Abstract
Object
Cavernous hemangiomas associated with epilepsy present an interesting surgical dilemma in terms of whether one should perform a pure lesionectomy or tailored resection, especially in the temporal lobe given the potential for cognitive damage. This decision is often guided by electrocorticography (ECoG), despite the lack of data regarding its value in cavernoma surgery. The purpose of the present study was several-fold: first, to determine the epilepsy outcome following resection of cavernomas in all brain regions; second, to evaluate the usefulness of ECoG in guiding surgical decision making; and third, to determine the optimum surgical approach for temporal lobe cavernomas.
Methods
The authors identified from their surgical database 173 patients who had undergone resection of cavernomas. One hundred two of these patients presented with epilepsy, and 61 harbored temporal lobe cavernomas. Preoperatively, all patients were initially evaluated by an epileptologist. The mean follow-up was 37 months.
Results
Regardless of the cavernoma location, surgery resulted in an excellent seizure control rate: Engel Class I outcome in 88% of patients at 2 years postoperatively. Of 61 patients with temporal lobe cavernomas, the mesial structures were involved in 35. Among the patients with temporal lobe cavernomas, those who underwent ECoG typically had a more extensive parenchymal resection rather than a lesionectomy (p < 0.0001). The use of ECoG in cases of temporal lobe cavernomas resulted in a superior seizure-free outcome: 79% (29 patients) versus 91% (23 patients) of patients at 6 months postresection, 77% (22 patients) versus 90% (20 patients) at 1 year, and 79% (14 patients) versus 83% (18 patients) at 2 years without ECoG versus with ECoG, respectively.
Conclusions
The surgical removal of cavernomas most often leads to an excellent epilepsy outcome. In cases of temporal lobe cavernomas, the more extensive the ECoG-guided resection, the better the seizure outcome. In addition to upholding the concept of kindling, the data in this study support the use of ECoG in temporal lobe cavernoma surgery in patients presenting with epilepsy.
Keywords: cavernoma, electrocorticography, epilepsy
Cerebral cavernous hemangiomas, or cavernomas, are benign, low-flow, arteriolar vascular malformations consisting of thin, loosely organized, and collagenous vascular channels with no intervening neural parenchyma. It is believed that the majority of cavernomas are clinically cryptogenic, as they constitute 16% of vascular malformations at autopsy.1 One of the common clinical manifestations is seizures or epilepsy, occurring in 40–70% of patients presenting with these lesions.2,15
One of the issues regarding the surgical treatment of cavernoma-related epilepsy is the extent to which surrounding parenchyma is resected beyond the confines of the cavernoma.3–7, 16–18 Although it is clear that the excision of these vascular lesions leads to good outcomes in patients with recent-onset seizures—with ∼ 90% demonstrating a decrease in seizure frequency and 60–80% gaining seizure freedom3,4,15,16—it is unclear how aggressive resections should be in patients with medically refractory epilepsy. The need for more extensive surgery in patients with intractable epilepsy may be attributable to the induction of secondary epileptic foci through repetitive stimulation, or kindling.2 Various authors have proposed that the best degree of excision in this population remains conservative, removing only the vascular lesion, whereas others have supported removal of the lesion plus the surrounding hemosiderin.3–7,16,17 Some experienced epilepsy surgeons have advocated “tailored excisions,” that is, the use of ECoG intraoperatively as a means of identifying and removing secondary epileptic foci with more aggressive resection.2,22,23
The goal of the present study was to evaluate the outcome of these 2 basic approaches: in cases of temporal lobe cavernomas, does ECoG alter the surgical approach or outcome? Furthermore, in temporal lobe cavernomas, does lesionectomy as opposed to more aggressive therapies (most often formal lobectomies) alter seizure outcome? We have analyzed our experience to answer these questions.
Methods
Inclusion Criteria
The Mayo Clinic medical and surgical index databases were searched from 1971 to July 2006, and 173 surgically removed cavernomas were identified. Among these 173 cases were 105 patients who had presented with epilepsy before undergoing a resection. All patients were screened for neuropathological confirmation of a cavernous hemangioma and a history of seizures intractable to medical therapy. Six-month neurological and surgical follow-up data at least were available for all patients.
Demographic Evaluation
Collected data included patient sex, age at operation, duration of preoperative epilepsy, lesion location, preoperative electroencephalographic recordings, lesion size, use of intraoperative ECoG, resection performed, and seizure outcome. A neurologist with a subspecialty in epilepsy had evaluated all patients before and after surgery. Both the location and size of the cavernoma, based on surgical and radiological records, respectively, were recorded.
Intraoperative ECoG
Subdural grids and strips (Ad-Tech Medical Instrument Corporation) were used as necessary to span the borders of the vascular malformation. The individual electrode contacts were 4-mm-diameter platinum-iridium discs with a center-to-center electrode distance of 10 mm. Continuous ECoG recordings were collected using clinical digital acquisition systems Xltek (128-channel, 16-bit analog to digital and a sampling frequency of 500 Hz, Xltek Corp.) or NCI (128-channel, 16-bit analog to digital and a sampling frequency of 250 Hz, Lamont Medical). All analyses were performed using a high-pass filter of 1.0 Hz and a low-pass filter of 100 Hz (dynamic range 1–100 Hz). Bipolar and referential electrode montages were reviewed, and a digital 60-Hz notch filter was utilized when necessary to eliminate line noise. Regions of active interictal spiking or electrographic seizure activity were resected. Postoperative recordings were not obtained.
Resection Designation
For ease of data analysis, a resection was typed as a lesionectomy if documentation indicated the removal of a cavernoma or a cavernoma plus surrounding hemosiderin, or as a lobectomy if a more extensive resection had been performed, including a tailored resection, anterior temporal or frontal lobectomy, and amygdalohippocampectomy.
Seizure Outcome
Seizure outcome was assessed by epileptologists at intervals of 6 months and 1, 2, > 2, and > 5 years after treatment, based on the Engel classification system. Engel classes were designated as Class I, completely seizure free since surgery; Class II, rare disabling and nondisabling seizures occurring within 2 years of follow-up and nocturnal seizures; Class III, worthwhile seizure reduction according to patient reports; and Class IV, no appreciable change in, or worse, seizures.13 For purposes of simplicity in this paper we considered seizure-free rates only (that is, Engel Class I); all other cases were considered treatment failures.
Statistical Analysis
Demographic tables where created and compared for group differences within each data set by using the chi-square test and ANOVA, as appropriate. A Kaplan-Meier analysis of seizure outcome was performed, as previously described by Cohen-Gadol et al.11 A probability level < 0.05 was considered statistically significant.
Results
Overall Patient Population
Data from 102 patients with cavernomas who had presented with medically intractable epilepsy were analyzed. Sixty-four surgeries were related to temporal lobe cavernomas (in 61 patients), and of these procedures 37 were undertaken in the mesial temporal lobe (35 patients) and 27 (26 patients) in the neocortical region. The mean age at surgery, mean size of the cavernoma, male/female ratios, and duration of epilepsy prior to surgery are presented in Table 1. Overall seizure freedom following the resection of a cavernoma was 85, 87, and 88% of patients at 6 months, 1 year, and 2 years posttreatment, respectively, regardless of the lesion location. Further division of the 64 procedures based on lesion location is available in Table 2. It is important to note that in terms of outcome and among the 64 procedures, 61 were unique; the repeat surgery data were not used to calculate outcome data. Demographic information with regard to the cavernoma location is presented in Table 1. The average follow-up was 37 months.
TABLE 1. Characteristics associated with resections of cavernomas due to epilepsy.
| Temporal | Extratemporal | |||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | Overall Total* | Total† | Mesial | Neocortical | Total‡ | Frontal | Parietal | Occipital |
| total no. of surgeries | 105 | 64 | 37 | 27 | 41 | 21 | 14 | 6 |
| M/F patients | 58/47 | 36/28 | 21/16 | 15/12 | 22/19 | 14/7 | 7/7 | 1/5 |
| mean age at surgery (yrs) | 35.4 ± 15 | 40.1 ± 15§ | 42.6 ± 14 | 36.7 ± 16 | 28.1 ± 14§ | 29.6 ± 15 | 29.1 ± 13 | 20.7 ± 14 |
| mean no. of mos w/ epilepsy before surgery | 9 ± 9 | 9 ± 10 | 9 ± 10 | 9 ± 11 | 8 ± 7 | 4 ± 3 | 11 ± 7 | 11 ± 8 |
| side of lesion (rt/lt) | 54/51 | 35/29 | 19/18 | 16/11 | 19/22 | 10/11 | 7/7 | 2/4 |
| mean lesion size (cm) | 1.8 ± 0.9 | 1.7 ± 0.9 | 1.6 ± 0.7 | 1.9 ± 1.1 | 2.0 ± 1.0 | 2.4 ± 1.2 | 1.6 ± 0.8 | 1.9 ± 0.7 |
Refers to total for cavernomas associated with epilepsy, as recorded in the institutional database.
Refers to total for temporal lobe cavernomas associated with epilepsy, as recorded in the institutional database.
Refers to total for extratemporal cavernomas associated with epilepsy, as recorded in the institutional database.
According to ANOVA, age was statistically significantly different between patients with temporal and those with extratemporal cavernomas (p < 0.001); otherwise, there were no statistically significant differences.
TABLE 2. Seizure outcome as measured by Engel Class, according to cavernoma location*.
| Region & FU | Engel Class (no. of unique procedures) | No. of Patients Lost to FU | % of Patients Seizure Free | ||||
|---|---|---|---|---|---|---|---|
| I | II | III | IV | Total | |||
| temporal | |||||||
| mesial | |||||||
| 6 mos | 25 | 0 | 3 | 1 | 29 | 6 | 86 |
| 1 yr | 21 | 2 | 2 | 0 | 25 | 10 | 84 |
| 2 yrs | 16 | 1 | 1 | 0 | 18 | 17 | 89 |
| neocortical | |||||||
| 6 mos | 21 | 1 | 1 | 2 | 25 | 1 | 84 |
| 1 yr | 17 | 0 | 1 | 2 | 20 | 6 | 85 |
| 2 yrs | 13 | 0 | 1 | 2 | 16 | 10 | 81 |
| total | |||||||
| 6 mos | 46 | 1 | 4 | 3 | 54 | 7 | 85 |
| 1 yr | 38 | 2 | 3 | 2 | 45 | 16 | 84 |
| 2 yrs | 29 | 1 | 2 | 2 | 34 | 27 | 85 |
| extratemporal | |||||||
| frontal | |||||||
| 6 mos | 12 | 2 | 0 | 2 | 16 | 5 | 75 |
| 1 yr | 8 | 1 | 0 | 0 | 9 | 12 | 89 |
| 2 yrs | 4 | 0 | 0 | 0 | 4 | 17 | 100 |
| parietal | |||||||
| 6 mos | 11 | 0 | 0 | 1 | 12 | 2 | 92 |
| 1 yr | 10 | 0 | 0 | 1 | 11 | 3 | 91 |
| 2 yrs | 7 | 0 | 0 | 1 | 8 | 6 | 88 |
| occipital | |||||||
| 6 mos | 5 | 0 | 0 | 0 | 5 | 1 | 100 |
| 1 yr | 3 | 0 | 0 | 0 | 3 | 3 | 100 |
| 2 yrs | 2 | 0 | 0 | 0 | 2 | 4 | 100 |
| total | |||||||
| 6 mos | 28 | 2 | 0 | 3 | 33 | 8 | 85 |
| 1 yr | 21 | 1 | 0 | 1 | 23 | 18 | 91 |
| 2 yrs | 13 | 0 | 0 | 1 | 14 | 27 | 93 |
| overall | |||||||
| 6 mos | 74 | 3 | 4 | 6 | 87 | 15 | 85 |
| 1 yr | 59 | 3 | 3 | 3 | 68 | 34 | 87 |
| 2 yrs | 42 | 1 | 2 | 3 | 48 | 54 | 88 |
FU = follow-up.
Lesionectomy Versus Lobectomy
Lesionectomy versus aggressive resection is most often a question in the temporal lobe; often one must clinically decide whether to perform an anterior temporal lobectomy or a pure lesionectomy. This dilemma also presents itself in some cases when considering the frontal lobe but fortunately so infrequently that analysis of such data would be fruitless in the present study. Among patients with preexisting epilepsy who had undergone lesionectomy as opposed to lobectomy of a temporal lobe cavernoma, 79 (29 patients) versus 91% of patients (23 patients) were seizure free at 6 months (p = 0.22), 77 (22 patients) versus 90% (20 patients) at 1 year (p = 0.26), and 80 (15 patients) versus 88% (17 patients) at 2 years (p = 0.28), respectively. None of these differences were statistically significant, although there appeared to be a trend toward an increased percentage of patients with a seizure-free status following a more aggressive resection (Fig. 1 upper). A Kaplan-Meier analysis of a seizure-free status with respect to time (months) demonstrated a tendency for more patients treated with lobectomy to be seizure free, but this difference was not statistically significant (p = 0.21; Fig. 1 lower). Statistically, there were no differences in patient age (39.4 ± 2.3 vs 43 ± 2.7 years, p = 0.29) or lesion size (1.7 ± 0.2 vs 1.7 ± 0.2 cm, p = 0.85) between those who underwent lesionectomy and those who underwent lobectomy, respectively, according to ANOVA (Table 3). It is notable that patients more frequently underwent an aggressive resection if the lesion was mesial rather than neocortical. There was a very strong correlation (p < 0.0001) between the use of ECoG and more aggressive resections. Of those patients who did not undergo ECoG (38 patients), only 9 had an aggressive resection; of those who did undergo ECoG analysis (23 patients), 17 underwent an aggressive resection involving at least an anterior temporal lobectomy.
Fig. 1.
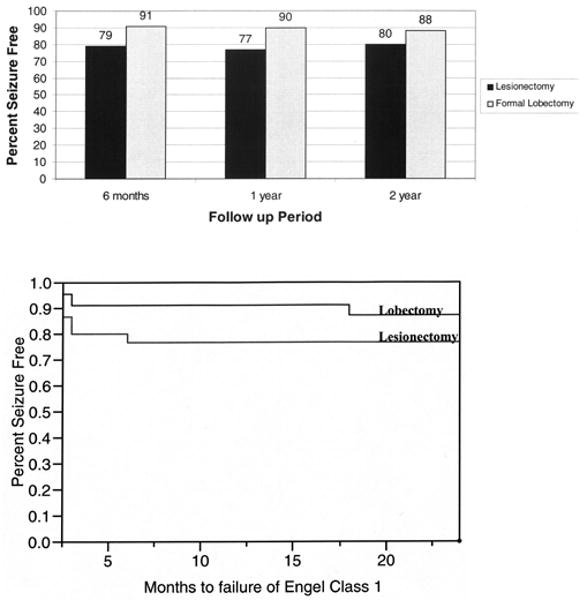
Upper: Bar graph demonstrating the seizure-free status in patients with preexisting epilepsy who have undergone temporal lobe cavernoma resection: lesionectomy versus lobectomy. None of the differences in seizure-free rates were statistically significant, although there appears to be a trend toward an increased seizure-free status with more aggressive resections. Lower: Kaplan-Meier plot showing the seizure-free status with respect to time (months). Note a trend in patients undergoing lobectomy to have higher seizure-free status rates, although the difference is not statistically significant.
TABLE 3. Demographics of patients who underwent surgical resection of temporal cavernomas with or without ECoG*.
| Parameter | Lesionectomy | Lobectomy | p Value | No ECoG | ECoG | p Value |
|---|---|---|---|---|---|---|
| total no. of patients | 35 | 26 | 38 | 23 | ||
| M/F | 22/13 | 13/13 | 0.3 | 22/16 | 13/10 | 0.3 |
| mean age at surgery (yrs) | 39.4 ± 2.2 | 43 ± 2.7 | 0.3 | 38.6 ± 2.2 | 44.9 ± 2.8 | 0.08 |
| mean no. of yrs before surgery | 8.5 ± 1.8 | 9.9 ± 2.1 | 0.6 | 7.7 ± 1.8 | 10.9 ± 2.1 | 0.3 |
| side of lesion (lt/rt) | 21/14 | 6/20 | <0.05 | 21/17 | 6/17 | <0.05 |
| mean lesion size (cm) | 1.7 ± 0.2 | 1.7 ± 0.2 | 0.9 | 1.9 ± 0.2 | 1.4 ± 0.2 | <0.05 |
Note that ECoG and lobectomy were significantly associated with right-sided lesions and that ECoG was more frequently used with smaller cavernomas. Ordinal and nominal variables were analyzed using chi-square analysis and continuous variables with ANOVA. p < 0.05 was considered statistically significant.
No ECoG Versus ECoG
Electrocorticography in our practice is most often used in cases of temporal lobe lesions given the clinical worries of associated mesial temporal seizure generation from associated cavernomas. In fact, only twice was ECoG used in cases of lesions outside the temporal lobe during the entire review period of this study. Among patients who underwent temporal lobe resection with or without ECoG 91 (23 patients) versus 79% of patients (29 patients) were seizure free at 6 months, 90 (20 patients) versus 77% (22 patients) at 1 year, and 83 (18 patients) versus 79% (14 patients) at 2 years, respectively (Fig. 2 upper). None of these differences was statistically significant; however, there appeared to be a trend toward an increased seizure-free status with the use of ECoG. A Kaplan-Meier analysis of seizure-free status with respect to time (months) demonstrated a trend toward higher seizure-free rates among patients who underwent ECoG, although this trend was not statistically significant (Fig. 2 lower). Patients who underwent ECoG had mean smaller cavernomas (1.4 ± 0.2 vs 1.9 ± 0.2 cm, p = 0.03), although there was no statistical difference between the sexes (Table 3). Electrocorticography was more frequently used in cases of right-sided lesions (p = 0.03), meaning that more patients' nondominant temporal lobes were subject to resection. Furthermore, we noted that those with less frequent seizures underwent ECoG more often (p = 0.0104).
Fig. 2.
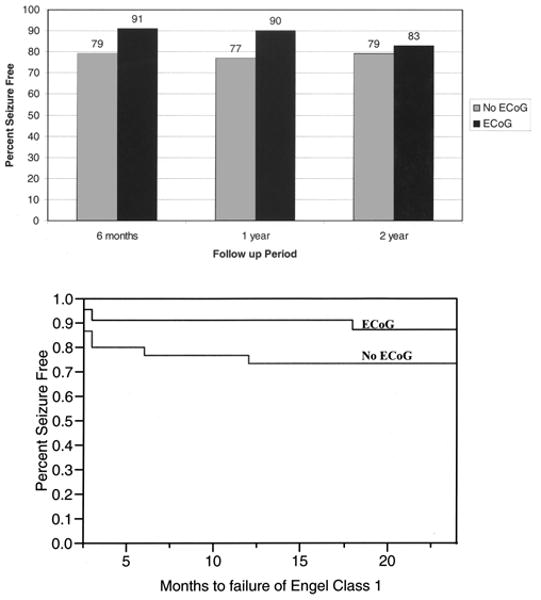
Upper: Bar graph revealing the seizure-free status in patients with preexisting epilepsy who have undergone temporal lobe cavernoma resection with or without ECoG. The differences between those who did or did not undergo ECoG were not statistically significant; however, there does appear to be a trend toward increased seizure-free status with the use of ECoG. Lower: Kaplan-Meier plot depicting the seizure-free status with respect to time (months). Note a trend in patients undergoing ECoG to have higher seizure-free status rates, although the difference is not statistically significant.
Discussion
Overall Outcome
Surgical removal of cavernous hemangiomas is an excellent means of treating the associated medically intractable epilepsy. In terms of demographics, the patients featured in the present report were on average between 5 and 10 years older at surgery than patients in the largest series reported to date.3,18 This difference seems to correspond to an older age in patients presenting with temporal cavernomas and an overall increased number of temporal lobe cavernomas among our series (Table 1). Moreover, the distribution of cavernomas in our series varied from that in the most recent metaanalysis of all reported cavernomas associated with epilepsy: temporal, 61 versus 34%; frontal, 20 versus 33%; and parietal, 13 versus 21%, respectively. 18 Note, however, that the seizure-free outcomes among our patients were similar overall and according to the location of resection.3,18 As documented by the literature, seizure freedom can be expected in 60–90% of patients.3,4,8,12,16,18 We recorded similar results, with overall seizure freedom in 85, 87, and 88% of patients at 6 months, 1 year, and 2 years after treatment, respectively.
Lesionectomy Versus Lobectomy
The mechanism of epileptic activity associated with cavernous malformations remains elusive but may be related to perilesional changes in amino acids, metabolic imbalance, edema, pH changes, or phenotypic changes in the glia. In cavernomas, however, the mechanism is believed to be primarily associated with changes in surrounding neural tissue due to the deposition of hemosiderin and iron products.14,21,24,25 The deposition of these compounds is most evident in the easily identifiable hemosiderin ring on T2-weighted MR imaging as well as in discolored brain during resection.4,15 The hemosiderin and iron products may be responsible for the transformation of adjacent neural tissue into permanent epileptic foci.15 Williamson et al.25 have demonstrated that remote mesial temporal cells increase the probability of generating prolonged and high-amplitude postsynaptic potentials along with hyperexcitable synaptic responses when comparing pericavernomas and peritumoral cells. As mentioned earlier, removing the lesion and adjacent brain parenchyma is an excellent treatment for new-onset cavernoma-related epilepsy but may be inadequate.4,9
Most studies of cavernomas have been focused on whether one should resect the cavernoma alone or include the surrounding hemosiderin-stained brain.4 Authors have detected an absolute increase of 5–15% in seizure-free survival when the hemosiderin-stained tissue as well as the cavernoma is resected and removal is confirmed on postoperative MR imaging, but these studies are underpowered as case numbers vary from 20–60 patients.4,6,8 In the present study we took the additional step of determining whether a more extensive resection (lobectomy) would lead to a seizure-free status when other testing had indicated that a lesionectomy alone likely would not result in seizure freedom. The finding of increased seizure freedom related to more aggressive resection has also been suggested by Hammen et al.17 as well as other authors;20,22 however, the Hammen et al. study included only 30 patients and relatively few aggressive resections (11 patients). A more aggressive resection does appear to improve seizure freedom—with 12, 13, and 8% absolute increases in seizure freedom at 6 months, 1 year, and 2 years after treatment, respectively—compared with patients who have undergone lesionectomy, although this difference is not statistically significant. As noted in our results, ECoG strongly and significantly correlates with a more extensive resection, and therefore alters the surgical approach at our institution.
Usefulness of ECoG
The excision of cavernomas and adjacent tissue in patients with long-standing epilepsy remains under debate because of the possible formation of secondary epileptic foci, particularly affecting the temporal cortex.2,10 Secondary epileptic foci can arise after long-term exposure to seizures through spontaneous transformation into hyperactive tissue.10 As demonstrated by Ferrier et al.,15 cavernomas associated with a long history of epilepsy display continuous spiking similar to that from highly epileptogenic neurodevelopmental lesions, supporting the proposal of newly formed secondary epileptic foci. Moreover, Sugano et el.23 have reported better outcomes when spike-positive parenchyma is excised in temporal lobe mass lesions. Thus, the removal of these regions of cortex would, in theory, offer better outcomes.19
In our own analysis we evaluated seizure freedom and the use of ECoG compared with non-ECoG cases specifically in the temporal cortex, as this location is where we most often clinically perform this procedure and where it seems to be the most useful. The intraoperative use of ECoG appeared to improve the chances of seizure freedom—with 12, 13, and 4% absolute increases in seizure freedom at 6 months, 1 year, and 2 years posttreatment, respectively—compared with cases not involving ECoG, although this difference was not statistically significant. Furthermore, the use of ECoG very strongly correlated with more aggressive resections and right-sided procedures. Although peripherally more aggressive resections did not lead to any significant differences with respect to complications, the excision of healthy brain parenchyma is a cause for concern as we did not perform extensive neurocognitive follow-up testing.
Despite fairly sizable increases in seizure-free survival, in 61 unique patients with a > 10% absolute increase at 6 months and 1 year for both the use of ECoG and the value of a more aggressive resection, these absolute increases did not reach statistical significance on formal testing. This finding is not surprising considering that we assessed a binomial variable, that is, seizure freedom versus no seizure freedom. In performing a power analysis, to obtain a 10% absolute difference in survival between these 2 groups, one would need at least 87 patients in each outcome group. To detect a difference of 5%, one would need at least 377 patients in either group. These numbers of course vary for specific percentage differences: if one wants to compare a 10% difference using a rate of 45 versus 55% in seizure-free survival, one would need 381 patients per group to have the minimum acceptable power (80%). If one is comparing a seizure-free survival rate of 89 versus 99%, 93 patients per group would be needed. In our series, an observed 10% difference for a reasonable seizure-free outcome of 75 versus 85% would require at least 254 patients per group. For 80 versus 85%, one would need a minimum of 908 patients per group. We believe that if there was in fact a 5% increase in seizure-free survival—or better yet, a 10% increase—most clinicians would perform ECoG. Due to the underpowering of the present study and based on our data, we believe that ECoG and more aggressive surgical therapy likely enhance seizure-free survival. However, we cannot advocate more aggressive surgical therapy as we did not accurately assess the complications in our review, and thus more aggressive surgery, especially in the left temporal lobe, may have decreased language or memory at the follow-up. If one extrapolates our data to current temporal lobectomy complication data, there is certainly adequate literature to base expected complications of more aggressive resections such as temporal lobectomy.
Conclusions
In summary, ECoG does alter surgical therapy, most often leading to a very significant change in the type of procedure performed. Our data suggest that ECoG does improve outcomes, and given the strong correlation between ECoG and aggressive resection, lobectomy appears to improve seizure-freedom rates as well. Note, however, that our study appears to be underpowered. We advocate the use of ECoG to guide surgical therapy for cavernomas, but we recognize that the potential complications of more aggressive therapy should be weighed against seizure freedom.
Abbreviations used in this paper
- ANOVA
analysis of variance
- ECoG
electrocorticography
Footnotes
Disclaimer: The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
References
- 1.Aiba T, Tanaka R, Koike T, Kameyama S, Takeda N, Komata T. Natural history of intracranial cavernous malformations. J Neurosurg. 1995;83:56–59. doi: 10.3171/jns.1995.83.1.0056. [DOI] [PubMed] [Google Scholar]
- 2.Awad I, Jabbour P. Cerebral cavernous malformations and epilepsy. Neurosurg Focus. 2006;21(1):E7. doi: 10.3171/foc.2006.21.1.8. [DOI] [PubMed] [Google Scholar]
- 3.Baumann CR, Acciarri N, Bertalanffy H, Devinsky O, Elger CE, Lo RG, et al. Seizure outcome after resection of supratentorial cavernous malformations: a study of 168 patients. Epilepsia. 2007;48:559–563. doi: 10.1111/j.1528-1167.2006.00941.x. [DOI] [PubMed] [Google Scholar]
- 4.Baumann CR, Schuknecht B, Lo RG, Cossu M, Citterio A, Andermann F, et al. Seizure outcome after resection of cavernous malformations is better when surrounding hemosiderin-stained brain also is removed. Epilepsia. 2006;47:563–566. doi: 10.1111/j.1528-1167.2006.00468.x. [DOI] [PubMed] [Google Scholar]
- 5.Bidzinski J, Marchel A. Lesionectomy in surgical treatment of epilepsy. Neurol Neurochir Pol. 1998;32(2 Suppl):69–79. [PubMed] [Google Scholar]
- 6.Cappabianca P, Alfieri A, Maiuri F, Mariniello G, Cirillo S, de Divitiis E. Supratentorial cavernous malformations and epilepsy: seizure outcome after lesionectomy on a series of 35 patients. Clin Neurol Neurosurg. 1997;99:179–183. doi: 10.1016/s0303-8467(97)00023-1. [DOI] [PubMed] [Google Scholar]
- 7.Casazza M, Avanzini G, Ciceri E, Spreafico R, Broggi G. Lesionectomy in epileptogenic temporal lobe lesions: preoperative seizure course and postoperative outcome. Acta Neurochir Suppl. 1997;68:64–69. doi: 10.1007/978-3-7091-6513-3_12. [DOI] [PubMed] [Google Scholar]
- 8.Casazza M, Broggi G, Franzini A, Avanzini G, Spreafico R, Bracchi M, et al. Supratentorial cavernous angiomas and epileptic seizures: preoperative course and postoperative outcome. Neurosurgery. 1996;39:26–32. doi: 10.1097/00006123-199607000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Cascino GD, Kelly PJ, Sharbrough FW, Hulihan JF, Hirschorn KA, Trenerry MR. Long-term follow-up of stereotactic lesionectomy in partial epilepsy: predictive factors and electroencephalographic results. Epilepsia. 1992;33:639–644. doi: 10.1111/j.1528-1157.1992.tb02340.x. [DOI] [PubMed] [Google Scholar]
- 10.Cohen DS, Zubay GP, Goodman RR. Seizure outcome after lesionectomy for cavernous malformations. J Neurosurg. 1995;83:237–242. doi: 10.3171/jns.1995.83.2.0237. [DOI] [PubMed] [Google Scholar]
- 11.Cohen-Gadol AA, Wilhelmi BG, Collignon F, White JB, Britton JW, Cambier DM, et al. Long-term outcome of epilepsy surgery among 399 patients with nonlesional seizure foci including mesial temporal lobe sclerosis. J Neurosurg. 2006;104:513–524. doi: 10.3171/jns.2006.104.4.513. [DOI] [PubMed] [Google Scholar]
- 12.D'Angelo VA, De Bonis C, Amoroso R, Cali A, D'Agruma L, Guarnieri V, et al. Supratentorial cerebral cavernous malformations: clinical, surgical, and genetic involvement. Neurosurg Focus. 2006;21(1):E9. doi: 10.3171/foc.2006.21.1.10. [DOI] [PubMed] [Google Scholar]
- 13.Engel J., Jr Epilepsy surgery. Curr Opin Neurol. 1994;7:140–147. doi: 10.1097/00019052-199404000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Engstrom ER, Hillered L, Flink R, Kihlstrom L, Lindquist C, Nie JX, et al. Extracellular amino acid levels measured with intracerebral microdialysis in the model of posttraumatic epilepsy induced by intracortical iron injection. Epilepsy Res. 2001;43:135–144. doi: 10.1016/s0920-1211(00)00191-1. [DOI] [PubMed] [Google Scholar]
- 15.Ferrier CH, Aronica E, Leijten FS, Spliet WG, Boer K, van Rijen PC, et al. Electrocorticography discharge patterns in patients with a cavernous hemangioma and pharmacoresistent epilepsy. J Neurosurg. 2007;107:495–503. doi: 10.3171/JNS-07/09/0495. [DOI] [PubMed] [Google Scholar]
- 16.Ferroli P, Casazza M, Marras C, Mendola C, Franzini A, Broggi G. Cerebral cavernomas and seizures: a retrospective study on 163 patients who underwent pure lesionectomy. Neurol Sci. 2006;26:390–394. doi: 10.1007/s10072-006-0521-2. [DOI] [PubMed] [Google Scholar]
- 17.Hammen T, Romstock J, Dorfler A, Kerling F, Buchfelder M, Stefan H. Prediction of postoperative outcome with special respect to removal of hemosiderin fringe: a study in patients with cavernous haemangiomas associated with symptomatic epilepsy. Seizure. 2007;16:248–253. doi: 10.1016/j.seizure.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Moran NF, Fish DR, Kitchen N, Shorvon S, Kendall BE, Stevens JM. Supratentorial cavernous haemangiomas and epilepsy: a review of the literature and case series. J Neurol Neurosurg Psychiatry. 1999;66:561–568. doi: 10.1136/jnnp.66.5.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paolini S, Morace R, Di Gennaro G, Picardi A, Grammaldo LG, Meldolesi GN, et al. Drug-resistant temporal lobe epilepsy due to cavernous malformations. Neurosurg Focus. 2006;21(1):E8. doi: 10.3171/foc.2006.21.1.9. [DOI] [PubMed] [Google Scholar]
- 20.Siegel AM, Roberts DW, Harbaugh RE, Williamson PD. Pure lesionectomy versus tailored epilepsy surgery in treatment of cavernous malformations presenting with epilepsy. Neurosurg Rev. 2000;23:80–83. doi: 10.1007/pl00021697. [DOI] [PubMed] [Google Scholar]
- 21.Singh R, Pathak DN. Lipid peroxidation and glutathione peroxidase, glutathione reductase, superoxide dismutase, catalase, and glucose-6-phosphate dehydrogenase activities in Fe-Cl3-induced epileptogenic foci in the rat brain. Epilepsia. 1990;31:15–26. doi: 10.1111/j.1528-1157.1990.tb05354.x. [DOI] [PubMed] [Google Scholar]
- 22.Stefan H, Hammen T. Cavernous haemangiomas, epilepsy and treatment strategies. Acta Neurol Scand. 2004;110:393–397. doi: 10.1111/j.1600-0404.2004.00333.x. [DOI] [PubMed] [Google Scholar]
- 23.Sugano H, Shimizu H, Sunaga S. Efficacy of intraoperative electrocorticography for assessing seizure outcomes in intractable epilepsy patients with temporal-lobe-mass lesions. Seizure. 2007;16:120–127. doi: 10.1016/j.seizure.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 24.Ueda Y, Willmore LJ, Triggs WJ. Amygdalar injection of FeCl3 causes spontaneous recurrent seizures. Exp Neurol. 1998;153:123–127. doi: 10.1006/exnr.1998.6869. [DOI] [PubMed] [Google Scholar]
- 25.Williamson A, Patrylo PR, Lee S, Spencer DD. Physiology of human cortical neurons adjacent to cavernous malformations and tumors. Epilepsia. 2003;44:1413–1419. doi: 10.1046/j.1528-1157.2003.23603.x. [DOI] [PubMed] [Google Scholar]


