruessner et al., 1999) as well as greater risk for MDD (Adam et al., 2008).
For youth who experienced high levels of feeling nervous/stressed however, they had lower than average wake-up levels. It seems that there were two different mechanisms at work, depending on the type of emotional strain experienced the day before. Feelings of lonely/sad caused the body to increase the cortisol awakening response, perhaps preparing and activating the body such that it can more effectively deal with the upcoming demands of the day, and helping to promote interaction with the social world (Adam et al., 2006). In contrast, feeling worried and stressed seemed to predict lower wakeup levels (but not the CAR); here we hypothesize that the body may be buffering against possible stress-related activations and thus the lower wake up values are promoting social withdrawal rather than engagement. In the Adam et al. (2006) study of older adults, low wakeup levels were associated with greater fatigue later that same day, suggesting that morning cortisol levels can have implications for the individuals’ experience of and approach to the upcoming day. Future research is needed, however, to test these hypotheses and to differentiate between the behavioral impact of variations in wakeup cortisol values as compared to variations in the CAR.
We also found partial support for our third hypothesis that momentary loneliness was associated with higher levels of momentary cortisol. Specifically, youth who experienced higher levels of chronic interpersonal stress were significantly more reactive to momentary feelings of lonely/sad—almost 20% larger response than youth who experienced average levels of interpersonal life stress. Youth who high levels of interpersonal stress in their life had significantly larger cortisol increases above their normal diurnal rhythm at moments when they were feeling particularly lonely or sad than youth with lower levels of interpersonal stress. Youth did not, on average, have significant increases in momentary cortisol in moments when they felt higher levels of nervous/stress. This was contrary to previous work which has shown that high levels of anger, tension, nervousness and stress were related to momentary increases in cortisol levels (van Eck et al., 1996; Peeters et al., 2003; Pressman et al., 2005; Adam, 2006) in both adolescent and adult populations.
Of great interest is the question of how the three time scales of associations between loneliness and cortisol may be related. Are these three independent mechanisms affecting the HPA, or might the small acute changes in cortisol in response to state loneliness contribute over time to the more dramatic changes to the diurnal rhythm we see in the association between trait loneliness and cortisol? For example, is it possible that repeated higher awakening responses, among individuals with frequent daily experiences of loneliness, might contribute to more long term alterations in the HPA axis such as flattened diurnal rhythms? Given prior associations between flattened diurnal rhythms and health outcomes (Rosmond and Bjorntorp, 2000; Sephton et al., 2000), these longer term changes may, in turn, be one mechanism by which loneliness is linked to poor physical or mental health outcomes.
In addition to our primary variables of interest (state and trait loneliness), several additional variables and covariates were significantly associated with HPA axis activity. Having current MDD was associated with a flattening of the diurnal rhythm independent of the effects of loneliness. Although these findings are consistent with past research (Dahl et al., 1991), there were only five youth in our sample with MDD. We therefore hesitate to draw strong conclusions regarding these findings, beyond the fact that the associations between loneliness and cortisol obtained in the current study were not attributable to the presence of clinical depression in some youth.
Recent research on HPA axis functioning has demonstrated the importance of looking at demographic variables, such as gender and race (e.g., see DeSantis et al., 2007), as well as the importance of looking at compliance with sampling procedures in naturalistic settings (Kudielka et al., 2003), and controlling for confounding health and lifestyle variables such as medication use and nicotine intake (Meulenberg et al., 1987; Kirschbaum, 1992; Jacks et al., 2002; Lovallo et al., 2005). In this analysis, few subgroup differences emerged. Males had a smaller cortisol awakening response than their female counterparts, about 30% smaller. Hispanics also had an approximately 30% smaller CAR than their non-Hispanic white counterparts (p <.10).
There are several limitations to this study. Our findings are longitudinal, but only over a very short time frame – three days. Future longitudinal research will need to examine whether short term momentary and daily alterations in cortisol might evolve into long term chronic pathways over the course of months and years. Secondly, we were restricted to only having three days of data within which to examine day-to-day variations in emotional experience and cortisol. Future research should work to gather cortisol, emotional and social environmental data on more than three days such that there is greater predictive power in modeling day-to-day variations. A last limitation is that our momentary construct of loneliness also included the emotion of sadness, and thus we can not disentangle the effect of feeling lonely and not sad. However, given that these constructs were highly correlated within our sample, it was not possible to parse out their individual contributions to cortisol activity in this study.
The evidence provided in this paper on the dynamic associations between loneliness and cortisol relates to work that has been done on social contacts and cortisol hormone regulation. Studies by Stetler and her colleagues (Stetler et al., 2004; Stetler and Miller, 2005) demonstrated that having higher levels of social contacts was associated with steeper cortisol slopes across the day. Similarly, Adam and Gunnar (2001) found that perceived quality of social relationships, including security of attachment, was associated with stronger diurnal cortisol slopes. Taken together, the findings on social contacts and the present study findings regarding loneliness illustrate that social regulation of the HPA axis stems not only from the external number of contacts, but the perception of the quality of these contacts.
This investigation illustrates one possible pathway through which our social environment and our interpretation of our social environment influence our physiology, in particular the HPA axis and cortisol. Future research should continue to unpack the biological underpinnings of various chronicities of loneliness and their subsequent impacts on health. This study of young adults found that feelings of loneliness and sadness can impact the HPA axis in differing ways across multiple time scales - in the moment, from day-to-day and over long periods of time. It will be vital to continue to measure, model and study these associations appropriately within naturalistic settings. By doing so, we can better understand how the biological pathways by which social experiences such as loneliness can relationship impact long term physical and mental health outcomes.
Acknowledgments
The authors would like to thank the participants of the Northwestern Sleep and Stress Study in the Youth Emotion Project for the time and effort they contributed to this research. We thank Katie Mendelsohn, Jennifer Cueto, Amy DeSantis and the undergraduate research assistants who worked on this study for their assistance, time and support. We would also like to thank Greg Duncan, Louise Hawkley and Susan Mineka for comments on earlier drafts of this manuscript. This research was conducted with the support of NIHM R01 MH65652 (Richard E. Zinbarg, Susan Mineka, Michelle G. Craske, Principal Investigators), a graduate fellowship from the Institute for Policy Research at Northwestern University to the primary author (L.D.D.) and a William T. Grant Foundation Scholars Award and an Institute for Policy Research Faculty Fellowship to the second author (E.K.A.).
Role of Funding Sources.
This research was financially supported by NIHM R01 MH65652 (R.E.Z., S.M., MG.C., Principal Investigators), a graduate fellowship from the Institute for Policy Research at Northwestern University (L.D.D.) and a William T. Grant Foundation Scholars Award and an Institute for Policy Research Faculty Fellowship to the second author (E.K.A.). The granting agencies involved had no further role in the study design, data collection, analysis and interpretation of the data, in the writing of the manuscript or the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest.None of the authors (L.D.D., E.K.A.) have any conflicts of interest to declare with respect to this manuscript.
Contributors.
Leah D. Doane collected and analyzed the data and wrote the paper. Emma K. Adam designed the study, collected and analyzed the data, contributed to the interpretation of the data and edited the manuscript.
Contributor Information
Leah D. Doane, Cells to Society Center, Institute for Policy Research, Northwestern University, 2120 Campus Drive, Evanston, IL 60208, USA, phone 773-315-5819 fax 847-491-8999, l-doane@northwestern.edu
Emma K. Adam, Program on Human Development and Social Policy, School of Education and Social Policy and the Cells to Society Center, Institute for Policy Research, Northwestern University, 2120 Campus Drive, Evanston, IL, 60208, USA, work 847-467-2010 fax 847-491-8999, ek-adam@northwestern.edu
References
- Adam EK. Transactions among trait and state emotion and adolescent diurnal and momentary cortisol activity in naturalistic settings. Psychoneuroendocrinology. 2006;31:664–679. doi: 10.1016/j.psyneuen.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Adam EK, Gunnar MR. Relationship functioning and home and work demands predict individual differences in diurnal cortisol patterns in women. Psychoneuroendocrinology. 2001;26:189–208. doi: 10.1016/s0306-4530(00)00045-7. [DOI] [PubMed] [Google Scholar]
- Adam EK, Hawkley LC, Kudielka BM, Cacioppo JT. Day-to-day dynamics of experience-cortisol associations in a population-based sample of older adults. The Periodicals of the National Academy of Sciences. 2006;103:17058–17063. doi: 10.1073/pnas.0605053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam EK, Sutton JM, Doane LD, Mineka S. Incorporating hypothalamic-pituitary-adrenal axis measures into preventive interventions for adolescent depression: Are we there yet? Development and Psychopathology. 2008;20:975–1001. doi: 10.1017/S0954579408000461. [DOI] [PubMed] [Google Scholar]
- Cacioppo J, Bernston G, Sheridan J, McClintock M. Multilevel integrative analyses of human behavior: Social neuroscience and the complementing nature of social and biological approaches. Psychological Bulletin. 2000;126:829–843. doi: 10.1037/0033-2909.126.6.829. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Enst JM, Burleson MH, McClintock MK, Malarkey WB, Hawkley LC, et al. Lonely traits and concomitant physiological processes: The MacArthur Social Neuroscience Studies. International Journal of Psychophysiology. 2000;35:143–154. doi: 10.1016/s0167-8760(99)00049-5. [DOI] [PubMed] [Google Scholar]
- Cacioppo J, Hawkley L, Berntson G. The anatomy of loneliness. Current Directions in Psychological Science. 2003;12:71–74. [Google Scholar]
- Cacioppo JT, Hawkley LC, Crawford LE, Ernst JM, Burleson JM, Kowalewski RB, et al. Loneliness and health: Potential mechanisms. Psychosomatic Medicine. 2002;64:407–417. doi: 10.1097/00006842-200205000-00005. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Hughes ME, Waite LJ, Hawkley LC, Thristed RA. Loneliness as a specific risk factor for depressive symptoms: cross-sectional and longitudinal analyses. Psychology and Aging. 2006;21:140–151. doi: 10.1037/0882-7974.21.1.140. [DOI] [PubMed] [Google Scholar]
- Clements AD, Parker CR. The relationship between salivary cortisol concentrations in frozen versus mailed samples. Psychoneuroendocrinology. 1998;23:613–616. doi: 10.1016/s0306-4530(98)00031-6. [DOI] [PubMed] [Google Scholar]
- Cohen S, Schwartz JE, Epel E, et al. Socioeconomic status, race, and diurnal cortisol decline in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Psychosomatic Medicine. 2006;68:41–50. doi: 10.1097/01.psy.0000195967.51768.ea. [DOI] [PubMed] [Google Scholar]
- Dahl RE, Ryan ND, Puig-Antich J, Nguyen NA, Al-Shabbout M, Meyer VA, Perel J. 24-Hour cortisol measures in adolescents with major depression: a controlled study. Biological Psychiatry. 1991;30:25–36. doi: 10.1016/0006-3223(91)90067-v. [DOI] [PubMed] [Google Scholar]
- DeSantis AS, Adam EK, Doane LD, Mineka S, Zinbarg R, Craske M. Racial and ethnic difference in cortisol diurnal rhythms in a community sample of adolescents. Journal of Adolescent Health. 2007;41:3–13. doi: 10.1016/j.jadohealth.2007.03.006. [DOI] [PubMed] [Google Scholar]
- DeSantis AS, Adam EK, Mendelsohn K, Doane LD. Concordance between reported and actual wake-up times in ambulatory salivary cortisol research: Implications for the cortisol response to awakening. International Journal of Behavioral Medicine. doi: 10.1007/s12529-009-9053-5. In press. [DOI] [PubMed] [Google Scholar]
- Dockray S, Bhattacharyya M, Molloy G, Steptoe A. The cortisol awakening response in relation to objective and subjective measures of waking in the morning. Psychoneuroendocrinology. 2008;33:77–82. doi: 10.1016/j.psyneuen.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Ehlert U, Gaab J, Heinrichs M. Psychoneuroendocrinological contributions to the etiology of depression, posttraumatic stress disorder, and stress-related bodily disorders: the role of the hypothalamus-pituitary-adrenal axis. Biological Psychology. 2001;57:141–152. doi: 10.1016/s0301-0511(01)00092-8. [DOI] [PubMed] [Google Scholar]
- Eysenck HJ, Eysenck SBJ. Manual of the Eysenck Personality Questionnaire (adult and junior) London: Hodder & Stoughton; 1975. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition. (SCID-I/NP) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Fox C, Harper A, Hyner G, Lyle R. Loneliness, emotional repression, marital quality, and major life events in women who develop breast cancer. Journal of Community Health. 1994;19:467–482. doi: 10.1007/BF02260327. [DOI] [PubMed] [Google Scholar]
- Gunnar M, Vazquez DM. Low cortisol and a flattening of the expected daytime rhythm: Potential indices of risk in human development. Development and Psychopathology. 2001;13:515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- Hagerty BM, Williams RA. The effects of sense of belonging, social support, conflict and loneliness on depression. Nursing Research. 1999;48:215–219. doi: 10.1097/00006199-199907000-00004. [DOI] [PubMed] [Google Scholar]
- Hammen C. Generation of stress in the course of unipolar depression. Journal of Abnormal Psychology. 1991;100:555–561. doi: 10.1037//0021-843x.100.4.555. [DOI] [PubMed] [Google Scholar]
- Hammen CL, Gordon D, Burge D, Adrian C. Maternal affective disorders, illness, and stress: Risk for children’s psychopathology. American Journal of Psychiatry. 1987;144:736–741. doi: 10.1176/ajp.144.6.736. [DOI] [PubMed] [Google Scholar]
- Hawkley L, Cacioppo J. Loneliness and pathways to disease. Brain, Behavior and Immunity. 2002;17:S98–S105. doi: 10.1016/s0889-1591(02)00073-9. [DOI] [PubMed] [Google Scholar]
- Hawkley LC, Masi CM, Berry JD, Cacioppo JT. Loneliness is a unique predictor of age-related differences in systolic blood pressure. Psychology and Aging. 2006;21:152–164. doi: 10.1037/0882-7974.21.1.152. [DOI] [PubMed] [Google Scholar]
- Heim C, Ehlert U, Hellhammer D. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25:1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- Herlitz J, Wiklund I, Caidahl K, Hardford M, Haglid M, Karlsson BW, et al. The feeling of loneliness prior to coronary artery bypass grafting might be a predictor of short-and long-term postoperative mortality. European Journal of Vascular and Endovascular Surgery. 1998;16:120–125. doi: 10.1016/s1078-5884(98)80152-4. [DOI] [PubMed] [Google Scholar]
- Hughes ME, Waite LJ, Hawkley LC, Cacioppo JT. A short scale for measuring loneliness in large surveys: Results from two population-based studies. Research on Aging. 2004;26:655–672. doi: 10.1177/0164027504268574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacks DE, Sowash J, Anning J, McGloughlin T, Andres F. Effect of exercise at three exercise intensities on salivary cortisol. Journal of Strength and Conditioning Research. 2002;16:286–289. [PubMed] [Google Scholar]
- Kaltsas GA, Chrousos GP. The neuroendocrinology of stress. In: Cacioppo J, Tassinary L, Bernston G, editors. Handbook of Psychophysiology. Cambridge University Press; 2007. [Google Scholar]
- Kirschbaum C, Wust S, Hellhammer D. Consistent sex differences in cortisol responses to psychological stress. Psychosomatic Medicine. 1992;54:648–657. doi: 10.1097/00006842-199211000-00004. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Broderick JE, Kirschbaum C. Compliance with saliva sampling protocols: Electronic monitoring reveals invalid cortisol daytime profiles in noncompliant subjects. Psychosomatic Medicine. 2003;65:313–319. doi: 10.1097/01.psy.0000058374.50240.bf. [DOI] [PubMed] [Google Scholar]
- Larson RW. The solitary side of life: An examination of the time people spend alone from childhood to old age. Developmental Review. 1990;10:155–183. [Google Scholar]
- Lovallo WR, Whitsett TL, Al’absi M, Sung BH, Vincent AS, Wilson MF. Caffeine stimulation of cortisol secretion across the waking hours in relation to caffeine intake levels. Psychosomatic Medicine. 2005;67:734–739. doi: 10.1097/01.psy.0000181270.20036.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. New England Journal of Medicine. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- Meulenberg PM, Ross HA, Swinkels LM, Benraad TJ. The effect of oral contraceptives on plasma-free and salivary cortisol and cortisone. Clinica chimica acta. 1987;165:379–385. doi: 10.1016/0009-8981(87)90183-5. [DOI] [PubMed] [Google Scholar]
- Miller G, Chen E, Zhou E. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenal axis in humans. Psychological Bulletin. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Nausheen B, Gidron Y, Gregg A, Tissarchondou H, Peveler R. Loneliness, social support and cardiovascular reactivity to laboratory stress. Stress. 2007;10:37–44. doi: 10.1080/10253890601135434. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Ahrens C. Age differences and similarities in the correlates of depressive symptoms. Psychology and Aging. 2002;17:115–124. doi: 10.1037//0882-7974.17.1.116. [DOI] [PubMed] [Google Scholar]
- Peeters F, Nicolson NA, Berkhof J. Cortisol responses to daily events in major depressive disorder. Psychosomatic Medicine. 2003;65:836–841. doi: 10.1097/01.psy.0000088594.17747.2e. [DOI] [PubMed] [Google Scholar]
- Peeters F, Nicolson N, Berkhof J. Levels and variability of daily life cortisol secretion in major depression. Psychiatry Research. 2004;126:1–13. doi: 10.1016/j.psychres.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Peplau L, Perlman D. Perspective on loneliness. In: Peplau LA, Perlman D, editors. Loneliness: A sourcebook of current theory, research and therapy. New York: Wiley; 1982. [Google Scholar]
- Pressman SD, Cohen S, Miller GE, Barkin A, Rabin BS, Treanor JJ. Loneliness, social network size and immune response to influenza vaccination in college freshmen. Health Psychology. 2005;24:297–306. doi: 10.1037/0278-6133.24.3.297. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Gaab J, Hellhammer DH, Lintz D, Schommer N, Kirschbaum C. Increasing correlations between personality traits and cortisol stress responses obtained by data aggregation. Psychoneuroendocrinology. 1997;22:615–625. doi: 10.1016/s0306-4530(97)00072-3. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Hellhammer DH, Kirschbaum C. Burnout, perceived stress, and cortisol responses to awakening. Psychosomatic Medicine. 1999;61:197–204. doi: 10.1097/00006842-199903000-00012. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical linear models: Application and data analysis methods. Thousand Oaks, CA: Sage Publications; 2002. [Google Scholar]
- Rokach A. Perceived causes of loneliness in adulthood. Journal of Social Behavior and Personality. 2001;15:67–84. [Google Scholar]
- Rosmond R, Bjorntorp P. The hypothalamic-pituitary-adrenal axis activity as a predictor of cardiovascular disease, type 2 diabetes, and stroke. Journal of Internal Medicine. 2000;247:188–197. doi: 10.1046/j.1365-2796.2000.00603.x. [DOI] [PubMed] [Google Scholar]
- Rotenberg K. Loneliness and interpersonal trust. Journal of Social and Clinical Psychology. 1994;13:152–173. [Google Scholar]
- Russell D, Peplau L, Cutrona C. The Revised UCLA Loneliness Scale: Concurrent and discriminant validity evidence. Journal of Personality and Social Psychology. 1980;39:472–480. doi: 10.1037//0022-3514.39.3.472. [DOI] [PubMed] [Google Scholar]
- Sadeh A, Acebo C. The role of actigraphy in sleep medicine. Sleep Medicine Review. 2002;6:113–124. doi: 10.1053/smrv.2001.0182. [DOI] [PubMed] [Google Scholar]
- Schulz P, Kirschbaum C, Pruessner J, Hellhammer D. Increased free cortisol secretion after awakening in chronically stressed individuals due to work overload. Stress Medicine. 1998;14:91–97. [Google Scholar]
- Schildkraut J, Myers R, Cupples L, Kiely K, Kannel WB. Coronary risk associated with age and sex of parental heart disease in the Framingham Study. American Journal of Cardiology. 1989;64:555–559. doi: 10.1016/0002-9149(89)90477-3. [DOI] [PubMed] [Google Scholar]
- Seeman TE. Health promoting effects of friends and family on health outcomes in older adults. American Journal of Health Promotion. 2000;14:362–370. doi: 10.4278/0890-1171-14.6.362. [DOI] [PubMed] [Google Scholar]
- Segrin C. Interpersonal communication problems associated with depression and loneliness. In: Andersen PA, Guerrero LK, editors. Handbook of communication and emotion: Research, theory, applications and contexts. San Diego, CA: Academic Press; 1998. [Google Scholar]
- Sephton S, Sapolsky R, Kraemer H, Spiegel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. Journal of the National Cancer Institute. 2000;92:994–1000. doi: 10.1093/jnci/92.12.994. [DOI] [PubMed] [Google Scholar]
- Shaver PR, Brennan KA. Measures of depression and loneliness. In: Robinson JP, Shaver PR, editors. Measures of social psychological attitudes. San Diego, CA: Academic Press; 1991. [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. Oxford: Oxford University Press; 2003. [Google Scholar]
- Skre I, Onstad S, Torgersen S, Kringlen E. High inter rater reliability for the structured clinical interview for DSM-III-R axis I (SCID-I) Acta Psychiatrica Scandinavica. 1991;84:167–173. doi: 10.1111/j.1600-0447.1991.tb03123.x. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Owne N, Kunz-Ebrecht SR, Brydon L. Loneliness and neuroendocrine, cardiovascular, and inflammatory stress responses in middle-aged men and women. Psychoneuroendocrinology. 2004;29:593–611. doi: 10.1016/S0306-4530(03)00086-6. [DOI] [PubMed] [Google Scholar]
- Stetler C, Dickerson S, Miller G. Uncoupling of social zeitgebers and diurnal cortisol secretion in clinical depression. Psychoneuroendocrinology. 2004;29:1250–1259. doi: 10.1016/j.psyneuen.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Stetler C, Miller G. Blunted cortisol response to awakening in mild to moderate depression: Regulatory influences of sleep patterns and social contacts. Journal of Abnormal Psychology. 2005;14:697–705. doi: 10.1037/0021-843X.114.4.697. [DOI] [PubMed] [Google Scholar]
- Stone AA, Siffman S. Ecological momentary assessment (EMA) in behavioral medicine. Annals of Behavioral Medicine. 1994;16:199–202. [Google Scholar]
- Uchino B, Cacioppo J, Kiecolt-Glaser J. The relationship between social support and psychological processes: A review with emphasis on underlying mechanisms and implications for health. Psychological Bulletin. 1996;119:488–531. doi: 10.1037/0033-2909.119.3.488. [DOI] [PubMed] [Google Scholar]
- van Eck M, Berkhof H, Nicolson N, Sulon J. The effects of perceived stress, traits, mood states, and stressful daily events on salivary cortisol. Psychosomatic Medicine. 1996;58:447–458. doi: 10.1097/00006842-199609000-00007. [DOI] [PubMed] [Google Scholar]
- Williams JB, Gibbon M, First MB, Spitzer RL, Davies M, Borus J, et al. The structured clinical interview for DSM-III-R (SCID).II: Multisite test-retest reliability. Archives of General Psychiatry. 1992;49:630–636. doi: 10.1001/archpsyc.1992.01820080038006. [DOI] [PubMed] [Google Scholar]
- Wüst S, Federenko I, Hellhammer DH, Kirschbaum C. Genetic factors, perceived chronic stress, and the free cortisol response to awakening. Psychoneuroendocrinology. 2000;25:707–720. doi: 10.1016/s0306-4530(00)00021-4. [DOI] [PubMed] [Google Scholar]