Abstract
Background
High insulin and IGF-I levels may be associated with an increased breast cancer risk and/or death. Given the need to identify modifiable factors that decrease insulin, IGF-I, and breast cancer risk and death, we investigated the effects of a 6-month randomized controlled aerobic exercise intervention vs. usual care on fasting insulin, IGF-I and its binding protein (IGFBP-3) in postmenopausal breast cancer survivors.
Methods
Seventy-five postmenopausal breast cancer survivors were identified from the Yale-New Haven Hospital Tumor Registry and randomly assigned to an exercise (n = 37) or usual care (n = 38) group. The exercise group participated in 150 min/wk of moderate-intensity aerobic exercise. The usual care group was instructed to maintain their current physical activity level. A fasting blood sample was collected on each study participant at baseline and 6 months. Blood levels of insulin and IGFs were measured with enzyme-linked immunosorbent assays.
Results
On average, exercisers increased aerobic exercise by 129 min/wk compared with 45 min/wk among usual care participants (p < .001). Women randomized to exercise experienced decreases in insulin, IGF-I, and IGFBP-3, whereas women randomized to usual care had increases in these hormones. Between-group differences in insulin, IGF-I and IGFBP-3 were 20.7% (p = 0.089), 8.9% (p = 0.026), and 7.9% (p = 0.006), respectively.
Conclusions
Moderate-intensity aerobic exercise, such as brisk walking, decreases IGF-I and IGFBP-3. The exercise-induced decreases in IGFs may mediate the observed association between higher levels of physical activity and improved survival in women diagnosed with breast cancer.
Keywords: physical activity, fitness, survival, recurrence, death, obesity, weight, glucose, diabetes, c-peptide
INTRODUCTION
There has been increasing evidence that high insulin levels increase the risk of breast cancer recurrence and death (1–3). Three recent studies have observed an approximate triple risk of all-cause mortality among women in the highest category of insulin or c-peptide, a marker of insulin secretion, relative to the lowest category of insulin or c-peptide (1–3). These studies have also shown that high insulin and c-peptide levels are strongly associated with obesity and low levels of physical activity (1,4), both adverse prognostic factors in women diagnosed with breast cancer (5–9). Specifically, several recent observational studies have suggested that women who participate in 2–3 hr/wk of moderate-intensity aerobic exercise, such as brisk walking, after a diagnosis of breast cancer have a 40–67% reduced risk of death (7–9). Although the mechanisms linking exercise to breast cancer prognosis are not well understood, insulin may prove to be the elusive link between low levels of physical activity and poor breast cancer prognosis. Thus, therapies to reduce insulin levels in breast cancer survivors could dramatically decrease breast cancer-related deaths. A lowering of insulin levels by 25% may improve survival by 5%, the same order of magnitude as the beneficial effect of adjuvant chemotherapy (1,10). Given that many existing breast cancer therapies are costly and have significant side effects that can result in long-term morbidity, non-pharmacologic methods to lower insulin levels and ultimately breast cancer recurrence and death, especially methods that are also associated with improvements in quality of life and other chronic diseases, may offer an attractive addition to the currently available treatment options.
Despite a growing body of research, evidence supporting the association between exercise and insulin in breast cancer survivors has remained somewhat inconclusive (11–13); yet, the possibility that exercise may act as a targeted therapy in breast cancer is intriguing and warrants further investigation. Therefore, the purpose of the Yale Exercise and Survivorship Study was to examine the effects of a six-month randomized controlled aerobic exercise intervention vs. usual care on fasting insulin levels in postmenopausal breast cancer survivors who completed adjuvant therapy.
Secondary aims were to examine the effect of exercise on insulin-like growth factor-I (IGF-I) and its primary binding protein (IGFBP-3). Similar to insulin, IGF-I has potent mitogenic and antiapoptotic properties in normal and malignant breast epithelial cells, whereas IGFBP-3 can either stimulate or suppress cellular proliferation by restricting IGF-I’s availability and biological activity (14). For insulin, some mitogenic effects may be mediated by interaction with IGF-I receptors, as hyperinsulinemia promotes the synthesis and activity of IGF-I (15). Although the data are not consistent, high levels of IGF-I and low levels of IGFBP-3 have been associated with an increased risk of breast cancer and adverse prognostic factors (16,17); however, a study by Goodwin and colleagues found high levels, rather than low levels, of IGFBP-3 predicted distant recurrence of breast cancer in postmenopausal women (18). In healthy women, the effect of exercise on IGFs has been somewhat inconsistent (19,20), and to date, only two trials have examined the impact of exercise on IGFs in breast cancer survivors (12,13), with only one study observing a significant effect of exercise on IGFs (12).
Lastly, our tertiary aim was to examine what factors may modify the effect of exercise on insulin and IGFs including stage at diagnosis, hormone therapy, age, body mass index, and change in body weight/fat. To our knowledge, no other study has examined whether certain prognostic or physiologic factors modify the effect of exercise on insulin and IGFs. We hypothesized that exercise would be associated with favorable changes in insulin and IGFs, and that stage at diagnosis, hormone therapy, age, body mass index (BMI), and weight/fat loss would modify the effect of exercise on insulin and IGFs.
METHODS
Participants were recruited into the Yale Exercise and Survivorship Study, which is described in detail elsewhere (21,22). All study procedures were reviewed and approved by the Yale University School of Medicine Human Investigation Committee.
Study Participants
Participants were physically inactive (< 60 min/wk of recreational physical activity reported in the past six months), postmenopausal women (i.e., cessation of menses for at least 12 months) diagnosed 1–10 years ago with Stage 0 to IIIA breast cancer and who had completed adjuvant treatment at least six months prior to enrollment. Smokers, women with type 2 diabetes, and women with a previous cancer, recurrence, or second cancer were excluded due to the potential effect of these factors on outcomes of interest.
Recruitment
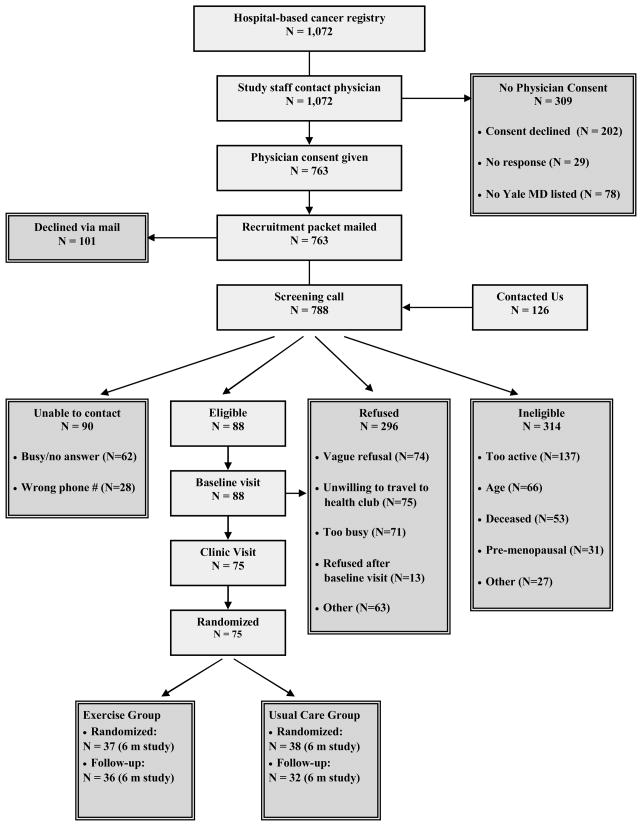
Study staff used the Yale-New Haven Tumor Registry to obtain the names of Connecticut women diagnosed with breast cancer by any Yale-affiliated physician from March 1994 to January 2006 (Figure 1). Staff contacted each patient’s physician to request permission to contact the participant. An invitation letter was mailed to the participant, followed by a telephone screening questionnaire, baseline and 6-month clinic visit. From 788 screening calls made, 75 (9.5%) women were eligible, interested, and randomized.
Figure 1.
Flow of participants through the YES Study.
Measures
Physical Activity
At baseline and 6-months, participants completed an interviewer-administered physical activity questionnaire, assessing the past 6 months of recreational activity (23), a seven-day physical activity log (PAL) (24), and a seven-day pedometer log (25). For the PAL, women recorded the type and duration of any recreational activity performed on each day. Hours/week spent in moderate-to-vigorous intensity aerobic activity were determined using Ainsworth’s Compendium of Physical Activities (26).
Demographics & Medical History
Information was collected via an interviewer-administered questionnaire at the baseline visit. Information regarding disease stage, hormone receptor status, adjuvant therapy, and surgery was provided by participants and later confirmed by their physician and review of the medical records.
Anthropometry
Height and weight were measured at baseline and 6-months. Participants were weighed on a digital scale in light clothing, without shoes; measurements were rounded up to the next 0.1 kilogram. Height without shoes was measured using a stadiometer, rounding up to the next 0.5 centimeter. Circumference measurements were taken at the waist (minimum circumference) and hips (greatest circumference). All measurements were taken twice in succession, by the same technician, and averaged for data entry.
DEXA Scans
A DEXA scan was completed for each participant at baseline and 6-months (27). The DEXA measurements were made with a Hologic scanner (Hologic 4500, Hologic Inc, Waltham, Mass) that was calibrated daily. The coefficient of variation for repeat assessment of body fat, lean mass, and BMD is less than 1%. All DEXA scans were evaluated by one radiologist blinded to the intervention group of the participant.
Food Frequency Questionnaire
All participants completed a 120-item validated food frequency questionnaire at baseline and 6-months (28). While participants were advised to maintain their current dietary habits, we measured their dietary habits in order to control for any changes in diet over the 6-month time period.
Blood Draws and Serological Assays
A fasting blood draw (≥ 12 hours) was performed on each study participant at baseline and 6 months. To reduce random or systematic variation in assay results, baseline and 6-month specimens collected from the same women were assayed simultaneously at the end of the study, and both intervention and control participants were included in each batch of assays. Samples were measured in duplicate to improve reliability. Quality control samples, measuring low and high ranges for each hormone, were included with each batch. Laboratory technicians were blinded to treatment assignment.
Insulin was measured in serum using an enzyme-linked immunosorbent assay (ELISA) kit (DSL-10-1600, Diagnostic Systems Laboratories, Inc., Webster, TX). The laboratory test was performed following the assay manual. The mean intra-assay coefficient of variation for the low and high range QC samples were 7.4% and 5.4%, respectively.
Commercial ELISA kits from Diagnostic Systems Laboratories, Inc (Webster, TX) were also used to measure plasma concentrations of total IGF-1 and IGFBP-3 following manufacturer instructions. For IGF-1, the mean intra-assay coefficient of variation was 2.8% for low values and 0.7% for high values. For IGFBP-3, the mean coefficients of variation for low and high range QC samples were 6.6% and 3.2%.
Exercise Intervention
The exercise intervention consisted of a combined supervised training program at a local health club and a home aerobic training program. Participants exercised at the health club during designated sessions three times per week and were instructed to exercise two days/week on their own, either at the health club or home. Participants were asked to perform three 15-minute sessions during Week 1, building to five 30-minute moderate-intensity sessions by Week 5, the current physical activity recommendation for adults (29). Participants wore heart rate monitors during each workout. Following each exercise session, participants recorded the type, duration, perceived intensity of activity, and average heart rate during exercise in physical activity logs. Participants returned logs to the exercise physiologists at the end of each week.
The intervention consisted primarily of walking, an activity preferred by breast cancer survivors (30), although participants could choose to meet the exercise goal through other forms of aerobic activity. Activities that did not involve sustained aerobic effort, such as resistance training and yoga, could be performed but did not count toward the exercise goal for each week.
Usual Care Group
Women in the usual care group were instructed to continue with their usual activities. If a participant wanted to exercise, she was told she could, but that our exercise program and training materials would not be offered to her until the end of the study. At the end of the study, women were offered three supervised training exercise sessions, a pedometer, exercise handouts, and results of clinical tests. All study participants also received quarterly newsletters that focused on issues related to breast cancer survivorship.
Randomization
After completion of all baseline measures, each participant was randomly assigned to either the exercise or usual care group using a random number generation.
Statistical Analyses
Data were analyzed using SAS version 9.1. Participants were grouped according to the intention-to-treat procedure in which all participants were grouped according to their intervention assignment at randomization; however, women with missing hormone values were excluded from analysis (n = 7). One woman’s 6-month insulin value was 224% greater than her baseline value (baseline insulin = 37.8 μU/mL, 6-month insulin = 122.5 μU/mL), and most likely associated with her recent chemotherapy-induced menopause. Therefore, this woman was excluded from the insulin analyses. We used t-tests and Chi-square analyses to assess between-group baseline differences. Intervention effects were evaluated by the differences in the mean changes at 6-months between the intervention and usual care groups using the generalized estimating equation modification to linear regression models to account for the longitudinal nature of the data. As the two study groups did not differ at baseline, analyses only adjusted for baseline insulin, IGF-I, or IGFBP-3 were performed. We also explored a priori effect modification by disease stage, hormone therapy, age, BMI, and change in body weight/fat. Lastly, for exercisers only, we examined changes in insulin and IGFs based upon adherence levels and change in pedometer steps to determine if a dose-response relationship exists.
RESULTS
Baseline Characteristics
Baseline demographic and physiologic data in the exercise and usual care groups were similar (Table 1). The average age of study participants was 56 yrs. The majority (84%) of participants were non-Hispanic white. Average time since diagnosis for participants was 3.3 yrs. Women were, on average, obese and physically inactive. Baseline levels of insulin, IGF-I and IGFBP-3 did not differ between the groups.
Table 1.
Baseline characteristics of randomized participants in the YES Study (N=68).*
| Exercisers Mean (SD) or % | Usual Care Mean (SD) or % | |
|---|---|---|
| N | 36 | 32 |
| Age (yrs) | 56.4 (9.5) | 55.6 (7.7) |
| Ethnicity | ||
| Non-Hispanic White | 83% | 90% |
| African-American | 17% | 7% |
| Asian/Pacific Islander | 0% | 3% |
| Education (%) | ||
| High school graduate | 17% | 19% |
| Some school after high school | 25% | 31% |
| College graduate + | 58% | 50% |
| Time since diagnosis (years) | 3.6 (2.2) | 3.3 (2.6) |
| Disease stage (%) | ||
| In Situ | 11% | 13% |
| Stage I | 56% | 25% |
| Stage II | 25% | 44% |
| Stage IIIA | 8% | 19% |
| Treatment (%) | ||
| None | 6% | 16% |
| Radiation only | 42% | 22% |
| Chemotherapy only | 19% | 22% |
| Radiation and chemotherapy | 33% | 41% |
| Hormone therapy | ||
| None | 42% | 28% |
| Tamoxifen | 31% | 25% |
| Aromatase Inhibitors | 28% | 47% |
| Weight (kg) | 81.0(16.8) | 79.3(21.3) |
| BMI (kg/m2) | 30.4(6.0) | 30.1(7.4) |
| Percent body fat (DEXA) | 41.3 (6.4) | 39.4 (5.9) |
| Physical Activity Questionnaire (min/wk recreational exercise) | 13.0 (24.0) | 12.0 (20.0) |
| Daily Activity Log (min/wk recreational exercise) | 30.0 (41.1) | 11.3 (24.8) |
| Pedometer steps/day | 5,083 (2,312) | 5,624 (2,744) |
No statistically significant differences between exercise and usual care groups at baseline.
Change in Physical Activity Levels and Adherence to the Exercise Intervention
Based on information reported in the 7-Day Physical Activity Log at baseline and 6-months, exercisers, on average, increased moderate- to vigorous-intensity recreational activity by 129 min/wk at 6-months (p < .001) compared to smaller increases among usual care participants (45 min/wk at 6 months) (data not shown). Exercisers also increased their pedometer steps/day, on average, by 1621 steps (0.9 of a mile per day) compared to 85 steps/day (0.05 of a mile per day) among usual care participants (data not shown).
Exercise group participants also completed physical activity logs each week of the intervention. They performed an average of 120 min/wk of moderate- to vigorous-intensity recreational activity over 6 months. A total of 73% were completing at least 80% of the exercise goal of 150 min/wk over 6 months (data not shown). Lastly, women randomized to exercise chose weight-bearing aerobic activities most often, with 82% walking. Few women reported doing resistance training (3%).
Effect of Exercise vs. Usual Care on Changes in Insulin and IGFs
We observed a 1.75 μU/mL (7.1%) reduction in insulin levels among exercisers and a 3.49 μU/mL (13.6%) increase among controls, resulting in a 20.7% between group difference over the course of the study, achieving borderline statistical significance (p=0.089) (Table 2). Significant differences between groups were observed for changes in both IGF-I and IGFBP-3. A 9% between group difference in IGF-I (p=0.026) was observed in exercisers compared to controls. IGF-I decreased by 7.36 ng/mL (3.4%) in the exercise group while controls experienced a 12.70 (5.5%) increase. Similarly, levels of IGFBP-3 decreased by 0.19 μg/mL (4.6%) in the intervention group versus a 0.15 μg/mL (3.3%) increase in the control group, resulting in an overall 7.9% between group difference (p=0.006).
Table 2.
Concentrations of insulin, IGF-I, and IGFBP-3 at baseline and 6 months for exercisers (n = 36) vs. usual care (n = 32) (Mean (SE))
| Baseline | 6 Months | Change over 6 Months1 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Exercisers | Usual Care | p-value | Exercisers | Usual Care | p-value | Exercisers | Usual Care | p-value | |
| Insulin (μU/mL) | 24.57 (3.85) | 25.69 (4.21) | 0.84 | 22.92 (3.25) | 31.98 (5.46) | 0.16 | −1.75 (2.32) | 3.49 (2.46) | 0.089 |
| IGF-1 (ng/mL) | 213.34 (12.57) | 232.34 (18.65) | 0.40 | 207.14 (11.20) | 243.73 (18.47) | 0.10 | −7.36 (6.02) | 12.70 (6.39) | 0.026 |
| IGFBP-3 (μg/mL) | 4.15 (0.16) | 4.48 (0.17) | 0.16 | 3.98 (0.16) | 4.61 (0.18) | 0.011 | −0.19 (0.08) | 0.15 (0.10) | 0.006 |
Adjusted for baseline value.
Effect of Exercise on Insulin and IGFs Stratified by Adherence
Table 3 presents the results of subgroup analyses examining adherence to the intervention among the exercise group only. Exercisers were stratified according to adherence (greater than vs. less than 120 min/week of recreational/sport activity assessed by7-Day PAL) and change in pedometer steps (greater than vs. less than one mile/day measured by pedometer). While a non-significant dose-response effect was observed, decreases in insulin and IGFs were observed for women exercising at lower and higher amounts.
Table 3.
Change over 6 months in insulin, IGF-I, and IGFBP-3 stratified by adherence and change in pedometer steps, exercisers only (Mean (SE))1
| Δ Insulin (μU/mL) | p-value | Δ IGF-1 (ng/mL) | p-value | Δ IGFBP-3 (μg/mL) | p-value | |
|---|---|---|---|---|---|---|
| Daily Activity Log over 6 months 2 | ||||||
| < 120 min/wk, N = 16 | −0.31(2.49) | 0.33 | −5.47 (8.24) | 0.91 | −0.16 (0.09) | 0.81 |
| ≥ 120 min/wk, N = 20 | −3.12 (2.13) | −6.79 (7.37) | −0.19 (0.08) | |||
| Change in Pedometer Steps 3 | ||||||
| < 1 mile/day, N=22 | −0.02 (1.98) | 0.20 | −4.29 (7.01) | 0.67 | −0.13 (0.07) | 0.38 |
| ≥ 1 mile/day, N=14 | −4.09 (2.43) | −9.20 (8.81) | −0.24 (0.09) | |||
Adjusted for baseline level.
Adherence was determined from daily activity logs kept for each week of the 6-month study. Women reported the type and duration of every moderate- to vigorous-intensity recreational activity performed daily with adherence defined as average min/wk.
Women wore pedomerters for seven consecutive days at baseline and 6-months. Average steps per day at baseline and 6-months, and the change in average steps per day between baseline and 6 months, were calculated. One mile = 1760 steps.
Effect of Exercise on Insulin and IGFs Stratified by Potential Effect Modifiers
Stratification by age, baseline BMI, change in body fat, disease stage, and hormone therapy did not modify the effect of exercise on insulin or IGFs (Table 4). However stronger effects of exercise on insulin and IGFs were observed among older women compared to younger women.
Table 4.
Stratified analysis of change in insulin, IGF-1 and IGFBP-3 ( Mean (SE))
| Δ Insulin | p-value | Δ IGF-1 | p-value | Δ IGFBP-3 | p-value | ||
|---|---|---|---|---|---|---|---|
| Age< 56 years | |||||||
| Exercisers, N=19 | 0.51 (3.41) | 0.50 | −0.64 (7.65) | 0.20 | −0.05 (0.08) | 0.24 | |
| Controls, N=15 | 4.06 (3.86) | 14.99 (8.66) | 0.11 (0.10) | ||||
| Age ≥ 56 years | |||||||
| Exercisers, N=17 | −4.51 (2.81) | 0.057 | −14.84 (9.33) | 0.064 | −0.36 (0.13) | 0.0047 | |
| Controls, N=17 | 3.38 (2.81) | 10.65 (9.33) | 0.20 (0.13) | ||||
| 0.53* | 0.60* | 0.22* | |||||
| BMI <30 kg/m2 | |||||||
| Exercisers, N=16 | 1.89 (4.60) | 0.57 | −12.81 (9.62) | 0.017 | −0.14 (0.12) | 0.0070 | |
| Controls, N=20 | 5.55 (4.06) | 19.70 (8.59) | 0.34 (0.11) | ||||
| BMI ≥30 kg/m2 | |||||||
| Exercisers, N=20 | −4.49 (2.06) | 0.18 | −2.49 (7.39) | 0.83 | −0.23 (0.10) | 0.78 | |
| Controls, N=12 | 0.23 (2.67) | 0.18 (9.55) | −0.18 (0.13) | ||||
| 0.40* | 0.098* | 0.062* | |||||
| No change or increased % body fat | |||||||
| Exercisers, N=12 | −3.36 (3.29) | 0.17 | −7.37 (7.10) | 0.0045 | −0.27 (0.09) | 0.0049 | |
| Controls, N=20 | 4.70 (4.59) | 30.11 (10.04) | 0.21 (0.13) | ||||
| Decreased % body fat | |||||||
| Exercisers, N=24 | −2.16 (3.30) | 0.59 | −5.37 (10.38) | 0.63 | −0.02 (015) | 0.53 | |
| Controls, N=12 | 2.19 (2.62) | 1.07 (8.03) | 0.10 (0.12) | ||||
| 0.25* | 0.11* | 0.16* | |||||
| Breast Cancer Stage 0 or 1 | |||||||
| Exercisers, N=24 | −3.17 (2.31) | 0.27 | 5.71 (6.88) | 0.30 | −0.19 (0.11) | 0.012 | |
| Controls, N=12 | 1.25 (3.21) | 18.34 (9.75) | 0.31 (0.15) | ||||
| Breast cancer stage 2 or 3 | |||||||
| Exercisers, N=12 | 2.65 (4.72) | 0.85 | −30.54 (10.56) | 0.0080 | −0.20 (0.13) | 0.16 | |
| Controls, N=20 | 3.83 (3.70) | 7.53 (8.18) | 0.04 (0.10) | ||||
| 0.34* | 0.15* | 0.37* | |||||
| Hormone Therapy | |||||||
| None | |||||||
| Exercisers, N=15 | −2.72 (2.54) | 0.16 | −7.78 (9.99) | 0.024 | −0.21 (0.14) | 0.0063 | |
| Controls, N=9 | 3.34 (3.28) | 34.20 (13.18) | 0.52 (0.18) | ||||
| Tamoxifen | |||||||
| Exercisers, N=11 | −5.85 (3.60) | 0.29 | 6.41 (9.53) | 0.80 | −0.25 (0.11) | 0.26 | |
| Controls, N=8 | 0.07 (4.02) | 2.58 (11.18) | −0.05 (0.13) | ||||
| Aromatase Inhibitors | |||||||
| Exercisers, N=10 | 4.83 (5.62) | 0.99 | −22.90 (10.42) | 0.044 | −0.14 (0.15) | 0.34 | |
| Controls, N=15 | 4.79 (4.72) | 5.88 (8.51) | 0.05 (0.12) | ||||
| 0.48* | 0.64* | 0.25* | |||||
P for interaction
DISCUSSION
In our study, moderate-intensity aerobic exercise, such as brisk walking, was associated with statistically significant decreases in IGF-I and IGFBP-3. Exercise was also associated with a borderline statistically significant decrease in insulin levels. If we assume the between-group difference in IGFs and insulin minimizes the adverse associations between IGFs, insulin and breast cancer risk/prognosis that have recently been reported (1–3), then the beneficial effect of exercise is comparable to that of many commonly used adjuvant chemotherapies (10). The approximate 9% between-group difference in IGF-I levels is clinically meaningful and roughly half the change that has been observed with a 20 mg/day dose of tamoxifen (31).
While three other studies have examined the effect of exercise on insulin (two of the studies also examined IGFs) in breast cancer survivors (11–13), only the study conducted by Ligibel and colleagues demonstrated a statistically significant decrease in insulin levels (11). Participants in their study were asked to participate in twice-weekly supervised strength training sessions and 90 min/wk of home-based aerobic exercise for 16 weeks. The intervention resulted in a significant 28% reduction in insulin levels; however, IGFs were not measured. Fairey and colleagues examined the impact of exercise on insulin and IGFs in breast cancer survivors randomized to a thrice-weekly supervised program of 30 min of stationary bicycling for 15 weeks (12). While participants in their study exercised for 98% of the prescribed exercise sessions, no significant differences between groups were observed for changes in insulin. However, significant differences between groups were observed for changes in IGF-I (−10.9%) and IGFBP-3 (−8.4%). Lastly, Schmitz and colleagues examined the impact of a twice-weekly resistance training program on insulin and IGFs (13). While participants were also highly adherent, there were no exercise effects on insulin, IGF-I or IGFBP-3. Given that Ligibel’s study and our study enrolled sedentary obese breast cancer survivors resulting in higher baseline insulin concentrations, compared to more active and leaner women enrolled in Fairey and Schmitz’s studies, resulting in lower baseline insulin concentrations, exercise may only have a beneficial effect among heavier, less active women, who also have higher insulin levels. A similar type and dose of weight-bearing aerobic exercise has been demonstrated to significantly lower insulin, but not IGF-I levels, in healthy, yet sedentary and obese, women (32). Lastly, our effect of exercise on insulin was stronger in older women. IGF-I and IGFBP-3 levels increase from birth until puberty, after which they continuously decline (33). Our finding of a stronger effect of exercise on insulin and IGFs in older women may partially be due to the natural decline in concentrations of these hormones with age. Larger, appropriately powered, studies are necessary to confirm the impact of aerobic and/or strength training exercise on insulin and IGF levels in breast cancer survivors. Special emphasis should be placed on the potential interaction of age and BMI.
An important finding of our study was the decrease in IGFBP-3 observed among exercisers. Comparison to the existing literature is difficult given the inconsistent results of previous studies. Although few studies have directly assessed breast cancer prognosis, a study by Goodwin and colleagues found that high levels of IGFBP-3 predicted distant recurrence of breast cancer in postmenopausal women (18). Tissue analysis also showed high levels of IGFBP-3 in breast tumors associated with poor prognosis (34). These results conflict, however, with earlier studies that reported a decreased risk of breast cancer associated with high levels of IGFBP-3 (35). Future studies need to examine the relationships among exercise, IGFBP-3, and breast cancer outcomes.
While we did not observe stronger effects of exercise on insulin or IGFs by weight or body fat loss, it has been proposed that changes in body fat mediate or modify the change in insulin and IGFs associated with increased physical activity. Our study was not appropriately powered to observe effects of exercise on insulin and IGFs stratified by weight/body fat change. Future studies need to address this critical question as to whether exercise has independent effects on insulin and IGFs or is mediated by changes in body fat. Several possible mechanisms may explain exercise-induced decreases in insulin and IGFs independent of changes in body fat including increased post-receptor insulin signaling, increased glucose transporter protein and mRNA, decreased release and increased clearance of free fatty acids, increased muscle glucose delivery, and changes in muscle composition favoring increased glucose disposal (36). Given that high insulin levels promote the synthesis and activity of IGF-I via increases in insulin-mediated changes in IGFBP-3 concentrations, decreases in insulin would favorably influence IGF levels.
The YES study improves upon prior research in several ways. In order to observe a maximal effect from the exercise intervention, physically inactive women were recruited for this study. The supervised exercise program allowed for improved participant education on how to exercise safely and at the appropriate intensity. Importantly, it also provided the ability to monitor adherence to the intervention and reduced potential bias by self-report. Other strengths of the YES Study include a population-based recruitment strategy, randomization to study groups, a physical activity prescription that met national guidelines, and long study duration. Our sample size is comparable to the earlier studies in breast cancer survivors; however it is still small in absolute terms, with implications for compromised statistical power overall and with regard to some of our stratified analyses. Additional limitations of the YES Study include a single hormone measurement used for the baseline and 6 month assessments, which may have resulted in misclassification of some participants. Also, as baseline and 6 month hormone specimens were assayed together to reduce variability, only those women with both samples were included in the analysis, resulting in the exclusion of seven women. Lastly, our sample was highly-educated and mostly non-Hispanic white. However, the 75 women enrolled in the study did not differ with regard to age, race, or educational status from the non-participants that comprised the majority of women diagnosed with breast cancer at Yale-New Haven Hospital in 2004 and 2005.
CONCLUSIONS
As evidence accumulates for an association between high levels of insulin, and potentially IGF-I and IGFBP-3, and breast cancer risk and/or death, it becomes increasingly important to identify modifiable factors that decrease insulin and IGF levels. The responsiveness of insulin and IGFs to lifestyle changes is key to novel strategies for improving prognosis. Physical activity is a modifiable behavior with a multitude of health benefits, including most recently a favorable association with breast cancer survival (7–9). The results of the YES Study demonstrate that the recommended level of moderate-intensity aerobic exercise, 30 minutes on 5 days/week, is well tolerated in breast cancer survivors and efficacious in decreasing levels of IGF-I and IGFBP-3. Repetition of this trial with a larger sample size is necessary to confirm this finding. Furthermore, given the four studies, including our own, that have examined the effects of exercise on insulin levels in breast cancer survivors have all been small-scale trials (< 100 participants), a larger-scale trial is necessary. Such a trial would help determine whether exercise after a diagnosis of breast cancer has clinically meaningful effects on insulin and other biological mechanisms that may mediate the observed association between physical activity and survival.
Acknowledgments
We thank Christian Stoddard, Linda Saucier, Eileen Mierzejewski, Rebecca Latka and Mary O’Neil for their assistance. We are indebted to the participants in the Yale Exercise and Survivorship Study for their dedication.
GRANT SUPPORT: American Cancer Society (MRSG-04-006-01-CPPB) and the Susan G. Komen Breast Cancer Foundation (BCTR0201916). Supported in part by a General Clinical Research Center grant from the National Center of Research Resources, National Institutes of Health (Grant # M01-RR00125) awarded to Yale University School of Medicine
References
- 1.Goodwin P, Ennis M, Pritchard K, et al. Fasting insulin and outcome in early-stage breast cancer: Results of a prospective cohort study. J of Clin Oncol. 2002;20(1):42–51. doi: 10.1200/JCO.2002.20.1.42. [DOI] [PubMed] [Google Scholar]
- 2.Pollak MN, Chapman JW, Shepherd L, et al. Insulin resistance, estimated by serum C-peptide level, is associated with reduced event-free survival for postmenopausal women in NCIC CTG MA.14 adjuvant breast cancer trial. J Clin Oncol. 2006;24(suppl 18S):9s. abstr 524. [Google Scholar]
- 3.Irwin ML, Ballard-Barbash R, McTiernan A, et al. Fasting C-peptide levels and breast cancer death. American Association of Cancer Research Frontiers in Cancer Prevention Meeting; December 2007; Abstract B99. [Google Scholar]
- 4.Irwin ML, McTiernan A, Bernstein L, et al. Relationship of obesity and physical activity with c-peptide, leptin, and insulin-like growth factors in breast cancer survivors. Cancer Epidemiol Biomarkers Prev. 2005;14(12):2881–8. doi: 10.1158/1055-9965.EPI-05-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abrahamson PE, Gammon MD, Lund MJ, et al. General and abdominal obesity and survival among young women with breast cancer. Cancer Epidemiol Biomarkers Prev. 2006;15(10):1871–7. doi: 10.1158/1055-9965.EPI-06-0356. [DOI] [PubMed] [Google Scholar]
- 6.Chlebowski R, Aiello E, McTiernan A. Weight loss in breast cancer patient management. J Clin Oncol. 2002;20(4):1128–43. doi: 10.1200/JCO.2002.20.4.1128. [DOI] [PubMed] [Google Scholar]
- 7.Holmes MD, Chen WY, Feskanich D, et al. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293(20):2479–86. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- 8.Holick CN, Newcomb PA, Trentham-Dietz A, et al. Physical activity and survival after diagnosis of invasive breast cancer. Cancer Epidemiol Biomarkers Prev. 2008 Feb;17(2):379–86. doi: 10.1158/1055-9965.EPI-07-0771. Epub 2008 Feb 4. [DOI] [PubMed] [Google Scholar]
- 9.Irwin ML, Smith A, McTiernan A, et al. Association between pre- and post-diagnosis physical activity and mortality in breast cancer survivors. J Clin Oncol. 2008 Aug 20;26(24):3958–64. doi: 10.1200/JCO.2007.15.9822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodwin PJ. Insulin in the adjuvant breast cancer setting: a novel therapeutic target for lifestyle and pharmacologic interventions? J Clin Oncol. 2008;26(6):833–34. doi: 10.1200/JCO.2007.14.7132. [DOI] [PubMed] [Google Scholar]
- 11.Ligibel JA, Campbell N, Partridge A, et al. Impact of a mixed strength and endurance exercise intervention on insulin levels in breast cancer survivors. J Clin Oncol. 2008;26(6):907–12. doi: 10.1200/JCO.2007.12.7357. [DOI] [PubMed] [Google Scholar]
- 12.Fairey AS, Courneya KS, Field CJ, Bell GJ, Jones LW, Mackey JR. Effects of exercise training on fasting insulin, insulin resistance, insulin-like growth factors, and insulin-like growth factor binding proteins in postmenopausal breast cancer survivors: a randomized controlled trial. Cancer Epidemiol Biomarkers Prev. 2003;12:721–727. [PubMed] [Google Scholar]
- 13.Schmitz K, Rehana Ahmed, Hannan P, Yee D. Safety and efficacy of weight training in recent breast cancer survivors to alter body composition, insulin, and insulin-like growth factor axis proteins. Cancer Epidemiol Biomarkers Prev. 2005;14(7):1672–80. doi: 10.1158/1055-9965.EPI-04-0736. [DOI] [PubMed] [Google Scholar]
- 14.Yu H, Rohan T. Role of the insulin-like growth factor family in cancer development and progression. Journal of Natl Cancer Inst. 2000;92 (18):1472–89. doi: 10.1093/jnci/92.18.1472. [DOI] [PubMed] [Google Scholar]
- 15.Werner H, Weinstein D, Bentov I. Similarities and differences between insulin and IGF-I: Structures, receptors, and signaling pathways. Arch Physiol Biochem. 2008;114(1):17–22. doi: 10.1080/13813450801900694. [DOI] [PubMed] [Google Scholar]
- 16.Hankinson SE, Willett WC, Colditz GA, Hunter DJ, Michaud DS, Deroo B, et al. Circulating concentrations of insulin-like growth factor-1 and risk of breast cancer. Lancet. 1998;351:1393–1396. doi: 10.1016/S0140-6736(97)10384-1. [DOI] [PubMed] [Google Scholar]
- 17.Vadgama JV, Wu Y, Datta G, Khan H, Chillar R. Plasma insulin-like growth factor-I and serum IGF-binding protein 3 can be associated with the progression of breast cancer, and predict the risk of recurrence and the probability of survival in African-American and Hispanic women. Oncology. 1999;57(4):330–40. doi: 10.1159/000012052. [DOI] [PubMed] [Google Scholar]
- 18.Goodwin PJ, Ennis M, Pritchard KI, Trudeau ME, Koo J, Hartwick W, Hoffman B, Hood N. Insulin-like growth factor binding proteins 1 and 3 and breast cancer outcomes. Breast Cancer Research Treat. 2002;74:65–76. doi: 10.1023/a:1016075709022. [DOI] [PubMed] [Google Scholar]
- 19.McTiernan A, Sorensen B, Yasui Y, Tworoger SS, Ulrich CM, Irwin ML, Rudolph RE, Stanczyk FZ, Schwartz RS, Potter JD. No effect of exercise on insulin-like growth factor 1 and insulin-like growth factor binding protein 3 in postmenopausal women: a 12-month randomized controlled trial. Cancer Epidemiol Biomarkers Prev. 2005;14:1020–1021. doi: 10.1158/1055-9965.EPI-04-0834. [DOI] [PubMed] [Google Scholar]
- 20.Orenstein MR, Friedenreich CM. Review of physical activity and the IGF family. Journal of Physical Activity and Health. 2004 [Google Scholar]
- 21.Irwin ML, Alvarez-Reeves M, Cadmus L, et al. Recruiting and retaining breast cancer survivors into a randomized controlled exercise trial: The Yale Exercise and Survivorship Study. Cancer. 2008;112(S11):2593–2606. doi: 10.1002/cncr.23446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Irwin ML, Alvarez-Reeves M, Cadmus L, et al. Effect of exercise on body fat, lean mass, and bone mineral density in breast cancer survivors. Obesity. doi: 10.1038/oby.2009.18. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kriska A, Knowler W, LaPorte R, et al. Development of a questionnaire to examine relationship of physical activity and diabetes in Pima Indians. Diabetes Care. 1990;13:401–411. doi: 10.2337/diacare.13.4.401. [DOI] [PubMed] [Google Scholar]
- 24.Irwin ML, Tworoger SS, Yasui Y, et al. Influence of demographic, physiologic, and psychosocial variables on adherence to a yearlong moderate-intensity exercise trial in postmenopausal women. Prev Med. 2004 Dec;39(6):1080–6. doi: 10.1016/j.ypmed.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 25.Bassett D, Ainsworth B, Leggett S, et al. Accuracy of five electronic pedometers for measuring distance walked. Med Sci Sport Exer. 1996;28:1071–7. doi: 10.1097/00005768-199608000-00019. [DOI] [PubMed] [Google Scholar]
- 26.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sport Exer. 2000;32(9 Suppl):S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 27.Van Loan MD, Mayclin PL. Body composition assessment: Dual energy x-ray absorptiometry for total body and regional bone mineral and soft tissue composition. Eur Journal of Clinical Nutrition. 1992;46:174–178. [PubMed] [Google Scholar]
- 28.Patterson Re, Kristal A, Tinker L, et al. Measurement characteristics of the Women’s Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9(3):178–87. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 29.Pate R, Ettinger W, Heath GW, King AC, Kriska A, Leon AS, Marcus BH, Morris J, Paffenbarger RS, Patrick K, Pollock ML, Rippes JM, Sallis J, Wilmore JH. Physical activity and public health: A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA. 1995;273:402–407. doi: 10.1001/jama.273.5.402. [DOI] [PubMed] [Google Scholar]
- 30.Irwin ML, McTiernan A, Bernstein L, et al. Physical activity levels among breast cancer survivors. Med Sci Sports Exerc. 2004;36(9):1484–91. [PMC free article] [PubMed] [Google Scholar]
- 31.Bonanni B, Johansson H, Gandini S, et al. Effect of low dose tamoxifen on the IGF system in healthy women. Breast Cancer Res Treat. 2001;69:21–27. doi: 10.1023/a:1012241505717. [DOI] [PubMed] [Google Scholar]
- 32.Frank LL, Sorensen BE, Yasui Y, Tworoger SS, Schwartz RS, Ulrich CM, Irwin ML, Rudolph RE, Rajan KB, Stanczyk F, Bowen D, Weigle DS, Potter JD, McTiernan A. Effects of exercise on metabolic risk variables in overweight postmenopausal women: a randomized clinical trial. Obesity Research. 2005;13:615–625. doi: 10.1038/oby.2005.66. [DOI] [PubMed] [Google Scholar]
- 33.Gomez JM. Serum leptin, insulin-like growth factor-I components and sex hormone binding globulin. Relationship with sex, age and body composition in healthy population. Protein Pept Lett. 2007;14(7):708–11. doi: 10.2174/092986607781483868. [DOI] [PubMed] [Google Scholar]
- 34.Yu H, Levesque MA, Khosravi MJ, et al. Insulin-like growth factor-binding protein-3 and breast cancer survival. Int J Cancer. 1998 Dec 18;79(6):624–8. doi: 10.1002/(sici)1097-0215(19981218)79:6<624::aid-ijc12>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 35.Allen NE, Roddam AW, Allen DS, et al. A prospective study of serum insulin-like growth factor-I, IGF-II, IGFBP-3 and breast cancer risk. Br J Cancer. 2005;92(7):1283–7. doi: 10.1038/sj.bjc.6602471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goodyear L, Kahn B. Exercise, glucose transport, and insulin sensitivity. Ann Rev Med. 1998;49:235–61. doi: 10.1146/annurev.med.49.1.235. [DOI] [PubMed] [Google Scholar]