Abstract
The purpose of the present study was to formulate effective and controlled release albendazole liposomal formulations. Albendazole, a hydrophobic drug used for the treatment of hydatid cysts, was encapsulated in nanosize liposomes. Rapid evaporation method was used for the preparation of albendazole-encapsulated conventional and PEGylated liposomes consisting of egg phosphatidylcholine (PC) and cholesterol (CH) in the molar ratios of (6:4) and PC:CH: polyethylene glycol (PEG) (5:4:1), respectively. In this study, PEGylated and conventional liposomes containing albendazole were prepared and their characteristics, such as particle size, encapsulation efficiency, and in vitro drug release were investigated. The drug encapsulation efficiency of PEGylated and conventional liposomes was 81% and 72%, respectively. The biophysical characterization of both conventional and PEG-coated liposomes were done by transmission electron microscopy and UV-vis spectrophotometry. Efforts were made to study in vitro release of albendazole. The drug release rate showed decrease in albendazole release in descending order: free albendazole, albendazole-loaded conventional liposomes, and least with albendazole-loaded PEG-liposomes. Biologically relevant vesicles were prepared and in vitro release of liposome-entrapped albendazole was determined.
Keywords: albendazole, PEGylated, in vitro release, liposomes, nanosize
Introduction
Liposomes are vesicles of varying size consisting of a spherical lipid bilayer and an aqueous inner compartment that are generated in vitro. In 1965, Bangham and colleagues first used a liposomal structure as a model to study the effect of narcotics on lipid bilayer membranes.1 These are useful in terms of biocompatibility, biodegradability, and low toxicity, and can control biodistribution by changing the size, lipid composition, and physical characteristics.2–4 Furthermore, liposomes can entrap both hydrophobic and hydrophilic drugs and are able to continuously release the entrapped substrate,5 thus being useful drug carriers.6–8 Polyethylene glycol (PEG) modification on the liposomal surface is known to be effective in preventing their uptake by the reticuloendothelial system (RES). Incorporation of PEG-lipids causes the liposome to remain in the blood circulation for extended periods of time (ie, t½ > 40 hours) and distribute through an organism relatively evenly with most of the dose remaining in the central compartment (ie, the blood) and only 10% to 15% of the dose being delivered to the liver.9–11 Long-circulating, PEGylated liposomes provide an attractive platform to improve the therapeutic index of a variety of drugs. A number of drugs have already been successfully encapsulated in liposomes, from antibacterials12 and interferons13 to antitumor drugs such as doxorubicin.14
Albendazole (ABZ), methyl [5-(propylthio)-1-H-benzimidazol-2yl] carbamate, is a benzimidazol derivative and it is effective in the treatment of echinococcosis, hydatid cysts, and neurocysticercosis. The therapeutic response of albendazole (20% to 50%) in cases of echinococcosis is variable and difficult to predict.15,16 Albendazole’s poor intestinal absorption (<5%) (due to its low aqueous solubility) is probably a major determinant of the variable response rate.17 In vitro and in vivo antifungal activity of albendazole was studied by Hardin and colleagues.18 Albenza® (albendazole) is an orally administered broad-spectrum anthelmintic and has been used for hydatid disease with promising results.19–21 Repeated dose therapy is the only treatment for this disease and a course of albendazole for prescribed period has to be completed by the patients. Therefore, preparation of drug formulations that facilitate controlled release of the drug to the target site is an important goal.
The objective of the present study was to prepare albendazole-encapsulated liposomal formulations. A comparison study was performed between conventional and PEG-coated or stealth liposomes to evaluate the in vitro performance of these formulations. Characterization of the prepared liposomes regarding physical morphology, particle size, and in vitro drug release was performed. A stability study was performed to investigate the release of drug from liposomes during storage.
Materials and methods
Materials
Albendazole was purchased from Cipla Pharma Co. (Mumbai, India). Egg phosphatidylcholine (PC), cholesterol (CH), and PEG were purchased from Sigma-Aldrich (St. Louis, MO, USA). All the chemicals and solvents used in the study were of analytical and high-pressure liquid chromatography (HPLC) grade and purchased from Sigma Chemicals Co. (St. Louis, MO, USA), Himedia Laboratories Ltd. (Mumbai, India), Bangalore Genei (Bengalaru, India), and deionized water was used throughout the experiments.
Methods
Preparation of conventional and stealth liposomes
The PC liposomes were prepared using a rapid evaporation method.22 The composition of the conventional liposomes prepared was albendazole-encapsulated PC:CH taken in the ratio of 6:4 and dissolved in a chloroform:methanol mixture (1:1, V/V). The PEG-liposomes were prepared by mixing albendazole-encapsulated PC:CH:PEG-PC in the ratio of 5:4:1. Lipids were dissolved in 50 mL of isopropyl methanol:chloroform solution taken in the ratio of 1:1. Albendazole was dissolved in the same solvent mixture and added to the lipid solution. The mixture was sonicated for four minutes in a bath-type sonicator (Soniprep 150; MSE, Van Nuys, CA, USA). The organic solvent was then removed using rotary evaporator at 40°C and 50 rpm (Metrex, Delhi, India). In the case of albendazole, the resulting thin lipid film was slowly hydrated using bicarbonate buffer (pH 9.0). The process of hydration was carried out by rotation at a low speed at 55°C in a rotary evaporator under atmospheric pressure followed by hand shaking for 15 minutes at 55°C in a thermostatically controlled water bath. The resulting liposomal dispersion was left to mature overnight at 4°C. All the above steps were performed under aseptic conditions. All glassware was sterilized by autoclaving, and the entire procedure was performed in a laminar flow hood (Lab Companion; JEIO Tech, Seoul, Korea).
Free drug separation
Free unentrapped drug was separated from albendazole-encapsulated conventional and stealth liposomes by centrifugation at 20,000 g for 1 hour at 4°C in a refrigerated centrifuge (model 3–18 K; Sigma-Aldrich). The pellets formed were washed twice with 10 mL bicarbonate buffer (pH 9.0) and recentrifuged again for 1 hour.
Determination of encapsulation efficiency
The percentage of drug encapsulated was determined after lysis of the prepared liposomes with absolute alcohol and sonication for 10 minutes.23 The concentration of albendazole in absolute alcohol was determined spectrophotometrically at 265 nm using a UV-visible spectrophotometer (model UV-1700 (E); Schimadzu, Kyoto, Japan) in triplicate. The encapsulation efficiency expressed as entrapment percentage was calculated through the following relationship:24
Characterization of albendazole iposomes
Liposomes vary in size from 20 nm to several μm. Using a transmission electron microscope, size of conventional and PEG-coated liposomes were determined. The liposomes were photographed at an original magnification ×1000 to ×25000, using a transmission electron microscope (TEM; JEOL, Tokyo, Japan).
Stability study
The stability of the albendazole-encapsulated PEG-liposomes was evaluated after storage at −20°C, +4°C and 25°C for three months. The particle size distribution and drug encapsulation efficiency of the samples were determined as a function of the storage time.
In vitro drug release studies
Drug release from liposomes was studied using a dialysis method. Dialysis bags were soaked before use in distilled water at room temperature for 12 hours to remove the preservative, followed by rinsing thoroughly in distilled water. In vitro release of albendazole from liposomes was conducted by dialysis in a dialysis sac (12,000 MW cut off; Sigma-Aldrich) with 150 mL of phosphate-buffered saline (PBS; pH 5.6) containing 7% (V/V) propylene glycol and 25% (V/V) methanol at 37°C following the method published previously.25 Three sacs were prepared containing free albendazole as control, albendazole in conventional liposomes, and albendazole in PEGcoated liposomes.
Liposomal concentrate (equivalent to 2 mg albendazole) dispersed in 1 mL of bicarbonate buffer (pH 9) was placed in a dialysis bag. Control bags were prepared and tested along with the liposomal dispersions. Each control bag contained 2 mg albendazole. Two ends of the dialysis sac were tightly bound with threads. The sac was hung inside a conical flask with the help of a glass rod so that the portion of the dialysis sac with the formulation dipped into the buffer solution. The flask was kept on a magnetic stirrer (Matrex) and stirring was maintained at 100 rpm at 37°C with a thermostatic control. Samples were collected every half an hour over a period of five hours and assayed spectrophotometrically for drug content at 295.7 nm.
Results and discussion
Albendazole-encapsulated liposomes
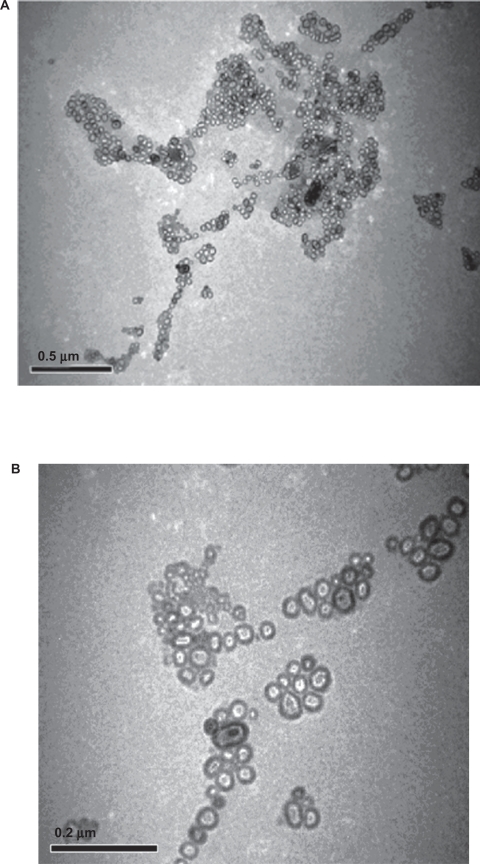
Drug-encapsulated PEG-liposomes were prepared by the combination of PC:CH:PEG-PC in the ratio of 5:4:1. On the other hand, conventional liposomes were prepared by mixing PC:CH in the ratio of 6:4 and lipids were dissolved in methanol:chloroform solution taken in the ratio (1:1 V/V). TEM micrograph was taken at 20,000 and 25,000 magnifications and clearly showed the formation of liposomes of moderate sizes ranging 50 to 150 nm. (Figures 1a and 1b). Most of the liposomes formed appeared spherical or slightly asymmetrical in shape and were mainly unilamellar in arrangement.
Figure 1.
Transmission electron microscopic photograph of liposome A) Albendazole-loaded non-PEGylated or conventional liposomes and B) Albendazole-loaded PEGylated liposomes.
Encapsulation efficiency
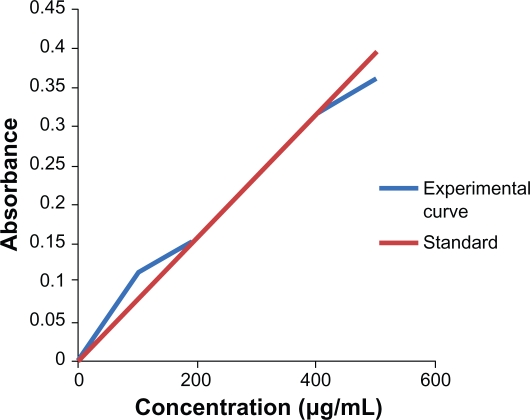
The absorbance at different concentration of albendazole was measured using a UV-vis spectrophotometer at 295.7 nm. The standard curve of the albendazole was made by plotting absorbance against the concentration (Figure 2). The absorbance is no longer linear at concentration 10–200 μg/mL and 400–500 μg/mL. The concentration of the nonencapsulated drug in the supernatant was determined using this curve. Consequently, the encapsulation efficiency of different types of liposomes entrapping albendazole was easily calculated. The encapsulation efficacy was obtained as the mass ratio between the amount of the drug incorporated in liposomes and this ratio was used in the liposome preparation. By inspection of Table 1, it is obvious that albendazole-encapsulation efficiency had higher values in cases of PEGylated liposomes than in conventional liposomes. The total drug used during the preparation of liposomes was 1 mL of 1 mM albendazole. So using the formula the encapsulation efficiency of non-PEGylated and albendazole-loaded PEGylated liposomes was found to be 72% and 81%, respectively.
Figure 2.
The standard curve of Albendazole drug at 295.7 nm.
Table 1.
Encapsulation efficiency of liposomes
| S no | Type of liposomes | Liposomal formulation composition (molar ratio) | Liposomal formulation charge | Encapsulation efficiency |
|---|---|---|---|---|
| 1 | Conventional liposomes | PC:CH (6:4) PC:CH (7:2) |
Neutral Neutral |
72% 50% |
| 2 | PEG-liposomes | PC:CH:PEG-PC (5:4:1) PC:CH:PEG-PC (6:2:1) |
Neutral Neutral |
81% 65% |
Abbreviations: PC, phosphatidylcholine; CH, cholesterol; PEG, polyethylene glycol.
The highest encapsulation efficiency (81%) was observed with the PEGylated liposomes composed of PC:CH:PEG-PC (5:4:1) molar ratio. Liposomes prepared with PC:CH (7:2) molar ratio, showed the lowest encapsulation efficiency and were excluded from further studies.
Absorbance at various concentrations of albendazole was measured using a UV-vis spectrophotometer at 295.7 nm. The standard curve for each drug release was established using the Beer–Lambert equation, which correlates the extinction coefficient (ɛ) of a substance with the absorbance (A): A = ɛ.c.b where: ɛ is extinction coefficient; c is concentration of solution (mol/L); b is thickness (cm).
Stability study
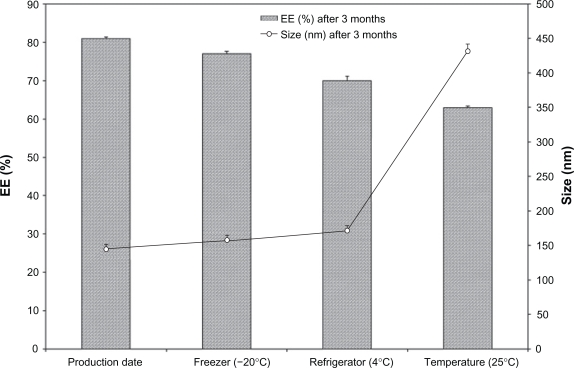
PEGylated liposomes were selected and their physical and chemical stability was evaluated at three different temperatures for 3 months (Table 2). Initially, the mean vesicle size was 150 nm and encapsulation efficiency was 81%, no significant changes in drug encapsulation efficiency was observed during the course of stability study for formulations stored at −20°C and 4°C (P > 0.05) but there was a significant decrease in albendazole encapsulation efficiency for liposomes stored at room temperature (P < 0.05). The mean vesicle size showed an increase at all storage temperatures (P < 0.05) while particle size of the formulations was increased up to fourfold after three months at room temperature. This extraordinary increase in the particle size of liposomes may be due to the aggregation or swelling of liposomes. Further experiments are required to explain this phenomenon. Results of the stability study are shown in (Figure 3).
Table 2.
Particle size of albendazole-loaded conventional and PEGylated liposomes mean size (nm)
| Liposomal formulation composition (molar ratio) | Liposomal formulation charge | Freshly prepared | 4°C |
After three months |
|
|---|---|---|---|---|---|
| −20°C | Room temperature | ||||
| PC:CH (6:4) | Neutral | 153 ± 6.4 | 160 ± 8.7 | 173 ± 9.5 | 425 ± 19.69 |
| PC:CH (7:2) | Neutral | 141 ± 5.2 | 155 ± 7.1 | 160 ± 7.9 | 397 ± 17.2 |
| PC:CH:PEG-PC (5:4:1) | Neutral | 145 ± 5.5 | 157 ± 7.6 | 171 ± 9.3 | 432 ± 20.35 |
| PC:CH:PEG-PC (6:2:1) | Neutral | 162 ± 8.5 | 176 ± 9.4 | 184 ± 10.3 | 450 ± 25.1 |
Abbreviations: PC, phosphatidylcholine; CH, cholesterol; PEG, polyethylene glycol.
Figure 3.
Albendazole encapsulated PEGylated liposomes stability after 3 months storage at three different temperatures.
Abbreviation: EE, encapsulation efficiency.
In vitro release studies
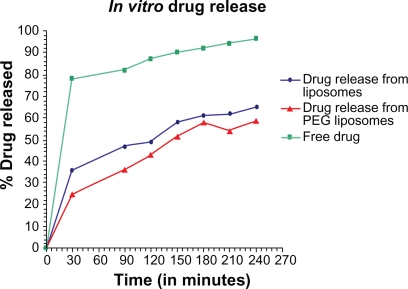
Evaluation of in vitro drug release from encapsulated liposome was done by dialysis method. The output obtained by the dialysis method provided a correlation with the in vivo release.26 The in vitro release behavior of the free albendazole, albendazole-loaded conventional liposomes, and PEGylated liposomes, is summarized in the cumulative percentage release shown in Figure 4. For the measurement of release rate of the free albendazole, and from the liposomes both PEG coated and uncoated, was measured at 37°C in PBS containing 7% (V/V) PEG and 25% (V/V) methanol. Over a period of five hours the measurement was taken after every half an hour at 295.7 nm. The value of concentration corresponding to the absorbance was calculated from the albendazole standard curve. The drug release rate of conventional liposomes and PEGylated liposomes or stealth liposomes is often comparable.
Figure 4.
In vitro release curve of albendazole -loaded non-PEGylated, PEGylated liposomes and free drug in phosphate buffer at 37°C.
The release rate of the drug was highest for the free drug while the rate of release from PEG liposomes was less than that of conventional liposomes. It seems that by adding PEG to the liposomes, the release rate of drug from the liposomes may decrease in its values. This confirms the fact that PEG acts as a barrier against diffusion of hydrophilic drugs. The hydrophobic long alkyl chains of the polymer may act as a barrier and the drug was effectively entrapped in the polymers.
Discussion
Among a variety of targeted drug carrier systems, liposomes have been studied extensively because of their capability to accommodate a large variety of drugs, alongside their good biocompatibility, low toxicity, and lack of immune system activation or suppression.27
The present study was designed to develop and compare albendazole containing nanovesicular conventional and PEGylated liposomes. Comparing the results of both conventional and PEGylated liposomal formulations of the same composition and molar ratio, it is obvious that PEGylated liposomes showed a more sustained action owing to the presence of PEGcoating on the surface, which release the drug slowly over a prolonged period of time. It is to be noted that the in vitro release results are consistent with those of the encapsulation efficiency, as the PEGylated liposomes with the highest cholesterol content PC:CH:PEG-PC (5:4:1) molar ratio and the highest encapsulation efficiency (ie, low leakage ability) showed the lowest drug release percentage. There was no significant difference between stability results of the liposome storage at 4°C or −20°C. The results of the stability study revealed that the prepared liposomes are stable for more than three months’ storage at 4°C or −20°C. This extraordinary increase in the particle size of liposomes at room temperature may be due to the aggregation or swelling of liposomes
As a rule of thumb, the drug concentration in the sink phase in release experiments should be kept below 10% saturation. If the drug is poorly soluble in water, nonaqueous solvents or solubilizing agents may be added to the sink. In case of albendazole-encapsulated liposomes, a release medium containing 7% (V/V) PEG and 25% (V/V) methanol was employed to provide sink conditions. The reproducibility and efficacy of the release study were ensured through a control sample containing drug in the free form. This could ensure that the dialysis membrane was not a barrier throughout the release study. The free albendazole (control sample) was released in about two hours.
The in vitro release behavior of free albendazole, albendazole-loaded conventional liposomes and PEGylated liposomes was summarized. In case of free albendazole more than 80% drug was released within the first sampling time (30 minutes) while both liposomal formulations produced an initial slower effect in which albendazole release was more than 25% for PEGylated and 35% for non-PEGylated liposomes within the first sampling time (30 minutes). The literature reports that drug release profiles from liposomes characteristically show an initial fast drug loss followed by slower rates of drug loss.28,29 The initial fast rate of release is commonly ascribed to drug detachment from liposomal surface while the later slow release results from sustained drug release from the inner lamellae. Albendazole is mainly associated within the bilayer lipid structure of the liposomes. The in vitro release study of albendazole showed no burst effect so that the drug transport out of the liposomes was driven mainly by a diffusion-controlled mechanism. The slight deviation at 210 minutes seems to be the effect of agglomeration of more PEG over liposome surface after a particular time interval which reshuffles soon it self due to intermolecular interaction with the surrounding environment.
Compared to the control data, albendazole release from PEGylated liposomes is prolonged. The slower release in PEGylated liposomes could be because of the fast hydration process at presenting the PEG on the surface of the particles. This result suggests that it takes time for albendazole to be released once encapsulated in the liposomes because lipid bilayer is stabilized by cholesterol. Thus a depot effect could be achieved using liposomes, especially in the PEGylated liposomal formulation. The above results, which suggest that the drug would be stable in the blood circulation and would be released slowly at the target site, are indications that our PEGylated liposomal formulation meets the requirements for an effective drug delivery system.30 Also the drug release data confirmed the drug entrapment efficiency results determined.
Conclusion
Evaluation of in the vitro release profile of hydrophobic drugs from liposomal formulations could be problematic. But this could be manipulated through employment of a proper release medium that could provide sufficient sink conditions without affecting the stability of the liposomal formulation. The results suggest that albendazole released for a prolonged period of time from the PEGylated liposomal formulations. Since they had nanodimensions, longer residence time in systemic circulation could help them reaching the target tissues. Nanosized PEGylated liposomes, would be a promising delivery systems for albendazole in the treatment of hydatid cyst. However, further studies including in vivo experiments are warranted.
Acknowledgments
The authors thank the technical support provided by State Biotechnology Programme, Biotech Bhawan, Haldi-263146, Uttarakhand. The authors also thank the Directorate of Research, G.B. Pant University of Agriculture and Technology, Pantnagar for providing laboratory facilities and Dr Rajendra Dobhal, Advisor, State Biotechnology Programme, Biotech Bhawan, Haldi-263146, Uttarakhand. The authors report no conflicts of interest in this work.
References
- 1.Bangham AD, Standish MM, Miller N. Cation permeability of phospholipid model membranes: effect of narcotics. Nature. 1965;208:1295–1297. doi: 10.1038/2081295a0. [DOI] [PubMed] [Google Scholar]
- 2.Anabousi S, Laue M, Lehr CM, Bakowsky U, Ehrhardt C. Assessing transferrin modification of liposomes by atomic force microscopy and transmission electron microscopy. Eur J Pharm Biopharm. 2005;60:295–303. doi: 10.1016/j.ejpb.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 3.Abraham SA, Waterhouse DN, Mayer LD, Cullis PR, Madden TD, Bally MB. The liposomal formulation of doxorubicin. Methods Enzymol. 2005;391:71–97. doi: 10.1016/S0076-6879(05)91004-5. [DOI] [PubMed] [Google Scholar]
- 4.Vyas SP, Quraishi S, Gupta S, Jaganathan KS. Aerosolized liposome-based delivery of amphotericin B to alveolar macrophages. Int J Pharm. 2005;296:2–25. doi: 10.1016/j.ijpharm.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Iinuma H, Maruyama K, Okinaga K, et al. Intracellular targeting therapy of cisplatin-encapsulated transferring-polyethyleneglycol liposome on peritoneal dissemination of gastric cancer. Int J Cancer. 2002;99:130–137. doi: 10.1002/ijc.10242. [DOI] [PubMed] [Google Scholar]
- 6.Hong RJ, Tseng YL. Phase I and pharmacokinetic study of a stable, polyethylene-glycolated liposomal doxorubicin in partients with solid tumors. Cancer. 2001;91:1826–1833. [PubMed] [Google Scholar]
- 7.Oku N, Doi K, Namba Y, Okada S. Therapeutic effect of adriamycin encapsulated in long-circulating liposomes on Meth-A-sarcoma-bearing mice. Int J Cancer. 1994;58:415–419. doi: 10.1002/ijc.2910580318. [DOI] [PubMed] [Google Scholar]
- 8.Tokudome Y, Oku N, Doi K, Namba Y, Okada S. Antitumor activity of vincristin encapsulated in glucuronide-modified long-circulating liposomes in mice bearing Meth A sarcoma. Biochim Biophys Acta. 1996;1279:70–74. doi: 10.1016/0005-2736(95)00242-1. [DOI] [PubMed] [Google Scholar]
- 9.Allen TM, Stuart DD. Liposomal pharmacokinetics Classical, sterically-stabilized, cationic liposomes and immunoliposomes. In: Janoff AS, editor. Liposomes: Rational design. New York, NY: Marcel Dekker, Inc; 2005. pp. 63–87. [Google Scholar]
- 10.Working PK, Newman MS, Stuart Y, et al. Pharmacokinetics, bio-distribution and therapeutic efficacy of doxorubicin encapsulated in STEALTH liposomes. J Liposome Res. 1994;46:667–687. [Google Scholar]
- 11.Newman MS, Colbern GT, Working PK, Engbers C, Amantea MA. Comparative sencapsulated in long-circulating, pegylated liposomes (SPI-077) in tumor-bearing mice. Cancer Chemother Pharmacol. 1999;43:1–7. doi: 10.1007/s002800050855. [DOI] [PubMed] [Google Scholar]
- 12.Budai L, Hajdu M, Budai M, et al. Gels and liposomes in optimized ocular drug delivery: Studies on ciprofloxacin formulations. Int J Pharm. 2007;343:34–40. doi: 10.1016/j.ijpharm.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 13.Vyas SP, Rawat M, Rawat A, Mahor S, Gupta PN. Pegylated protein encapsulated multivesicular liposomes: a novel approach for sustained release of Interferon α. Drug Dev Ind Pharm. 2006;32:699–707. doi: 10.1080/03639040500528954. [DOI] [PubMed] [Google Scholar]
- 14.Han HD, Lee A, Hwang T, et al. Enhanced circulation time and antitumor activity of doxorubicin by comblike polymer-incorporated liposomes. J Control Release. 2007;120:161–168. doi: 10.1016/j.jconrel.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 15.Davis A, Dixon H, Pawlowski ZS. Multicentre clinical trials of benzimidazole-carbamates in human cystic echinococcosis (phase 2) Bull World Health Organ. 1989;67:503–508. [PMC free article] [PubMed] [Google Scholar]
- 16.Horton RJ. Albendazole in treatment of human cystic echinococcosis: 12 years of experience. Acta Trop. 1997;64:79–93. doi: 10.1016/s0001-706x(96)00640-7. [DOI] [PubMed] [Google Scholar]
- 17.Jung H, Medina L, Garcia L, Fuentes I, Moreno-Esparza R. Absorption studies of albendazole and some physicochemical properties of the drug and its metabolite albendazole sulphoxide. J Pharm Pharmacol. 1998;50:43–48. doi: 10.1111/j.2042-7158.1998.tb03303.x. [DOI] [PubMed] [Google Scholar]
- 18.Hardin TC, Najvar LK, Rizzo J, Fothergill AW, Rinaldi MG, Graybill JR. Discrepancy between in vitro and in vivo antifungal activity of albendazole. J Med Vet Mycol. 1998;35:153–158. [PubMed] [Google Scholar]
- 19.Ramos G, Orduna A, Garcia-Yuste M. Hydatid cyst of the lung: diagnosis and treatment. World J Surg. 2001;25:46–57. doi: 10.1007/s002680020007. [DOI] [PubMed] [Google Scholar]
- 20.Anadol D, Ozcelik U, Kiper N, Gocmen A. Treatment of hydatid disease. Paediatr Drugs. 2001;3:123–135. doi: 10.2165/00128072-200103020-00005. [DOI] [PubMed] [Google Scholar]
- 21.Keshmiri M, Baharvahdat H, Fattahi SH, Davachi B, Dabiri RH, Baradaran H. Albendazole versus placebo in treatment of echinococcosis. Trans R Soc Trop Med Hyg. 2001;95:190–194. doi: 10.1016/s0035-9203(01)90162-2. [DOI] [PubMed] [Google Scholar]
- 22.Alexander M, Biren PM, Daniel TC, Owe O, Richard NZ. Rapid preparation of giant unilamellar vesicles. Proc Natl Acad Sci U S A. 1996;93:11443–11447. doi: 10.1073/pnas.93.21.11443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Law SL, Shih CL. Characterization of calcitonin-containing liposomes formulations for intranasal delivery. J Microencapsul. 2001;18:211–221. doi: 10.1080/02652040010000334. [DOI] [PubMed] [Google Scholar]
- 24.Gulati M, Grover M, Singh M, Singh S. Study of azathioprine encapsulation into liposomes. J Microencapsul. 1998;15:485–494. doi: 10.3109/02652049809006875. [DOI] [PubMed] [Google Scholar]
- 25.Zhang JA, Anyarambhatla G, Ma L, et al. Development and characterization of a novel Cremophor EL free liposome-based paclitaxel (LEPETU) formulation. Eur J Pharm Biopharm. 2005;59:177–187. doi: 10.1016/j.ejpb.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 26.Woo BH, Kostanski JW, Gebrekidan S, et al. Preparation characterization and in vivo evaluation of 120-day poly (D,L-lactide) leuprolide microspheres. J Control Release. 2001;75:307–315. doi: 10.1016/s0168-3659(01)00403-5. [DOI] [PubMed] [Google Scholar]
- 27.Metselaar JM, Mastrobattista E, Storm G. Liposomes for I.V. drug targeting: Design and applications. Mini Rev Med Chem. 2002;2:319–329. doi: 10.2174/1389557023405873. [DOI] [PubMed] [Google Scholar]
- 28.Henriksen I, Sande SA, Smistad G, Agren T, Karlsen J. In vitro evaluation of drug release kinetics from liposomes by fractional dialysis. Int J Pharm. 1995;119:231–238. [Google Scholar]
- 29.Paveli Z, Kalko Basnet N, Schubert R. Liposomal gels for vaginal drug delivery. Int J Pharm. 2001;219:139–149. doi: 10.1016/s0378-5173(01)00637-8. [DOI] [PubMed] [Google Scholar]
- 30.Song H, Zhang J, Han Z, et al. Pharmacokinetic and cytotoxic studies of pegylated liposomal daunorubicin. Cancer Chemother Pharmacol. 2006;57:591–598. doi: 10.1007/s00280-005-0076-6. [DOI] [PubMed] [Google Scholar]