Abstract
BACKGROUND
The absence of a gold standard test for Trypanosoma cruzi antibodies represents a problem not only for the evaluation of screening tests, but also for appropriate blood donor counseling. The aim of this study was to estimate the sensitivity and specificity of multiple blood donor screening tests for T. cruzi antibodies in Argentina.
STUDY DESIGN AND METHODS
From June 2006 to March 2007 a sample of 1455 blood donors was recruited from two blood banks in Chaco province, an area of Argentina with highly endemic T. cruzi infection. Samples were tested by three epimastigote lysate enzyme immunoassays (EIAs), one recombinant antigen EIA, two indirect hemagglutination assay (IHA) tests, a particle agglutination assay (PA), and a research trans-sialidase inhibition assay (TIA). Sensitivity and specificity were estimated using latent class analysis (LCA).
RESULTS
LCA estimated the consensus prevalence of T. cruzi infection at 24.5%. Interassay correlation was higher among the four EIA tests and TIA compared to IHA tests. Assay sensitivities varied from 96 to 99.7 for different EIAs, 91% for TIA, 84% for PA, and 66 to 74% for IHA tests. Relative to the LCA, assay specificities were from 96% to almost 100%.
CONCLUSION
Based on the comparison of several tests in a large population from an endemic area for T. cruzi infection, our data showed an adequate sensitivity for EIA tests in contrast to PA and IHA assays. The latter tests should no longer be used for blood donor screening.
Chagas’ disease or American trypanosomiasis is a chronic disease caused by the parasite Trypanosoma cruzi and represents a significant health problem in Latin American countries. The parasite is transmitted to humans or other mammals by triatomine bugs of the family Reduviidae. The implementation of vectorial control has been successful in the interruption of this way of transmission in some Latin American countries;1 however, blood transfusion remains as an alternative route. In Argentina, the geographic region of vectorial transmission is at parallel 44° 45′S north and Triatoma infestans is the main vector.2,3 Blood transfusion and transplantation have increasingly been reported as the cause of new infections outside the foci of natural transmission.4 Asymptomatic carriers who migrate to nonendemic countries represent a source for potential transmission of T. cruzi by blood transfusion in such countries. The concern about this infection has even reached the United States and in some European countries, where routine blood donor screening for T. cruzi was implemented in some blood centers.5–7
During the acute phase circulating parasites are easily detectable. After a brief period, the titers of T. cruzi antibodies increase markedly and parasites become rarely detectable. Carriers remain asymptomatic for several years but after 20 or more years postinfection, approximately 30% of them develop cardiomyopathy or megaviscera. Due to the low level of parasitemia, direct detection of the parasite is difficult during the chronic phase of the infection even with molecular techniques such as polymerase chain reaction (PCR). Furthermore, PCR for T. cruzi has been not completely standardized; it shows different sensitivity depending on the procedures employed.8–10 As a consequence it cannot yet be implemented as a confirmatory test or as a suitable method for blood donor screening given that a PCR-negative result could not rule out an infected blood donor.
The detection of antibodies to T. cruzi remains the main method for infection diagnosis and blood donor screening. Serologic tests employ whole or semipurified antigenic fractions of epimastigote forms, which are easily grown in cultures, even though the human immune response is directed against the trypomastigote and amastigote forms of the parasite. Different assays using recombinant or synthetic antigens have also been developed to improve test performance.11,12 Among them, trans-sialidase inhibition assay (TIA), which detects neutralizing antibodies against trans-sialidase (TS), an enzyme expressed by T. cruzi, was also developed.13
The sensitivity and specificity of the currently licensed tests are known to be suboptimal, but have been difficult to asses due to the fact there is no recognized gold standard method to detect T. cruzi infection in blood donors. In 2002, the WHO Expert Committee established the evaluation of diagnostic tests available as a research priority.14
At present in Argentina, blood bank regulations require two parallel methods for T. cruzi antibody screening of blood donors.15,16 In the absence of an accepted reference test, discordant results are considered inconclusive, which represents a problem for appropriate counseling of donors or for establishing algorithms for reentry of blood donors reactive by only one test.
To evaluate the sensitivity and specificity of different methods for T. cruzi antibodies, most comparative studies have been conducted using panels of selected sera,17,18 which could introduce bias in the final results because these panels might not include the natural spectrum of antibody response. The main objective of this study was the evaluation of eight tests for T. cruzi infection detection in a large sample of blood donors from an endemic area. Six available licensed routine tests in Argentina were used, together with a locally developed test (Chaco Province) and TIA.
MATERIALS AND METHODS
Study design and subjects
From June 2006 to March 2007, we recruited a sample of 1455 donors from two blood banks located in a region of Argentina considered as highly endemic for T. cruzi infection (Chaco province, Northeast Argentina). Two public hospital–based blood banks were the centers of blood donor recruitment: the “Hospital Dr. Ramón Carrillo” blood bank (in the city of Roque Saenz Peña) and the “Hospital Dr. J.C.Perrando-Castelán” (in the city of Resist-encia). The coordinating center of the study was the “Servicio de Hemoterapia” at “Hospital de Pediatría Prof. Dr. Juan P. Garrahan” (City of Buenos Aires).
The project was approved by the institutional review board of the coordinating center. Blood donors who agreed to participate in the project received an explanation about the study before giving written informed consent. Age and sex data were obtained as part of the routine blood donor questionnaire. A serum sample was collected from each donor and stored at –20°C until being sent to the coordinating center. Samples were received at the coordinating center once a month and were aliquoted in four vials and frozen at −80°C.
Serologic assays
Screening tests were done in the coordinating center laboratory, with the exception of the TIA, which was performed in the Department of Microbiology, School of Medicine, University of Buenos Aires.
The following available assays were employed: Chagatek (EIA BioM; Lemos Laboratory, commercialized by BioMerieux Lab., Buenos Aires, Argentina), Chagas BiosChile (EIA BiosCh; BiosChile, Santiago, Chile) and BioZima Chagas (EIA BioZ; Lemos Laboratory, Buenos Aires, Argentina),19 Chagatest rec. (EIA Wrec; Wiener Lab, Rosario, Argentina),20 Chagas Serodia (particle agglutination assay [PA]; Serodia, Fujirebio, Tokyo, Japan), Chagas HAI (indirect hemagglutination assay [IHA]; PolyChaco, Buenos Aires, Argentina), and HAI LC (IHA LC; Ministry of Health Laboratory, Province of Chaco, Argentina; Table 1). Agglutination tests were considered reactive with the cutoff serum titer recommended by the manufacturer (8, 16, and 16 for Chagas HAI, HAI LC, and Chagas Serodia, respectively). An optical density value of the sample/cutoff as 0.90 or greater was considered reactive by all EIAs. Borderline samples were repeated by duplicate, and samples were considered reactive if two of three results were reactive, as current practice in blood donor screening.
TABLE 1.
Characteristics of the various T. cruzi serologic assays used in this comparative study
| Assay | Manufacturer | Type of antigen | Assay principle | Licensed for blood donor screening in Argentina |
|---|---|---|---|---|
| Chagatek (EIA BioM) | Lemos Lab., commercialized by BioMerieux, Argentina |
Epimastigote lysate | EIA | Yes |
| Chagas BiosChile (EIA BiosCh) | BiosChile, Chile | Epimastigote lysate | EIA | Yes |
| BioZima Chagas (EIA BioZ) | Lemos Lab., Argentina | Epimastigote lysate | EIA | Yes |
| Chagatest rec. (EIA Wrec) | Wiener Lab., Argentina | Recombinant, from epimastigote and trypomastigote forms |
EIA | Yes |
| Chagas Serodia (PA) | Serodia, Fujirebio, Japan | Epimastigote lysate | Gelatin particle agglutination | Yes |
| TIA | No commercial | Recombinant TS | Antibody-mediated inhibition of T. cruzi TIA |
No, only for research |
| Chagas HAI (IHA) | PolyChaco, Lemos Lab., Argentina |
Epimastigote lysate | Indirect hemagglutination | Yes |
| HAI LC (IHA LC) | Ministry of Health, Chaco | Epimastigote lysate | Indirect hemagglutination | No |
EIA BioM = Chagatek; EIA BiosCh = Chagas BiosChile; EIA BioZ = BioZima Chagas; EIA Wrec = Chagatest rec. 3.0; PA = Chagas Serodia; IHA = Chagas HAI; IHA LC = HAI LC.
TIA
Serum samples (10 µL) were incubated with recombinant TS21 enzyme for 20 minutes at room temperature as was previously described.13,22 The remnant TS activity was evaluated in a final volume of 30 µL containing 1 mmol/L sialyllactose (Sigma, St Louis, MO), 12 µmol/L [d-glucose-1-14C]-lactose (GE Healthcare; 54.3 mCi/ mmol), 20 mmol/L Tris buffer, pH 7.6. After 30 minutes of incubation at room temperature, 1 mL of bidistilled water was added to stop the reaction. A dense slurry of quaternary aminoethyl-Sepharose A-25 (Sigma) was added to bind sialyl-[14C]lactose reaction product. After briefly being mixed vigorously, beads were washed three times with bidistilled water, the bound material was eluted with 800 µL of 0.5 mol/L NaCl, and the counts per minute were quantified. TIA results were expressed as the percentage of TS activity inhibition. Pooled normal human sera were used as negative controls, and the inhibition value obtained was taken as 0%. A sample was considered TIA positive when a percentage of inhibition of 50% or more was obtained.
Statistical analysis
Data were entered in Epi Info 2000 (3.3.2 version, CDC, Atlanta, GA). Data were double checked and verified for completeness and consistency. The database was transferred to Stata 10.0 (College Station, TX) for statistical analysis. Descriptive frequencies were first generated for demographic and test result data. Descriptive concordance between pairs of assays was calculated as
Agreement between each pair of methods was measured by Cohen’s kappa coefficient, which incorporates a correction for chance agreement among the pairs of tests being compared.
In the absence of a gold standard test, latent class analysis (LCA) has been suggested as one method by which researchers can obtain provisional estimates of sensitivity, specificity, and prevalence. Infection (measured as reactivity) was the latent class variable in a two-latent-class model that assumes the sample of sera to be conformed in two groups: infected donors and non-infected donors. Global LCA model fit was assessed via a posterior predictive p-value via Markov Chain Monte Carlo estimation in SPSS AMOS 16 (Chicago, IL). The LEM program (Jeroen K.Vermunt, 1997, http://www.uvt.nl/faculteiten/fsw/organisatie/departementen/mto/software2.html) was then used to obtained estimates and 95% confidence intervals (CIs) for the LCA sensitivity and specificity estimates. Finally, each specimen was classified as donors infected and donors not infected according to the a posteriori probabilities. The sensitivity and specificity of these posterior probabilities were then compared with the sensitivity and specificity values from three and four reactive tests, respectively.
RESULTS
Study population
A total of 1455 blood donors were recruited in two blood banks in Chaco province, in the Northeast of Argentina. Fifty-five percent of blood donors were recruited in Saenz Peña city and the rest in Resistencia. Seventy-nine percent were male and the median of age was 36 years (Q25% = 29, Q75% = 45). More than 90% of the blood donors were born and lived in the same province. Sixteen percent of blood donors were born in Resistencia; 18% in Saenz Peña (main cities in the province); and 66% in different towns, villages, and cities from different regions of the province. Based on site characteristics the majority of blood donors were first-time, replacement donors.
Test evaluation
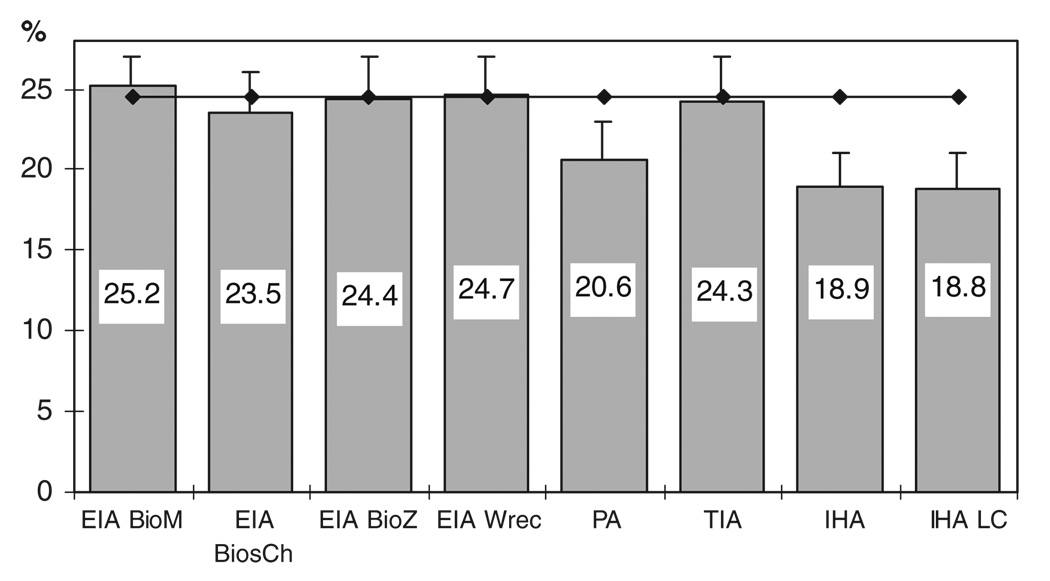
The percentage of positive results for each test is shown in Fig. 1 in comparison with the overall prevalence obtained by LCA. Given the different antibody response patterns by each donor sample to the eight tests used in the study, the maximum theoretical number of antibody responses expected is 256 (= 28). These are the total possible combinations of antibody responses considering two classes of responses (reactive and nonreactive) to eight tests. In this study, we observed a total of 51 patterns of antibody response. The highest frequency observed was 68.6% (998/1455) followed by 14.4% (209/1455) corresponding to samples nonreactive and reactive by all the tests, respectively.
Fig. 1.
Percentage of reactivity of each test in the blood donor population studied. Blood donor population (n = 1455), Chaco Province, Argentina (2006–2007). The overall prevalence of infection estimated by LCA (24.5%) is represented by a horizontal line. The bars above the columns represent the upper bound of the 95% CI for the proportion. EIA BioM = Chagatek (25.2%); EIA BiosCh = Chagas BiosChile (23.5%); EIA BioZ = BioZima Chagas (24.4%); EIA Wrec = Chagatest rec. 3.0 (24.7%); PA = Chagas Serodia (20.6%); TIA = trans-sialidase inhibition assay (24.3%); IHA = Chagas HAI (18.9%); IHA LC = HAI LC (18.8%).
Concordance and agreement of test
Table 2 summarizes the interassay concordance among different tests. EIA tests showed a high degree of concordance among themselves. IHA assays showed less concordance with EIAs and TIA. An intermediate concordance with the rest of the assays was observed for PA and TIA. Kappa agreement values ranged from 0.80 to 0.96 for pairs of different EIAs, EIAs with PA, or EIAs with TIA. Kappa index values ranged from 0.62 to 0.79 when IHAs were correlated with other assays.
TABLE 2.
Interassay concordance in the analysis of blood donor samples (n = 1455) from Chaco Province, Argentina (2006–2007)*
| EIA BioM | EIA BiosCh | EIA BioZ | EIA Wrec | PA | TIA | IHA | IHA LC | |
|---|---|---|---|---|---|---|---|---|
| EIA BioM | ||||||||
| EIA BiosCh | 98.1 | |||||||
| EIA BioZ | 98.4 | 97.7 | ||||||
| EIA Wrec | 97.0 | 97.3 | 96.6 | |||||
| PA | 95.2 | 96.3 | 95.8 | 95.1 | ||||
| TIA | 95.1 | 94.4 | 95.1 | 94.4 | 93.1 | |||
| IHA | 92.1 | 93.6 | 92.7 | 92.6 | 94.6 | 90.4 | ||
| IHA LC | 88.0 | 89.6 | 89.0 | 88.3 | 91.6 | 87.1 | 93.3 |
For each pair of assays compared, concordance was defined as (Number positive by both assays + Number negative by both assays)/ Number tested (= 1455).
EIA BioM = Chagatek; EIA BiosCh = Chagas BiosChile; EIA BioZ = BioZima Chagas; EIA Wrec = Chagatest rec. 3.0; PA = Chagas Serodia; TIA = trans-sialidase inhibition assay; IHA = Chagas HAI; IHA LC = HAI LC.
Estimation of sensitivity and specificity
Our first approach to estimate the sensitivity and specificity of the different tests was as follows. We considered as “true positive” those samples that tested as reactive in: 1) at least three of eight tests and 2) at least four of eight tests (Table 3). A third estimation was also done with LCA. The overall LCA prevalence in the sample population was 24.5%. The posterior predictive p-value was 0.62, indicating overall satisfactory model-data fit. The estimated values of sensitivity and specificity are shown in Table 3. According to the three estimations mentioned, we classify tests in three groups showing similar sensitivity: EIAs (high sensitivity, >95%), TIA (intermediate, 90%-95%), and agglutination assays (low sensitivity, <90%). Specificities were higher than 96% for all the evaluated tests.
TABLE 3.
Estimation of sensitivity and specificity of various assays, in comparison positive samples defined by reactivity on three tests, reactivity on four tests, and by LCA*
| Three reactive tests |
Four reactive tests |
LCA |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Se (%) | 95% CI | Sp (%) | 95% CI | Se (%) | 95% CI | Sp (%) | 95% CI | Se (%) | 95% CI | Sp (%) | 95% CI | |
| EIA BioM | 99.2 | 98.1–100.0 | 99.1 | 98.5–99.7 | 99.7 | 99.0–100.0 | 98.2 | 97.4–99.0 | 99.7 | 99.0–100.0 | 99.0 | 98.5–99.5 |
| EIA BiosCh | 94.7 | 92.3–97.2 | 99.8 | 99.5–100.0 | 97.1 | 95.2–99.0 | 99.5 | 99.1–100.0 | 95.6 | 93.3–97.9 | 99.8 | 99.2–100.0 |
| EIA BioZ | 97.2 | 95.4–99.1 | 99.5 | 99.0–99.9 | 97.4 | 95.6–99.2 | 98.5 | 97.7–99.2 | 97.5 | 95.9–99.2 | 99.3 | 98.7–99.9 |
| EIA Wrec | 95.3 | 92.9–97.6 | 98.4 | 97.6–99.2 | 98.0 | 96.4–99.6 | 98.2 | 97.4–99.0 | 95.9 | 93.7–98.1 | 98.3 | 97.5–99.1 |
| TIA | 90.3 | 87.0–93.5 | 97.3 | 96.3–98.3 | 91.4 | 88.3–94.5 | 96.7 | 95.6–97.8 | 90.5 | 87.4–93.6 | 97.1 | 96.1–98.1 |
| PA | 83.6 | 79.6–87.5 | 100 | 99.9–100.0 | 86.2 | 82.4–89.9 | 99.9 | 99.7–100.0 | 84.1 | 80.2–88.0 | 99.9 | 99.7–100.0 |
| IHA | 73.5 | 68.8–78.2 | 99.0 | 98.4–99.6 | 75.8 | 71.1–80.4 | 98.9 | 98.3–99.6 | 73.9 | 69.3–78.5 | 98.9 | 98.3–99.5 |
| IHA LC | 65.2 | 60.1–70.2 | 96.4 | 95.2–97.5 | 67.1 | 62.1–72.2 | 96.3 | 95.1–97.5 | 65.5 | 60.5–70.5 | 96.3 | 95.2–97.4 |
Blood donor samples (n =1455) from Chaco Province Argentina (2006–2007).
Se = sensitivity; Sp = specificity; EIA BioM = Chagatek; EIA BiosCh = Chagas BiosChile EIA BioZ = BioZima Chagas EIA Wrec = Chagatest rec. 3.0 PA = Chagas Serodia; TIA = transsialidase inhibition assay; IHA = ChagasHAI; IHA LC = HAI LC
DISCUSSION
Our study assessed the accuracy of T. cruzi screening tests based on different principles (EIA, agglutination, enzyme activity inhibition) in blood donors from endemic northeastern Argentina. Ideally, the evaluation of tests should be developed with panels of sera that represent the whole natural spectrum of reactivity that can be found in individuals exposed to the parasite. In our work, this point was faced with the analysis of sera from a large, nonpreselected, unbiased, blood donor population that might represent a wide variety of antibody titers. However, as the population under study was focused at an endemic region of Argentina, the parasite epitope’s responses was restricted to the human and parasite genetic background given by the geographic area.
Our results showed a higher sensitivity for EIA compared to the other methods evaluated. Lower sensitivity for hemagglutination tests has also been reported by other authors.18,23–25 At the same time, IHA tests showed a bad concordance with different EIAs and TIA. In contrast, specificities were high (>96%) for all the assays in our study.
The prevalence for TIA and each EIA was similar to the one estimated by LCA (approx. 25%). IHA showed a lower prevalence value (19%) in agreement with the result described above. The fact that TIA shows the same prevalence as EIA tests even when the sensitivity estimated by LCA was lower could be explained by the ability of TIA to recognize infected individuals that are not detected by conventional serology.22,26 Alternatively, TIA might recognize chronic infections but not recent (or acute) ones.13
This is the first time that TIA is evaluated in a large nonpreselected blood donor population from an Argentinian endemic area. At present Argentina Blood Bank regulations require two parallel methods to screen for T. cruzi infection. In this setting the recommended combination is to use an EIA along with an IHA test. However, our data support discontinuing the use of agglutination tests in blood donor screening due to its low sensitivity and lower concordance with the other tests evaluated. Moreover the national recommended screening strategy for T. cruzi antibody detection should be revised. One proposed strategy is to use an EIA based on parasite lysate together with a recombinant EIA that in theory could detect a wider spectrum of reactive donors, although our findings suggest that each EIA performs with similar sensitivity and specificity. In fact, based on EIA’s high sensitivity and specificity, we consider that moving toward a single test strategy is reasonable. For this purpose, a national pilot study including cost–benefit analysis should be developed.
Our findings are subject to some limitations. One of these limitations is that without a gold standard comparator assay it is not possible to definitively determine the sensitivity and specificity of any test. We can only give an estimation of these variables from the assays used in the study.27,28 Future research studies should validate the robustness of our findings once a gold standard for T.cruzi antibodies has been defined. However, LCA has also been employed by other authors in similar conditions,29,30 despite the fact that the conditional independence assumption may not always be true in practice because some indicators may be correlated for different reasons. In our case, this correlation could be due to the recognition of antibodies to similar parasite antigens in assays with similar principles (parasite homogenates). These limitations can affect the sensitivity of recombinant assays in the analysis. In this sense, it was communicated that TIA remains reactive in samples from patients treated and parasitologically cured, Amerindians who live at high risk of infection as well as in individuals with idiopathic mega-viscera and megacardiopathy, but negative by routine tests.22,26 We cannot completely rule out the possibility that some reactive EIA results but negative by recombinant EIA or TIA could be due to cross-reactivity with other protozoan parasites as Leishmania spp., which are also present in this geographical area of the country.26,31
In conclusion, we hope these data will contribute to the implementation of better T. cruzi tests in Argentinian blood banks. Better screening approaches would improve not only blood safety, but also blood donor counseling. We would like to emphasize the importance of results obtained from studies like this one that are performed in unselected blood donor populations. Further studies should also include comparison with PCR testing as a potential gold standard, assuming that this method may be improved in sensitivity as well as adequately standardized.32
ACKNOWLEDGMENTS
We thank Zhana Kaidarova, Blood Center Research Institute (BSRI), for support in statistical analysis. We thank Ester Sabino for critical revision of the manuscript. The authors acknowledge the technical assistance of personnel at the participating blood centers in Chaco and Hospital de Pediatría Prof. Dr. J.P. Garrahan.
The present work was supported by a research grant from Blood Systems Research Institute and by training grants to ELM (NHLBI K24-HL-75036), the University of California, San Francisco, Center for AIDS Prevention Studies (U.S. National Institute of Mental Health (NIMH), P30 MH062246); the International Traineeships in AIDS Prevention Studies (U.S. NIMH, R25MH064712); and the AIDS International Training in Research Program (AITRP; Fogarty International Center, D43TW00003).
ABBREVIATIONS
- IHA(s)
indirect hemagglutination assay(s)
- LCA
latent class analysis
- PA(s)
particle agglutination assay(s)
- TIA(s)
trans-sialidase inhibition assay(s)
- TS
trans-sialidase
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
REFERENCES
- 1.Moncayo A. Chagas disease: current epidemiological trends after the interruption of vectorial and transfusional transmission in the Southern Cone countries. Mem Inst Oswaldo Cruz. 2003;98:577–591. doi: 10.1590/s0074-02762003000500001. [DOI] [PubMed] [Google Scholar]
- 2.Gurtler RE, Kitron U, Cecere MC, Segura EL, Cohen JE. Sustainable vector control and management of Chagas disease in the Gran Chaco, Argentina. Proc Natl Acad Sci U S A. 2007;104:16194–16199. doi: 10.1073/pnas.0700863104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biancardi MA, Conca Moreno M, Torres N, Pepe C, Altcheh J, Freilij H. [Seroprevalence of Chagas disease in 17 rural communities of “Monte Impenetrable”, Chaco Province] Medicina (B Aires) 2003;63:125–129. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Blood donor screening for chagas disease—United States, 2006–2007. MMWR Morb Mortal Wkly Rep. 2007;56:141–143. [PubMed] [Google Scholar]
- 5.Leiby DA, Herron RM, Jr, Read EJ, Lenes BA, Stumpf RJ. Try-panosoma cruzi in Los Angeles and Miami blood donors: impact of evolving donor demographics on seroprevalence and implications for transfusion transmission. Transfusion. 2002;42:549–555. doi: 10.1046/j.1537-2995.2002.00077.x. [DOI] [PubMed] [Google Scholar]
- 6.Tobler LH, Contestable P, Pitina L, Groth H, Shaffer S, Blackburn GR, Warren H, Lee SR, Busch MP. Evaluation of a new enzyme-linked immunosorbent assay for detection of Chagas antibody in US blood donors. Transfusion. 2007;47:90–96. doi: 10.1111/j.1537-2995.2007.01068.x. [DOI] [PubMed] [Google Scholar]
- 7.Flores-Chavez M, Fernandez B, Puente S, Torres P, Rodriguez M, Monedero C, Cruz I, Garate T, Canavate C. Transfusional chagas disease: parasitological and serological monitoring of an infected recipient and blood donor. Clin Infect Dis. 2008;46:e44–e47. doi: 10.1086/527448. [DOI] [PubMed] [Google Scholar]
- 8.Schijman AG, Vigliano C, Burgos J, Favaloro R, Perrone S, Laguens R, Levin MJ. Early diagnosis of recurrence of Trypanosoma cruzi infection by polymerase chain reaction after heart transplantation of a chronic Chagas’ heart disease patient. J Heart Lung Transpl. 2000;19:1114–1117. doi: 10.1016/s1053-2498(00)00168-6. [DOI] [PubMed] [Google Scholar]
- 9.Solari A, Ortiz S, Soto A, Arancibia C, Campillay R, Contreras M, Salinas P, Rojas A, Schenone H. Treatment of Trypanosoma cruzi-infected children with nifurtimox: a 3 year follow-up by PCR. J Antimicrob Chemother. 2001;48:515–519. doi: 10.1093/jac/48.4.515. [DOI] [PubMed] [Google Scholar]
- 10.Schijman AG, Altcheh J, Burgos JM, Biancardi M, Bisio M, Levin MJ, Freilij H. Aetiological treatment of congenital Chagas’ disease diagnosed and monitored by the polymerase chain reaction. J Antimicrob Chemother. 2003;52:441–449. doi: 10.1093/jac/dkg338. [DOI] [PubMed] [Google Scholar]
- 11.Umezawa ES, Bastos SF, Coura JR, Levin MJ, Gonzalez A, Rangel-Aldao R, Zingales B, Luquetti AO, da Silveira JF. An improved serodiagnostic test for Chagas’ disease employing a mixture of Trypanosoma cruzi recombinant antigens. Transfusion. 2003;43:91–97. doi: 10.1046/j.1537-2995.2003.00279.x. [DOI] [PubMed] [Google Scholar]
- 12.da Silveira JF, Umezawa ES, Luquetti AO. Chagas disease: recombinant Trypanosoma cruzi antigens for serological diagnosis. Trends Parasitol. 2001;17:286–291. doi: 10.1016/s1471-4922(01)01897-9. [DOI] [PubMed] [Google Scholar]
- 13.Leguizamon MS, Campetella O, Russomando G, Almiron M, Guillen I, Ganzalez Cappa SM, Frasch AC. Antibodies inhibiting Trypanosoma cruzi trans-sialidase activity in sera from human infections. J Infect Dis. 1994;170:1570–1574. doi: 10.1093/infdis/170.6.1570. [DOI] [PubMed] [Google Scholar]
- 14.WHO. Control of Chagas disease: second report of the WHO expert committee. WHO Tech Rep Ser. 2002;905:1–109. [PubMed]
- 15.WHO. Control of Chagas disease: report of a WHO expert committee. WHO Tech Rep Ser. 1991;811:1–95. [PubMed]
- 16.Schmunis GA, Zicker F, Segura EL, del Pozo AE. Transfusion-transmitted infectious diseases in Argentina, 1995 through 1997. Transfusion. 2000;40:1048–1053. doi: 10.1046/j.1537-2995.2000.40091048.x. [DOI] [PubMed] [Google Scholar]
- 17.Berrizbeitia M, Ndao M, Bubis J, Gottschalk M, Ache A, Lacouture S, Medina M, Ward BJ. Field evaluation of four novel enzyme immunoassays for Chagas’ disease in Venezuela blood banks: comparison of assays using fixed-epimastigotes, fixed-trypomastigotes or trypomastigote excreted-secreted antigens from two Trypanosoma cruzi strains. Transfus Med. 2006;16:419–431. doi: 10.1111/j.1365-3148.2006.00703.x. [DOI] [PubMed] [Google Scholar]
- 18.Caballero ZC, Sousa OE, Marques WP, Saez-Alquezar A, Umezawa ES. Evaluation of serological tests to identify Trypanosoma cruzi infection in humans and determine cross-reactivity with Trypanosoma rangeli and Leishmania spp. Clin Vaccine Immunol. 2007;14:1045–1049. doi: 10.1128/CVI.00127-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oelemann WM, Teixeira MD, Verissimo Da Costa GC, Borges-Pereira J, De Castro JA, Coura JR, Peralta JM. Evaluation of three commercial enzyme-linked immunosorbent assays for diagnosis of Chagas’ disease. J Clin Microbiol. 1998;36:2423–2427. doi: 10.1128/jcm.36.9.2423-2427.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanchez Negrette O, Mora MC, Basombrio MA. High prevalence of congenital Trypanosoma cruzi infection and family clustering in Salta, Argentina. Pediatrics. 2005;115:668–672. doi: 10.1542/peds.2004-1732. [DOI] [PubMed] [Google Scholar]
- 21.Buschiazzo A, Frasch AC, Campetella O. Medium scale production and purification to homogeneity of a recombinant trans-sialidase from Trypanosoma cruzi. Cell Mol Biol (Noisy-le-grand) 1996;42:703–710. [PubMed] [Google Scholar]
- 22.Leguizamon MS, Russomando G, Luquetti A, Rassi A, Almiron M, Gonzalez-Cappa SM, Frasch AC, Campetella O. Long-lasting antibodies detected by a trans-sialidase inhibition assay of sera from parasite-free, serologically cured chagasic patients. J Infect Dis. 1997;175:1272–1275. doi: 10.1086/593697. [DOI] [PubMed] [Google Scholar]
- 23.Leiby DA, Wendel S, Takaoka DT, Fachini RM, Oliveira LC, Tibbals MA. Serologic testing for Trypanosoma cruzi: comparison of radioimmunoprecipitation assay with commercially available indirect immunofluorescence assay, indirect hemagglutination assay, and enzyme-linked immunosorbent assay kits. J Clin Microbiol. 2000;38:639–642. doi: 10.1128/jcm.38.2.639-642.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moncayo A, Ortiz Yanine MI. An update on Chagas disease (human American trypanosomiasis) Ann Trop Med Parasitol. 2006;100:663–677. doi: 10.1179/136485906X112248. [DOI] [PubMed] [Google Scholar]
- 25.Gadelha AA, Vercosa AF, Lorena VM, Nakazawa M, Carvalho AB, Souza WV, Ferreira AG, Silva ED, Krieger MA, Goldenberg S, Gomes YM. Chagas’ disease diagnosis: comparative analysis of recombinant ELISA with conventional ELISA and the haemagglutination test. Vox Sang. 2003;85:165–170. doi: 10.1046/j.1423-0410.2003.00340.x. [DOI] [PubMed] [Google Scholar]
- 26.Buchovsky AS, Campetella O, Russomando G, Franco L, Oddone R, Candia N, Luquetti A, Gonzalez Cappa SM, Leguizamon MS. trans-sialidase inhibition assay, a highly sensitive and specific diagnostic test for Chagas’ disease. Clin Diagn Lab Immunol. 2001;8:187–189. doi: 10.1128/CDLI.8.1.187-189.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uebersax JS, Grove WM. Latent class analysis of diagnostic agreement. Stat Med. 1990;9:559–572. doi: 10.1002/sim.4780090509. [DOI] [PubMed] [Google Scholar]
- 28.Yang I, Becker MP. Latent variable modeling of diagnostic accuracy. Biometrics. 1997;53:948–958. [PubMed] [Google Scholar]
- 29.Pirard M, Iihoshi N, Boelaert M, Basanta P, Lopez F, Van der Stuyft P. The validity of serologic tests for Trypanosoma cruzi and the effectiveness of transfusional screening strategies in a hyperendemic region. Transfusion. 2005;45:554–561. doi: 10.1111/j.0041-1132.2005.04214.x. [DOI] [PubMed] [Google Scholar]
- 30.Pellett PE, Wright DJ, Engels EA, Ablashi DV, Dollard SC, Forghani B, Glynn SA, Goedert JJ, Jenkins FJ, Lee TH, Neipel F, Todd DS, Whitby D, Nemo GJ, Busch MP. Multi-center comparison of serologic assays and estimation of human herpesvirus 8 seroprevalence among US blood donors. Transfusion. 2003;43:1260–1268. doi: 10.1046/j.1537-2995.2003.00490.x. [DOI] [PubMed] [Google Scholar]
- 31.Leguizamon MS, Russomando G, Rojas de Arias A, Samudio M, Cabral M, Gonzalez-Cappa SM, Frasch AC, Campetella O. Use of trans-Sialidase inhibition assay in a population serologically negative for Trypanosoma cruzi but at a high risk of infection. Clin Diagn Lab Immunol. 1998;5:254–255. doi: 10.1128/cdli.5.2.254-255.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leiby DA, Herron RM, Jr, Garratty G, Herwaldt BL. Trypanosoma cruzi parasitemia in US blood donors with serologic evidence of infection. J Infect Dis. 2008;198:609–613. doi: 10.1086/590159. [DOI] [PubMed] [Google Scholar]