Abstract
The existence of bacterial K+/H+ antiporters preventing the over-accumulation of potassium in the cytoplasm was predicted by Peter Mitchell almost fifty years ago. The importance of K+/H+ antiport for bacterial physiology is widely recognized but its molecular mechanisms remain underinvestigated. Here, we demonstrate that a putative Na+/H+ antiporter, Vc-NhaP2, protects cells of Vibrio cholerae growing at pH 6.0 from high concentrations of external K+. Resistance of V. cholerae to Na+ was found to be independent of Vc-NhaP2. When assayed in inside-out membrane vesicles derived from antiporter-deficient Escherichia coli, Vc-NhaP2 catalyzed the electroneutral K+(Rb+)/H+ exchange with pH optimum at ~7.75 with an apparent Km for K+ of 1.62 mM. In the absence of K+ it exhibited Na+/H+ antiport, albeit rather weakly. Interestingly, while Vc-NhaP2 cannot exchange Li+ for protons, elimination of functional Vc-NhaP2 resulted in a significantly higher Li+ resistance of V. cholerae cells growing at pH 6.0, suggesting the possibility of Vc-NhaP2-mediated Li+/K+ antiport. The peculiar cation specificity of Vc-NhaP2 and the presence of its two additional paralogues in the same genome make this transporter an attractive model for detailed analysis of structural determinants of the substrate specificity in alkali cation exchangers.
Potassium is the major monovalent cation of the bacterial cytoplasm. It regulates internal pH, activates many intracellular enzymes and functions as an important osmotic solute (1). However, excessive amounts of internal K+ are detrimental (2–4). Therefore, bacteria tightly regulate their cytoplasmic K+ through the activity of a number of different transport systems (reviewed in (1)). Kdp, Trk and Kup systems import K+ either at the expense of ATP hydrolysis (TrkA and Kdp) or symporting it with a proton (Kup) (5–8). In addition, tetracycline antiporters TetL in Bacillus subtilis and TetK in Staphylococcus aureus that are able to exchange monovalent cations, may contribute to the net K+ uptake (9–11). Export of K+ can be mediated by (a) the glutathione adduct-activated “emergency” KefB/KefC systems of Gram-negative organisms (12); (b) mechanosensitive channels under severe hypoosmotic stress (13–15), although they are thought to play only a minor role in overall K+ homeostasis (1); and (c) the MdfA multidrug resistance transporter, which at external pH >9.0 import protons in exchange for extracellular Na+ or K+ (16).
All the above potassium-expelling systems seem to be mobilized only in specific stressful situations. Paradoxically, the identity of system(s) responsible for routine energy-dependent K+ extrusion remains poorly understood. Almost fifty years ago, Peter Mitchell postulated the existence of “housekeeping” K+/H+ and Na+/H+ antiporters, that can directly use the proton motive force to prevent the dangerous over-accumulation of alkali cations (17). Typically, growing bacteria employ a variety of primary proton pumps to maintain a high transmembrane electrical potential difference, ΔΨ (negative inside) over a wide range of external pH. As a result, K+ (or any other monovalent cation), if allowed to equilibrate with the ΔΨ, would accumulate inside the cell at poisonous concentrations. At −120 mV of ΔΨ and a moderate external K+ concentration of 30 mM, at equilibrium the cell would accumulate as much as 3 M K+, a concentration that clearly is beyond the physiological limit. A K+/H+ antiporter would allow H+ expelled by the primary pumps to return into the cytoplasm in exchange for internal K+, thus solving the problem.
Although several families of bacterial Na+/H+ antiporters have been identified and studied in great detail (18–22), identification of specific K+/H+ antiporters in bacteria remains elusive. K+/H+ antiport activity as such has been demonstrated in everted membrane vesicles from E. coli a long time ago (23). Some Na+/H+ antiporters, exemplified by well-studied Ec-NhaA and Ec-NhaB (22), are highly discriminative against K+, while others exhibit more or less pronounced K+/H+ exchange as a concomitant activity, such as the multi-subunit Vc-Mrp in Vibrio cholerae (24), or the alkali-activated Aa-NhaP from Alkalimonas amylolytica that transports Na+, K+ and possibly NH4+, but not Li+ (25).
Recently, Radchenko and co-authors reported that Vp-NhaP2 from V. parahaemolyticus might be a K+-specific antiporter (4). If confirmed, this would set a valuable precedent, because in spite of the widely recognized importance of K+/H+ antiporters for bacterial ion and pH homeostasis (1), no transporter exclusively specific for K+ has been identified thus far. The authors assayed inside-out vesicles obtained from antiporter-deficient E. coli overexpressing the cloned Vp-NhaP2. The antiporter displayed a rather modest activity with K+ even at its pH optimum of 9.0; in the absence of K+, Na+ seemed to be a substrate as well, albeit poorer than K+ (see Fig. 5B in (4)). Unfortunately, the authors did not examine the effect of Na+ concentration on the Na+/H+ antiport activity. Therefore, definitive conclusions about the specificity of Vp-NhaP2 were hard to make at the moment. Also, one more pressing question remained: would the chromosomal deletion of nhaP2 gene produce a potassium-sensitive phenotype in its native host, V. parahaemolyticus?
Inspired by the work of Radchenko and colleagues, we undertook a search for other possible antiporters exclusively transporting K+. In the course of this search we cloned, functionally expressed and examined a homologue of Vp-NhaP2 from Vibrio cholerae O395, Vc-NhaP2, encoded by the open reading frame VC2703. We also engineered and characterized the Vp-NhaP2 chromosomal deletion mutant of V. cholerae. Data presented in this article define Vc-NhaP2 as an electroneutral K+/H+ antiporter, which in vitro is able to catalyze K+/H+, Rb+/H+, Na+/H+ and, possibly, Li+/K+ (but not Li+/H+) exchange, but in situ operates as a Mitchellian K+/H+ antiporter, protecting V. cholerae cells growing at pH 6.0 from high concentrations of K+. The peculiar behavior of Vc-NhaP2 in relation to the general problem of search for specific K+/H+ antiporters in bacteria is discussed.
EXPERIMENTAL PROCEDURES
Bacterial Strains and Culture Conditions
The Na+/H+ antiporter-deficient strain of E. coli TO114 (F l IN (rrnD-rrnE) nhaA::KmR nhaB::EmR chaA::CmR) was kindly provided by Dr. H. Kobayashi (Faculty of Pharmaceutical Sciences, Chiba University, Chiba, Japan) (31). For routine cloning and plasmid construction, DH5α (supE44 hsdR17 recA1 endA1, gyrA96 thi-1 relA1) (US Biochemical Corporation) or TOP10 (F- mcrA Δ(mrr-hsdRMS-mcrBC) ϕ80lacZΔM15 ΔlacX74 recA1 araD139 Δ(araleu) 7697 galU galK rpsL (StrR) endA1 nupG) (Invitrogen) were used as hosts. The Vibrio cholerae strain used in this study was O395-N1 (26), which is the classical Ogawa strain with partial deletion of the ctxAB operon (O1 classical biotype; SmR, ΔctxA1). If not otherwise indicated, TO114 cells were grown aerobically at 37°C in LBK medium (modified L broth in which NaCl was replaced with KCl (27)) supplemented with 100 μg/ml ampicillin, 30 μg/ml kanamycin, 34 μg/ml chloramphenicol, 100 μg/ml erythromycin and 0.05% (w/v) arabinose. V. cholerae cells were grown aerobically at 37°C in LB supplemented with 100 μg/ml carbenicillin, 100 μg/ml streptomycin and 0.0002% (w/v) arabinose.
Cloning and Expression of Vc-NhaP2
Sequence data for V. cholerae was obtained from the Institute of Genomic Research website at http://www.jcvi.org. The putative Vc-nhaP2 ORF was amplified by high-fidelity PCR, using chromosomal DNA of V. cholerae O395-N1 as a template and directly cloned into pBAD-TOPO vector (Invitrogen) under the arabinose-induced promoter (PBAD), yielding pVc-NhaP2. Primers used for cloning were: forward primer VcNhaP2expF 5′-GAGGAA-TAATAAGTGGACGCCGTTACGATTAAC-3′ and reverse primer VcNhaP2expR 5′-TTACTCCGCGCCTTCTTGTAGCTC-3′. The forward primer was designed to achieve expression of native enzyme without adding the N-terminal leader sequence usually introduced by this vector. The primer contains an in-frame stop codon and a translation re-initiation sequence, which consists of a ribosome-binding site and the first GTG of the protein. In the reverse primer, the native stop codon of Vc-nhaP2 was maintained.
PCR Conditions
Platinum PCR Supermix High Fidelity DNA polymerase (Invitrogen) was used to amplify the 1.75 Kb fragment corresponding to Vc-nhaP2. A hanging adenine was added by incubating the DNA in the presence of 1 unit of Taq DNA polymerase (Fermentas). A 5 μl aliquot of the PCR reaction was run on a gel to verify the product size and the remaining PCR reaction was purified using the QIAquick PCR Purification Kit (Qiagen). The DNA was then introduced into pBAD-TOPO vector according to the manufacturer’s protocol (Invitrogen). The ligation mixture was then used to transform TOP10 competent cells, which were then plated onto LB agar plates containing 100 μg/ml ampicillin and incubated overnight at 37°C. Transformants were screened by PCR for the correct orientation by using a forward primer for the plasmid (pBAD Forward) and the 3′ expression primer for the gene. Transformants with the gene in the correct orientation were grown in LB broth containing 100 μg/ml ampicillin overnight at 37°C and plasmid DNA was isolated using the QIAprep Spin Miniprep Kit (Qiagen). The fidelity of the PCR was confirmed by DNA sequencing at the Oregon State University Center for Genome Research and Biocomputing core lab facility. The pVc-NhaP2 construct was then introduced into E. coli TO114 by chemical transformation and into V. cholerae ΔNhaP2 by electroporation as described in (28).
Chromosomal Deletion of the Vc-nhaP2 Gene
Chromosomal deletion of Vc-nhaP2 was constructed by homologous recombination. The in-frame deletion construct was made using overlap extension PCR (29). A 1 Kb fragment upstream of the start codon was amplified from genomic DNA by PCR using the following primer pair: 1, 5′-GGGGGACTAGTGGTTCTGGAGT-AGTAACGATCTCCG-3′ and 2, 5′-GACTGACTGACTGACTGACTGACTCACTCTACCTCC-CAGTCTGCGATTAACG-3′. A 1 Kb fragment downstream of the stop codon was amplified from genomic DNA by PCR using the primer pair: 3, 5′-AGTCAGTCAGTCAGTCAGTCAGTCTAA-CGATCGTTTGCGCCTTGACGTTGAGG-3′ and 4, 5′-GGGGGGAGCTCGGAACGCGCA-AGGCGAGCCAGTACCG-3′. The 1 Kb products of these two PCR reactions are able to anneal together due to complementary sequences engineered into the primers and were used as a template for a third PCR reaction using primers 1 and 4, resulting in a 2 Kb PCR product encompassing 1 Kb upstream of the start codon and 1 Kb downstream of the stop codon with the gene itself removed. This was cloned into the suicide vector pWM91 (30) by restriction sites engineered into the primers and introduced into the chromosome of V. cholerae O395-N1 following sucrose selection as previously described (30). The above results in an in-frame deletion of 580 amino acids. This mutant strain (VcΔP2) along with its isogenic parent (VcWT) and VcΔP2/pVc-NhaP2 over-expressing Vc-NhaP2 in trans were used to assess the possible physiological role of Vc-NhaP2 in V. cholerae.
Analysis of Growth Phenotypes
For growth analysis of V. cholerae transformants, LBB medium (non-cationic L broth) was supplemented with antibiotics, arabinose (see above) and varying concentrations of KCl, LiCl or NaCl. The initial pH was adjusted to pH 6.0, pH 7.2 and pH 8.5 by the addition of 60 mM Bis-Tris-Propane (BTP)-HCl. Cells were inoculated at a starting optical density at 600 nm (O.D. (600 nm)) of 0.05 into 200 μl of liquid media placed in 96 deep-well plates (Whatman) and grown at 37°C for 18 hours with vigorous aeration. Growth was then measured as the O.D. of the bacterial suspension at 600 nm by scanning the plates on a Biotek Instruments plate reader using the Gen5 program. All experiments were repeated at least three times in triplicate.
Isolation of Membrane Vesicles and Assays of Antiporter Activity
TO114/pVc-NhaP2 and TO114/pBAD24 transformants were grown in LBK medium supplemented with 100 μg/ml ampicillin, 30 μg/ml kanamycin, 34 μg/ml chloramphenicol, 100 μg/ml erythromycin and 0.05% arabinose. Cells were harvested at an O.D. (600 nm) of 1.5 to 1.8 and immediately used for isolation of inside-out membrane vesicles essentially as described previously (24). Briefly, overnight cultures of TO114 transformants were grown in LBK medium containing the above antibiotics. These cultures were then used to inoculate the growth medium at a concentration of 1:100. For ΔpH measurements, after harvesting, the cells were washed three times in buffer containing 140 mM choline-chloride, 10% (w/v) glycerol and 20 mM Tris-HCl, pH 7.5. After the last wash, the bacterial pellet was resuspended in the same buffer containing 1 mM 1,4-dithiothreitol (DTT), 1 μg/ml pepstatin-A, 0.1 mM phenylmethylsulfonyl fluoride (PMSF) and approximately 5 mg/L DNase. The bacterial suspension was then passed twice through a French Press (Aminco), the unbroken cells were pelleted at 12,000 × g for 10 min at 4°C and the supernatant was ultracentrifuged at 184,000 × g for 90 min at 4°C. The resulting membrane pellets were then resuspended and stored in the same buffer containing all the additions except DNase until assay for cation/proton antiport activity. For ΔΨ measurements, vesicles were isolated in the above buffer, but it was made Cl−-free by the elimination of choline-chloride and replacing it with 280 mM sorbitol. Membrane vesicles were isolated and all experiments were performed at least two times.
Measurement of Transmembrane ΔpH
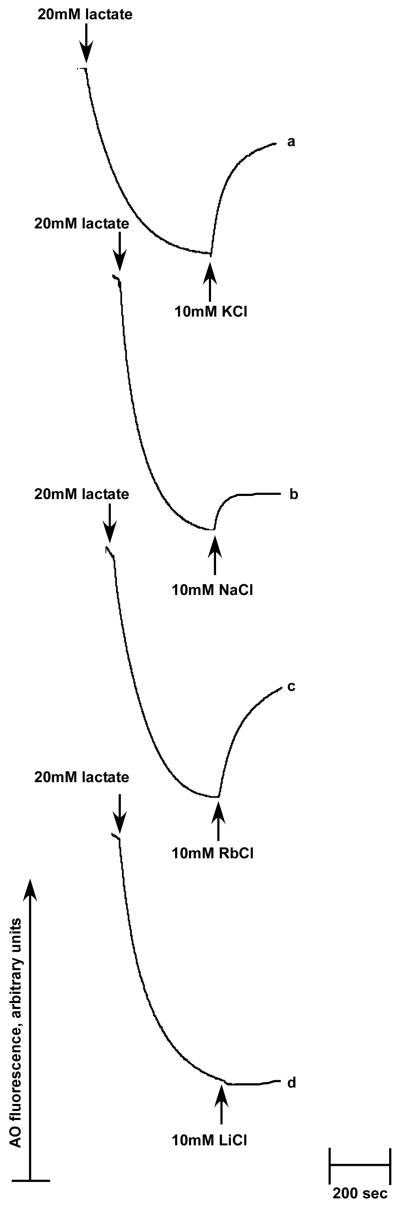
For ΔpH measurements, aliquots of vesicles (200 μg of protein) were added to 2 ml of buffer containing 140 mM choline chloride, 5 mM MgCl2, 10% (w/v) glycerol, 4 μM acridine orange and 50 mM BTP-HCl adjusted to the indicated pH. The cation/H+ antiport activity was then registered using the acridine orange fluorescence quenching/dequenching assay. Respiration-dependent generation of ΔpH was initiated by the addition of 20 mM Tris-D-lactate and the resulting quenching of acridine orange fluorescence was monitored in a Shimadzu RF-1501 spectrofluorophotometer (excitation at 492 nm and emission at 528 nm). Antiport activity was estimated based on its ability to dissipate the established ΔpH in response to the addition of NaCl, LiCl or KCl at the indicated concentrations. 10 mM of each was used in the determination of the pH profile of activity and 0.05 mM to 100 mM was used in the determination of half-maximal effective cation concentration (apparent Km). The antiport activities are expressed as percent restoration of lactate-induced fluorescence quenching. Each experiment was carried out in duplicate on at least two separate isolations of membrane vesicles. The traces shown in Figure 2 represent typical experimental results.
FIGURE 2.
Vc-NhaP2 antiport activities in sub-bacterial vesicles. Inside-out membrane vesicles were isolated from TO114 cells transformed with pVc-NhaP2 and assayed with 10 mM of the specified salt in standard choline chloride buffer adjusted to pH 7.5. At the indicated time, respiration-dependent formation of the transmembrane pH gradient was initiated by the addition of 20 mM Tris-D-lactate. After steady-state ΔpH was reached, cation/H+ antiporter activity was detected upon the addition of 10 mM KCl (a), 10 mM NaCl (b), 10 mM RbCl (c) and 10 mM LiCl (d). Acridine orange fluorescence is shown in arbitrary units.
Measurement of Transmembrane ΔΨ
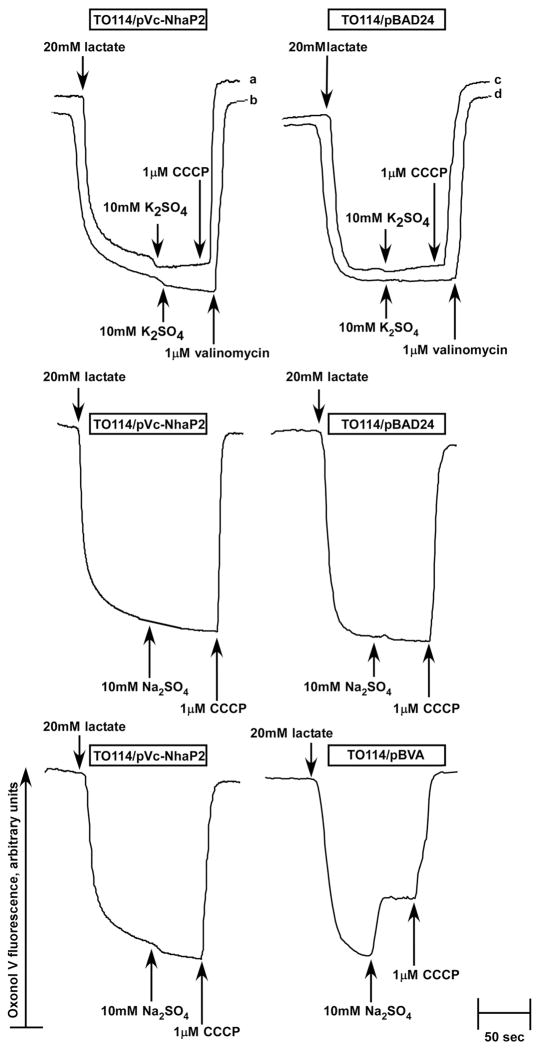
The ΔΨ-sensitive dye, Oxonol V, was used to examine whether the Vc-NhaP2-mediated cation/proton antiport has any effect on the respiration-generated formation of ΔΨ. In this case, vesicles were isolated in Cl−-free buffer as described above, resuspended in 2 ml of the same medium supplemented with 5 mM MgSO4 as well as 20 mM diethanolamine, pH 7.5 and pre-incubated at room temperature for 5 min before the addition of 8.0 μM Oxonol V. Excitation and emission were at 595 nm and at 630 nm, respectively. For some control experiments, vesicles were isolated from TO114 cells expressing Vc-NhaA (38). Each experiment was carried out in duplicate on at least two separate isolations of membrane vesicles. The traces shown in Figure 6 represent typical experimental results.
FIGURE 6.
Probing the stoichiometry of Vc-NhaP2. Inside-out membrane vesicles were isolated from TO114 transformants and assayed for ΔΨ at pH 7.5 in sorbitol-based medium devoid of K+ and Cl-. Diethanolamine at 20 mM was added to the experimental mixture 5 min prior to the addition of Oxonol V. At the indicated time, respiration-dependent formation of ΔΨ was initiated by the addition of 20 mM Tris-D-lactate. After steady-state ΔΨ was reached, cation/H+ antiport was initiated by the addition of 10 mM K2SO4 and 10 mM Na2SO4 (as indicated). The protonophore CCCP (traces marked “a”, “c”) or valinomycin in the presence of K+ (traces “b”, “d”) was added at the end of each measurement to collapse the generated ΔΨ for the control. The control experiment shown in the two lower panels compares side by side behavior of the electrogenic antiporter, Vc-NhaA (right lower panel, TO114/pBVA vesicles) and Vc-NhaP2 (left lower panel). Fluorescence of Oxonol V is shown in arbitrary units.
Protein Determination
Protein content in preparations of inside-out membrane vesicles was measured with the DC Protein Assay Kit (Bio-Rad) following the manufacturer’s instructions for membrane proteins.
Materials
All chemicals were purchased from Sigma-Aldrich or Fisher Scientific. Restriction endonucleases and DNA modifying enzymes were purchased from Invitrogen, MBI Fermentas or New England Biolabs.
RESULTS
Cloning and Expression of Vc-NhaP2
A BLAST search of the Vibrio cholerae O395 genome revealed three open reading frames homologous to Vp-nhaP2, namely VC0389, VC0689 and VC2703. Of these paralogues, VC2703 showed the highest degree of identity/similarity to Vp-nhaP2 at the level of translated sequence (76.7 and 83.4%, respectively), so we therefore designated it Vc-nhaP2. This putative Vc-nhaP2 ORF was amplified by PCR and cloned into pBAD-TOPO (Invitrogen) expression vector as described above. This construct was then introduced into E. coli TO114 and into V. cholerae O395-N1 after the chromosomal Vc-nhaP2 gene was deleted. Gene expression in pBAD-TOPO is under the control of the tightly regulated arabinose promoter (PBAD). Expression of Vc-NhaP2 was induced by the addition of 0.05% arabinose to the growth medium in the case of E. coli TO114 during the middle of log phase and in the case of Vibrio cholerae by the addition of 0.0002% arabinose at the beginning of growth. These concentrations of arabinose did not have any toxic effect on the bacterial cells, but allowed for adequate expression of Vc-NhaP2 as judged by activity in membrane vesicles and the results obtained from growth experiments performed in V. cholerae (see below).
The Physiological Role of Vc-NhaP2 in Vibrio cholerae
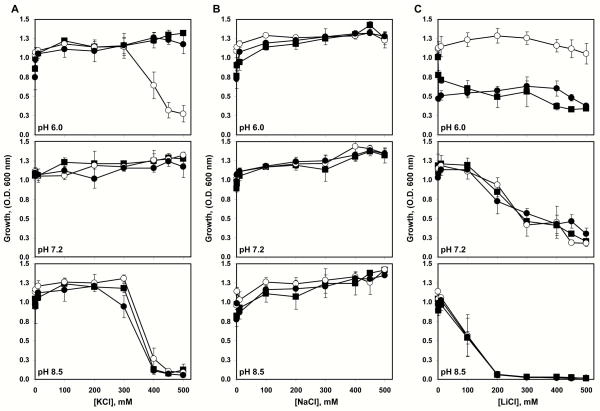
The VcΔP2 mutant strain of V. cholerae bearing the chromosomal Vc-nhaP2 deletion along with its isogenic parent, VcWT, and the VcΔP2/pVc-NhaP2 strain over-expressing Vc-NhaP2 in trans were used to assess the possible physiological role of Vc-NhaP2 in V. cholerae. Each strain was analyzed in LB-based medium (non-cationic L broth), containing increasing concentrations of K+ (Fig. 1A), Na+ (Fig. 1B) and Li+ (Fig. 1C) at three different pHs (6.0, 7.2, 8.5).
FIGURE 1.
Vc-NhaP2 protects V. cholerae from high concentrations of external potassium at acidic pH. The pVc-NhaP2 plasmid, containing Vc-NhaP2, was used to transform V. cholerae strain VcΔNhaP2, bearing deletion of Vc-nhaP2 gene (closed circles). Wild-type cells (closed squares) and VcΔNhaP2 (open circles) were transformed with “empty” pBAD24. Cells were grown aerobically for 18 hours in 96-deep well plates as described in the Experimental Procedures. In all cases the LBB medium, adjusted to the desirable pH, was supplemented with 0.0002% (w/v) arabinose and the indicated concentrations of KCl (A), NaCl (B), or LiCl (C). Growth was measured as the O.D. (600 nm) of the bacterial suspension. The starting O.D. (600 nm) was approximately 0.05 in all cases. Plotted are the averages of three separate experiments, each performed in tripicate. Bars show the standard deviation.
Presence of functional Vc-NhaP2 encoded by either chromosomal gene or plasmid-born Vc-nhaP2 is critical for the survival of V. cholerae at acidic pH in the presence of high concentrations of potassium (Fig. 1A, upper panel). In contrast, deletion of this antiporter does not affect the Na+ resistance of V. cholerae (Fig. 1B). This suggests that the actual physiological role of Vc-NhaP2 in V. cholerae is to maintain the internal concentration of potassium under toxic levels, by expelling cytoplasmic K+ ions. This suggestion is further supported by the fact that the growth of the VcΔP2 strain is unimpeded in the presence of highly toxic Li+ (which is a close chemical analog of Na+) compared to the wild-type strain and VcΔP2/pVc-NhaP2 at any pH tested (Fig. 1C). Actually, at pH 6.0 deletion of Vc-NhaP2 had a rather beneficial effect on growth (Fig. 1C, upper panel).
Ion Specificity of Vc-NhaP2
To measure the activity of Vc-NhaP2 directly, we expressed it in trans in cells of the antiport-deficient strain of E. coli, TO114. Membranes of this triple deletion mutant are devoid of both specific Na+/H+ antiporters, Ec-NhaA and Ec-NhaB, as well as Na+(Ca2+, K+)/H+ antiporter, Ec-ChaA (31,32). Consequently, the inside-out membrane vesicles derived from this strain display practically no Na+/H+ or Li+/H+ antiport activity at pH 6.0 to 7.75, and only at pH 8.0 and higher, a minor background Na+(Li+)/H+ exchange is detectable (4). Importantly, the same seems to be true about K+/H+ exchange in TO114 vesicles, as well (4), which makes TO114 the host of choice for the heterologous expression and analysis of cation-proton antiporters. We therefore employed inside-out membrane vesicles prepared from TO114/pVc-NhaP2 and TO114/pBAD24 (“empty”) cells. The cation/H+ antiport activities were detected by the standard acridine orange fluorescence dequenching technique and expressed as percent restoration of lactate-induced fluorescence quenching. The background activity mentioned above was measured at every pH tested in separate control experiments and subtracted from the levels obtained in Vc-NhaP2 containing vesicles to yield the data plotted in Figs. 3–6. However, it must be noted that this activity was below 2% at pH 7.5 (where most of our measurements were done) and even at pH 9.5 it did not exceed 8% when it was initiated by the standard addition of 10 mM of substrate cation.
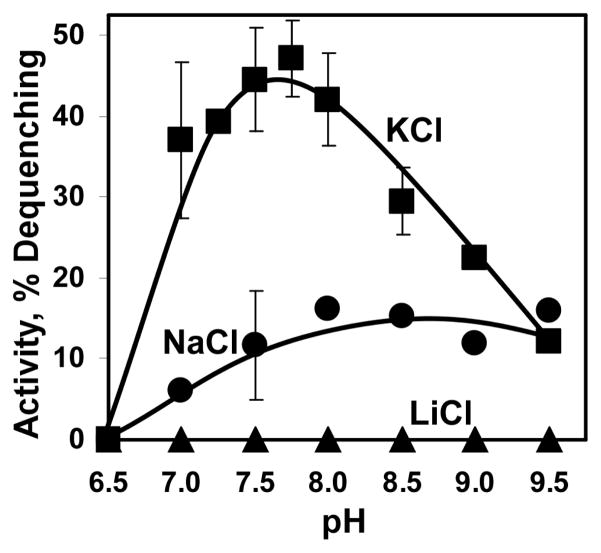
FIGURE 3.
pH profile of Vc-NhaP2 activity. Inside-out membrane vesicles were isolated from TO114 cells transformed with pVc-NhaP2 or “empty” pBAD24 and assayed with the specified salt in standard choline chloride buffer adjusted to the indicated pH with 50 mM BTP-HCl. In each case, residual non-specific activity measured in “empty” vesicles was substracted from that registered in Vc-NhaP2-containing vesicles and the resulting Vc-NhaP2-dependent activity was plotted as a function of pH. All other conditions as in Fig. 2. Plotted are the averages of six measurements (carried out in duplicate with three separate isolations of vesicles).
In accord with the growth data presented in Fig. 1, it turned out that K+ (Fig. 2, trace a) and its analog, Rb+ (Fig. 2, trace c), are preferable substrates of the Vc-NhaP2-mediated cation-proton exchange. In the absence of potassium, Vc-NhaP2 could also exchange Na+ (Fig. 2, trace b), but the overall antiport was much weaker (compare traces a and b in Fig. 2). Noticeably, addition of 10 mM Li+ failed to initiate H+ translocation in the membranes of TO114 cells (Fig. 2, trace d) and even at concentrations as high as 50–100 mM, Li+ was not exchangeable with proton (data not shown).
pH Profile of Vc-NhaP2 Activity
When assayed in inside-out membrane vesicles by the addition of 10 mM of alkali cations, Vc-NhaP2 demonstrated a bell-shaped pH profile of K+/H+ antiport activity with a maximum at pH from 7.5 to 7.75 (Fig. 3, squares). At pH 7.75, the activity of Vc-NhaP2 with 10 mM of potassium reached almost 50% of dequenching (Fig. 3, squares). No activity was detected at pH 6.5 and below with any of the probed cations. When probed with 10 mM of sodium, the antiporter showed gradual increase of activity up to pH of 8.0, reaching a plateau at approximately 15% of dequenching (Fig. 3, circles). With lithium, Vc-NhaP2 did not show any activity at all the pHs tested (Fig. 3, triangles), even at Li+ concentrations as high as 50–100mM (not shown). As expected, Rb+ behaved identically to K+ (data not shown). Thus K+/H+ antiport seems to be a major mode of activity of Vc-NhaP2.
The Affinity of Vc-NhaP2 for Alkali Cations
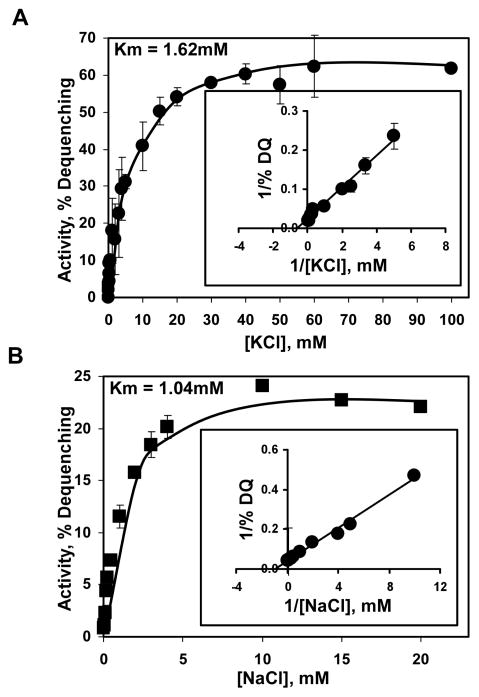
In order to assess the affinity of Vc-NhaP2 for transported potassium and sodium, we initiated the dequenching response in membrane vesicles isolated from TO114/pVc-NhaP2 by varying concentrations of K+ or Na+ at pH 7.5. These measurements yielded the concentrations of K+ and Na+ required for half-maximal response (Fig. 4). Although only indirectly related to the actual Km values of the antiport, these easily assessable parameters are by convention used as a measure of affinity of cation-proton antiporters and termed apparent Km for the corresponding substrate (see, for example, (33–35) and references therein). For Vc-NhaP2, [K+]1/2 and [Na+]1/2 are 1.62 mM (Fig. 4A) and 1.04 mM (Fig. 4B), respectively. Therefore, it is not the poor affinity to sodium as such that is responsible for the relative kinetic incompetence of Vc-NhaP2 in Na+/H+ antiport compared to K+/H+ antiport.
FIGURE 4.
Determination of kinetic parameters of Vc-NhaP2 in inside-out membrane vesicles isolated from TO114 transformants (inset, double recipricol plot). Measurements were done in standard choline chloride buffer adjusted to pH 7.5 with the final concentration of added KCl (A) and NaCl (B) varying from 0.05 to 50 mM. Each point represents the average of four measurements (carried out in duplicate with two separate isolations of vesicles). Bars show the standard deviation.
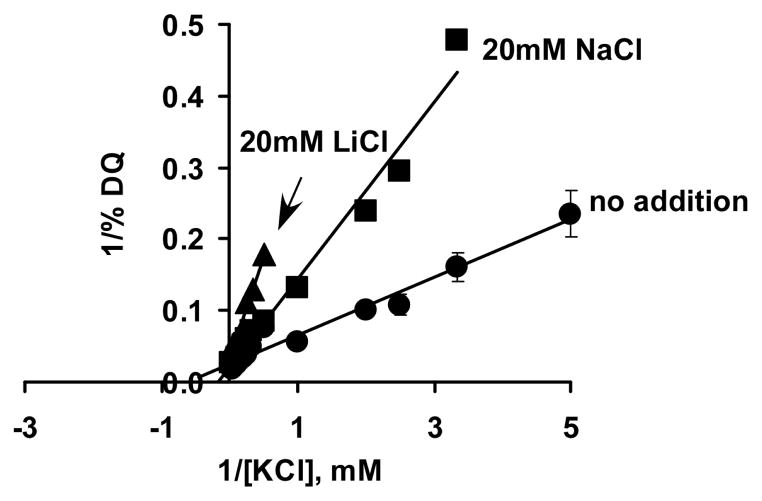
In the next series of experiments, the K+/H+ antiport activity was analyzed in the presence of 20 mM NaCl or 20 mM LiCl at pH 7.5 (Fig. 5). It turned out that both Na+ and Li+ worsened the affinity of Vc-NhaP2 toward potassium ions: when 20 mM NaCl was added to the experimental buffer, its Km for potassium changed from 1.62 to 5.95 mM and 20 mM of LiCl affected the Km even more, increasing it to 9.00 mM (Fig. 5). These competition assays clearly show that Li+ ions, as well as Na+, indeed compete with K+ for binding to the antiporter, affecting its apparent Km for K+, despite the fact that Vc-NhaP2, as it is evident from Fig. 2 and 3, does not catalyze Li+/H+ antiport per se (see Discussion).
FIGURE 5.
Na+ and Li+ compete with K+ ions for the Vc-NhaP2 antiporter. Dequenching of acridine orange in response to varying concentrations of KCl was monitored at pH 7.5 in standard choline chloride buffer with or without the indicated concentrations of NaCl or LiCl. Each point represents the average of four measurements (carried out in duplicate with two separate isolations of vesicles). Data are shown in reciprocal coordinates. Bars show the standard deviation.
Vc-NhaP2 is not Electrogenic
The above kinetic data do not determine the overall Vc-NhaP2 stoichiometry (i.e., the number of protons exchanged per each alkali cation). The V. cholerae growth results, however, indicates that Vc-NhaP2 probably requires ΔpH to extrude K+. Indeed, Vc-NhaP2 protects the growth from K+ in acidic (pH 6.0), but not in alkaline (pH 8.5) medium (Fig. 1A, compare upper and lower panels). This implies that it may catalyze electroneutral exchange of 1K+ per 1H+, because while at low external pHs ΔpH is the major component of the proton motive force on the bacterial membrane, at pH 8.5 it is close to zero or even may have an inverted polarity (cytoplasm more acidic than the culture medium) (36).
To probe the stoichiometry of Vc-NhaP2, inside-out membrane vesicles were isolated from TO114 transformants and assayed for ΔΨ in chloride-free, potassium-free (sorbitol-based) buffer. To maximize the respiration-generated ΔΨ, 20 mM diethaolamine was added to the vesicle suspension five minutes prior to addition of the ΔΨ-sensitive dye, Oxonol V. As a control, “empty” (TO114/pBAD24 without the Vc-nhaP2 insert) vesicles were isolated and assayed in the same way (Fig. 6). Energization by lactate led to the rapid generation of respiratory ΔΨ in both “empty” and TO114/pVc-NhaP2 vesicles (Fig. 6). Yet, the addition of K+ (Fig. 6, upper panels) or Na+ (Fig. 6, middle panels) resulted in no depolarization, clearly indicating the electroneutral nature of the cation/H+ exchange mediated by Vc-NhaP2 i.e., exchanging one alkali cation per one proton. Addition of the protonophore CCCP or valinomycin in the presence of potassium completely dissipated respiratory ΔΨ (the last addition in each trace of Fig. 6). As a positive control in these experiments, vesicles were isolated from TO114/pBVA cells expressing the electrogenic Vc-NhaA, which we cloned and functionally expressed in E. coli previously (38). Depolarization in response to the addition of its substrate cation differs this electrogenic antiporter from Vc-NhaP2 (Fig. 6, compare two lower panels). As another positive control, we pre-treated “empty” TO114 vesicles with very high (2.0 to 5.0 μM) concentrations of nigericin, an artificial ion exchanger which at sub-micromolar concentrations catalyzes the electroneutral K+/H+ antiport, but at concentrations exceeding 1.0 μM acts as an electrogenic antiporter (9, 37). As expected, addition of potassium to the nigericin-treated vesicles energized by lactate resulted in considerable depolarization (not shown).
Thus, although the experimental approach used here is a qualitative one, it provides means for the reliable distinguishing of an electroneutral process from an electrogenic one. Definitively, in order to measure the stoichiometry of electrogenic antiport, a more refined experimental model of reconstituted proteoliposomes would be required, as it has been done for Ec-NhaA (3) and Ec-NhaB (40).
DISCUSSION
The data presented above define Vc-NhaP2 as a non-electrogenic antiporter (Fig. 6) exchanging internal alkali cations for extracellular protons with clear maximum of activity at neutral pH (Fig. 3). Not only K+ and Rb+, but also Na+ ions were found to be the substrates (Fig. 2–3), binding to the antiporter with similar affinities (Fig. 4–5), displaying Km values in the low-millimolar range that are typical for bacterial Na+/H+ antiporters (35, 41–42). Although Li+ was not a substrate of cation-proton exchange (Fig. 2–3), Vc-NhaP2 obviously provided the route of entry for toxic Li+ ions into V. cholerae cells growing at pH 6.0: the Vc-NhaP2 deletion mutant showed much better Li+ resistance compared to both its wild type parent and the mutant expressing Vc-NhaP2 in trans (Fig. 1C, upper panel). What could account for such apparent discrepancy?
In addition to antiport with proton, Vc-NhaP2 also must be able to catalyze homo-ion (i.e., Na+/Na+, K+/K+, or Li+/Li+) and hetero-ion exchange, such as K+/Na+, Li+/Na+, and Li+/K+ exchange. When lithium is added to the growth medium, the latter of these processes will inevitably result in Li+ entry thus explaining the Vc-NhaP2-dependent Li+ sensitivity shown in the upper panel of Fig. 1C. The effect is only evident in acidic medium, where the major Li+(Na+) extruding system, electrogenic antiporter Vc-NhaA, is virtually inactive (Winogrodzki and Dibrov, unpublished observations; also see (43)). Higher pHs cause a steep activation of NhaA, so it is not surprising that the presence of Vc-NhaP2 does not affect overall Li+ sensitivity (Fig. 1C, middle and lower panels). As external pH rises, toxicity of Li+ ions increases dramatically (Fig. 1C, empty symbols in all three panels). This phenomenon has been observed in several species and is thought to reflect higher permeability of the membrane for Li+ ions in more alkaline media (18–20). As Fig. 1C shows, however, Vc-NhaP2 is not responsible for this massive alkali-stimulated Li+ leakage.
The inability of Vc-NhaP2 to catalyze Li+/H+ exchange (Fig. 2–3) despite the fact that the exchange of Li+ for other alkali cations is apparently in place (Fig. 1C), is one of the most curious features of this antiporter. It is widely accepted that in Na+/H+ antiporters all substrate alkali cations and protons share the same cation-binding site of the protein. This could be demonstrated by kinetic analysis (see (24) for a recent example) as well as inferred from structural data (22). Of course, in a general case translocated ions may use different subsets of ligands available at the cation-binding site; thus while H+ requires only one electronegative atom to bind to, the optimal number for coordination of Na+ ion by polypeptides is six (see (44) and references therein for a detailed discussion). Therefore, if in Vc-NhaP2, Li+ ion happens to bind, in addition to its other ligands, to a group normally employed by H+, it would out-compete proton thus preventing “normal” Li+/H+ exchange without affecting the exchange of Li+ with other alkali cations. X-ray diffraction-based structural data (co-crystallization with different substrates) could provide an ultimate test to this supposition, but kinetic analysis of mutant forms of Vc-NhaP2 appears to be a viable complementing, if not alternative, approach. On a more general note, the broad substrate specificity together with the peculiar behavior of Li+ make Vc-NhaP2 a promising experimental subject for detailed studies on structural determinants of cation specificity in ion exchangers.
The alkali cation exchange via Vc-NhaP2 in the cells of V. cholerae bearing functional Vc-NhaP2 should lead to the net uptake of not only Li+ but also Na+ ions. Nevertheless, Vc-NhaP2 does not diminish the Na+ resistance even at pH 6.0, where NhaA is silenced: V. cholerae cells grow well in Na+-rich media irrespectively of the presence of Vc-NhaP2 at any pH (Fig. 1B). It should be noted in this connection that one of V. cholerae primary Na+ pumps, namely the Na+-translocating NADH:ubiquinone oxidoreductase, NQR (45) could contribute to the net export of Na+. Indeed, despite the presence of the array of different Na+/H+ antiporters in the membrane, elimination of functional NQR renders growth of V. cholerae somewhat more sensitive to Na+ (Häse et al., unpublished observations; also Winogrodzki and Dibrov, unpublished results). Furthermore, in the presence of HQNO, a potent inhibitor of NQR, the growth of cells possessing Vc-NhaP2, but not the deletion Vc-NhaP2 mutant also became severely compromised at high concentrations of NaCl (data not shown). It is also worth mentioning that, in contrast to NhaA, Li+ is not a substrate for NQR.
But perhaps the most interesting result of this work is the direct demonstration of an ion antiporter with a potentially broad substrate specificity acting as a specific K+/H+ antiporter expelling K+ in vivo. It is evident from the potassium- but not sodium-sensitive phenotype of the Vc-NhaP2 deletion mutant (Fig. 1A,B) as well as the kinetic data obtained in the in vitro system. Despite comparable affinities to alkali cations (Fig. 4 and 5), the much lower levels of Na+/H+ antiport compared to K+/H+ antiport and the lack of Li+/H+ antiport mediated by Vc-NhaP2 in inside-out membrane vesicles (Fig. 2 and 3), clearly points towards K+/H+ exchange as the only physiological function of this antiporter.
In a sense, this finding may effectually end the ongoing quest for “specific” K+/H+ antiporters. Indeed, based on the ion radii of K+ (1.38 Å) and Na+(1.02 Å), one may argue that only Na+/H+ antiporters could efficiently discriminate K+ ions by simple size exclusion, however K+/H+ antiporters can hardly avoid binding smaller Na+ as a substrate.
Acknowledgments
This work was supported by grants from the National Institutes of Health (grant no. AI-063121–02) and the Natural Sciences and Engineering Research Council of Canada (grant no. 227414–04).
Abbreviations
- Aa-NhaP
Na+/H+ antiporter of NhaP type from Alkalimonas amylolytica
- BTP
Bis-Tris-propane
- CCCP
carbonyl cyanide m-chlorophenylhydrazone
- DTT
1,4-dithiothreitol
- Ec-NhaA and Ec-NhaB
bacterial Na+/H+ antiporters of NhaA type and NhaB type from Escherichia coli, respectively
- ORF
open reading frame
- Vc-Mrp
Na+/H+ antiporter of Mrp type from Vibrio cholerae
- Vc-NhaP2
Na+/H+ antiporter of NhaP2 type from Vibrio cholerae
- pmf
proton motive force on the membrane
- PMSF
phenylmethylsulfonyl fluoride
- ΔpH
pH difference across the membrane
- ΔpNa
chemical gradient of Na+ across the membrane
- ΔΨ
transmembrane difference of electric potentials
Footnotes
This work was supported by grants from the National Institutes of Health (no. AI-063121-02) and the Natural Sciences and Engineering Research Council of Canada (no. 227414-04).
References
- 1.Epstein W. The roles and regulation of potassium in bacteria. Progress in Nucleic Acid Research. 2003;75:293–320. doi: 10.1016/s0079-6603(03)75008-9. [DOI] [PubMed] [Google Scholar]
- 2.Putnoky P, Kereszt A, Nakamura T, Endre G, Grosskopf E, Kiss P, Kondorosi A. The pha gene cluster of Rhizobium meliloti involved in pH adaptation and symbiosis encodes a novel type of K+ efflux system. Mol Microbiol. 1998;28:1091–101. doi: 10.1046/j.1365-2958.1998.00868.x. [DOI] [PubMed] [Google Scholar]
- 3.Benito B, Garciadeblás B, Rodriquez-Navarro A. Potassium- or sodium-efflux ATPase, a key enzyme in the evolution of fungi. Microbiology. 2002;148:933–941. doi: 10.1099/00221287-148-4-933. [DOI] [PubMed] [Google Scholar]
- 4.Radchenko MV, Waditee R, Oshimi S, Fukuhara M, Takabe T, Nakamura T. Cloning, functional expression and primary characterization of Vibrio parahaemolyticus K+/H+ antiporter genes in Escherichia coli. Mol Microbiol. 2006;59:651–663. doi: 10.1111/j.1365-2958.2005.04966.x. [DOI] [PubMed] [Google Scholar]
- 5.Epstein W, Kim BS. Potassium transport loci in Escherichia coli K-12. J Bacteriol. 1971;108:639–644. doi: 10.1128/jb.108.2.639-644.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bossemeyer D, Schlösser A, Bakker EP. Specific cesium transport via the Escherichia coli Kup(TrkD) K+ uptake system. J Bacteriol. 1989;171:2219–2221. doi: 10.1128/jb.171.4.2219-2221.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bossemeyer D, Borchard A, Dosch DC, Helmer GC, Epstein W, Booth IR, Bakker EP. K+-transport protein TrkA of Escherichia coli is a peripheral membrane protein that requires other trk gene products for attachment to the cytoplasmic membrane. J Biol Chem. 1989;264:16403–16410. [PubMed] [Google Scholar]
- 8.Dosch DC, Helmer GL, Sutton SH, Salvacion FF, Epstein W. Genetic analysis of potassium transport loci in Escherichia coli: evidence for three constitutive systems mediating uptake of potassium. J Bacteriol. 1991;173:687–696. doi: 10.1128/jb.173.2.687-696.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guffanti AA, Cheng J, Krulwich TA. Electrogenic antiport activities of the Gram-positive Tet proteins include a Na+(K+)/K+ mode that mediates net K+ uptake. J Biol Chem. 1998;273:26447–26454. doi: 10.1074/jbc.273.41.26447. [DOI] [PubMed] [Google Scholar]
- 10.Wang W, Guffanti AA, Wei W, Ito M, Krulwich TA. Two types of Bacillus subtilis tetA(L) deletion strains reveal the physiological importance of TetA(L) in K+ acquisition as well as in Na+, alkali and tetracycline resistance. J Bacteriol. 2000;182:2088–2095. doi: 10.1128/jb.182.8.2088-2095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krulwich TA, Jin J, Guffanti AA, Bechhofer H. Functions of tetracycline efflux proteins that do not involve tetracycline. J Mol Microbiol Biotechnol. 2001;3:237–246. [PubMed] [Google Scholar]
- 12.Bakker EP, Booth IR, Dinnbier U, Epstein W, Gajewska A. Evidence for multiple K+ export systems in Escherichia coli. J Bacteriol. 1987;169:3743–3749. doi: 10.1128/jb.169.8.3743-3749.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sukharev SI, Blount P, Martinac B, Blattner FR, Kung C. A large-conductance mechanosensitive channel in Escherichia coli encoded by mscL alone. Nature. 1994;363:265–268. doi: 10.1038/368265a0. [DOI] [PubMed] [Google Scholar]
- 14.Levina N, Tötemeyer S, Stokes NR, Louis P, Jones MA, Booth IR. Protection of Escherichia coli cells against extreme turgor by activation of MscS and MscL mechanosensitive channels: identification of genes required for MscS activity. EMBO J. 1999;18:1730–1737. doi: 10.1093/emboj/18.7.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y, Moe PC, Chandrasekaran S, Booth IR, Blount P. Ionic regulation of MscK, a mechanosensitive channel from Escherichia coli. EMBO J. 2002;21:5323–5330. doi: 10.1093/emboj/cdf537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewinson O, Padan E, Bibi E. Alkalitolerance: a biological function for a multidrug transporter in pH homeostasis. Proc Natl Acad Sci USA. 2004;101:14073–14078. doi: 10.1073/pnas.0405375101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitchell P. Coupling of phosphorylation to electron and hydrogen transfer by a chemiosmotic type of mechanism. Nature. 1961;191:144–148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- 18.Padan E, Schuldiner S. Bacterial Na+/H+ antiporters - molecular biology, biochemistry and physiology. In: Konings W, Kaback R, Lolkema J, editors. The Handbook of Biological Physics, Vol II, Transport Processes in Membranes. Elsevier Science; Amsterdam, The Netherlands: 1996. pp. 501–531. [Google Scholar]
- 19.Padan E, Krulwich TA. Sodium Stress. In: Storz G, Hengge-Aronis R, editors. Bacterial Stress Responses. ASM Press; Washington, D. C: 2000. pp. 117–130. [Google Scholar]
- 20.Padan E, Venturi M, Gerchman Y, Dover N. Na+/H+ antiporters. Biochim Biophys Acta. 2001;1505:144–157. doi: 10.1016/s0005-2728(00)00284-x. [DOI] [PubMed] [Google Scholar]
- 21.Krulwich TA, Lewinson O, Padan E, Bibi E. Do physiological roles foster persistence of drug/multidrug-efflux transporters? A case study. Nat Rev Microbiol. 2005;3:566–572. doi: 10.1038/nrmicro1181. [DOI] [PubMed] [Google Scholar]
- 22.Padan E. The enlightening encounter between structure and function in the NhaA Na+–H+ antiporter. Trends in Biochem Sci. 2008;33:435–443. doi: 10.1016/j.tibs.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 23.Brey RN, Rosen BP, Sorensen EN. Cation/proton antiporter systems in Escherichia coli. Properties of the potassium/proton antiporter. J Biol Chem. 1980;255:39–44. [PubMed] [Google Scholar]
- 24.Dzioba-Winogrodzki J, Winogrodzki O, Krulwich TA, Boin MA, Häse CC, Dibrov P. Characterization of the Vibrio cholerae multifunctional mrp-encoded cation/proton antiporter conferring resistance to bile salts in a heterologous host. J Mol Microbiol Biotechnol. 2009;16:176–186. doi: 10.1159/000119547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wei Y, Liu J, Ma Y, Krulwich TA. Three putative cation/proton antiporters from the soda lake alkaliphile Alkalimonas amylolytica N10 complement an alkali-sensitive Escherichia coli mutant. Microbiology. 2007;153:2168–2179. doi: 10.1099/mic.0.2007/007450-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mekalanos JJ, Swartz DJ, Pearson GD, Harford N, Groyne F, de Wilde M. Cholera toxin genes: nucleotide sequence, deletion analysis and vaccine development. Nature. 1983;306:551–557. doi: 10.1038/306551a0. [DOI] [PubMed] [Google Scholar]
- 27.Padan E, Maisler N, Taglicht D, Karpel R, Schuldiner S. Deletion of ant in Escherichia coli reveals its function in adaptation to high salinity and an alternative Na+/H+ antiporter system(s) J Biol Chem. 1989;264:20297–202302. [PubMed] [Google Scholar]
- 28.Hamashima H, Iwasaki M, Arai T. A simple and rapid method for transformation of Vibrio species by electroporation. Methods Mol Biol. 1995;47:155–160. doi: 10.1385/0-89603-310-4:155. [DOI] [PubMed] [Google Scholar]
- 29.Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 30.Metcalf WW, Jiang W, Daniels LL, Kim SK, Haldimann A, Wanner BL. Conditionally replicative and conjugative plasmids carrying lacZ alpha for cloning, mutagenesis and allele replacement in bacteria. Plasmid. 1996;35:1–13. doi: 10.1006/plas.1996.0001. [DOI] [PubMed] [Google Scholar]
- 31.Ohyama T, Igarashi K, Kobayashi H. Physiological role of chaA gene in sodium and calcium circulations at high pH in Escherichia coli. J Bacteriol. 1994;176:4311–4315. doi: 10.1128/jb.176.14.4311-4315.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Radchenko MV, Tanaka K, Waditee R, Oshimi S, Matsuzaki Y, Fukuhara M, Kobayashi H, Takabe T, Nakamura T. Potassium/proton antiport system of Escherichia coli. J Biol Chem. 2006;281:19822–19829. doi: 10.1074/jbc.M600333200. [DOI] [PubMed] [Google Scholar]
- 33.Galili L, Rothman A, Kozachkov L, Rimon A, Padan E. Transmembrane domain IV is involved in ion transport activity and pH regulation of the NhaA Na+/H+ antiporter of Escherichia coli. Biochemistry. 2002;41:609–617. doi: 10.1021/bi011655v. [DOI] [PubMed] [Google Scholar]
- 34.Padan E, Tzubery T, Herz K, Kozachkov L, Rimon A, Galili L. NhaA of Escherichia coli, as a model of a pH-regulated Na+/H+ antiporter. Biochim Biophys Acta. 2004;1658:2–13. doi: 10.1016/j.bbabio.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 35.Habibian R, Dzioba J, Barrett J, Galperin MY, Loewen PC, Dibrov P. Functional analysis of conserved polar residues in Vc-NhaD, Na+/H+ antiporter of Vibrio cholerae. J Biol Chem. 2005;280:39637–39649. doi: 10.1074/jbc.M509328200. [DOI] [PubMed] [Google Scholar]
- 36.Padan E, Zilberstein D, Schuldiner S. pH homeostasis in bacteria. Biochim Biophys Acta. 1981;650:151–166. doi: 10.1016/0304-4157(81)90004-6. [DOI] [PubMed] [Google Scholar]
- 37.Gómez-Puyou A, Gómez-Lojero C. The use of ionophores and channel formers in the study of the function of biological membranes. Curr Top Bioenerg. 1977;6:221–257. [Google Scholar]
- 38.Dibrov P, Rimon A, Dzioba J, Winogrodzki A, Shalitin Y, Padan E. 2-Aminoperimidine, a specific inhibitor of bacterial NhaA Na+/H+ antiporters. FEBS Lett. 2005;579:373–37. doi: 10.1016/j.febslet.2004.11.098. [DOI] [PubMed] [Google Scholar]
- 39.Taglicht D, Padan E, Schuldiner S. Proton-sodium stoichiometry of NhaA, an electrogenic antiporter from Escherichia coli. J Biol Chem. 1993;268:5382–5387. [PubMed] [Google Scholar]
- 40.Pinner E, Padan E, Schuldiner S. Kinetic properties of NhaB, a Na+/H+ antiporter from Escherichia coli. J Biol Chem. 1994;269:26274–26279. [PubMed] [Google Scholar]
- 41.Tzubery T, Rimon A, Padan E. Mutation E252C increases drastically the Km value for Na+ and causes an alkaline shift of the pH dependence of NhaA Na+/H+ antiporter of Escherichia coli. J Biol Chem. 2004;279:3265–3272. doi: 10.1074/jbc.M309021200. [DOI] [PubMed] [Google Scholar]
- 42.Waditee R, Buaboocha T, Kato M, Hibino T, Suzuki S, Nakamura T, Takabe T. Carboxyl-terminal hydrophilic tail of a NhaP type Na+/H+ antiporter from cyanobacteria is involved in the apparent affinity for Na+ and pH sensitivity. Arch Biochem Biophys. 2006;450:113–121. doi: 10.1016/j.abb.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 43.Herz K, Vimont S, Padan E, Berche P. Roles of NhaA, NhaB, and NhaD Na+/H+ antiporters in survival of Vibrio cholerae in a saline environment. J Bacteriol. 2003;185:1236–1244. doi: 10.1128/JB.185.4.1236-1244.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mulkidjanian AY, Dibrov P, Galperin MY. The past and present of sodium energetics: May the sodium-motive force be with you. Biochim Biopys Acta. 2008;1777:985–992. doi: 10.1016/j.bbabio.2008.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barquera B, Hellwig P, Zhou W, Morgan J, Häse C, Gosink K, Nilges M. Purification and characterization of the recombinant Na+-translocating NADH:quinone oxidoreductase from Vibrio cholerae. Biochemistry. 2002;41:3781–3789. doi: 10.1021/bi011873o. [DOI] [PubMed] [Google Scholar]