Abstract
Production of nitric oxide by macrophages is believed to be an important microbicidal mechanism for a variety of intracellular pathogens, including Toxoplasma gondii. Mice with a targeted disruption of the inducible nitric oxide synthase gene (iNOS) were infected orally with T. gondii tissue cysts. Time to death was prolonged compared with parental controls. Histologic analysis of tissue from infected mice showed scattered small foci of inflammation with parasites in various tissues of iNOS−/− mice, whereas tissue from the parental C57BL/6 mice had more extensive tissue inflammation with few visible parasites. In particular, extensive ulceration and necrosis of distal small intestine and fatty degeneration of the liver was seen in the parental mice at day 7 postinfection, as compared with the iNOS−/− mice where these tissues appeared normal. Serum interferon γ and tumor necrosis factor α levels postinfection were equally elevated in both mouse strains. Treatment of the parental mice with a NO synthase inhibitor, aminoguanidine, prevented early death in these mice as well as the hepatic degeneration and small bowel necrosis seen in acutely infected control parentals. These findings indicate that NO production during acute infection with T. gondii can kill intracellular parasites but can be detrimental, even lethal, to the host.
The importance of cytokines in host resistance against infection with Toxoplasma gondii is well recognized (1, 2). A number of cytokines, including interleukin (IL)-7 (2), IL-12 (3), IL-15 (4), and interferon (IFN)γ (5) appear to be involved in modulating host resistance against acute parasite infection. The common pathway by which these cytokines increase survival involves the induction of IFNγ. This cytokine plays a pivotal role in both acute and recrudescent chronic infection in mice. Mice treated with an antibody that blocks the activity of IFNγ are unable to survive infection with an Toxoplasma strain of low virulence (5). During chronic infection, administration of antibody to IFNγ results in the development of toxoplasmic encephalitis (6).
The mechanism by which IFNγ protects is not completely understood. However, at least two possible mechanisms have been reported. First, IFNγ activates oxidative killing of the parasite by macrophages through increased production of reactive oxygen metabolites (OH) (7–9). The synergistic action of IFNγ together with a second effector [e.g., tumor necrosis factor (TNF) α] stimulates the production of nitric oxide (10–12). The tumoricidial and microbicidal activity of NO against a number of pathogens, including T. gondii, has been reported (10, 13, 14). We recently have observed the role of NO during acute T. gondii infection in mice with a targeted disruption in the gene expressing interferon-regulating factor (IRF-1) (15). This gene is up-regulated when macrophages are stimulated with IFNγ. The product of IRF-1 binds to the promotor of the inducible nitric oxide synthase (iNOS) gene, which culminates in the production of NO. IRF-1−/− mice are unable to produce detectable levels of NO when infected with T. gondii (15). These mice are more susceptible to acute T. gondii infection when compared with their genetically matched parental control. However, in addition to an alteration in NO production, it has been reported that IRF-1−/− mice have fewer CD8+ T cells (16). Because CD8+ T cells are essential for host protection against T. gondii, the contribution of this immunologic deletion to increased susceptibility of IRF-1−/− mice could not be separated from the effects of NO. To clarify the importance of NO in host resistance to T. gondii, mice with a specific deletion in the synthesis pathway of NO were used. Mice with a targeted disruption of the iNOS gene (17) were orally infected with T. gondii. In spite of uncontrolled hematogenous dissemination of the parasite in the iNOS−/− mice, these mice survived significantly longer with less evidence of pathologic changes to their liver and gut than the parental controls.
MATERIALS AND METHODS
Parasites and Mice.
The 76K strain of T. gondii in this study was provided by Daniel Bout, Tours, France. This strain is maintained by continuous oral passage of cysts as previously described (18, 19). Cysts were isolated from infected tissue, enumerated, and used to orally infect mice. Control and test mice were challenged with 50 cysts, except as otherwise noted. Control mice were challenged via oral administration with an equal quantity of uninfected mouse brain tissue. For quantitation of parasite burden we used RH strain tachyzoites that are regularly maintained in our laboratory by serial passage in human fibroblast cells.
Breeding pairs of mice (C57BL/6X129) were kindly provided by John MacMicking (Cornell University Medical College, NY), John Mudgett (Merck Research Laboratories, Rahway, NJ), and Carl Nathan (Cornell University Medical College, NY). These mice have a targeted deletion of the iNOS gene as previously described (17). The mice were backcrossed for five generations to wild-type C57BL/6. The absence of iNOS gene was confirmed by PCR.
In preliminary experiments C57BL/6X129 were used as the control. We observed histological changes in both the liver and intestine at the microscopic and gross level. An identical observation in the histology of the liver and intestine was confirmed in uncrossed C57BL/6 mice. Based on these observations, C57BL/6 mice were used as the control in these studies because of their greater availability. These mice were bred under approved conditions in the Animal Research Facility at Dartmouth Medical School. Before the study, offspring were analyzed for expression of iNOS (20). Five- to six-week-old female mice were used in these studies. Control C57BL/6 mice of the same parental lineage were age and sex matched (The Jackson Laboratory). Mice were evaluated daily for clinical evidence of infection (weight loss, ruffled fur).
Aminoguanidine (Sigma) was used to block production of NO. The drug was titrated in mice to establish a dosage that was nontoxic over the time of the experiment. Aminoguanidine was administered by intraperitoneal injection once daily (100 mg/kg body weight). The treatment was started at the time of infection and continued up to day 14 postchallenge.
Cytokine and iNOS Analysis.
Expression of both mRNA and protein for various cytokines was measured after infection of the mice. For protein determination, sera (0.25 ml/mouse) was obtained by tail bleed and analyzed for level of protein by ELISA. The serum levels of IFNγ were tested by using a commercially available kit (Endogen, Cambridge, MA) according to the manufacturer’s instructions. Serum level of TNFα were determined by ELISA as described (21).
Expression of mRNA for cytokines was performed by using quantitative PCR (2). Briefly, splenocytes were isolated from infected mice at indicated times postinfection (p.i.), and the message for either IFNγ or IL-10 was determined by the PQRS quantitative method. The splenocytes from uninfected mice were used to establish a baseline value of 1.0 against which the level of message for cytokine in the test mice was quantitated.
For the measurement of iNOS expression, C57BL/6 mice were infected orally. At various time points p.i. the mice were sacrificed, and tissues were stored at −70°C. The pooled tissues from three mice per group were homogenized in Trizol and meshed in a tissue homogenizer. The RNA was extracted from these samples, and reverse transcriptase–PCR was performed as above. Each tissue from uninfected mice were used to establish a baseline value of 1.0 against which the level of message for this gene was quantitated.
Quantitation of Tissue Parasite Burden.
Organs were recovered fresh from mice at 7 days p.i. Genomic DNA was prepared according to standard protocol (22). Tissue was homogenized in 0.1 M NaCl/10 mM Tris⋅HCl, pH 8/10 mM EDTA/0.5% sodium deoxysulfate/0.2 mg/ml proteinase K. The homogenate was incubated at 55°C until the lysate was clear. Nucleic acids were collected after phenol-chloroform extraction and ethanol precipitation. The RNA was digested in a final concentration of 0.5 mg/ml RNase A for 30 min at 37°C. The isolated DNA was collected after phenol extraction and ethanol precipitation. To quantify the number of parasites in the tissue, Toxoplasma DNA was isolated from a known number of cell culture-derived extracellular tachyzoites.
The Toxoplasma B1 gene sequence was used as a probe to quantify tissue parasite load. This gene, previously used for detection of this parasite (23), is a 35-fold repetitive sequence and is found in all parasite strains, including 76K and RH. A 1,018-bp B1 sequence was amplified by using primers 5′-GTTGGTTCCGCCTCCTTCGTC and 3′-CGAATCAACGGAACTGTAATG with a cycle of 1 min denaturation at 93°C, 1 min. annealing at 55°C and 1.5 min. extension at 72°C. A corresponding 1-kb band observed on 1% agarose gel was purified by QIA Gel Extraction Kit (Qiagen, Chatsworth CA) and [32P]dATP tagged with a random-primed labeling kit (Boehringer).
Slot blot hybridization was carried out as described (22). Five micrograms of genomic DNA from each tissue was denatured with 0.4 N NaOH and 0.2 mM EDTA and then blotted onto a Hybond-N+ positive charged membrane. The membranes were analyzed by autoradiography and scanned by a Silverscanner III flatbed scanner Model G550A (Epson, Torrance, CA). Data analysis was performed by using the public domain NIH Image program (developed at the U.S. National Institutes of Health and available from the Internet at zippy.nimh.nih.gov).
Histopathological Analysis.
Tissues were immediately fixed in buffered 10% formalin, embedded, sectioned, and stained with hematoxylin and eosin for routine histological examination. Sections were examined and photographed on Kodak TMax film with an Olympus VanOx photomicrosope.
Statistical Analysis.
Statistical analysis for the survival of mice was performed by Fisher’s exact test, and difference in survival time was determined by log rank test.
RESULTS
iNOS−/− Mice Survive Primary Oral Infection with T.
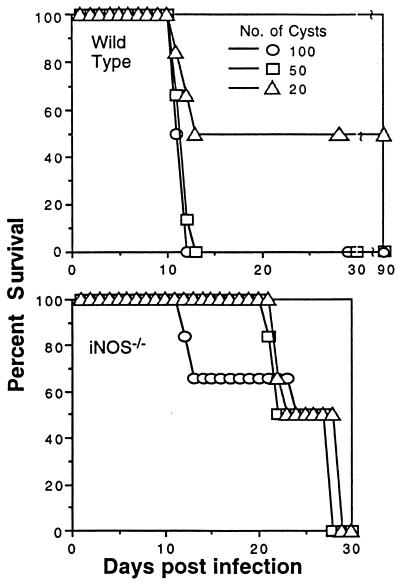
gondii. To determine if deletion of the iNOS gene altered innate resistance to the parasite, iNOS−/− mice or their genetically matched parental controls were infected orally with tissue cysts containing bradyzoites. A significant difference in time to death was observed between the parental and iNOS−/− strain. As shown in Fig. 1, all iNOS−/− mice that were infected with 50 or 100 76K strain tissue cysts per mouse survived the acute phase of infection (day 1–12 p.i.). At no time during the initial period of infection (day 0–21 days p.i.) did these manifest evidence of clinical illness. In contrast, none of the parental control mice survived beyond day 12 p.i. (P = .0001). When 20 cysts per mouse were used, the overall mortality in the parental strain was reduced by 50% during the first 12 days p.i. All of the iNOS−/− mice infected with same cyst load (20 cysts) survived beyond 12 days.
Figure 1.
Survival of iNOS−/− mice from oral T. gondii challenge. iNOS−/− and parental mice (n = 8/group) were orally infected with different number of cysts containing bradyzoites of T. gondii and mortality monitored. This study was performed three times with similar findings. Data presented are representative of one experiment.
On day 27 p.i. the iNOS−/− mice infected with T. gondii began to die. By day 30, all of the iNOS−/− mice were dead. The difference in survival time p.i. was independent of the number of cysts that were used for infection. In contrast, parental mice that survived the initial stages of infection with 20 cysts survived to day 90 when the experiment was terminated. This observation suggested that the ability to produce NO in the acute phase of infection may be detrimental to the infected host, but that survivors could control chronic infection better than mice that lack the ability to produce NO.
Analysis of Cytokine Levels P.I.
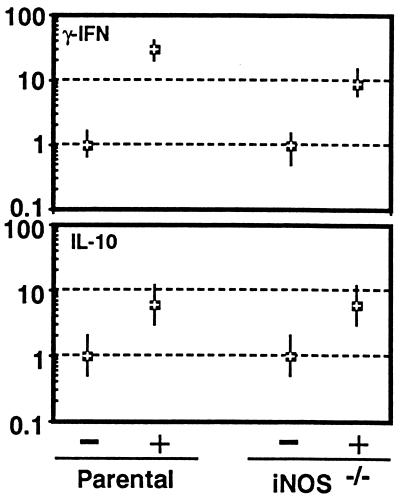
We measured cytokine levels in spleen and serum to determine if an alteration in the expression of mRNA or serum concentration of cytokine proteins could account for the difference in susceptibility between the parental and iNOS−/− mouse strains. For this analysis, IFNγ and IL-10, two cytokines that are important in mediating host immunity to this parasite, were studied. IFNγ is the principal mediator responsible for induction of NO in the host. IL-10 was measured because of its known association with immune down-regulation after T. gondii infection in mice (24). Mice were infected orally with cysts, and their splenocytes were isolated at day 7 p.i. These cells were analyzed for cytokine message expression by quantitative PCR. As shown in Fig. 2, the level of IFNγ mRNA for both the wild-type and iNOS−/− mice was increased at least 10-fold over baseline. The parental mice exhibited a several-fold increase in message for IFNγ when compared with the iNOS−/− mice but there was no appreciable difference in the level of message for IL-10 between the two mouse strains.
Figure 2.
Cytokine mRNA expression after T. gondii infection in iNOS−/− and parental mice. Mice were uninfected (−) or infected orally (+) with 50 cysts and on day 7 p.i. splenocytes from three mice were harvested and pooled, and mRNA expression for IFNγ and IL-10 was assayed by reverse transcriptase–PCR. The differences in the transcriptional level for both the genes are expressed relative to those mice treated with saline (level designated as 1). The cDNA concentration examined at each point was standardized to the hypoxanthine phosphoribosyltransferase mRNA level (not shown).
Serum level of IFNγ and TNFα were quantitated p.i. because induction of NO is dependent on costimulation of effector cells with IFNγ and another molecule such as TNFα. Mice were infected orally with tissue cysts containing bradyzoites. Sera from infected mice was collected 7 days p.i. and assayed by ELISA for the level of IFNγ and TNFα protein. As shown in Table 1, there were increases in the levels of IFNγ in the sera of both parental and iNOS−/− mice p.i. Oral infection with cysts also resulted in a significant rise in the level of circulating TNFα in both mouse strains. However, there was no significant difference in the levels of either of these cytokines between the parental and iNOS−/− mice.
Table 1.
Serum cytokine levels of T. gondii-infected iNOS −/− mice
| Mice | IFNγ, pg/ml | TNFα, pg/ml |
|---|---|---|
| Parental uninfected | 15 ± 3.7 | 10.9 ± 2.5 |
| Parental infected | 2,650 ± 158 | 1,480 ± 211 |
| INOS −/− uninfected | 16 ± 4.7 | 13.6 ± 6.4 |
| INOS −/− infected | 2,735 ± 250 | 1,217 ± 35.9 |
iNOS −/− and parental mice (n = 3/group) were infected with 50 cysts of T. gondii and sera collected at day 7 p.i. The sera was quantitated for both IFNγ and TNFα by ELISA.
Histologic Analysis.
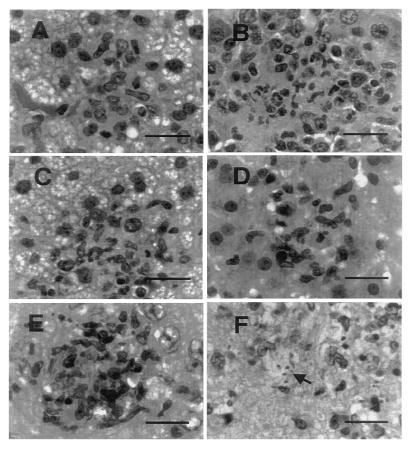
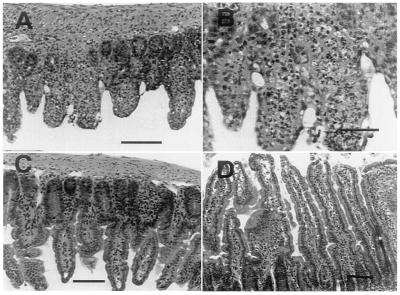
Examination of tissues of T. gondii-infected parental mice at day 7 showed small scattered nodules composed of lymphocytes and a few polymorphonuclear neutrophils in the liver, spleen, pancreas, and brain, without easily demonstrated parasites. As seen in Fig. 3A, the liver had evidence of extensive fatty degeneration. There were only scattered islands of preserved hepatocytes. The distal small bowel was extensively necrotic, with full thickness inflammation and ulceration (Fig. 4 A and B). The brains of parental mice had small foci of encephalitis scattered in the cortex and periventricular gray matter, with predominant lymphocyte infiltration and little necrosis (Fig. 3E).
Figure 3.
Photomicrographs of mouse tissues 7 days p.i. with T. gondii, processed as specified in Materials and Methods. Bars equal 30 μM. (A) Liver of C57BL/6 parental mouse showing an inflammatory nodule surrounded by hepatocytes with marked fatty degeneration. (B) Liver of iNOS−/− mouse showing acute inflammatory nodule among largely preserved hepatocytes. (C) Liver of sham-treated C57BL/6 parental mouse with changes similar to the untreated parental mouse. (D) Liver of aminoguanidine-treated parental mouse with inflammation but preservation of hepatocyte morphology. (E) Brain of parental mouse with acute inflammation and necrosis. (F) Brain of iNOS−/− mouse, with acute inflammation. Arrow indicates T. gondii tachyzoites.
Figure 4.
Photomicrographs of mouse distal ileum 7 days p.i. with T. gondii. (A and B) Parental C57BL/6 mouse showing acute necrosis of small bowel villi with acute inflammatory infiltration and ulceration. (A) Bar equals 100 μM. (B) Bar equals 200 μM. (C) Parental mouse treated with aminoguanidine showing preservation of small bowel epithelium. Bar equals 100 μM. (D) iNOS−/− mouse with normal small bowel epithelium. Bar equals 75 μM.
In contrast, although the iNOS−/− mice showed scattered foci of lymphocytes and polymorphonuclear neutrophils in similar tissue distribution to that seen in parentals, the liver was morphologically intact, with little evidence of hepatocellular dysfunction (Fig. 3B). Liver enzyme value was two times greater in the infected parental compared with iNOS−/− mice. The distal small bowel was normal (Fig. 4D). The brains of iNOS−/− mice had larger, looser foci of encephalitis. These brains included more extensive polymorphonuclear neutrophil infiltration, necrosis, and demonstrable parasites (Fig. 3F).
At day 27, both iNOS−/− and parental mice exhibited foci of necrosis and inflammation in brain and liver (data not shown). The infected foci observed in the iNOS−/− were more extensive, compared with parental mice, suggesting that iNOS−/− mice have decreased ability to control hematogenous spread of the parasite and parasite multiplication in the chronic infection before their death at day 27–30 p.i.
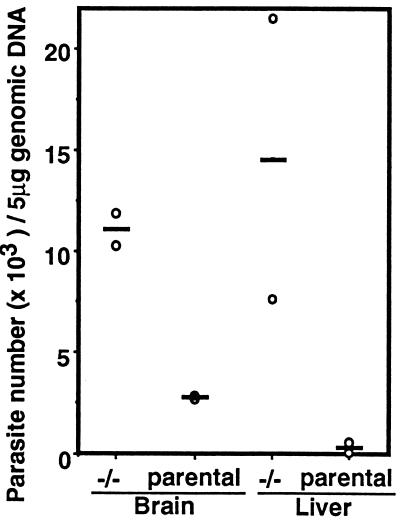
A Southern blot analysis was done to quantitate the parasite burden in the brain and liver tissue at day 7 p.i. Mice orally infected with cysts were sacrificed at day 7 p.i., and brains and liver were harvested for assay of the relative abundance of B1, a T. gondii marker gene. Analysis of brain and liver tissue from the two strains of mice demonstrated a significant increase in the number of parasites in the iNOS−/− mice compared with the parental mice. As shown in Fig. 5, the number of parasites in the brain of iNOS−/− was 3.5 times over that observed in the parental mice. An even greater difference in parasite burden was observed in the liver where 15 times more parasites were quantified in the iNOS−/− compared with the parentals.
Figure 5.
Levels of parasite DNA in the organs of iNOS−/− and parental mice infected with T. gondii. Mice (n = 3/group) were infected orally with 50 cysts of T. gondii. DNA was prepared from the brain and liver of mice 7 days p.i. A slot blot analysis was probed with T. gondii-specific B1 sequence. The scanned auto radiogram was quantitated by NIH Image that used DNA from a known number of extracellular tachyzoites as control. Bars represent the average number of parasites detected in each tissue.
Treatment of Parental Mice with Aminoguanidine Prolongs Time to Death.
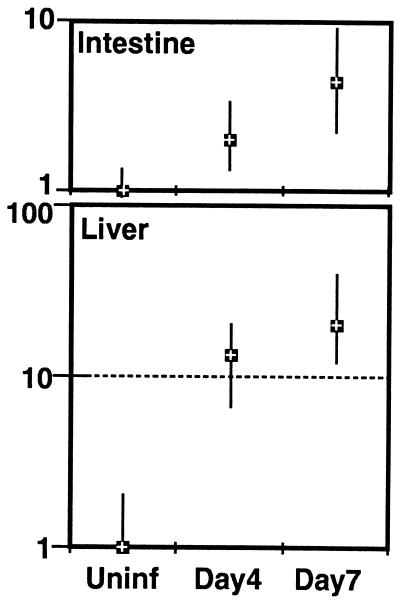
An analysis of the iNOS response in the wild-type mice was undertaken to determine if this molecule was up-regulated in response to infection. For this study, mice were orally infected with cysts, and message for this molecule was measured in the gut and liver at day 4 and 7 p.i. There is a substantial increase in the expression of this molecule after oral infection in the wild-type C57BL/6 mice (Fig. 6). The expression of this molecule is at least 5-fold in the gut and 20-fold in the liver above baseline at day 7 p.i.
Figure 6.
Levels of iNOS message in the intestine and liver of C57BL/6 mice infected with T. gondii. Mice were infected orally with cysts. On day 0, 4, and 7 p.i. the liver and distal ileum were isolated and stored at −70°C. The pooled tissues from three mice at each time point were homogenized, and mRNA expression for iNOS was assayed by reverse transcription–PCR. The transcriptional level is expressed relative to uninfected mice (designated as 1). The cDNA concentration examined at each point was standardized to the hypoxanthine phosphoribosyltransferase mRNA level (not shown).
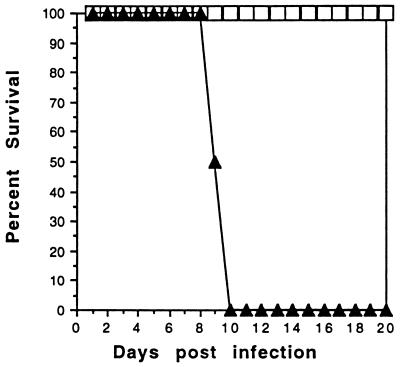
To determine if NO was the factor responsible for the increased susceptibility and marked pathologic changes in the parental mice, we treated mice with the NO inhibitor aminoguanidine. Parental mice were infected orally with 50 cysts of 76K strain and treated daily with aminoguanidine beginning the day of infection. Treatment with aminoguanidine was continued for 2 weeks. Administration of this drug resulted in the complete reversal of susceptibility to infection in the parental mice (Fig. 7). The aminoguanidine-treated mice survived beyond 20 days p.i. whereas all of the untreated control mice died by day 8–9 p.i. Histological examination of tissues from parental mice treated with aminoguanidine showed scattered inflammatory liver lesions resembling the iNOS−/− mice, with preservation of hepatocyte morphology (Fig. 3D). In contrast, the sham-treated infected controls showed the same extensive fatty degeneration of hepatocytes previously described (Fig. 3C).
Figure 7.
Effect of aminoguanidine treatment on the susceptibility of mice infected with T. gondii. Parental mice (n = 6/group) were infected with 50 cysts and treated daily with 100 mg/kg body weight of aminoguanidine. Control mice were infected and administered saline. The experiment was performed twice with similar findings. The data presented is representative of one experiment.
A analysis of serum cytokine levels in infected parental mice was performed to determine if aminoguanidine treatment altered the cytokine response. Both aminoguanidine-treated and untreated control mice were bled on day 7 p.i., and serum was analyzed for presence of cytokine. As shown in Table 2 the level of IFNγ production in the two groups of mice was similar. Further, there was no difference in the production of TNFα between the aminoguanidine-treated and control groups.
Table 2.
Serum cytokine levels of T. gondii-infected mice treated with aminoguanidine
| Mice | IFNγ pg/ml | TNFα pg/ml |
|---|---|---|
| Uninfected + saline | 28 ± 12 | 30 ± 17 |
| Infected + saline | 2,764 ± 216 | 1,336 ± 44 |
| Uninfected + AG | 35 ± 16 | 28 ± 16 |
| Infected + AG | 2,865 ± 192 | 1,320 ± 161 |
Parental mice (n = 3/group) were treated daily with 100 mg/kg body weight of aminoguanidine starting on the day of infection. The sera was collected at day 7 p.i. and the cytokine levels of IFNγ and TNFα were determined by ELISA. AG, aminoguanidine.
DISCUSSION
In this report we demonstrate that mice with a targeted deletion of the iNOS gene are significantly more resistant to death during acute infection with the intracellular protozoan, T. gondii than their control parentals. Mice with a disruption of this gene are unable to produce NO in response to microbial pathogen exposure (17). Oral infection of these mice with tissue cysts containing bradyzoites resulted in longer survival than the parental control mice. At 7–11 days p.i., morphologic changes were observed in the liver and distal small intestine of parental, but not iNOS−/−, mice. At the same time, we also observed a significant increase in parasite burden in the brain and liver tissue of the iNOS mice compared with parentals. Treatment of parental mice with aminoguanidine, an inhibitor of NO synthase, rendered the mice resistant to early death after oral parasite infection. Moreover, aminoguanidine treatment reversed the pathologic changes noted in the liver and gut of the parental mice. These findings suggest that NO has both a microbicidal role and a detrimental role in stimulating immunopathologic changes in tissues, which may lead to the death of the infected host.
Although the importance of IFNγ in stimulating host immunity to T. gondii is well appreciated, the mechanism by which this cytokine prevents death of the host in the murine model is not well understood. The importance of IFNγ-mediated NO production in host immunity against acute T. gondii has been observed. Recent studies by Scharton-Kursten et al. (25) have suggested that NO is more important in host immunity against chronic infection. We observed that NO may not be an essential mediator of protection against acute T. gondii in mice that have a targeted deletion in the gene for IRF (15). After intraperitoneal infection with T. gondii tachyzoites, these mice express significant quantity of mRNA for IFNγ in their splenocytes, but are unable to produce measurable amounts of nitrite, a metabolic product of NO biosynthesis. These mice were observed to be significantly more susceptible to acute Toxoplasma infection, which we presumed to be secondary to the loss of their ability to produce NO in response to microbial pathogens. However, it now has been shown that IRF−/− mice have an inherent defect in both the production of CD8+ T cells (16) and functionally adequate NK cells. Because both of these cell types are associated with host immunity to Toxoplasma it is possible that the increased susceptibility of the IRF−/− mice to T. gondii infection could be attributed to either immunologic defect.
To better evaluate the role of NO during acute T. gondii infection, mice with a targeted disruption in the iNOS gene were used (17). Current evidence suggests that these mice are immunologically intact except for their inability to produce inducible NO. These mice are unable to produce NO in response to infection with the intracellular bacteria, Listeria monocytogenes (17). Although iNOS−/− mice were able to survive acute infection, none of the mice survived beyond day 30, and all mice had histologic evidence of tissue infection and chronic inflammatory changes. The observation that chronically infected iNOS−/− eventually succumb to fatal T. gondii infection is consistent with the recent report that NO is an important modulator of long-term immunity against this parasite (25). In the absence of NO, these mice eventually die from chronic, progressive infection.
During acute infection, however, NO also appears to be a modulator of immunopathogenesis. In the most susceptible strain of mouse, C57BL/6 (26), histopathological analysis at day 7 p.i. demonstrated marked changes in the liver and intestine. Quantitative measure of iNOS expression in the wild-type C57BL/6 mice showed a substantial rise in the production of this molecule in response to oral infection. The histologic analysis included many foci of inflammatory cells with little evidence of either intracellular or extracellular Toxoplasma. In the liver, hepatic degeneration was characterized by fatty infiltration throughout the parenchyma. Intestinal inflammation was evident in the distal ileum with loss of the brush border, necrosis, and acute inflammatory infiltration. Although it has been suggested that NO may not be essential for host protection against acute infection (15, 25) a quantitative assay of tissue parasite burden were provided. The histologic and quantitative analysis that NO production can reduce, but not eliminate, parasite burden during acute infection. However, the price to pay for this exuberant immunologic response can be detrimental to the host.
Our observations indicate that the degenerative changes noted in the liver and bowel of parental mice are secondary to NO rather than a direct effect of cytokines. The role of TNFα and IFNγ in stimulating immunopathologic changes during intracellular infections has been reported (27, 28). TNFα increases susceptibility during murine Trypanosome cruzii infection (29). The observation that IL-10-deleted mice are more susceptible to acute T. gondii infection has been attributed to increased levels of TNFα and IFNγ production (30, 31). Treatment of susceptible C57BL/6 mice with anti-IFNγ antibody prevents intestinal necrosis because of T. gondii infection (29). Our data suggests that it is more likely a local tissue abundance of NO and not the level of IFNγ produced by the host that is responsible for the pathologic changes in the gut (28) and possibly in the liver. We observed that the pathologic changes in the liver and gut are preventable and appear to be independent of the systemic level of either IFNγ or TNFα. This observation has suggested to us and others (25) that IFNγ may stimulate host immunity to this parasite by alternative pathways.
Acknowledgments
We thank Drs. Dominique Buzoni-Gatel, Carl Nathan, John MacMicking, and Jacqueline Channon for their helpful thoughts in the preparation of this manuscript. This work was supported by National Institutes of Health Grants AI19613, AIAI33325, and AI35956.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: iNOS, inducible nitric oxide synthase; IFN, interferon; TNF, tumor necrosis factor; IL, interleukin; p.i., postinfection; IRF, interferon-regulating factor.
References
- 1.Gazzinelli R T, Wysocka M, Hayashi S, Denkers E Y, Hieny S, Caspar P, Trinchieri G, Sher A. J Immunol. 1994;153:2533–2543. [PubMed] [Google Scholar]
- 2.Kasper L H, Matsuura T, Khan I A. J Immunol. 1995;155:4798–4804. [PubMed] [Google Scholar]
- 3.Khan I A, Matsuura T, Kasper L H. Infect Immun. 1994;62:1639–1642. doi: 10.1128/iai.62.5.1639-1642.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khan I A, Kasper L H. J Immunol. 1996;157:2103–2108. [PubMed] [Google Scholar]
- 5.Suzuki Y, Orellana R, Schreiber R D, Remington J S. Science. 1988;240:516–518. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki Y, Remington J S. J Immunol. 1990;144:1954–1956. [PubMed] [Google Scholar]
- 7.Mcabe R E, Luft B J, Remington J S. J Infect Dis. 1984;150:961. doi: 10.1093/infdis/150.6.961. [DOI] [PubMed] [Google Scholar]
- 8.Nathan C F, Murray H W, Weibe M E, Rubin B Y. J Exp Med. 1983;158:670. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murray H W, Spitalny G L, Nathan C F. J Immunol. 1985;134:1619. [PubMed] [Google Scholar]
- 10.Green S J, Nacy C A, Meltzer M S. J Leukocyte Biol. 1991;50:93–103. doi: 10.1002/jlb.50.1.93. [DOI] [PubMed] [Google Scholar]
- 11.Mauel J, Ransijn A, Buchmuller-Rouiller Y. J Leukocyte Biol. 1991;49:73–82. doi: 10.1002/jlb.49.1.73. [DOI] [PubMed] [Google Scholar]
- 12.Roach T I, Kiderlen A F, Blackwell J M. Infect Immun. 1991;59:3935–3944. doi: 10.1128/iai.59.11.3935-3944.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gazzinelli R T, Eltoum I, Wynn T A, Sher A. J Immunol. 1993;151:3672–3681. [PubMed] [Google Scholar]
- 14.Liew F Y, Cox F E. Immunol Today. 1991;12:A17–A21. doi: 10.1016/S0167-5699(05)80006-4. [DOI] [PubMed] [Google Scholar]
- 15.Khan I A, Matsuura T, Fonseka S, Kasper L H. J Immunol. 1996;156:636–643. [PubMed] [Google Scholar]
- 16.Kamijo R, Harada H, Matsuyama T, Bosland M, Gerecitano J, Shapiro D, Le J, Koh S I, Kimura T, Green S J, Mak T W, Taniguchi T, Vilček J. Science. 1994;263:1612–1615. doi: 10.1126/science.7510419. [DOI] [PubMed] [Google Scholar]
- 17.MacMicking J D, Nathan C, Hom G, Chartrain N, Fletcher D S, Trumbauer M, Stevens K, Xie Q W, Sokol K, Hutchinson N, Chen H, Mudgett J S. Cell. 1995;81:641–650. doi: 10.1016/0092-8674(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 18.Chardes T, Buzoni-Gatel D, Lepage A, Bernard F, Bout D. J Immunol. 1994;153:4596–4603. [PubMed] [Google Scholar]
- 19.Chardes T, Velge-Roussel F, Mevelec P, Mevelec M N, Buzoni-Gatel D, Bout D. Immunology. 1993;78:421–429. [PMC free article] [PubMed] [Google Scholar]
- 20.MacMicking J D, North R J, LaCourse R, Mudgett J S, Shah S K, Nathan C F. Proc Natl Acad Sci USA. 1997;94:5243–5248. doi: 10.1073/pnas.94.10.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tachibana K, Chen G J, Huang D S, Scuderi P, Watson R R. J Leukocyte Biol. 1992;51:251–255. doi: 10.1002/jlb.51.3.251. [DOI] [PubMed] [Google Scholar]
- 22.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. In: Current Protocols in Molecular Biology. Ausubel F M, editor. New York: Wiley; 1987. pp. 204–214. [Google Scholar]
- 23.Burg J L, Grover C M, Pouletty P, Boothroyd J C. J Clin Microbiol. 1989;27:1789–1792. doi: 10.1128/jcm.27.8.1787-1792.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan I A, Matsuura T, Kasper L H. Parasite Immunol. 1995;17:185–195. doi: 10.1111/j.1365-3024.1995.tb00888.x. [DOI] [PubMed] [Google Scholar]
- 25.Scharton-Kersten T M, Yap G, Magram J, Sher A. J Exp Med. 1997;185:1261–1273. doi: 10.1084/jem.185.7.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McLeod R, Eisenhauer P, Mack D, Brown C, Filice G, Spitalny G. J Immunol. 1989;142:3247–3255. [PubMed] [Google Scholar]
- 27.Lucas R, Magez S, Songa B, Darji A, Hamers R, de Baetselier P. Res Immunol. 1993;144:370–376. doi: 10.1016/s0923-2494(93)80082-a. [DOI] [PubMed] [Google Scholar]
- 28.Liesenfeld O, Kosek J, Remington J S, Suzuki Y. J Exp Med. 1996;184:597–607. doi: 10.1084/jem.184.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Truyens C, Torrico F, Angelo-Barrios A, Lucas R, Heremans H, De Baetselier P, Carlier Y. Parasite Immunol. 1995;17:561–568. doi: 10.1111/j.1365-3024.1995.tb00999.x. [DOI] [PubMed] [Google Scholar]
- 30.Gazzinelli R T, Wysocka M, Hieny S, Scharton-Kersten T, Cheever A, Kuhn R, Muller W, Trinchieri G, Sher A. J Immunol. 1996;157:798–805. [PubMed] [Google Scholar]
- 31.Neyer E L, Grunig G, Fort M, Remington J S, Rennick D, Hunter C A. Infect Immun. 1997;65:1675–1682. doi: 10.1128/iai.65.5.1675-1682.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]