Abstract
While the potential impact of magnetic nanoparticles (MNPs) has been widely explored in almost all medical fields, including cardiology, one question remains; that is whether MNPs interfere with cardiac physiological processes such as the expression and function of ion channels, especially in vivo. KCNQ1 channels are richly expressed in cardiac myocytes and are critical to the repolarization of cardiac myocytes. In this study, we evaluated the effects of Fe3O4-magnetic nanoparticles (MNPs-Fe3O4) on the expression of KCNQ1 in cardiac muscle of mice at rest and at different times following a single bout of swimming (SBS). Firstly, we demonstrated that the expression levels of KCNQ1 channels are significantly up-regulated in mice following a SBS by means of reverse transcription polymerase chain reaction (RT-PCR) and western-blot. After treating mice with normal saline or pure MNPs-Fe3O4 separately, we studied the potential effect of MNPs-Fe3O4 on the expression profile of KCNQ1 in mouse cardiac muscle following a SBS. A SBS increased the transcription of KCNQ1 at 3 hours post exercise (3PE) 164% ± 24% and at 12 hours post exercise (12PE) by 159% ± 23% (P < 0.05), and up-regulated KCNQ1 protein 161% ± 27% at 12PE (P < 0.05) in saline mice. In MNPs-Fe3O4 mice, KCNQ1 mRNA increased by 151% ± 14% and 147% ± 12% at 3 and 12 PE, respectively (P <0.05). Meanwhile, an increase of 152% ± 14% in KCNQ1 protein was also detected at by 12PE. These results indicated that the administration of MNPs-Fe3O4 did not cause any apparent effects on the expression profile of KCNQ1 in rested or exercised mice cardiac muscle. Our studies suggest a novel path of KCNQ1 current adaptations in the heart during physical exercise and in addition provide some useful information for the biomedical application of MNPs which are imperative to advance nanomedicine.
Keywords: KCNQ1, cardiac muscle, magnetic nanoparticles of Fe3O4
Introduction
Nanotechnology is at the leading edge of the rapidly developing, new therapeutic and diagnostic concepts in all areas of medicine.1 Nanoscale materials are a broadly defined set of substances in which at least one critical dimension is less than 100 nanometers.2 The unique ability of magnetic nanoparticles (MNPs) to be functionalized with bioactive agents and respond to a magnetic field has made them a useful tool for: magnetic resonance imaging; targeted drug and gene delivery; magnetic hyperthermia cancer therapy; tissue engineering, and cell tracking to name a few.3–5 Our previous study also proposed that daunorubicin-loaded magnetic nanoparticles of Fe3O4 (MNPs-Fe3O4) can overcome the multi-drug resistance of certain carcinoma cells, both in vitro and in vivo.6–8 While mounting evidence suggests that MNPs have numerous advanced applications and the benefits of nanotechnology are widely publicized, in vivo data on the potential effects of pure MNPs on mammals are limited. An understanding of the interface between the MNPs and specific organs, for to their application and safety, are imperative to advance nanomedicine. Although there are several reports showing that MNPs may be accumulated in tissues when administered at clinically relevant concentrations, via relevant routes, show unremarkable histological changes in vital organs, suggesting their safety in the respective formulations to some degree.9,10 However information on the physiological function of vital organs after the administration of MNPs is rarely reported.
The heart is a rhythmic electro-mechanical pump, the functioning of which depends on action potential (AP) generation and propagation, followed by relaxation and a period of refractoriness until the next impulse is generated. Cardiac repolarization is a key cellular function. Disruption of cardiac repolarization leads to potentially lethal ventricular tachyarrhythmias. Delayed rectifier K+ current (IK) is the major outward current involved in ventricular repolarization. Two components of IK, the rapid voltage-gated potassium current, IKr and slow voltage-gated potassium current, IKs, have been identified. A prolongation of APs under a variety of conditions would favor the activation of IKs so as to prevent excessive repolarization delay, causing early depolarization. The pore-forming subunit of the voltage-dependent K+ channel underlying IKs is KCNQ1.11
The KCNQ1 is a 6-transmembrane protein that can either form a functional homomeric potassium channel or coassemble with the single transmembrane domain protein encoded by the KCNE family of genes to form a heteromeric channel, the slow voltage-gated potassium channel (IKs channel).12,13 Mutations in the cardiac KCNQ1 have been linked to the long QT syndrome (LQTS)1, which predisposes patients to arrhythmia during exercise and emotional stress, conditions that involve high levels of sympathetic nervous system activity.14,15
Stimulation of the sympathetic nervous system (SNS) in response to exercise or emotional stress results in a rapid and dramatic increase in heart rate. To ensure adequate diastolic filling time between beats, the ventricular action potential duration (APD) exhibits a concomitant shortening at the cellular level, which results in a reduction in the corresponding QT interval of the electrocardiography (ECG). The enhancement of slow voltage-gated potassium current IKs plays a major role in this process.16 Although some reports show that the activity of IKs channel can be upregulated in the face of elevated SNS activity.16,17 It is currently unknown whether the remodeling of IKs channel expression may be induced by the stimulation of exercise.
In this study, we investigated the expression profile of KCNQ1 in mouse cardiac muscle following a single bout of swimming (SBS). In order to assess the potential effects of pure MNPs on mammal heart, we also treated mice with normal saline or MNPs-Fe3O4 separately and then examined the effects of MNPs-Fe3O4 on the expression of KCNQ1 in exercised and non-exercised mouse heart. MNPs-Fe3O4 have not been found to downregulate the apoptosis-associated genes in certain carcinoma cells.7
Material and methods
Preparation of MNPs-Fe3O4
MNPs-Fe3O4 were produced by electrochemical deposition under oxidizing conditions in a 0.1 mol/L tetraheptylammonium-2-propanol solution, in which the magnetization and particle size of MNPs-Fe3O4 were found to be 25.6 × 10−3 emu/mg and 30 nm, respectively.18 The deposited clusters were capped with tetraheptylammonium-2-propanol, which acts as a stabilizer of the colloidal nanocrystallites.19 Before being applied in this experiment, the magnetized nanoparticles of Fe3O4 were well distributed in 0.9% NaCl solution by using ultrasound treatment in order to obtain colloidal suspension of MNPs-Fe3O4.18
Animals
Four-week-old, male BALB/c-nu/nu mice were purchased from Beijing National Center for Laboratory Animals, the Chinese Academy of Medical Sciences prior to the beginning of our experiments. Both food and water were provided ad libitum.
Exercise protocols
Forty-two mice were randomly assigned to either the non-exercised control group (n = 6) or exercise groups (n = 36). Thirty-six mice were placed into plastic cylinders (23 cm tall × 14 cm diameter) containing water (22–24°C) filled to a depth of 15 cm for a 30-minute swim. Non-exercised controls were kept in their home cage for the duration of the test. Mice completing the exercise were euthanized via cervical dislocation immediately or at 1, 3, 6, 12, or 24 hour after exercise. Until euthanasia, food and water were provided ad libitum. The ventricles were quickly dissected from each mouse, frozen in liquid nitrogen, and stored at −80°C until assayed.
For the study of the MNPs-Fe3O4, 48 mice were assigned randomly into 2 groups (n = 24) and treated with normal saline 0.2 mL or MNPs-Fe3O4 (0.2 mL, 0.58 mg/kg) every other day for 14 day by vena caudalis injection, respectively. Mice treated with normal saline or MNPs-Fe3O4 were then randomly assigned to either non-exercised control group (n =6) or the exercise groups (n = 18) respectively. Mice were exposed to a 30-minute swim 1 day after the last injection. All experiments were conducted at approximately the same time of day. After completing the exercise the mice were euthanized via cervical dislocation at 3, 12, or 24 hour after exercise (Table 1). Until euthanasia, food and water were provided ad libitum.
Table 1.
Number of mice in each group for the study of the MNPs-Fe3O4
| Groups | Treated with saline (n) | Treated with MNPs-Fe3O4 (n) | |
|---|---|---|---|
| Non-exercised control group | 6 | 6 | |
| Exercise groups | Euthanized at 3PE | 6 | 6 |
| Euthanized at 12PE | 6 | 6 | |
| Euthanized at 24PE | 6 | 6 | |
Abbreviation: PE, post exercise.
Semiquantitative polymerase chain reaction (PCR)
The dissected left ventricles were crushed into a fine powder using a mortar and pestle. Total RNA was extracted using TRIzol® (Invitrogen, Carlsbad, CA, USA), and RNA concentrations were determined spectrophotometrically at 260 nm. RNA was treated with DNase to eliminate potential genomic DNA contamination, and reverse transcription and polymerase chain reaction (PCR) were performed with 1 μg of total RNA using ReverTraAce® a (Toyobo, Osaka, Japan). cDNAs were amplified by PCR in a Bio-Rad Mycycler thermal cycler. PCR was undertaken with triplicate samples prepared from each animal, in each group. Each reaction contained 500 ng cDNA. The PCR primers used were as: KCNQ1 (525bp) forward primer, 5′-CACTTCACCGTCTT CCTCATT-3′; reverse, 5′-TCTGCGTAGC TGCCAAACT-3′; β-actin (360bp) forward primer, 5′-TTGCAGCTCCTTC GTTGCCG-3′; reverse, 5′-GCCTCGGTG AGCAGCACAGG-3′. Cycle times used for each primer pair were within the linear increase in PCR product. PCR products were separated and visualized in 1.5% agarose gels with ethidium bromide. Band intensities of KCNQ1 were quantitated using ImageQuant™ software and normalized to β-actin.
Western blot analysis
Left ventricular tissue was homogenized with 500 μL lysis buffer (100 mM/L Tris [pH 6.8], 4% sodium dodecyl sulfate [SDS], 20% glycerol) containing the protease inhibitor phenylmethanesulfonyl fluoride 0.1 mM/L. We separated 50 μg of proteins on SDS–polyacrylamide gel electrophoresis 10%. Proteins were transferred to nitrocellulose membrane (Bio-Rad Laboratories, Hercules, CA, USA) blocked with 5% dry milk and exposed to rabbit polyclonal antibody anti-KCNQ1 (H-130: sc-20816, [Santa Cruz Biotechnology, Santa Cruz, CA USA]; dilution 1:2,000 in bovine serum albumin (BSA) 5%), rabbit polyclonal antibody anti-β-actin (ab8227, [Abcam, Cambridge, MA, USA]; dilution 1:5,000 in BSA 5%). Immunodetection was accomplished using goat anti-rabbit IgG (Sigma-Aldrich, St. Louis, MO, USA) secondary antibodies (dilution 1:4,000 in BSA 5%), and a chemiluminescence kit (Amersham Biosciences, Little Chalfont, Buckinghamshire, UK). Band intensities were analyzed by densitometry. Band intensities of KCNQ1 were normalized to β-actin.
Statistical analysis
All data are expressed as means ± SE. The effect of swimming on expression of KCNQ1 was analyzed using one-way analysis of variance (ANOVA). Two-way ANOVA was used for Fe3O4 administration studies.
Results
Acute exercise enhanced KCNQ1 mRNA and protein expression in mouse cardiac muscle
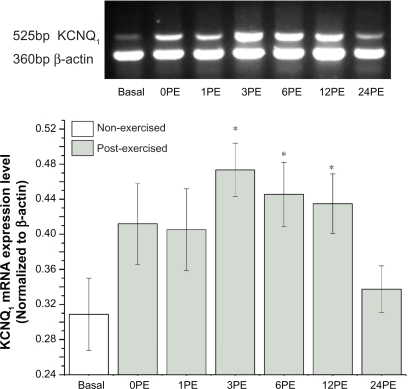
Compared to mice in the non-exercise control group (basal), a 30-minute single bout of swimming enhanced the transcription of KCNQ1 mRNA in the heart at 3, 6, and 12 hour post exercise (PE) (P < 0.05, Figure 1). The expression level of KCNQ1 mRNA returned to approximately control levels within 24-PE.
Figure 1.
Effect of a single bout of exercise on the transcription of KCNQ1 mRNA.
Notes: All values are reported as means ± SE (n = 6). *P < 0.05, the expression levels of KCNQ1 at 3PE, 6PE and 12PE were significantly different from the basal group.
Abbreviation: PE, post exercise.
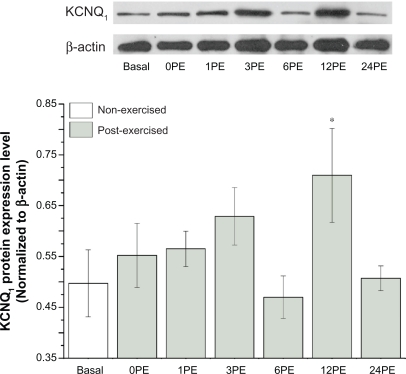
Expression of KCNQ1 protein significantly increased with exercise in mice at 12PE compared with the non-exercised basal animals (P < 0.05, Figure 2), and the amount of KCNQ1 protein decreased nearly to base level at 24PE.
Figure 2.
Effect of a single bout of exercise on the expression of KCNQ1 protein.
Notes: All values are reported as means ± SE (n = 6). *P < 0.05, the expression level of KCNQ1 at 12PE was significantly different from the basal group.
Abbreviation: PE, post exercise.
Effect of Fe3O4 magnetic nanoparticles on acute exercise enhanced KCNQ1 expression in mouse cardiac muscle
Over the course of the 14-day treatment with normal saline or MNPs-Fe3O4, mice showed no significant health problems. Drug administration also did not appear to affect exercise performance.
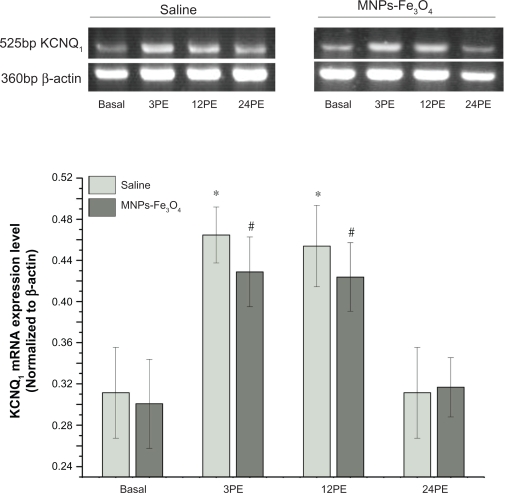
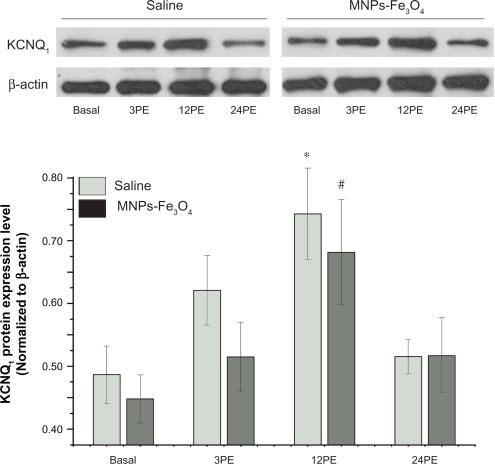
The amount of KCNQ1 mRNA increased significantly at 3PE and 12PE compared to basal animals (P < 0.05) both in saline and MNPs-Fe3O4 group (Figure 3, Table 2). Expression of KCNQ1 protein also increased significantly at 12PE when compared to basal animals (P < 0.05) both in saline and MNPs-Fe3O4 group (Figure 4, Table 2).
Figure 3.
Effect of Fe3O4-magnetic nanoparticles on the transcription of acute exercise enhanced KCNQ1 mRNA in mouse cardiac muscle.
Notes: All values are reported as means ± SE (n = 6). *P < 0.05, the transcriptions level of KCNQ1 at 3PE and 12PE in saline mouse was significantly different from the basal group. #P < 0.05, the transcriptions level of KCNQ1 at 3PE and 12PE in mouse treated with MNPs-Fe3O4 were significantly different from the basal group.
Abbreviation: PE, post exercise.
Table 2.
Effect of MNPs-Fe3O4 on change of KCNQ1 expression in mouse cardiac muscle
| Group | Change of KCNQ1 mRNA (%) | Change of KCNQ1 protein (%) | ||||
|---|---|---|---|---|---|---|
| Saline | MNPs-Fe3O4 | P value | Saline | MNPs-Fe3O4 | P value | |
| 3PE | 164 ± 24 | 151 ± 14 | 0.64 | 129 ± 11 | 136 ± 21 | 0.80 |
| 12PE | 159 ± 23 | 147 ± 12 | 0.66 | 161 ± 27 | 152 ± 14 | 0.76 |
| 24PE | 119 ± 17 | 110 ± 10 | 0.67 | 112 ± 9 | 116 ± 15 | 0.84 |
Notes: All values of change are compared to corresponding basal and are reported as means ± SE (n = 6). P > 0.05. P values are for comparison of MNPs-Fe3O4 group with corresponding saline group.
Abbreviations:SE, standard error; PE, post exercise.
Figure 4.
Effect of Fe3O4-magnetic nanoparticles on the expression of acute exercise enhanced KCNQ1 protein in mouse cardiac muscle.
Notes: All values are reported as means ± SE (n = 6). *P < 0.05, the expression level of KCNQ1 at 12PE in saline mouse was significantly different from the basal group. #P < 0.05, the expression level of KCNQ1 protein at 12PE in mouse treated with MNPs-Fe3O4 was significantly different from the basal group.
Abbreviation: PE, post exercise.
No significant differences were found in KCNQ1 expression profile between MNPs-Fe3O4 mice and control saline mice at rest or post exercise (Figures 3 and 4, Table 2).
Discussion
The present study examined firstly the effect of a single bout of swimming on the expression of KCNQ1 in mouse cardiac muscle. Our data shows a 30-minute bout of swimming enhanced KCNQ1 expression at 3PE and 12PE. Administrations of MNPs-Fe3O4 had no significant influence or enhancement effect of acute exercise on KCNQ1 expression in mouse cardiac muscle.
Cardiac APs are driven by ionic currents flowing through specific channels and exchangers across cardiomyocyte membranes. Voltage-gated K+ currents play a major role in repolarization of APs. KCNQ1 is a 6-transmembrane domain protein that can form the slow voltage-gated potassium channel (IKs channel) constituting the slowly activating potassium current IKs. There is a growing number of studies that suggest that the IKs is important for AP repolarization and APD adaptation to changes in heart rate.16,17 The importance of the KCNQ1 channel at fast heart rates is especially evident from the fact that exercise or emotional stress conditions, which involve high levels of beta-adrenergic stimulation, trigger arrhythmias in more than 80% of patients with the long QT syndrome (LQTS) which arise from mutations in KCNQ1.20 The activation of β-adrenergic receptors can regulate select ion channel proteins via cAMP-dependent protein kinase A (PKA) or by direct binding of cAMP to channel subunits. One key substrate for PKA-dependent phosphorylation in the regulation of the cardiac action potentials is the IKs channel.21 Augmentation of IKs current by β-adrenergic stimulation plays an important role in mediating cardiac electrophysiological response during exercise and emotional stress.16 Despite the elucidation of the macromolecular signaling complex and striking clinical data, the possibility that exercise might induce remodeling of IKs channel expression has not been tested.
Our present results show that the transcription and expression of KCNQ1 are increased following a 30-minute swimming. These data indicate the remodeling of KCNQ1 expression that might partially compensate for the shortening of cardiac action potential duration; which results in a reduction in the corresponding QT interval of the elctrocardiogram.22
The potential benefits of nanotechnology in medicine are inevitable, owing to refined, highly targeted: blood-brain barrier-crossing drug delivery and imaging platforms; unique transfection; labeling and bioseparation, together with analytical and tissue engineering approaches. The versatility of MNPs is due to their capability of responding to an external magnetic field and can be functionalized with bioactive agents at the same time and/or differentially.23 For successful application of MNPs, it is essential to understand the real effect of the pure MNPs on vital organs and cells.
MNP size, charge, surface chemistry and route of delivery each influence their effective time and biodistribution patterns in the body.25 Large (>200 nm) particles are usually sequestered by the spleen via mechanical filtration followed by phagocytosis, whereas smaller (<10 nm) particles are rapidly removed through extravasation and renal clearance, with particles 10–100 nm believed to be optimal for intravenous administration.25 In this study, pure MNPs-Fe3O4 (30 nm) were used for the evaluation of their potential effect on the protein expression in the heart as well as exercise ability. The results showed that pure MNPs did not cause any apparent effects on swimming behavior nor in the KCNQ1 expression profile before or after exercise.
Conclusion
In conclusion, our results indicate a novel path of IKs current adaptations in the heart during physical exercise. Compared with mice in the non-exercise control group expression of KCNQ1 in the heart were significantly increased following exercise. Therefore, not only the increased activity but also the enhanced expression of KCNQ1 channel might contribute to current MNP-Fe3O4 adaptations in the face of elevated sympathetic nervous system activity. Further study shows that the administration of MNPs-Fe3O4 has no significant influence on the expression profile of KCNQ1 in rested or in exercised mice cardiac muscle. These data might provide some useful information required to improve MNP formulations and biomedical applications which are imperative to advance nanomedicine.
Acknowledgments
This work was supported by Special-Purpose Science Research Fund for the Doctoral Program of Higher Education of China (No. 20070286042) and National Nature Science Foundation of People’s Republic of China (No. 30770537, 30872970). The authors report no conflicts of interest in this work.
Footnotes
Disclosures
The authors report no conflicts of interest relevant to this study.
References
- 1.Whitesides GM. The ‘right’ size in nanobiotechnology. Nat Biotechnol. 2003;21(10):1161–1165. doi: 10.1038/nbt872. [DOI] [PubMed] [Google Scholar]
- 2.Jeng HA, Swanson J. Toxicity of metal oxide nanoparticles in mammalian cells. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2006;41(12):2699–2711. doi: 10.1080/10934520600966177. [DOI] [PubMed] [Google Scholar]
- 3.Gupta AK, Gupta M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials. 2005;26(18):3995–4021. doi: 10.1016/j.biomaterials.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 4.McCarthy JR, Kelly KA, Sun EY, Weissleder R. Targeted delivery of multifunctional magnetic nanoparticles. Nanomed. 2007;2(2):153–167. doi: 10.2217/17435889.2.2.153. [DOI] [PubMed] [Google Scholar]
- 5.Naqvi S, Samim M, Dinda AK, et al. Impact of magnetic nanoparticles in biomedical applications. Recent Pat Drug Deliv Formul. 2009;3(2):153–161. doi: 10.2174/187221109788452249. [DOI] [PubMed] [Google Scholar]
- 6.Lai BB, Chen BA, Cheng J, et al. Daunorubicin-loaded magnetic nanoparticles of Fe(3)O(4) greatly enhance the responses of multidrug-resistant K562 leukemic cells in a nude mouse xenograft model to chemotherapy. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2009;17(2):345–351. [PubMed] [Google Scholar]
- 7.Jiang Z, Chen BA, Wu Q, et al. Reversal effect of Fe3O4-magnetic nanoparticles on multi-drug resistance of ovarian carcinoma cells and its correlation with apoptosis-associated genes. Chin J Cancer. 2009;28(11):1158–1162. doi: 10.5732/cjc.009.10090. [DOI] [PubMed] [Google Scholar]
- 8.Chen B, Sun Q, Wang X, et al. Reversal in multidrug resistance by magnetic nanoparticle of Fe3O4-loaded with adriamycin and tetrandrine in K562/A02 leukemic cells. Int J Nanomedicine. 2008;3(2):277–286. doi: 10.2147/ijn.s2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muldoon LL, Sàndor M, Pinkston KE, Neuwelt EA. Imaging, distribution, and toxicity of superparamagnetic iron oxide magnetic resonance nanoparticles in the rat brain and intracerebral tumor. Neurosurgery. 2005;57(4):785–796. doi: 10.1093/neurosurgery/57.4.785. [DOI] [PubMed] [Google Scholar]
- 10.Dunning MD, Lakatos A, Loizou L, et al. Superparamagnetic iron oxide-labeled Schwann cells and olfactory ensheathing cells can be traced in vivo by magnetic resonance imaging and retain functional properties after transplantation into the CNS. J Neurosci. 2004;24(44):9799–9810. doi: 10.1523/JNEUROSCI.3126-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nerbonne JM, Kass RS. Molecular physiology of cardiac repolarization. Physiol Rev. 2005;85(4):1205–1253. doi: 10.1152/physrev.00002.2005. [DOI] [PubMed] [Google Scholar]
- 12.Takumi T, Ohkubo H, Nakanishi S. Cloning of a membrane protein that induces a slow voltage-gated potassium current. Science. 1988;242(4881):1042–1045. doi: 10.1126/science.3194754. [DOI] [PubMed] [Google Scholar]
- 13.Charpentier F, Mérot J, Loussouarn G. Delayed rectifier K(+) currents and cardiac repolarization. J Mol Cell Cardiol. 2009. Aug 14, [Epub ahead of print] [DOI] [PubMed]
- 14.Yang WP, Levesque PC, Little WA, Conder ML, Shalaby FY, Blanar MA. KvLQT1, a voltage-gated potassium channel responsible for human cardiac arrhythmias. Proc Natl Acad Sci U S A. 1997;94:4017–4021. doi: 10.1073/pnas.94.8.4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Q, Curran ME, Splawski I, et al. Positional cloning of a novel potassium channel gene: KVLQT1 mutations cause cardiac arrhythmias. Na Genet. 1996;12:17–23. doi: 10.1038/ng0196-17. [DOI] [PubMed] [Google Scholar]
- 16.Walsh KB, Kass RS. Regulation of a heart potassium channel by protein kinase A and C. Science. 1988;242:67–69. doi: 10.1126/science.2845575. [DOI] [PubMed] [Google Scholar]
- 17.Terrenoire C, Clancy CE, Cormier JW, Sampson KJ, Kass RS. Autonomic control of cardiac action potentials: role of potassium channel kinetics in response to sympathetic stimulation. Circ Res. 2005;96(5):e25–e34. doi: 10.1161/01.RES.0000160555.58046.9a. [DOI] [PubMed] [Google Scholar]
- 18.Chen BA, Lai BB, Jiang J, et al. Daunorubicin-loaded magnetic nanoparticles of Fe3O4 overcome multidrug resistance and induce apoptosis of K562-n/VCR cells in vivo. Int J Nanomedicine. 2009;4:201–208. doi: 10.2147/ijn.s7287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, Zhang R, Wu C, et al. The application of Fe3O4 nanoparticles in cancer research: a new strategy to inhibit drug resistance. J Biomed Mater Res A. 2007;80(4):852–860. doi: 10.1002/jbm.a.30901. [DOI] [PubMed] [Google Scholar]
- 20.Jespersen T, Grunnet M, Olesen SP. The KCNQ1 potassium channel: from gene to physiological function. Physiology (Bethesda) 2005;20:408–416. doi: 10.1152/physiol.00031.2005. [DOI] [PubMed] [Google Scholar]
- 21.Chen L, Kurokawa J, Kass RS. Phosphorylation of the A-kinase-anchoring protein Yotiao contributes to protein kinase A regulation of a heart potassium channel. J Biol Chem. 2005;280(36):31347–31352. doi: 10.1074/jbc.M505191200. [DOI] [PubMed] [Google Scholar]
- 22.Swan H, Toivonen L, Viitasalo M. Rate adaptation of QT intervals during and after exercise in children with congenital long QT syndrome. European Heart Journal. 1998;19:508–513. doi: 10.1053/euhj.1997.0764. [DOI] [PubMed] [Google Scholar]
- 23.Shubayev VI, Pisanic TR, Jin S. Magnetic nanoparticles for theragnostics. Adv Drug Deliv Rev. 2009;61(6):467–477. doi: 10.1016/j.addr.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jain TK, Reddy MK, Morales MA, Leslie-Pelecky DL, Labhasetwar V. Biodistribution, clearance, and biocompatibility of iron oxide magnetic nanoparticles in rats. Mol Pharm. 2008;5(2):316–327. doi: 10.1021/mp7001285. [DOI] [PubMed] [Google Scholar]
- 25.Duguet E, Vasseur S, Mornet S, Devoisselle JM. Magnetic nanoparticles and their applications in medicine. Nanomed. 2006;1(2):157–168. doi: 10.2217/17435889.1.2.157. [DOI] [PubMed] [Google Scholar]