Abstract
Object
Patients with normal MR imaging (nonlesional) findings and medically refractory extratemporal epilepsy make up a disproportionate number of nonexcellent outcomes after epilepsy surgery. In this paper, the authors investigated the usefulness of intracranial electroencephalography (iEEG) in the identification of surgical candidates.
Methods
Between 1992 and 2002, 51 consecutive patients with normal MR imaging findings and extratemporal epilepsy underwent intracranial electrode monitoring. The implantation of intracranial electrodes was determined by seizure semiology, interictal and ictal scalp EEG, SPECT, and in some patients PET studies. The demographics of patients at the time of surgery, lobar localization of electrode implantation, duration of follow-up, and Engel outcome score were abstracted from the Mayo Rochester Epilepsy Surgery Database. A blinded independent review of the iEEG records was conducted for this study.
Results
Thirty-one (61%) of the 51 patients who underwent iEEG ultimately underwent resection for their epilepsy. For 28 (90.3%) of the 31 patients who had epilepsy surgery, adequate information regarding follow-up (> 1 year), seizure frequency, and iEEG recordings was available. Twenty-six (92.9%) of 28 patients had frontal lobe resections, and 2 had parietal lobe resections. The most common iEEG pattern at seizure onset in the surgically treated group was a focal high-frequency discharge (in 15 [53.6%] of 28 patients). Ten (35.7%) of the 28 surgically treated patients were seizure free. Fourteen (50%) had Engel Class I outcomes, and overall, 17 (60.7%) had significant improvement (Engel Class I and IIAB with ≥ 80% seizure reduction). Focal high-frequency oscillation at seizure onset was associated with Engel Class I surgical outcome (12 [85.7%] of 14 patients, p = 0.02), and it was uncommon in the nonexcellent outcome group (3 [21.4%] of 14 patients).
Conclusions
A focal high-frequency oscillation (> 20 Hz) at seizure onset on iEEG may identify patients with nonlesional extratemporal epilepsy who are likely to have an Engel Class I outcome after epilepsy surgery. The prospect of excellent outcome in nonlesional extratemporal lobe epilepsy prior to intracranial monitoring is poor (14 [27.5%] of 51 patients). However, iEEG can further stratify patients and help identify those with a greater likelihood of Engel Class I outcome after surgery.
Keywords: electroencephalography, epilepsy surgery, high-frequency oscillation
Partial epilepsy represents the most common type of intractable epilepsy, and partial seizures originating from neocortex are notoriously difficult to localize and treat.11,44 Presently, treatment options for patients with medically refractory epilepsy are limited to implantation of a vagus nerve stimulator, which has similar efficacy to medical therapy.13 Epilepsy surgery has the best chance of producing a cure, that is, complete seizure freedom, but it is a viable option only if the brain region generating seizures can be accurately localized and safely removed.10,17,18,26 Thus, accurate localization of epileptogenic brain is of paramount importance.
Recent studies investigating neuronal activity in humans and animals have described a wide dynamic range of EEG activity, from gamma oscillations (~ 30–60 Hz) that are believed to play a role in learning and memory21,25 and ripple oscillations (~ 100–200 Hz) that may be important for memory consolidation,7–9 to somatosensory-evoked potential oscillations (~ 600 Hz).10,14,15 In addition to normal brain function, high-frequency oscillations have been described in human epileptic foci at seizure onset.3–6,20,39 To date, with the exceptions discussed later in this paper, human iEEG studies performed as part of presurgical evaluations have focused on a limited dynamic range (~ 0.1–40 Hz) of iEEG activity.35–38 This limited frequency range may reflect the fact that oscillations with frequencies higher than ~ 30 Hz are usually very low amplitude, and they are obscured by cerebral activity in the lower frequency (0.1–20 Hz) range28 when using standard clinical reviewing parameters.45,48 In fact, the use of broadband digital EEG recordings and spectral analysis has allowed detection of high-frequency activity (> 40 Hz) at the onset of neocortical seizures in adult3,4,20,45,48 and pediatric39 patients. There is now accumulating evidence to support the idea that pathological HFEOs are an electrophysiological signature of epileptogenic brain, and they may play a role in neocortical ictogenesis.39,45,48
Although preliminary results from pediatric and adult patients with refractory neocortical epilepsy are interesting, the clinical significance and usefulness of the aforementioned findings are yet to be determined. Unfortunately, information about the overall prevalence of HFEOs in neocortical epilepsy is limited. In a retrospective study of consecutive patients, we previously showed that high-frequency oscillations are a common seizure onset pattern in neocortical epilepsy, independent of the cause of epilepsy.48 However, the total number of patients (6) in that study was small, and all the other studies cited above have described only small numbers of highly selected patients.3,4,20 There is even less information regarding accurate localization of epileptogenic brain by interictal and ictal iEEG, but the preliminary data support this concept in neocortical partial epilepsy.4,48 Presently, it is not known whether ictal and interictal high-frequency oscillations offer improved localization of epileptogenic brain and whether resection of the regions with high-frequency activity leads to improved surgical outcomes. In this study, we investigate the prevalence of HFEOs, here defined as seizure onset frequency > 20 Hz, and their role in achieving good surgical outcomes in a cohort of consecutive patients with normal MR imaging findings who were undergoing evaluation for epilepsy surgery.
Methods
Patient Population
Between 1992 and 2002, 51 consecutive patients with normal MR imaging findings underwent iEEG monitoring for presurgical evaluation of intractable extratemporal epilepsy. These patients were identified by a retrospective review of the Mayo Clinic electronic record system. Each patient gave informed consent for participation, and the Mayo Clinic Internal Review Board approved the study. An electrophysiologist and epileptologist (G.A.W.) reviewed each patient’s iEEG recordings.
Electroencephalography Electrodes
Each patient had intracranial electrodes placed according to standard presurgical evaluation protocols.17,35,36 All patients underwent comprehensive evaluation, including asleep and awake EEG with standard activating procedures, MR imaging, neuropsychological studies, visual perimetry, and long-term video-EEG monitoring. Magnetic resonance imaging was performed according to a standardized seizure protocol with a 1.5-T Signa unit (GE Medical Systems).22 This protocol included a spin echo T1-weighted whole-brain volumetric series consisting of 124 contiguous 1.5-mm-thick slices, acquired either coronally or perpendicularly along the long axis of the hippocampal formation. Axial T1-weighted images, coronal and axial T2-weighted and proton density images, and coronal FLAIR images were acquired with a 3-mm slice thickness and a 2-mm interslice gap. Ictal and interictal SPECT was performed in all patients, and in 15 patients, SISCOM was performed.29–33 Ad-Tech subdural grids, strips, and depth electrodes (Ad-Tech Medical Instrument Corp.) were used for all studies. The location of subdural electrode placement was decided based on findings from the noninvasive studies (interictal and ictal scalp EEG and SPECT, and seizure semiology). The individual electrode contacts of the subdural grids were 4.0-mm-diameter platinum-iridium discs with a center-to-center electrode distance of 10 mm.
Intracranial EEG Data Collection and Storage
Continuous video-EEG and iEEG were collected using 1 of 2 clinical digital acquisition systems: the XLTEK (XLTEK Corp.; 128-channel 16-bit A/D, and sampling frequency 500 Hz) or the NCI (Lamont Medical; 128-channel, 16-bit A/D, and sampling frequency of 250 Hz). All analyses were performed using a high-pass filter of 1.0 Hz and low-pass filter of 100 Hz (1–100 Hz dynamic range). Bipolar and referential electrode montages were reviewed, and in the majority of cases the bipolar montage was used to eliminate common mode artifact. A digital 60-Hz notch filter was used when necessary to eliminate line noise.
Determination of Seizures and Seizure Onset Zone
Seizure onset times were determined by visually identifying an electrographic seizure discharge and then looking backward in the record for the earliest EEG change from baseline associated with the seizure. The earliest EEG change was selected as the seizure onset time. Only the neocortical onset seizures were selected for analysis. The seizure onset zone, defined by the electrode(s) with the earliest onset of seizure activity, was documented for each seizure and was classified as frontal, parietal, or occipital. The seizure onset iEEG pattern was characterized by the waveform morphology, frequency of discharge, and spatial extent (focal [≤ 4 contacts] or regional [4–8 contacts], or multilobar or diffuse).
Coregistration of Subdural Electrodes and MR Imaging
To evaluate the concordance of the aforementioned EEG features and to localize the epileptogenic zone, the subdural grids were coregistered with the patient’s postresection MR image. The epileptogenic zone is the region that, when resected, produced excellent seizure control. The epileptogenic zone is thus defined in relation to surgical outcome and not electrographic onset pattern (seizure onset zone). We have previously used this technique to correlate the seizure onset zone with functional imaging modalities (Fig. 1).47 Coregistration of subdural electrodes with the postresection MR images allowed detailed correlation of the interictal and ictal EEG measurements used to define epileptogenic brain (size of area involved, focal, extended, or multifocal) and anatomical structures (frontal, parietal, and occipital lobes). This degree of anatomical detail allows the completeness of resection of epileptogenic brain, as determined by the ictal features, to be compared with seizure-free outcome.
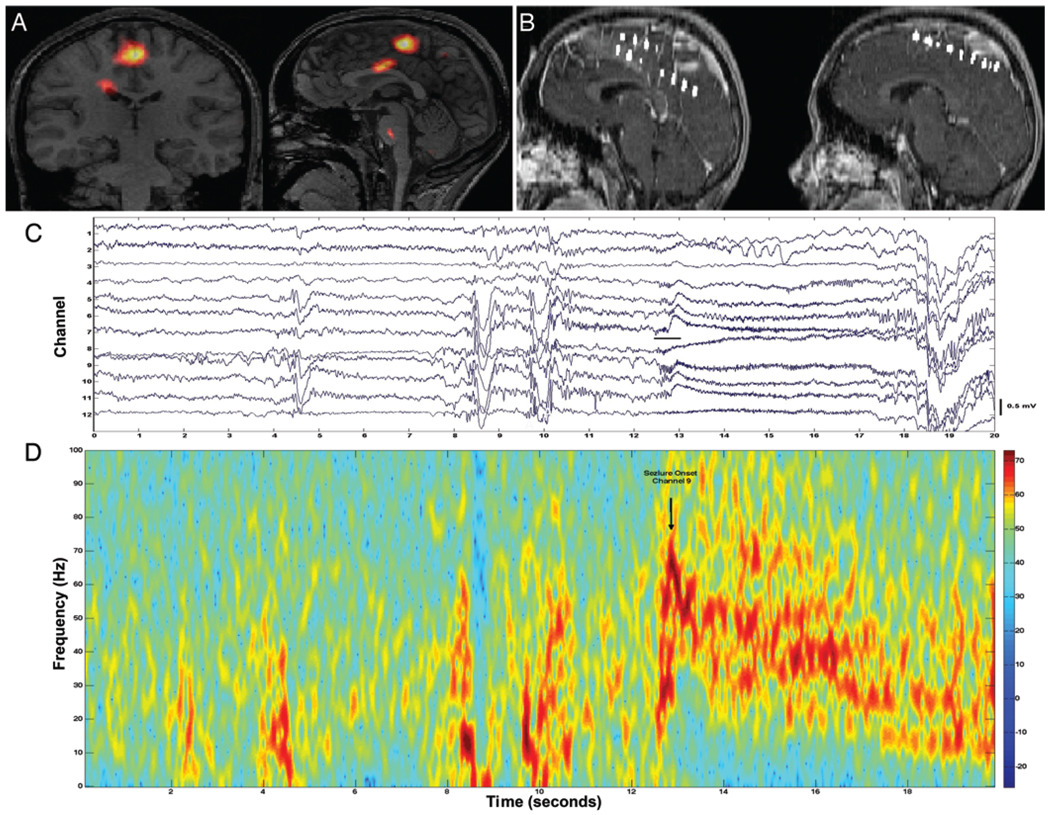
FIG 1.
A: A SISCOM image showing the localization of right mesial frontal epileptogenic zone. B: Sagittal MR images showing interhemispheric electrodes in position over the SISCOM localization of the frontal epileptogenic zone. C: Tracing showing seizure onset with focal high-frequency activity. D: Spectrogram showing the neocortical seizure onset in C.
Data Analysis
Neocortical seizures were analyzed provided the seizure onset was preceded by ≥ 1 minute of uninterrupted artifact-free interictal EEG. Seizure onset times and the seizure onset zone (electrode location and number) were both determined by visual review and spectral analysis. The frequency of oscillation at seizure onset was then determined by spectral analysis, selecting the frequency of the dominant spectral peak obtained from the initial 3 ± 5 seconds at seizure onset or exclusively by visual review. The seizure onset was analyzed using short-time spectrogram (MATLAB, Inc.) by using a 0.25-second sliding window and a 0.2-second overlap (Fig. 1). Because of the limited number of patients identified (51), we combined all seizures with onset frequency discharges > 20 Hz (20–100 Hz) into a single group (HFEO group). A neuropathologist confirmed the pathological diagnosis of resected tissue.
Statistical Analysis
The association among seizure onset pattern, SPECT location, and seizure outcome (Engel Class42) was tested using the chi-square and Fisher exact tests when there were < 5 patients within each group. An association between total number of intracranial electrodes and seizure outcome was tested using the rank-sum statistic. A probability value ≤ 0.05 was considered statistically significant. All data were recorded and entered into a spreadsheet (MS Excel, Microsoft Corp.), and statistical analyses were performed using JMP (version 6.0, SAS Institute).
Results
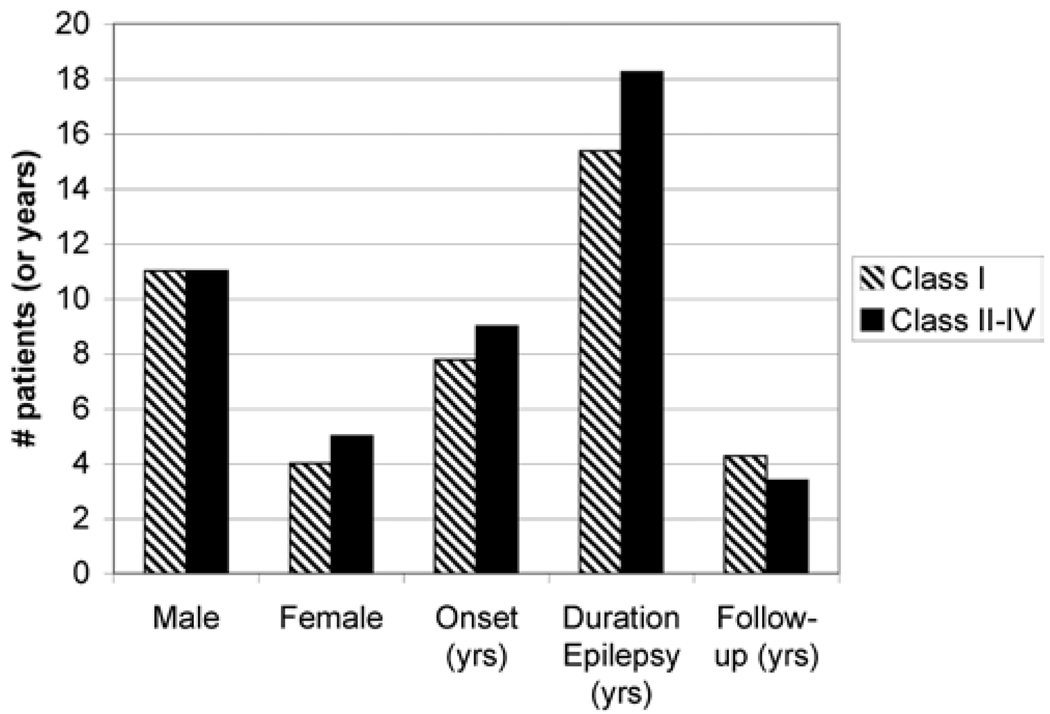
Thirty-one (61%) of the 51 patients undergoing iEEG had epilepsy surgery. The decision for resection of epileptic brain was based on the results of iEEG monitoring. In 20 patients (39%), the evaluation did not yield adequate localizing information for seizure origin, and these patients were not considered suitable candidates for resection. The precise locations of grid placement varied with each patient depending on seizure semiology and the results of the scalp EEG, SPECT, and SISCOM31 studies. For 28 (90.3%) of the 31 patients (22 men and 9 women) who underwent epilepsy surgery, adequate information regarding follow-up (> 1 year), seizure frequency, and iEEG recordings was available. The average age at seizure onset was 8.5 years, and the average duration of seizures was 16.5 years (Fig. 2).
FIG 2.
Bar graph showing the demographic and surgical outcome data based on sex, age at seizure onset, duration of epilepsy, and length of follow-up of 31 consecutive patients with nonlesional extratemporal epilepsy undergoing resection. Differences in seizure outcomes were not statistically significant among the categories.
The number of subdural electrodes varied widely across patients, from 24 to 128 electrodes (mean 43 ± 24.5 electrodes), and reflected the degree of confidence of localization based on the noninvasive presurgical evaluation. The number of subdural electrodes did not correlate with seizure outcome from surgery (p = 0.7, rank-sum).
All patients in this study underwent interictal and ictal SPECT studies. In 15 of the 31 patients undergoing resection, a region of SPECT abnormality was identified, and subdural electrodes were placed directly over this region. However, in this study we did not find a correlation between a regional SPECT abnormality and seizure-free outcome (p = 0.75).
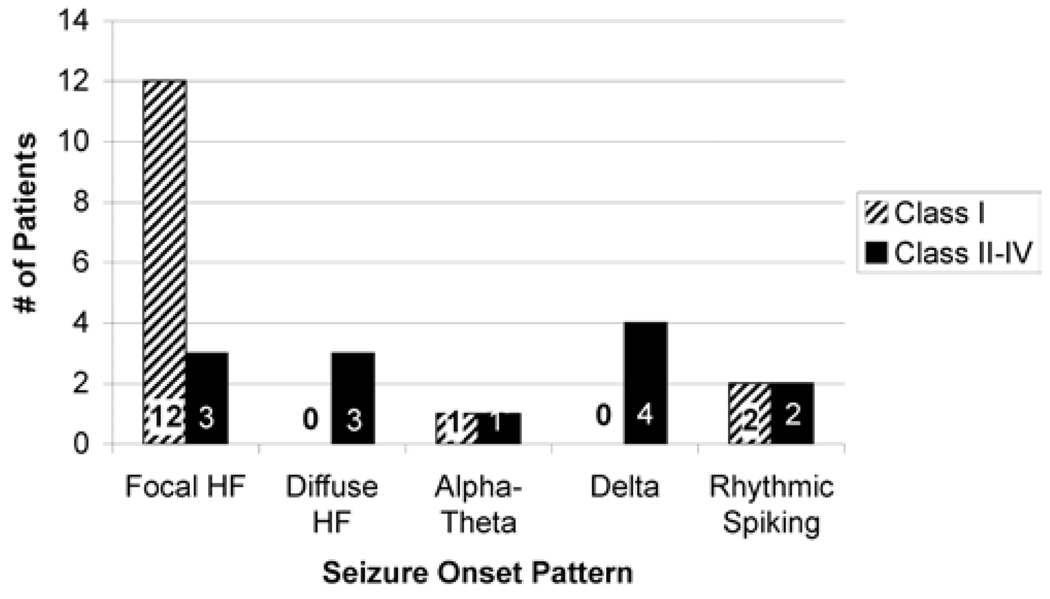
The most common iEEG pattern at seizure onset in the surgically treated group was a focal HFEO (in 15 [53.6%] of 28 patients), and 3 patients had diffuse HFEO. Four patients each had rhythmic spiking or δ wave activity at seizure onset, and 2 had α-θ activity (5–12 Hz). Twenty-six patients (92.9%) had frontal lobe neocortical resections, and 2 patients had parietal lobe resections. In most patients (25 [89.3%] of 28) who underwent resections, gliosis was noted on pathological examination, and the remainder had cortical dysplasia. There was no significant association between seizure onset type and region of seizure onset. All patients with cortical dysplasia had high-frequency seizure onset, but an association between the two was not statistically significant. On repeated review of the MR images in patients with cortical dysplasia, there was no abnormality apparent.
Ten (35.7%) of the 28 surgically treated patients were seizure free. Fourteen (50%) had Engel Class I outcomes,42 and overall 17 (60.7%) had significant improvement (Class I and IIAB with ≥ 80% seizure reduction). A focal HFEO at seizure onset was associated with Engel Class I surgical outcome (12 [85.7%] of 14 patients, p = 0.02), and it was uncommon in the Engel Class II–IV out-come group (3 [21.4%] of 14 patients, p = 0.02). Twelve (66.7%) of 18 patients with HFEO onset had Engel Class I surgical outcome, and all of these patients had focal on-sets that could be completely resected (Fig. 3). No patient with diffuse high-frequency onset or δ wave activity at onset had a good surgical outcome. There was no relationship between pathological findings or location of seizure onset and seizure outcome.
FIG 3.
Bar graph showing the relationship of seizure onset pattern on iEEG to surgical outcome. The presence of a focal high-frequency onset was correlated with Engel Class I outcome.
Discussion
Seizure outcomes are favorable in the majority of patients with mesial temporal lobe epilepsy and lesional neocortical epilepsy. Concordant results of electrophysiological and MR imaging studies have a predictive value for excellent surgical outcome (up to 80–90% in some series).41 However, MR imaging is ineffective in 20–30% of patients in localizing a lesion, particularly in those with cortical dysplasia and gliosis, and an increasing number of patients referred to epilepsy surgery centers have normal MR imaging results. Patients with intractable epilepsy and normal MR imaging results or nonlesional, neocortical substrate now represent 20–30% of patients undergoing presurgical evaluation at major epilepsy centers.12,16,38 The rate of seizure freedom in this challenging group of patients after surgery has been poor. Even in those deemed surgical candidates, only 30–50% achieve seizure freedom, thus demonstrating the present limitation of epilepsy surgery.19,27,40,43,49 Characteristics of neocortical epilepsy, such as widespread epileptogenic process, rapid propagation of ictal rhythm, and the presence of eloquent areas, contribute to a poor prognosis. In this study we provided further evidence that patients with nonlesional extratemporal neocortical epilepsy are more likely to have poor surgical outcomes. Only 35.7% of patients were seizure free after surgery, and only 50% had Engel Class I outcomes.
Previous studies that included patients with or without lesions on MR imaging and extratemporal partial epilepsy have shown a correlation between outcome and complete resection of a SISCOM abnormality.31 In the present study of patients with nonlesional MR imaging, we did not find an association between a localizing SPECT study and seizure outcome. However, it should be noted that only 15 of the patients had SISCOM performed in the current study. The current study is also biased by the fact that patients with positive findings on SPECT and SISCOM studies are more likely to be considered for epilepsy surgery.
Intracranial monitoring has been found to be indispensable in the diagnostic workup of patients with extratemporal epilepsy and normal MR imaging findings. The intracranial seizure onset pattern48 and spread24 have been suggested to be important in predicting seizure outcome. High-frequency discharges on iEEG are a frequent observation at the onset of neocortical seizures. We observed these discharges in 15 (53.6%) of 28 patients who underwent surgical evaluation for intractable nonlesional neocortical epilepsy. Although high-frequency oscillations have been previously described in human epileptic foci at seizure onset,1–4,6,20,23,34,48 most iEEG studies performed to date in humans as part of a presurgical evaluation have reported only a limited dynamic range (~ 0.1–40 Hz) of activity.35–38
Recently, the use of broadband digital EEG recordings and spectral analysis has allowed detection of high-frequency activity at the onset of neocortical seizures in adult3,4,20,48 and pediatric1,2,34,39 patients. Clinically, it appears beneficial to identify areas of focal high-frequency onset to improve surgical outcome. We identified 15 (53.6%) of 28 investigated patients with focal HFEOs at seizure onset and an additional 3 patients with diffuse high-frequency oscillations. Twelve of 14 patients with Engel Class I surgical outcome underwent resection of the region in which these focal high-frequency oscillations at seizure onset occurred. Resection of the area of seizure onset has been shown to improve seizure outcome. Identification of a high-frequency onset zone may be an important seizure onset pattern to identify to achieve this goal.
Conclusions
A focal high-frequency (> 20 Hz) seizure onset on iEEG may identify nonlesional extratemporal epilepsy patients likely to have an excellent (Engel Class I) outcome after epilepsy surgery. Overall, the chance of an excellent outcome in nonlesional extratemporal lobe epilepsy prior to intracranial monitoring is poor (14 [27%] of 51 patients). However, iEEG can further stratify patients and identify those with a high likelihood of Class I outcome after surgery. In fact, multiple studies4,5,23,46,49 have pointed to potentially useful information at frequencies > 100 Hz. Determination of the optimal clinical bandwidth for iEEG will have to wait for the results of ongoing clinical trials.
Abbreviations used in this paper
- HFEO
high-frequency epileptiform oscillation
- iEEG
intracranial electroencephalography
- SISCOM
subtracted ictal�interictal SPECT coregistered with MR imaging
Footnotes
Disclaimer
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
References
- 1.Akiyama T, Otsubo H, Ochi A, Galicia EZ, Weiss SK, Donner EJ, et al. Topographic movie of ictal high-frequency oscillations on the brain surface using subdural EEG in neocortical epilepsy. Epilepsia. 2006;47:1953–1957. doi: 10.1111/j.1528-1167.2006.00823.x. [DOI] [PubMed] [Google Scholar]
- 2.Akiyama T, Otsubo H, Ochi A, Ishiguro T, Kadokura G, Ramachandrannair R, et al. Focal cortical high-frequency oscillations trigger epileptic spasms: confirmation by digital video subdural EEG. Clin Neurophysiol. 2005;116:2819–2825. doi: 10.1016/j.clinph.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 3.Alarcon G, Binnie CD, Elwes RD, Polkey CE. Power spectrum and intracranial EEG patterns at seizure onset in partial epilepsy. Electroencephalogr Clin Neurophysiol. 1995;94:326–337. doi: 10.1016/0013-4694(94)00286-t. [DOI] [PubMed] [Google Scholar]
- 4.Allen PJ, Fish DR, Smith SJ. Very high-frequency rhythmic activity during SEEG suppression in frontal lobe epilepsy. Electroencephalogr Clin Neurophysiol. 1992;82:155–159. doi: 10.1016/0013-4694(92)90160-j. [DOI] [PubMed] [Google Scholar]
- 5.Bragin A, Engel J, Jr, Wilson CL, Fried I, Buzsaki G. High-frequency oscillations in human brain. Hippocampus. 1999;9:137–142. doi: 10.1002/(SICI)1098-1063(1999)9:2<137::AID-HIPO5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 6.Bragin A, Engel J, Jr, Wilson CL, Fried I, Mathern GW. Hippocampal and entorhinal cortex high-frequency oscillations (100–500 Hz) in human epileptic brain and in kainic acid–treated rats with chronic seizures. Epilepsia. 1999;40:127–137. doi: 10.1111/j.1528-1157.1999.tb02065.x. [DOI] [PubMed] [Google Scholar]
- 7.Buzsaki G. The hippocampo-neocortical dialogue. Cereb Cortex. 1996;6:81–92. doi: 10.1093/cercor/6.2.81. [DOI] [PubMed] [Google Scholar]
- 8.Buzsaki G. Memory consolidation during sleep: a neurophysiological perspective. J Sleep Res. 1998;7:17–23. doi: 10.1046/j.1365-2869.7.s1.3.x. [DOI] [PubMed] [Google Scholar]
- 9.Buzsaki G. Two-stage model of memory trace formation: a role for “noisy” brain states. Neuroscience. 1989;31:551–570. doi: 10.1016/0306-4522(89)90423-5. [DOI] [PubMed] [Google Scholar]
- 10.Cascino G, Boon P, Fish D. Surgically remedial lesional syndromes. In: Engel J Jr, editor. Surgical Treatment of the Epilepsies. New York: Raven Press; 1993. pp. 77–86. [Google Scholar]
- 11.Cascino GD. Commentary: how has neuroimaging improved patient care? Epilepsia. 1994;35:S103–S104. doi: 10.1111/j.1528-1157.1994.tb05983.x. [DOI] [PubMed] [Google Scholar]
- 12.Cascino GD, O’Brien TJ. Resection for epilepsy in the setting of a nonlocalizing MRI. In: Wyllie E, editor. Treatment of Epilepsy. Philadelphia: Lippincott Williams & Williams; 2001. pp. 1135–1145. [Google Scholar]
- 13.Cohen-Gadol AA, Britton JW, Wetjen NM, Marsh WR, Meyer FB, Raffel C. Neurostimulation therapy for epilepsy: current modalities and future directions. Mayo Clin Proc. 2003;78:238–248. doi: 10.4065/78.2.238. [DOI] [PubMed] [Google Scholar]
- 14.Curio G. Ain’t no rhythm fast enough: EEG bands beyond beta. J Clin Neurophysiol. 2000;17:339–340. doi: 10.1097/00004691-200007000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Curio G. Linking 600-Hz “spikelike” EEG/MEG wavelets (“sigma-bursts”) to cellular substrates: concepts and caveats. J Clin Neurophysiol. 2000;17:377–396. doi: 10.1097/00004691-200007000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Engel J., Jr . Outcome with respect to seizures. In: Engel J Jr, editor. Surgical Treatment of the Epilepsies. New York: Raven Press; 1987. pp. 553–571. [Google Scholar]
- 17.Engel J., Jr . Principles of epilepsy surgery. In: Shorvon S, Dreifuss F, Fish D, editors. The Treatment of Epilepsy. Oxford: Blackwell; 1996. pp. 519–529. [Google Scholar]
- 18.Engel J., Jr . Surgical Treatment of the Epilepsies. New York: Raven Press; 1987. [Google Scholar]
- 19.Engel J, Jr, Van Ness P, Rasmussen T. Outcome with respect to epileptic seizures. In: Engel J Jr, editor. Surgical Treatment of the Epilepsies. New York: Raven Press; 1993. pp. 609–621. [Google Scholar]
- 20.Fisher RS, Webber WR, Lesser RP, Arroyo S, Uematsu S. High-frequency EEG activity at the start of seizures. J Clin Neurophysiol. 1992;9:441–448. doi: 10.1097/00004691-199207010-00012. [DOI] [PubMed] [Google Scholar]
- 21.Gray CM. Synchronous oscillations in neuronal systems: mechanisms and functions. J Comput Neurosci. 1994;1:11–38. doi: 10.1007/BF00962716. [DOI] [PubMed] [Google Scholar]
- 22.Jack CR., Jr Magnetic resonance imaging. Neuroimaging and anatomy. Neuroimaging Clin N Am. 1995;5:597–622. [PubMed] [Google Scholar]
- 23.Jirsch JD, Urrestarazu E, LeVan P, Olivier A, Dubeau F, Gotman J. High-frequency oscillations during human focal seizures. Brain. 2006;129:1593–1608. doi: 10.1093/brain/awl085. [DOI] [PubMed] [Google Scholar]
- 24.Kutsy RL, Farrell DF, Ojemann GA. Ictal patterns of neocortical seizures monitored with intracranial electrodes: correlation with surgical outcome. Epilepsia. 1999;40:257–266. doi: 10.1111/j.1528-1157.1999.tb00702.x. [DOI] [PubMed] [Google Scholar]
- 25.Llinas R. Intrinsic electrical properties of nerve cells and their role in network oscillation. Cold Spring Harb Symp Quant Biol. 1990;55:933–938. doi: 10.1101/sqb.1990.055.01.087. [DOI] [PubMed] [Google Scholar]
- 26.Lüders H. Epilepsy Surgery. New York: Raven Press; 1992. [Google Scholar]
- 27.Mosewich RK, So EL, O’Brien TJ, Cascino GD, Sharbrough FW, Marsh WR, et al. Factors predictive of the outcome of frontal lobe epilepsy surgery. Epilepsia. 2000;41:843–849. doi: 10.1111/j.1528-1157.2000.tb00251.x. [DOI] [PubMed] [Google Scholar]
- 28.Nuñez PL. Electric Fields of the Brain: The Neurophysics of EEG. New York: Oxford University Press; 1981. [Google Scholar]
- 29.O’Brien TJ, O’Connor MK, Mullan BP, Brinkmann BH, Hanson D, Jack CR, et al. Subtraction ictal SPET co-registered to MRI in partial epilepsy: description and technical validation of the method with phantom and patient studies. Nucl Med Commun. 1998;19:31–45. doi: 10.1097/00006231-199801000-00006. [DOI] [PubMed] [Google Scholar]
- 30.O’Brien TJ, So EL, Cascino GD, Hauser MF, Marsh WR, Meyer FB, et al. Subtraction SPECT coregistered to MRI in focal malformations of cortical development: localization of the epileptogenic zone in epilepsy surgery candidates. Epilepsia. 2004;45:367–376. doi: 10.1111/j.0013-9580.2004.54703.x. [DOI] [PubMed] [Google Scholar]
- 31.O’Brien TJ, So EL, Mullan BP, Cascino GD, Hauser MF, Brinkmann BH, et al. Subtraction peri-ictal SPECT is predictive of extratemporal epilepsy surgery outcome. Neurology. 2000;55:1668–1677. doi: 10.1212/wnl.55.11.1668. [DOI] [PubMed] [Google Scholar]
- 32.O’Brien TJ, So EL, Mullan BP, Hauser MF, Brinkmann BH, Bohnen NI, et al. Subtraction ictal SPECT co-registered to MRI improves clinical usefulness of SPECT in localizing the surgical seizure focus. Neurology. 1998;50:445–454. doi: 10.1212/wnl.50.2.445. [DOI] [PubMed] [Google Scholar]
- 33.O’Brien TJ, So EL, Mullan BP, Hauser MF, Brinkmann BH, Jack CR, Jr, et al. Subtraction SPECT co-registered to MRI improves postictal SPECT localization of seizure foci. Neurology. 1999;52:137–146. doi: 10.1212/wnl.52.1.137. [DOI] [PubMed] [Google Scholar]
- 34.Ochi A, Otsubo H, Donner EJ, Elliott I, Iwata R, Funaki T, et al. Dynamic changes of ictal high-frequency oscillations in neocortical epilepsy: using multiple band frequency analysis. Epilepsia. 2007;48:286–296. doi: 10.1111/j.1528-1167.2007.00923.x. [DOI] [PubMed] [Google Scholar]
- 35.Quesney LF. Intracranial EEG investigation in neocortical epilepsy. Adv Neurol. 2000;84:253–274. [PubMed] [Google Scholar]
- 36.Quesney LF, Constain M, Rasmussen T, Olivier A, Palmini A. Presurgical EEG investigation in frontal lobe epilepsy. Epilepsy Res Suppl. 1992;5:55–69. [PubMed] [Google Scholar]
- 37.Schiller Y, Cascino GD, Busacker NE, Sharbrough FW. Characterization and comparison of local onset and remote propagated electrographic seizures recorded with intracranial electrodes. Epilepsia. 1998;39:380–388. doi: 10.1111/j.1528-1157.1998.tb01390.x. [DOI] [PubMed] [Google Scholar]
- 38.Spencer S, Sperling M, Shewmon D. Intracranial electrodes. In: Engel J Jr, Pedley T, editors. Epilepsy: A Comprehensive Textbook. Philadelphia: Lippincott-Raven Publishers; 1997. pp. 1719–1747. [Google Scholar]
- 39.Traub RD, Whittington MA, Buhl EH, LeBeau FE, Bibbig A, Boyd S, et al. A possible role for gap junctions in generation of very fast EEG oscillations preceding the onset of, and perhaps initiating, seizures. Epilepsia. 2001;42:153–170. doi: 10.1046/j.1528-1157.2001.26900.x. [DOI] [PubMed] [Google Scholar]
- 40.Van Ness P. Surgical outcome for neocortical focal epilepsy. In: Lüders H, editor. Epilepsy Surgery. New York: Raven Press; 1992. pp. 613–624. [Google Scholar]
- 41.Wetjen NM, Cohen-Gadol AA, Maher CO, Marsh WR, Meyer FB, Cascino GD. Frontal lobe epilepsy: diagnosis and surgical treatment. Neurosurg Rev. 2002;25:119–140. doi: 10.1007/s101430100174. [DOI] [PubMed] [Google Scholar]
- 42.Wieser HG, Blume WT, Fish D, Goldensohn E, Hufnagel A, King D, et al. ILAE Commission Report. Proposal for a new classification of outcome with respect to epileptic seizures following epilepsy surgery. Epilepsia. 2001;42:282–286. [PubMed] [Google Scholar]
- 43.Williamson P, Van Ness P, Weizer H, Quesney LF. Surgically remediable extratemporal syndromes. In: Engel J Jr, editor. Surgical Treatment of the Epilepsies. ed 2. New York: Raven Press; 1993. pp. 65–76. [Google Scholar]
- 44.Williamson P, Wieser H, Delgado-Escueta A. Clinical characteristics of partial seizures. In: Engel J Jr, editor. Surgical Treatment of the Epilepsies. New York: Raven Press; 1987. pp. 101–120. [Google Scholar]
- 45.Worrell GA, Cranstoun S, Echauz J, Litt B. Evidence for self-organized criticality in human epileptic hippocampus. Neuroreport. 2002;13:2017–2021. doi: 10.1097/00001756-200211150-00005. [DOI] [PubMed] [Google Scholar]
- 46.Worrell GA, Gardner A, Stead SM, Hu S, Goerss S, Cascino GJ, et al. High-frequency oscillations in human temporal lobe: simultaneous microwire and clinical macroelectrode recordings. Brain. 2008;131:928–937. doi: 10.1093/brain/awn006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Worrell GA, Lagerlund TD, Sharbrough FW, Brinkmann BH, Busacker NE, Cicora KM, et al. Localization of the epileptic focus by low-resolution electromagnetic tomography in patients with a lesion demonstrated by MRI. Brain Topogr. 2000;12:273–282. doi: 10.1023/a:1023407521772. [DOI] [PubMed] [Google Scholar]
- 48.Worrell GA, Parish L, Cranstoun SD, Jonas R, Baltuch G, Litt B. High-frequency oscillations and seizure generation in neocortical epilepsy. Brain. 2004;127:1496–1506. doi: 10.1093/brain/awh149. [DOI] [PubMed] [Google Scholar]
- 49.Worrell GA, So EL, Kazemi J, O’Brien TJ, Mosewich RK, Cascino GD, et al. Focal ictal beta discharge on scalp EEG predicts excellent outcome of frontal lobe epilepsy surgery. Epilepsia. 2002;43:277–282. doi: 10.1046/j.1528-1157.2002.37501.x. [DOI] [PubMed] [Google Scholar]