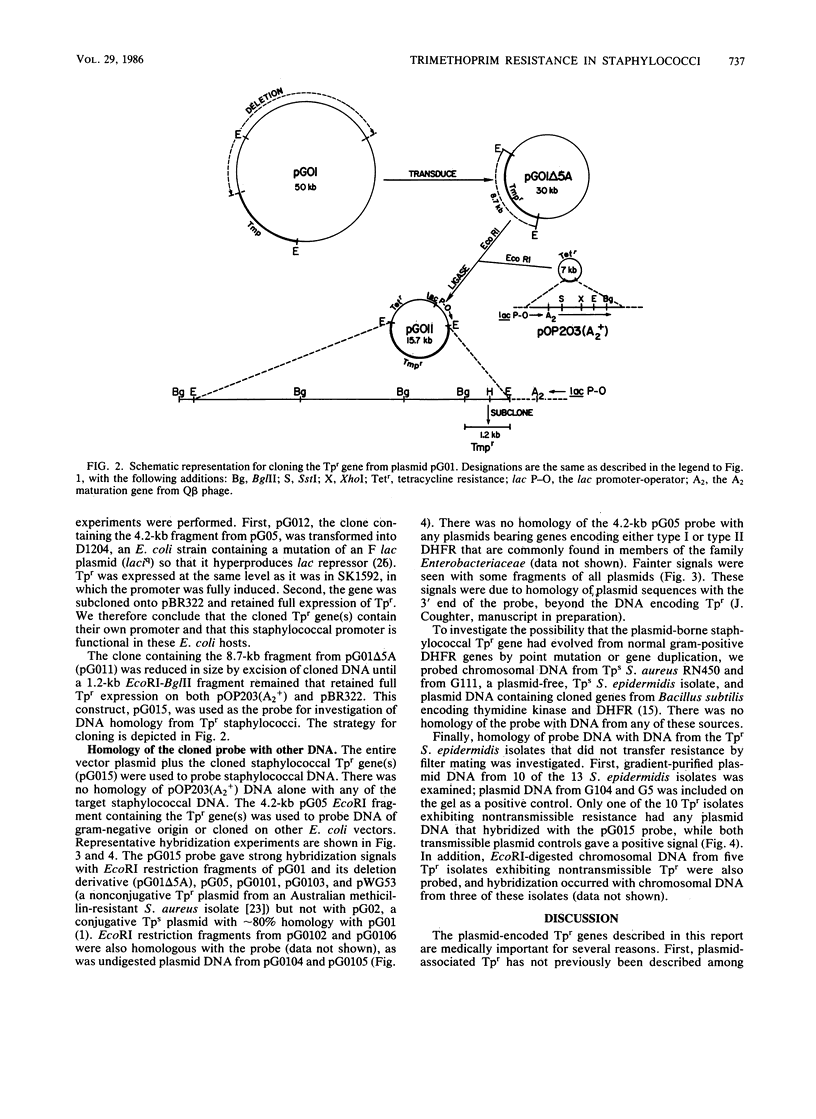
Abstract
High-level (greater than 1,000 micrograms/ml) resistance to the antimicrobial agent trimethoprim was found in 17 of 101 (17%) coagulase-negative staphylococci and 5 of 51 (10%) Staphylococcus aureus from a number of different hospitals in the United States. Resistance was plasmid encoded and could be transferred by conjugation in 4 of the 17 (24%) Tpr coagulase-negative staphylococci and 3 of the 5 (60%) Tpr S. aureus. A 1.2-kilobase segment of plasmid DNA from one of the plasmids (pG01) was cloned on a high-copy-number vector in Escherichia coli and expressed high-level Tpr (MIC, 1,025 micrograms/ml) in the gram-negative host. In situ filter hybridization demonstrated homology between the cloned Tpr gene probe and plasmid DNA from each conjugative Tpr plasmid, a single nonconjugative plasmid from a United States Staphylococcus epidermidis isolate, a nonconjugative plasmid from an Australian methicillin-resistant S. aureus isolate, and chromosomal DNA from three Tpr S. epidermidis isolates that did not contain any plasmid DNA that was homologous with the probe. No homology was seen between the probe and staphylococcal plasmids not mediating Tpr, plasmid DNA from 12 Tpr S. epidermidis isolates not transferring Tpr by conjugation, or plasmid-encoded Tpr genes derived from gram-negative bacteria. Plasmid-encoded Tpr appears to be a relatively new gene in staphylococci and, because it can be transferred by conjugation, could become more prevalent in nonsocomial isolates.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archer G. L., Dietrick D. R., Johnston J. L. Molecular epidemiology of transmissible gentamicin resistance among coagulase-negative staphylococci in a cardiac surgery unit. J Infect Dis. 1985 Feb;151(2):243–251. doi: 10.1093/infdis/151.2.243. [DOI] [PubMed] [Google Scholar]
- Archer G. L., Johnston J. L. Self-transmissible plasmids in staphylococci that encode resistance to aminoglycosides. Antimicrob Agents Chemother. 1983 Jul;24(1):70–77. doi: 10.1128/aac.24.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer G. L., Mayhall C. G. Comparison of epidemiological markers used in the investigation of an outbreak of methicillin-resistant Staphylococcus aureus infections. J Clin Microbiol. 1983 Aug;18(2):395–399. doi: 10.1128/jcm.18.2.395-399.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer G. L., Vishniavsky N., Stiver H. G. Plasmid pattern analysis of Staphylococcal epidermidis isolates from patients with prosthetic valve endocarditis. Infect Immun. 1982 Feb;35(2):627–632. doi: 10.1128/iai.35.2.627-632.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchall J. J., Elwell L. P., Fling M. E. Molecular mechanisms of resistance to trimethoprim. Rev Infect Dis. 1982 Mar-Apr;4(2):246–254. doi: 10.1093/clinids/4.2.246. [DOI] [PubMed] [Google Scholar]
- Ferone R., Bushby S. R., Burchall J. J., Moore W. D., Smith D. Identification of Harper-Cawston factor as thymidine phosphorylase and removal from media of substances interfering with susceptibility testing to sulfonamides and diaminopyrimidines. Antimicrob Agents Chemother. 1975 Jan;7(1):91–98. doi: 10.1128/aac.7.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fling M. E., Walton L., Elwell L. P. Monitoring of plasmid-encoded, trimethoprim-resistant dihydrofolate reductase genes: detection of a new resistant enzyme. Antimicrob Agents Chemother. 1982 Nov;22(5):882–888. doi: 10.1128/aac.22.5.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe H. W., Sweeney H. M., Weinstein R. A., Kabins S. A., Nathan C., Cohen S. Structural and phenotypic varieties of gentamicin resistance plasmids in hospital strains of Staphylococcus aureus and coagulase-negative staphylococci. Antimicrob Agents Chemother. 1982 May;21(5):773–779. doi: 10.1128/aac.21.5.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitz R. E., Quintiliani R. Trimethoprim-sulfamethoxazole for bacterial meningitis. Ann Intern Med. 1984 Jun;100(6):881–890. doi: 10.7326/0003-4819-100-6-881. [DOI] [PubMed] [Google Scholar]
- Lewis E. L., Lacey R. W. Present significance of resistance to trimethoprim and sulphonamides in coliforms, Staphylococcus aureus, and Streptococcus faecalis. J Clin Pathol. 1973 Mar;26(3):175–180. doi: 10.1136/jcp.26.3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley S. L., Falkow S. Nucleotide sequence homology between the heat-labile enterotoxin gene of Escherichia coli and Vibrio cholerae deoxyribonucleic acid. J Bacteriol. 1980 Oct;144(1):444–446. doi: 10.1128/jb.144.1.444-446.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myoda T. T., Lowther S. V., Funanage V. L., Young F. E. Cloning and mapping of the dihydrofolate reductase gene of Bacillus subtilis. Gene. 1984 Jul-Aug;29(1-2):135–143. doi: 10.1016/0378-1119(84)90174-4. [DOI] [PubMed] [Google Scholar]
- Nakhla L. S. Resistance of Staphylococcus aureus to sulphamethoxazole and trimethoprim. J Clin Pathol. 1972 Aug;25(8):708–712. doi: 10.1136/jcp.25.8.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology. 1967 Sep;33(1):155–166. doi: 10.1016/0042-6822(67)90105-5. [DOI] [PubMed] [Google Scholar]
- Richardson J. F. Frequency of resistance to trimethoprim among isolates of Staphylococcus epidermidis and Staphylococcus saprophyticus. J Antimicrob Chemother. 1983 Feb;11(2):163–167. doi: 10.1093/jac/11.2.163. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tennent J. M., Lyon B. R., Gillespie M. T., May J. W., Skurray R. A. Cloning and expression of Staphylococcus aureus plasmid-mediated quaternary ammonium resistance in Escherichia coli. Antimicrob Agents Chemother. 1985 Jan;27(1):79–83. doi: 10.1128/aac.27.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend D. E., Ashdown N., Greed L. C., Grubb W. B. Transposition of gentamicin resistance to staphylococcal plasmids encoding resistance to cationic agents. J Antimicrob Chemother. 1984 Aug;14(2):115–124. doi: 10.1093/jac/14.2.115. [DOI] [PubMed] [Google Scholar]
- Ward T. T., Winn R. E., Hartstein A. I., Sewell D. L. Observations relating to an inter-hospital outbreak of methicillin-resistant Staphylococcus aureus: role of antimicrobial therapy in infection control. Infect Control. 1981 Nov-Dec;2(6):453–459. doi: 10.1017/s0195941700055715. [DOI] [PubMed] [Google Scholar]
- Winter R. B., Gold L. Overproduction of bacteriophage Q beta maturation (A2) protein leads to cell lysis. Cell. 1983 Jul;33(3):877–885. doi: 10.1016/0092-8674(83)90030-2. [DOI] [PubMed] [Google Scholar]