Abstract
BACKGROUND
Syphilis screening of blood donors is a common practice worldwide, but very little is known about the meaning of a positive serologic test for syphilis in blood donors and the risk profile of these donors. The aim of this study was to determine the demographic characteristics and risk behaviors of blood donors with recent and past syphilis and their implications for blood bank testing and deferral strategies.
STUDY DESIGN AND METHODS
Demographic characteristics, category of donation, number of previous donations, sexual behavior, and history of sexually transmitted diseases were reviewed comparing blood donors with recent and past syphilis from January 1, 1999, to December 31, 2003.
RESULTS
A total of 2439 interviews were reviewed, including 2161 (88.6%) donors with past and 278 (11.4%) with recent syphilis infection. Factors associated with recent infection included younger age (≤20 years odds ratio [OR], 36.5; 95% confidence interval [CI], 15.8–84.1), two previous donations (OR, 2.7; 95% CI, 1.9–3.9), male-male sex (homosexual OR, 8.2; 95% CI, 3.2–20.8; and bisexual OR, 11.4; 95% CI, 3.6–36.3), two or more partners in the past 12 months (OR, 2.3; 95% CI, 1.3–4.0), symptoms for syphilis (OR, 4.5; 95% CI, 2.8–7.1), and human immunodeficiency virus (HIV) seropositivity (OR, 39.6; 95% CI, 4.6–339.8). Community donors were also associated with recent syphilis infection (OR, 1.5; 95% CI, 1.2–1.9) compared to replacement donors.
CONCLUSION
Sexual history, including male-male sex and multiple partners, were strongly associated with recent syphilis infection, which in turn was strongly associated with HIV. Continuous and vigilant surveillance that includes assessing sexual history and other factors associated with syphilis are needed to guide blood safety policies.
Syphilis, an ancient disease, is still a public health problem worldwide. The World Health Organization (WHO) estimated that there are 12 million new cases of syphilis each year, with more than 90 percent occurring in developing nations.1 In Brazil, the National Program for Sexually Transmitted Diseases and AIDS Control estimated there were 937,000 cases of syphilis in the sexually active population.2 Moreover, in the past 30 years, through its association with an increased risk of human immunodeficiency virus (HIV) infection, syphilis has acquired a new potential for morbidity and mortality.3
At the same time, transfusion-transmitted syphilis has decreased all over the world. In the past 35 years, only three cases of transfusion-transmitted syphilis have been reported in the English literature.4–6 Improved donor selection, serologic screening of donors for the presence of syphilis, and the shift from transfusion of fresh blood components to refrigerated blood components have greatly decreased rates of transfusion-transmitted syphilis.7
The declining of syphilis rates in developed countries raised the question of whether syphilis screening was still necessary for blood donors. However, in developing countries, the prevalence of positive serologic tests for syphilis (STS) can be as high as 13.5 percent,8 as in Ghana. In such settings, the potential for laboratory or human error missing infections highlights the continued need for universal blood donor screening for syphilis and ongoing assessment of blood donor risk for infection to help ensure a safer blood supply.9 While many questions about syphilis and blood donation are discussed, little systematic information is available on the profile of blood donors with a positive STS, including differences between donors with recent versus past infection. For example, one question that remains is whether blood from donors with histories of past syphilis with low serologic titers should be transfused. While excluding these donations is wasteful, past history of syphilis may be amarker of continuing and undisclosed high-risk sexual behavior, which could lead to HIV infection.
Currently, in Brazil, only one screening test for syphilis is mandatory. Blood banks may choose Venereal Disease Research Laboratory (VDRL), rapid plasma reagin (RPR), or enzyme immunoassay (EIA). VDRL and RPR are sensitive for recent syphilis infection, but not for past infection. EIA can detect past or recent infection, but may result in rejecting noninfectious blood with distant past infection. The aim of this study is to characterize the profile of blood donors with recent and past syphilis and discuss improvements blood banks may introduce to maintain the safety of the blood supply.
MATERIALS AND METHODS
Overall study design and setting
We conducted a cross-sectional study in which blood donors with recent syphilis were compared to blood donors with past syphilis. Demographic characteristics, category of donation, number of previous donations, sexual behavior, and history of sexually transmitted diseases (STDs) were reviewed for all blood donors who confirmed positive STS and returned for notification and counseling from January 1, 1999, to December 31, 2003. Fundação Pró-Sangue (FPS) in São Paulo, Brazil, located in the largest public hospital of the city, collects roughly 175,000 units of blood annually, representing 14 percent of the blood donated in all of Brazil. The study was approved by the Ethical Committee of Hospital das Clínicas from University of São Paulo, the ethical review board of the FPS.
Study subjects
Blood donors participating in this study were a subsample of the routine blood donor recruitment process at FPS; all were volunteers, between 18 and 65 years old, and weighed more than 50 kg, according to the standard blood donation requirements. Candidates for blood donation were submitted to a rapid physical examination and a predonation interview, carried out face-to-face by a trained nurse in a private room under a qualified physician’s direction, according to FPS standard operational procedures. Predonation interview included questions about sexual behavior and risk factors for HIV/AIDS and other STDs. At the time data were collected, deferral criteria included risk factors for HIV/AIDS and STD, namely: 1) having five or more sex partners in the past 12 months, 2) ever having male-male sexual behavior, 3) ever using intravenous (IV) drugs, 4) receiving or paying money for sex in the past 12 months, 5) having sex with a partner who had more than five partners or sex worker contact in the past 12 months, 6) ever having an HIV-positive sexual partner, 7) ever having an IV drug user partner, 8) women ever having a male partner who had sex with a male partner, and 9) having any STD in the past 12 months. After the predonation interview and before blood collection, donors were asked to privately answer, in a confidential unit exclusion process, if they have been at risk for AIDS. All blood units were screened for syphilis, HIV types 1 and 2, human T-lymphotropic virus (HTLV)-1 and -2, hepatitis B virus (HBV), hepatitis C virus (HCV), and Chagas disease. Blood donors who presented any reagent screening test were recalled for additional laboratory test. If the retest presented any reagent result, the donor was recalled for notification and counseling.
Persons eligible for the study were those who qualified for blood donation and who returned for counseling and retest and met the following serologic criteria: 1) a reactive EIA at serologic blood donation screening; 2) fluorescent treponemal antibody absorption test (FTA-ABS) and EIA tests reactive at retest; and 3) a reactive VDRL, with titers of 16 or greater, or a VDRL nonreactive at retest. Recent syphilis was defined as a VDRL titer of 16 or greater and both FTA-ABS– and EIA-positive tests. We chose higher titers of VDRL because they appear in the early phases of infection, mainly during primary and secondary syphilis.10 Past syphilis was defined as a VDRL negative and both FTA-ABS and EIA positive, which includes donors who presented serologic markers of previous treated disease and those who had late latent infection.10,11
In the present data, only blood donors who donated at the main blood center and mobile units of FPS, which collect approximately 75 percent of donations, were included. The three different hospital-based sites that collect the other 25 percent were not included in the study because their blood donors’ data were not available in the same computer-based system.
Measures
Data were collected from blood donor interviews conducted after donation, when they returned to be notified about their serologic status. Interviews at the return or recall visit were carried out by physicians trained according to the standard operational protocols of FPS, through a face-to-face process, applying a standardized questionnaire in a private room. The interview included 1) demographic characteristics; 2) category of donation (community, replacement, and autologous); 3) number of previous donations; 4) sexual orientation; 5) number of sexual partners in the past 12 months; 6) ever had sex with a sex worker; 7) sex with a sex worker in the past 12 months; 8) history of previous STD; 9) history of past chancre or skin rash; 10) previous diagnosis of syphilis and when it was made; 11) previous treatment for syphilis and when it was treated; and 10) past history of IV drug use. Of note, many of the questions repeat criteria that would have resulted in deferral of the donor if reported at the time of predonation. Data of eligible blood donors were collected and entered in a computer spreadsheet (Excel, Microsoft Corp., Redmond, WA) by two different data managers with reconciliation against the originally completed forms and interviewer.
Statistical analysis
Data were first analyzed as a series of separate bivariate analyses comparing demographic characteristics, category of donation, number of previous donations, history of IV drug use, history of STD, and behaviors between donors with recent versus past syphilis infection using the chi-square test. Predictors and potential confounders significant in bivariate analyses at p values of less than 0.05 were included in multivariate analysis. Nonsignificant variables were eliminated from the final multivariate model in a stepwise fashion, unless they substantially confounded associations between syphilis and other variables. We used the Pearson’s goodness-of-fit test to assess the fit of the final model. All statistical tests were performed with statistical software (STATA 8.0, StataCorp, College Station, TX).
Laboratory methods
Laboratory testing was performed at the Serology Division of FPS following usual blood bank procedures for HBV surface antigen, HBV core antibody, anti-HCV, anti-HTLV-I/-II, Chagas disease, and anti-HIV-1 and -2 (details available upon request). The screening test for syphilis was EIA. If positive, blood donors were invited to return in 30 days and collect a new sample. If the EIA was still positive in the new sample, FTA-ABS and VDRL were performed. Serologic tests changed over time, although all kits used were approved by the Brazilian Ministry of Health and had sensitivity higher than 99 percent as described in the manufacturers’ information. Before being used at FPS, all kits are tested with an in-house panel of syphilis serum and are accepted only if all positive samples are detected. The most recent testing algorithm for syphilis included a competitive EIA (Enzygnost* syphilis, Dade Behring, Newark, DE), VDRL test (Wiener lab, Rosario, Argentina), and Imunopallidum (biolab-Mérieux S/A, Rio de Janeiro, Brazil).
RESULTS
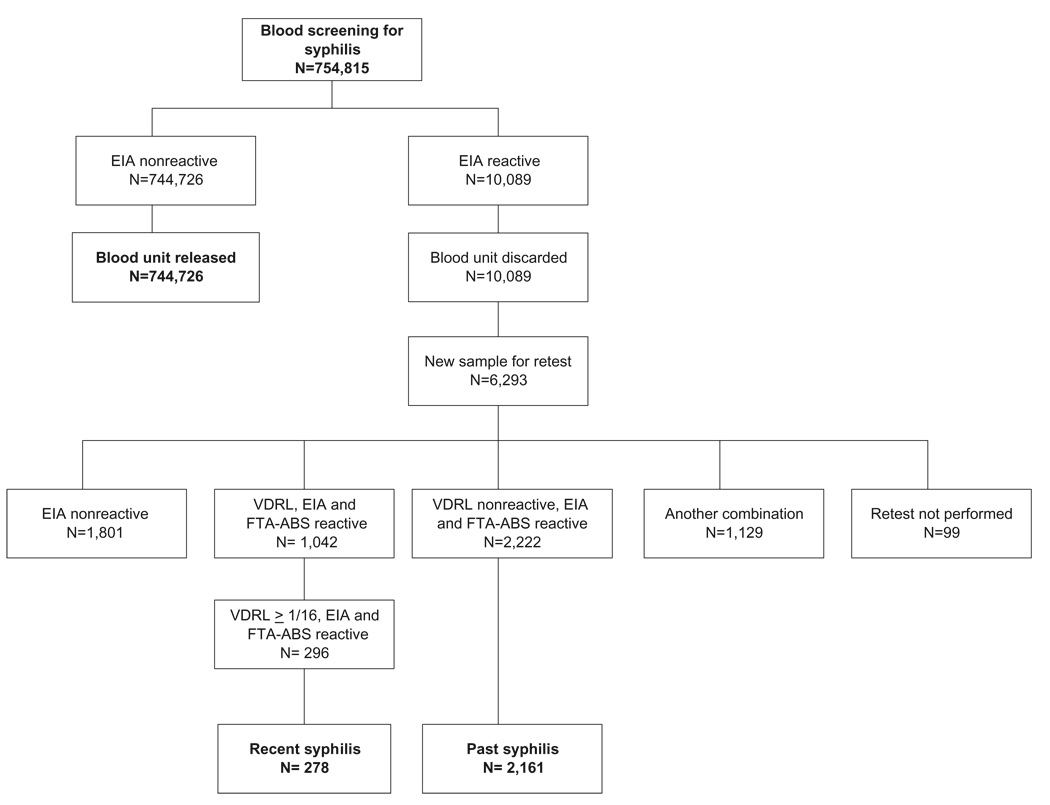
The algorithm for syphilis screening and the distribution of blood donors according to the confirmatory blood tests can be seen in Fig. 1. There were 754,815 blood donations during the study period. A total of 10,089 (1.3%) units were discarded due to being reactive to STS. For these units, 6293 (62.4%) blood donors returned to give a new blood sample. Upon retesting, 1042 (16.6%) blood donors were reactive on EIA, VDRL, and FTA-ABS; 2222 (35.3%) were VDRL-nonreactive, EIA- and FTA-ABS–reactive; 1801 (28.6%) had a negative result; in 99 (1.6%) the retest was not performed; and 1129 (17.9%) presented another STS combination. The study included 296 (4.7%) donors classified with “recent syphilis” who presented at retest with VDRL titers of 16 or greater, and 2222 (35.3%) donors classified with “past syphilis” with VDRL-negative and EIA- and FTA-ABS–reactive results. Of those, 2439 were interviewed, 2161 (88.6%) with past syphilis and 278 (11.4%) with recent syphilis.
Fig. 1.
Algorithm for syphilis screening and retest at FPS.
In the FPS overall population of donors, replacement, community, and autologous donors represent approximately 43, 57, and 0.2 percent, respectively. Of all donors, 52 percent are first-time donors. In the current study, replacement blood donors represented 44.2 percent of those with recent syphilis and 54.0 percent of those with past syphilis (p = 0.02; Table 1). First-time donors represented 61.2 percent of those with recent syphilis and 59.4 percent of those with past infection (p < 0.001). The majority of donors with recent syphilis (72.7%) and past syphilis (67.8%) were men.
TABLE 1.
Association between reported demographic and exposure variables and recent syphilis infection among blood donors in São Paulo, Brazil (n = 2439)
| Demographic or exposure variable | Recent syphilis (n = 278)* | Past syphilis (n = 2161)* | p Value | OR (95% CI) |
|---|---|---|---|---|
| Male gender | 202 (72.7) | 1464 (67.8) | 0.1 | 1.3 (1.0–1.7) |
| Age (years)† | <0.0001 | |||
| ≤20 | 20 (7.2) | 16 (0.7) | 36.5 (15.8–84.1) | |
| 21–30 | 129 (46.4) | 198 (9.2) | 19.0 (10.9–33.3) | |
| 31–40 | 75 (27.0) | 638 (29.5) | 3.4 (1.9–6.1) | |
| 41–50 | 39 (14.0) | 870 (40.3) | 1.3 (0.7–2.4) | |
| >50 | 15 (5.4) | 438 (20.3) | 1.0 | |
| Donor category | 0.02 | |||
| Replacement | 123 (44.2) | 1166 (54.0) | 1.0 | |
| Community | 155 (55.8) | 993 (46.0) | 1.5 (1.2–1.9) | |
| Autologous | 0 (0) | 1 (0.05) | ||
| Number of previous donations† | <0.001 | |||
| 0 | 170 (61.2) | 1284 (59.4) | 1.0 | |
| 1 | 18 (6.5) | 221 (10.2) | 0.7 (0.4–1.0) | |
| 2 | 46 (16.6) | 129 (6.0) | 2.7 (1.9–3.9) | |
| ≥3 | 40 (14.4) | 516 (23.9) | 0.6 (0.4–0.8) | |
| Sexual orientation | <0.001 | |||
| Homosexual | 9 (3.2) | 9 (0.4) | 8.2 (3.2–20.8) | |
| Bisexual | 7 (2.5) | 5 (0.2) | 11.4 (3.6–36.3) | |
| Heterosexual | 261 (94.0) | 2132 (98.7) | 1.0 | |
| Two or more sexual partners in the past 12 months | 85 (30.6) | 274 (12.7) | 0.01 | 2.3 (1.3–4.0) |
| Ever had sex with a sex worker | 34 (12.2) | 412 (19.1) | 0.006 | 1.7 (1.2–2.5) |
| History of other STD ever | 37 (13.3) | 596 (27.6) | <0.001 | 0.4 (0.3–0.6) |
| HIV-positive‡ | 5 (1.8) | 1 (0.05) | <0.001 | 39.6 (4.6–339.8) |
| Treatment for syphilis | 21 (7.6) | 591 (27.4) | <0.001 | 0.2 (0.1–0.3) |
| Symptoms for syphilis | 29 (10.4) | 55 (2.6) | <0.001 | 4.5 (2.8–7.1) |
Data are reported as number (%).
Totals may be less due to missing data.
Confirmed by two other EIAs and Western blot in another sample.
Table 1 also shows bivariate comparisons of demographic characteristics, sexual behavior, and STD history for donors with recent and past syphilis. Donors with recent infection were more likely to be between 21 and 30 years old (46.4%), homosexual (3.2%), and bisexual (2.5%) and report two or more partners in the past 12 months (30.6%) and symptoms of primary and secondary syphilis (10.4%) when compared with those with past infection. Compared to donors with recent infection, those with past syphilis were more likely to have been diagnosed and treated for syphilis (27.4% vs. 7.6%), to have had another STD (27.6% vs. 13.3%), and to have had sex with a sex worker (19.1% vs. 12.2%). Only 11 (6.2%) donors with recent syphilis and 21 (1.0%) with past syphilis admitted previously unreported sex with a sex worker in the past 12 months (p < 0.001). Coinfection with HIV infection was found in 5 (1.8%) donors with recent syphilis and in 1 (0.05%) with past disease (p < 0.001). There was no association of syphilis with other serologic markers.
In multivariate analyses, factors independently associated with recent infection for syphilis included younger age (≤20 years, adjusted odds ratio [OR], 36.5; 95% CI, 15.8–84.1), two previous donations (OR, 2.7; 95% CI, 1.9–3.9), male-male sex (homosexual OR, 8.2; 95% CI, 3.2–20.8; and bisexual OR, 11.4; 95% CI, 3.6–36.3), two or more sexual partners in the past 12 months (OR, 2.3; 95% CI, 1.3–4.0), symptoms for syphilis (OR, 4.5; 95% CI, 2.8–7.1), treatment for syphilis (OR, 0.2; 95% CI, 0.1–0.3), and HIV seropositivity (OR, 39.6; 95% CI, 4.6–339.8). Community donors, as opposed to replacement donors, were associated with recent syphilis infection (OR, 1.5; 95% CI 1.2–1.9). It is noteworthy that blood donors who had syphilis 5 or fewer years ago were more likely to report male-male sex than those who had had syphilis more than 5 years ago.
DISCUSSION
In this systematic analysis of interviews ofmore than 2000 blood donors testing positive for the presence of syphilis, we were able to distinguish strong associations with recent versus past infection. Recent syphilis infection was associated with younger age, male-male sex, two or more sex partners, past syphilis treatment, past syphilis history, HIV seropositivity, and, contrary to expectation, two previous donations and community—not replacement—donations. A higher prevalence of past syphilis relative to recent infection in older groups is expected, since older donors manifest a cumulative exposure over their longer past.12 In contrast, recent infections were found in younger groups manifesting current sexual behaviors.
We also wish to highlight the additional public health benefit of the STS screening in our blood bank. As in other developing countries,7,13 all blood donors with reactive serologic blood tests were notified and referred for specialized medical care in public centers for STD treatment and prevention for themselves and their partners. As noted, we found that less than one-third of blood donors with past syphilis infection had previously been referred to treatment; very few remembered having any symptoms. In contrast, Orton and colleagues14 in the United States found that half of blood donors screened with an automated specific treponemal test for syphilis (PK-TP) and with a confirmed-positive FTA-ABS reported a past and treated syphilis infection. Blood donors with past disease who have not received medical treatment can be in a late latent phase and, if not treated, can evolve to a tertiary syphilis. Likewise, the great majority of our donors with recent infection had not yet received medical treatment nor recognized symptoms.
It is controversial whether the blood of donors with confirmed STS and serologically recent infection are truly infectious for syphilis. Analysis of syphilis outbreaks in the United States found that Treponema pallidum DNA could be detected in the blood of untreated, infected individuals during all phases of syphilis infection.15,16 In contrast, a study conducted with 196 samples either RPR-positive or RPR-negative suggested that blood donors with confirmed-positive results on STS are unlikely to have circulating T. pallidum in their blood.17
It is noteworthy that 6 percent of blood donors with recent infection and 0.6 percent with past infection admitted to male-male sex after having previously denied the behavior at predonation interview. An increase in syphilis infections since the mid-1990s has been observed especially in men who have sex with another man, in different European and North American cities, with high rates of HIV coinfection.18,19 Although the number of HIV cases was small in our study, the association of recent syphilis with HIV infection was nearly 40-fold higher than with past infection and nearly 50-fold higher than in the general blood donor population.20 Both diseases are sexually transmitted and high-risk sexual behavior is associated with one or both infections. Indeed, HIV has enhanced the potential morbidity of syphilis making the treatment and cure more difficult.11 Although there is a strong association of reactive STS and HIV, the usefulness of STS as a surrogate marker for HIV infection in seronegative blood donors in United States seems to be negligible.21 Herrera and colleagues22 published the most extensive evaluation of syphilis screening as a HIV surrogate marker. Among 4,468,570 donations, STS-reactive donations were 12 times more likely to be HIV-positive; however, of an estimated 13 infectious window-period donations, 0.2 would have been removed because of a reactive STS, at a cost of more than $16 million. Studies to assess the role of STS as a surrogate marker for window-period HIV infection in populations with higher rates of syphilis and HIV, such as found in many parts of the developing world, may be a promising area of research.
Although most blood donors with a positive STS were first-time donors, our study found higher recent infection among persons who had donated two times previously. One logical explanation for this finding could be related with the serologic donor’s status in the previous donation. If donors were positive for the presence of syphilis in the past, then they may be less likely to donate again (e.g., their letter would indicate they should wait or the counseling would discourage them), whereas donors who were negative for the presence of syphilis in the past would be more likely to donate again and therefore would have acquired their syphilis since the previous donation; therefore, it would be more recent.
Potentially, a large number of blood donors with past infection could be safe donors, but we found that there was still a small group among them who presented some risk factors in common with those with recent infection that were just identified because an EIA test was applied as serologic screening. Blood donors screening with VDRL and RPR have the advantage of identifying mostly recent infections and increase the blood supply, but they can lose past infection, sometimes in a latent phase, which deserves treatment and may be an undisclosed risk for other STDs including HIV. Moreover, VDRL and RPR cannot be automated and are time-consuming to be done in a large scale. On the other hand, EIA and PK-TP are automated and detect recent and past infections, but more blood units will be discarded. In a conservative way, it seems EIA and PK-TP screenings are still safer measures for blood screening in developing countries with low and intermediate-low prevalence of reactive STS. Those countries that have high rates of reactive STS in blood donors probably will need to adopt VDRL or RPR as screening, but they must be aware that this measure is necessary to avoid blood shortages but is not as safe as EIA or PK-TP.
An unexpected finding of our study was the association between recent syphilis and community donation. Contrary to what many would assume, our study showed that community donors are not necessarily safer than replacement donors with respect to recent syphilis infection and by extension potentially for HIV. A possible explanation for this finding is that community-recruited donors may have a higher proportion of persons seeking HIV testing who are at risk for HIV or other STDs. An implication of this phenomenon is that qualified replacement donors must be retained in the pool of repeated donors as a safe resource for the blood supply.23
We recognize that our study has limitations. Ideally, the diagnosis of primary syphilis is based on the presence of typical lesions and either a reactive nontreponemal test and no history of past syphilis or a fourfold increase in titer on a quantitative nontreponemal test when results of past test are compared with the most recent results. A presumption diagnosis of secondary syphilis is based on the presence of typical lesions and a reactive nontreponemal test titer of 8 or greater and no previous history of syphilis or, for persons with a history of syphilis, a fourfold increase in the most recent titer compared with past test results.10 Laboratory methods alone, especially when applied at blood donor screening, are sometimes not enough to establish a diagnosis. We selected blood donors based only in serologic findings and defined recent infection as a VDRL titer of 16 or greater in an additional blood sample collected after donation. Although a positive VDRL with high titer alone does not necessarily mean recent infection, in the majority of cases, VDRL titers of more than 8 and a positive treponemal test as FTA-ABS and EIA define this profile. The rate of false-positive results for syphilis is always a matter of concern. Positive predictive values are dependent on the population screened and the method used. False-positive STS results have been associated with hepatitis, mononucleosis, viral pneumonia, chicken pox, measles, other viral infections, immunizations, pregnancy, connective tissue diseases, narcotic addiction, aging, leprosy, malignancy, diseases associated with hypergammaglobulinemia, and laboratory error.12 Although we cannot rule out STS false-positive results, we selected blood donors who returned to a confirmatory test and were retested and in two different samples and presented consistent STS results.
In conclusion, the epidemiologic profile of blood donors with reactive syphilis differs between those with current versus remote serologic patterns. For blood banks, findings can help target recruitment, predonation interview questions, laboratory screening procedures, and counseling strategies. For public health, syphilis testing and risk assessment helps to treat patients who otherwise may not seek care, as well as direct the implementation of prevention activities. Preventing syphilis in the donor population has further benefits to their partners, children, and society at large. Syphilis persists in developing nations and is resurging in parts of the world where the rates of disease were once lower.24 A continuous and vigilant syphilis screening program, including the monitoring of the profile of blood donors with infection, remains relevant in the developed and developing world.
Acknowledgments
This study was funded by a grant from the Blood Systems Foundation, San Francisco, CA, and the Center for AIDS Prevention Studies, University of California, San Francisco, CA.
ABBREVIATIONS
- FPS
Fundação Pró-Sangue, Blood Center of São Paulo
- FTA-ABS
fluorescent treponemal antibody absorption test
- PK-TP
automated specific treponemal test for syphilis
- RPR
rapid plasma reagin
- STD(s)
sexually transmitted disease(s)
- STS
serologic tests for syphilis
- VDRL
Venereal Disease Research Laboratory
REFERENCES
- 1.WHO Department of HIV/AIDS. Global prevalence and incidence of selected curable sexually transmitted infections: overview and estimates. Geneva, Switzerland: WHO; 2001 Available from: http://www.who.int/docstore/hiv/GRSTI/005.htm.
- 2.DST em números. Brasília—DF: PN-DST/AIDS. 2003 Available from: http://www.aids.gov.br/data/Pages/LUMISD1F318A3PTBRIE.htm.
- 3.Zeltser R, Kurban AK. Syphilis. Clin Dermatol. 2004;22:461–468. doi: 10.1016/j.clindermatol.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 4.Soendjojo A, Boedisantoso M, Ilias MI, Rahardjo D. Syphilis d’emblee due to blood transfusion. Case report. Br J Vener Dis. 1982;58:149–150. doi: 10.1136/sti.58.3.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Risseeuw-Appel IM, Kothe FC. Transfusion syphilis: a case report. Sex Transm Dis. 1983;10:200–201. doi: 10.1097/00007435-198311000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Chambers RW, Foley HT, Schmidt PJ. Transmission of syphilis by fresh blood components. Transfusion. 1969;9:32–34. doi: 10.1111/j.1537-2995.1969.tb04909.x. [DOI] [PubMed] [Google Scholar]
- 7.Gardella C, Marfin AA, Kahn RH, Swint E, Markowitz LE. Persons with early syphilis identified through blood or plasma donation screening in the United States. J Infect Dis. 2002;185:545–549. doi: 10.1086/338829. [DOI] [PubMed] [Google Scholar]
- 8.Ampofo W, Nii-Trebi N, Ansah J, Abe K, Naito H, Aidoo S, Nuvor V, Brandful J, Yamamoto N, Ofori-Adjei D, Ishikawa K. Prevalence of blood-borne infectious diseases in blood donors in Ghana. J Clin Microbiol. 2002;40:3523–3525. doi: 10.1128/JCM.40.9.3523-3525.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmunis GA, Zicker F, Pinheiro F, Brandling-Bennett D. Risk for transfusion-transmitted infectious diseases in Central and South America. Emerg Infect Dis. 1998;4:5–11. doi: 10.3201/eid0401.980102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larsen SA, Steiner BM, Rudolph AH. Laboratory diagnosis and interpretation of tests for syphilis. Clin Microbiol Rev. 1995;8:1–21. doi: 10.1128/cmr.8.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh AE, Romanowski B. Syphilis: review with emphasis on clinical, epidemiologic, and some biologic features. Clin Microbiol Rev. 1999;12:187–209. doi: 10.1128/cmr.12.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aberle-Grasse J, Orton SL, Notari E, 4th, Layug LP, Cable RG, Badon S, Popovsky MA, Grindon AJ, Lenes BA, Williams AE. Predictive value of past and current screening tests for syphilis in blood donors: changing from a rapid plasma reagin test to an automated specific treponemal test for screening. Transfusion. 1999;39:206–211. doi: 10.1046/j.1537-2995.1999.39299154737.x. [DOI] [PubMed] [Google Scholar]
- 13.Wendel S. Current concepts on transmission of bacteria and parasites by blood components. Vox Sang. 1994;67 Suppl 3:161–174. doi: 10.1111/j.1423-0410.1994.tb04568.x. [DOI] [PubMed] [Google Scholar]
- 14.Orton SL, Dodd RY, Williams AE. Absence of risk factors for false-positive test results in blood donors with a reactive test result in an automated treponemal test (PK-TP) for syphilis. Transfusion. 2001;41:744–750. doi: 10.1046/j.1537-2995.2001.41060744.x. [DOI] [PubMed] [Google Scholar]
- 15.Marfin AA, Liu H, Sutton MY, Steiner B, Pillay A, Markowitz LE. Amplification of the DNA polymerase I gene of Treponema pallidum from whole blood of persons with syphilis. Diagn Microbiol Infect Dis. 2001;40:163–166. doi: 10.1016/s0732-8893(01)00275-9. [DOI] [PubMed] [Google Scholar]
- 16.Pietravalle M, Pimpinelli F, Maini A, Capoluongo E, Felici C, D’Auria L, Di Carlo A, Ameglio F. Diagnostic relevance of polymerase chain reaction technology for T. pallidum in subjects with syphilis in different phases of infection. New Microbiol. 1999;22:99–104. [PubMed] [Google Scholar]
- 17.Orton SL, Liu H, Dodd RY, Williams AE. Prevalence of circulating Treponema pallidum DNA and RNA in blood donors with confirmed-positive syphilis tests. Transfusion. 2002;42:94–99. doi: 10.1046/j.1537-2995.2002.00023.x. [DOI] [PubMed] [Google Scholar]
- 18.Vall-Mayans M, Casals M, Vives A, Loureiro E, Armengol P, Sanz B. Reemergence of infectious syphilis among homosexual men and HIV coinfection in Barcelona, 2002–2003. Med Clin (Barc) 2006;126:94–96. doi: 10.1157/13083877. [DOI] [PubMed] [Google Scholar]
- 19.Brant LJ, Bukasa A, Davison KL, Newham J, Barbara JA. Increase in recently acquired syphilis infections in English, Welsh and Northern Irish blood donors. Vox Sang. 2007;93:19–26. doi: 10.1111/j.1423-0410.2007.00923.x. [DOI] [PubMed] [Google Scholar]
- 20.Salles NA, Sabino EC, Barreto CC, Barreto AM, Otani MM, Chamone DF. The discarding of blood units and the prevalence of infectious diseases in donors at the Pro-Blood Foundation/Blood Center of Sao Paulo, Sao Paulo, Brazil. Rev Panam Salud Publica. 2003;13:111–116. doi: 10.1590/s1020-49892003000200011. [DOI] [PubMed] [Google Scholar]
- 21.Orton S. Syphilis and blood donors: what we know, what we do not know, and what we need to know. Transfus Med Rev. 2001;15:282–291. doi: 10.1053/tmrv.2001.26956. [DOI] [PubMed] [Google Scholar]
- 22.Herrera GA, Lackritz EM, Janssen RS, Raimondi VP, Dodd RY, Aberle-Grasse J, Petersen LR. Serologic test for syphilis as a surrogate marker for human immunodeficiency virus infection among United States blood donors. Transfusion. 1997;37:836–840. doi: 10.1046/j.1537-2995.1997.37897424407.x. [DOI] [PubMed] [Google Scholar]
- 23.de Almeida Neto C, McFarland W, Murphy EL, Chen S, Nogueira FA. Risk factors for human immunodeficiency virus infection among blood donors in Sao Paulo, Brazil, and their relevance to current donor deferral criteria. Transfusion. 2007;47:608–614. doi: 10.1111/j.1537-2995.2007.01161.x. [DOI] [PubMed] [Google Scholar]
- 24.Hook EW, 3rd, Peeling RW. Syphilis control—continuing challenge. N Engl J Med. 2004;351:122–124. doi: 10.1056/NEJMp048126. [DOI] [PubMed] [Google Scholar]