Summary
Purpose
To determine the long-term efficacy of anterior temporal lobectomy for medically refractory temporal lobe epilepsy in patients with nonlesional magnetic resonance imaging (MRI).
Methods
We identified a retrospective cohort of 44 patients with a nonlesional modern “seizure protocol” MRI who underwent anterior temporal lobectomy for treatment of medically refractory partial epilepsy. Postoperative seizure freedom was determined by Kaplan-Meyer survival analysis. Noninvasive preoperative diagnostic factors potentially associated with excellent surgical outcome were examined by univariate analysis in the 40 patients with follow-up of >1 year.
Results
Engel class I outcomes (free of disabling seizures) were observed in 60% (24 of 40) patients. Preoperative factors associated with Engel class I outcome were: (1) absence of contralateral or extratemporal interictal epileptiform discharges, (2) subtraction ictal single photon emission computed tomography (SPECT) Coregistered to MRI (SISCOM) abnormality localized to the resection site, and (3) subtle nonspecific MRI findings in the mesial temporal lobe concordant to the resection.
Discussion
In carefully selected patients with temporal lobe epilepsy and a nonlesional MRI, anterior temporal lobectomy can often render patients free of disabling seizures. This favorable rate of surgical success is likely due to the detection of concordant abnormalities that indicate unilateral temporal lobe epilepsy in patients with nonlesional MRI.
Keywords: Partial seizures, Epilepsy surgery, Temporal lobe, Nonlesional–MRI
Partial seizure disorders account for the majority of epilepsy (Sander et al., 1990). In one tertiary center cohort of 2,200 patients, partial epilepsy was more than twice as common as symptomatic, cryptogenic, and idiopathic generalized epilepsies combined. Moreover, in that study, two-thirds of the partial seizure disorders localized to the temporal lobe (Semah et al., 1998). Unfortunately, despite optimal medical therapy, approximately 30% of patients continue to experience recurrent seizures (Sander et al., 1990 Wiebe, et al. 2001). In a recent well-designed trial studying patients who failed initial medication trials, seizure freedom was achieved in only 8% of patients with continued medical therapy compared to 58% of patients undergoing anterior temporal lobectomy (Wiebe, et al. 2001).
However, physicians may be reluctant to consider surgery for temporal lobe epilepsy when structural neuroimaging appears normal. Temporal lobectomy has been shown to render about 80% of patients seizure-free in the setting of a magnetic resonance imaging (MRI)–apparent structural abnormality concordant to the seizure onset zone, such as mesial temporal sclerosis (MTS) (Cascino, 2004). On the other hand, patients with temporal lobe epilepsy and normal MRI have received less attention. In order to demonstrate the absence of a potentially epileptogenic structural lesion, it is critical that patients have high-resolution seizure protocol MRI with both T1- and T2-weighted images (Jack, 1996; Cascino, 2004). There are relatively few studies examining temporal lobectomy in patients with normal modern seizure protocol MRI. Existing research suggests significantly disparate rates of successful surgery in patients with nonlesional MRI, ranging from 18–63% of patients becoming seizure-free (Berkovic et al., 1995; Theodore et al., 1997; Sylaja et al., 2004; Cohen-Gadol et al., 2005; Jeha et al., 2006). Many of these studies were conducted during the 1980s and early 1990s (Berkovic et al., 1995; Radhakrishnan et al., 1998; McIntosh et al., 2004), prior to the widespread use of epilepsy neuroimaging protocols that are more sensitive for detecting MTS (Jack, 1996; Jack et al., 1996). Some of these studies used pathologic findings to categorize patients (McIntosh et al., 2004; Cohen-Gadol et al., 2006), information that is not available preoperatively for clinical prognostication. Other studies include heterogeneous patient populations and only small numbers of patients with nonlesional MRI (Holmes et al., 2000; Sylaja et al., 2004; Cohen-Gadol et al., 2005). Therefore, we sought to examine the efficacy of epilepsy surgery and noninvasive predictors of favorable outcome for patients with medically refractory temporal lobe epilepsy and a nonlesional high-resolution seizure protocol MRI.
Methods
Subjects
Following Mayo Clinic Investigational Review Board approval, we retrospectively identified 272 patients ≥13 years of age with temporal lobe epilepsy, who underwent anterior temporal lobectomy between January 1997 and June 2005 at the Mayo Clinic in Rochester, MN, U.S.A. From these 272 patients we identified 44 patients who had a nonlesional preoperative “seizure protocol” MRIs interpreted by a board-certified neuroradiologist at our institution. Nonlesional MRI was defined as being normal or as showing only nonspecific white matter abnormalities and/or diffuse cerebral atrophy.
Charts were reviewed to determine patient characteristics including (Table 1): age at surgery, duration of epilepsy, gender, history of febrile seizures, significant head trauma, meningitis, encephalitis, perinatal distress, family history of epilepsy, and history of generalized seizures or status epilepticus. Noninvasive presurgical studies included (Table 2): neuropsychological evaluation, seizure protocol MRI, interictal and ictal scalp electroencephalography (EEG), single photon emission computed tomography (SPECT), and positron emission tomography (PET). Lastly, a subset of patients had invasive presurgical studies that included the Wada test (Trenerry & Loring, 2005), chronic intracranial EEG monitoring, and intraoperative electrocorticography.
Table 1. Characteristics of the 44 patients in the cohort.
| n (%) | Average | Range | |
|---|---|---|---|
| Age at surgery, years | 36 | 13–62 | |
| Duration of epilepsy prior to surgery, years | 16 | 1–36 | |
| Female | 27 (61%) | ||
| History of febrile seizures | 5 (11%) | ||
| History of significant head traumaa | 8 (18%) | ||
| History of meningitis or encephalitis | 2 (5%) | ||
| History of perinatal distressb | 1 (2%) | ||
| Family history of epilepsy | 13 (30%) | ||
| No known epilepsy risk factors | 19 (43%) | ||
| History of generalized tonic–clonic seizure | 34 (77%) | ||
| History of status epilepticus | 0 | ||
| Patients with <1 year follow-upc | 4 (9%) |
Resulting in loss of consciousness.
Born premature (34 weeks of gestation).
Excluded from study except Kaplan-Meyer survival analysis.
Table 2. Preoperative factors of 40 patients with Engel class I surgical outcome (free of disabling seizures) versus Engel class II–IV (continued disabling seizures)a.
| Class I (n = 24) | Class II–IV (n = 16) | p-valueb | |
|---|---|---|---|
| Scalp EEG | |||
| Extratemporal/contralateral discharges | 4 | 7 | 0.04 |
| No extratemporal/contralateral discharges | 20 | 9 | |
| Ictal EEG | |||
| Type 1 pattern | 20 | 13 | 1.0 |
| Type 2 or 3 pattern | 4 | 3 | |
| SISCOM | |||
| Localized to resection | 18 | 9 | 0.04 |
| Not localized to resection | 1 | 5 | |
| PET | |||
| Localized to resection | 9 | 4 | 1.0 |
| Not localized to resection | 3 | 2 | |
| MRI re-review | |||
| Subtle concordant abnormality | 10 | 1 | 0.03 |
| No concordant abnormality | 14 | 15 | |
| Neuropsychological testing | |||
| Localized or lateralized to resection | 8 | 4 | 0.73 |
| Nonlateralized | 14 | 10 | |
| Side of resection | |||
| Dominant (average 36 mm resection) | 15 | 8 | 0.52 |
| Nondominant (average 42 mm resection) | 9 | 8 |
Excluded from this analysis if <1 year postoperative follow-up (four patients).
Fisher's exact test. Using the Bonferroni correction, significance requires p ≤ 0.007.
Presurgical noninvasive testing
Neuropsychological testing (Table 3)
Table 3. Neuropsychological testing before and after temporal lobectomy.
| Presurgery | Postsurgery | |
|---|---|---|
| VCa,b,c | ||
| Left (12) | 85.5 | 81 |
| Right (13) | 97.5 | 101 |
| COWAT | ||
| Left (16) | 27.8 | 27 |
| Right (12) | 29.1 | 29 |
| TMT | ||
| Left (16) | 83.1 | 90.9 |
| Right (11) | 81.4 | 71.5 |
| SFTb,c | ||
| Left (12) | 30.6 | 27.3 |
| Right (11) | 40.5 | 43.4 |
| BNTb,c | ||
| Left (12) | 44.8 | 31.9 |
| Right (15) | 50.4 | 52.9 |
| AVLTa,b | ||
| Left (11) | 5.5 | 1.1 |
| Right (5) | 12.8 | 12.2 |
Statistically significant difference prior to surgery.
Statistically significant difference after surgery.
Statistically significant change associated with surgery.
VC, Verbal Comprehension Test; COWAT, Controlled Oral Word Association Test; TMT, Trails Making Test (Trails B); SFT, Semantic Fluency Test (animals, fruits, vegetables); BNT, Boston Naming Test; AVLT, Auditory Verbal Learning Test.
Neuropsychological testing of memory, language, and intelligence was obtained prior to temporal lobectomy and repeated at 6 months after surgery. Selected neuropsychological testing results reviewed included: verbal comprehension quotients from a Wechsler intelligence scale, a test of lexical verbal fluency (Controlled Oral Word Association), Trail Making Test, a semantic fluency test (animals, fruits, vegetables), Boston Naming Test, and the Auditory Verbal Learning Test.
Structural MRI
The seizure protocol MRIs were performed on 1.5 or 3 T magnets and included coronal T1-spoiled gradient (SPGR), coronal fluid-attenuated inversion-recovery (FLAIR), sagittal T1, and axial T2 spin-echo sequences. Thin slices through the temporal lobes were obtained, with 1.6-mm slice thickness for the coronal T1 and 4-mm slices for the coronal FLAIR (Jack, 1996). Patients with radiographically apparent MTS or other structural abnormalities or lesions mentioned in the neuroradiologist's report were excluded.
MRI re-review
All patients had presented at our institution's multidisciplinary epilepsy surgical conference. At the conference, neurologists, neurosurgeons, and neuroradiologists with expertise in epilepsy reviewed the clinical, neuroimaging, and prolonged inpatient video-EEG recordings. The conference attendees then discussed the risks and benefits of surgery, and consensus recommendations were subsequently conveyed to the patient.
Conference records were examined to determine if the second review of the neuroimaging identified abnormalities not mentioned in the initial radiology reports (initial interpretations were by board-certified neuroradiologist). If probable MTS or a focal lesion was appreciated at the conference the patient was excluded from the study. Patients who had subtle indeterminate abnormalities appreciated during MRI re-review, however, were not excluded from the study. These subtle indeterminate abnormalities included: (1) possible hippocampal atrophy without FLAIR hyperintensity (Jack, 1996), (2) hippocampal FLAIR hyperintensity without atrophy, and (3) amygdala FLAIR signal or volume asymmetry. Each of the preceding indeterminate MRI abnormalities were believed to be of uncertain significance at the time of surgery conference. Subsequent quantitative hippocampal volume studies for these patient showed that none had hippocampal atrophy or asymmetry greater than one standard deviation of control populations (Jack, 1996).
Scalp EEG recording
All patients had routine interictal scalp EEG and continuous video scalp EEG monitoring to record seizures. Scalp EEG recordings were obtained with 31 electrodes [modified 10–20 montage with subtemporal electrodes (Sharbrough et al., 1991)] using a Cz reference electrode and a sampling rate of 200 Hz. Interictal epileptiform discharges were characterized as temporal (ipsilateral or contralateral to surgery), extratemporal, or generalized.
Ictal EEG seizure patterns were categorized as previously described by Ebersole and Pacia (1996). Type I: seizures characterized by a progressive buildup of a regular 5–9 Hz EEG rhythm of uniform morphology persisting for greater than 5 s with the same morphology and localized principally to the subtemporal and temporal electrodes. Type IIA&B: Type IIA seizures characterized by irregular EEG rhythms in the 2–5 Hz range that were lateralized to one hemisphere, but less often were localized only to the temporal electrodes. These rhythms exhibited little stability, and changes in morphology and frequency occurred every few seconds. If type IIA pattern was followed by type I seizure pattern it was termed type IIB. Type III: seizures without a distinct EEG ictal discharge. In these patients, clinical seizure activity was accompanied by EEG showing an interruption of normal background activity and often diffuse irregular slowing. Type 1 seizure patterns have been reported to be associated with seizures originating in the hippocampus, and type II and III patterns with temporal neocortical seizures.
Functional neuroimaging studies
Ictal and interictal SPECT (O'Brien et al., 1998) and interictal [18F] Fluoro-deoxyglucose PET (Theodore et al., 1997) studies were performed. Subtraction ictal SPECT Co-registered to MRI (SISCOM) (O'Brien et al., 1998) and PET studies were characterized as unilateral or bilateral temporal, extratemporal, or indeterminate.
Invasive presurgical evaluation, surgery, pathology, and outcome
Wada testing
Patients with unclear language lateralization, and those likely to be at risk for significant postoperative memory decline (i.e., dominant temporal lobe resections) underwent Wada testing (Trenerry & Loring, 2005).
Intracranial EEG
Patients with discordant noninvasive testing had chronic intracranial EEG monitoring with bilateral temporal depth electrodes and subdural grid electrodes.
Surgery
All patients had standard anterior temporal lobectomies that included anterior temporal neocortex and amygdalohippocampal structures. The surgical resection included the entire amygdalohippocampal structure and 45–50 mm of the anterior temporal neocortex for nondominant temporal lobe, and 30–35 mm of anterior temporal neocortex for dominant temporal lobe surgeries (Radhakrishnan et al., 1998).
Pathology
Pathologic tissue analysis was reviewed by a board-certified neuropathologist (CG) (Table 4). Hippocampal subfields were qualitatively reviewed for neuronal cell loss and evidence of MTS. Immunohistochemical stains (glial fibrillary acidic protein, neuronal nuclear antigen (NeuN), and in selected cases neurofilament and synaptophysin) were used as ancillary studies to identify pathologic gliosis, MTS, and cortical dysplasia.
Table 4. Pathologic findings of 40 patients with Engel class I outcome (free of disabling seizures) versus Engel class II–IV (continued disabling seizures).
| Class I (n=24) | Class II–IV (n=16) | p-valuea | |
|---|---|---|---|
| Pathology | |||
| Mesial temporal sclerosis | 4 | 3 | |
| Gliosis only | 19 | 13 | 1.0 |
| Focal malformation of cortical development | 1 | 0 |
Fisher’s exact test comparing outcomes of patients withmesial temporal sclerosis (MTS) versus gliosis.
Outcome assessment
Prior to data collection, Engel class I outcome (free of disabling seizures) versus Engel class II–IV was selected as the primary endpoint. Patient outcome and follow-up was obtained by chart review, mail out questionnaire, and telephone contact. Patients with less than 1 year of postoperative follow-up were included in the Kaplan-Meyer Engel class I survival analysis to eliminate selection bias, but they were excluded from the analysis of presurgical studies and seizure outcome to avoid confounding results due to inadequate follow-up.
Statistical methods
Because of the cohort size, multivariate analysis and more detailed distinction between Engel outcome class was not feasible. The relationship of preoperative patient characteristics to outcome was analyzed by univariate analysis using the Fisher's exact test. We prospectively selected seven noninvasive preoperative factors for investigation to determine if they relate to outcome in this cohort (Table 2). Using the Bonferroni correction statistic, significance required p ≤ 0.007. Evidence for significant difference in the neuropsychometric scores of patients with left and right temporal lobe epilepsy, and change in neuropsychometric scores after surgery was investigated using analysis of variance (ANOVA). Specifically, we looked for a significant difference in mean scores across all groups (right, left, presurgical, and postsurgical) using ANOVA.
Results
Of the 44 patients meeting study inclusion criteria, four had less than a year of postoperative follow-up and were excluded from the study, except for the Kaplan-Meyer survival analysis. Mean follow-up duration of the remaining 40 patients was 4.2 years [median 3.4 years, range (1.5–10.5 years)].
Of 40 patients with 1 year or more of follow-up, 24 (60%) remained free of disabling seizures at terminal follow-up (Engel class I). Of the 24 patients with Engel class I outcome [International League Against Epilepsy (ILAE) Class I & II (Wieser et al., 2001)], 19 (47.5%) were free of all seizures including auras (ILAE Class I). Fig. 1 shows a Kaplan-Meyer curve depicting Engel class I survival. All patients who did not have recurrent seizures until after 3 months following surgery ultimately had only rare disabling seizures (Engel class II outcome). This occurred in six patients (15%). The 10 patients (25%) with Engel class III or IV outcomes all had seizure recurrence within the first 3 months after surgery. Of the 24 patients (60%) who were free of disabling seizures, only one patient (4%) had successfully discontinued anticonvulsant medications.
Figure 1.
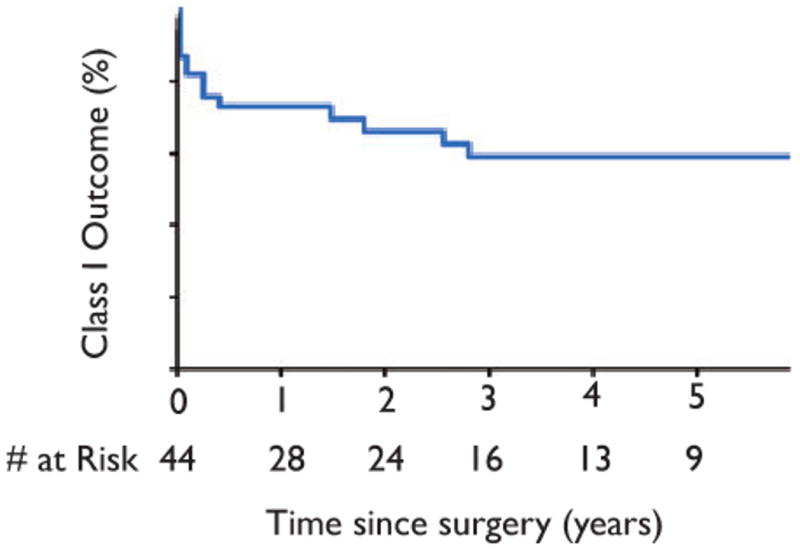
Kaplan–Meyer curve of Engel class I survival.
Epilepsia © ILAE
Patient characteristics
Table 1 depicts the patient characteristics of the cohort. The study involved teens and adults, with a mean age of 36 years. Most patients had a long history of epilepsy, averaging 16 years, perhaps underscoring a reluctance to pursue epilepsy surgery in these patients. Most patients had previously had generalized tonic–clonic seizures. Prior generalized seizures did not correlate with poor outcome, as has been previously reported in patients with temporal lobe epilepsy related to MTS (Hennessy et al., 2001). None of the study patients had a history of status epilepticus.
More than half of the cohort had an epilepsy risk factor; however, risk factors related to MTS were infrequent. Only five patients had a history of febrile seizures, and only two had prior meningitis or encephalitis (Table 3).
Neuropsychological testing
The available neuropsychological data for these patients varied, with different testing paradigms used over the decade of patient enrollment (Table 3). Nonetheless, on an individual basis the same neuropsychological tests were applied before and after surgery.
The mean Verbal Comprehension test (VC) showed a difference across evaluations. Prior to surgery the VC for right temporal patients was higher than that for the left temporal patients [right = 97.5, left = 85.5, F(1,23) = 5.24, p < 0.05]. Surgery was associated with a significant change, and the difference between right and left temporal patients widened [right = 101.2, left = 81.0, F(1,23) = 13.3, p < 0.05] resulting in a significant interaction term [F(1,23) = 10.13, p < .005].
The mean semantic fluency test (animals, fruits, vegetables) showed a difference across evaluations. Prior to surgery the mean performance was not significantly different [right M = 40.46, left M = 32.6, F(1,21) = 2.61, p > 0.05]. But after surgery the tests show a significant decline in patients undergoing left temporal lobectomy [right M = 43.36, left M = 27.33, F(1,21) = 18.6, p < 0.05], and the interaction term was significant [F(1,21) = 8.35, p < .01].
The mean Boston Naming Test (BNT) showed a significant difference between patients with right and left temporal epilepsy after surgery. Prior to surgery, patients with right temporal epilepsy had a slightly higher score compared to those with left temporal epilepsy, but this difference was not statistically significant [right = 50.4, left = 44.8, F(1,25) = 3.55, p > 0.05]. After surgery the difference widened and patients with right temporal lobe epilepsy had a significantly higher score than did patients with left temporal epilepsy [right = 52.9, left = 31.9, F(1,25) = 36.99, p < 0.0001]. The interaction term was significant [F(1,25) = 16.66, p < .0005].
The mean Auditory Verbal Learning Test (AVLT) showed a significant difference across evaluations. The patients with right temporal lobe epilepsy recalled more words at the 30 min delay before surgery than did the patients with left temporal epilepsy [right = 12.8, left = 5.5, F(1,14) = 14.15, p < 0.05]. After surgery, this difference widened [right = 12.2, left = 1.09, F(1,14) = 110.58, p < 0.05], but the interaction term was not statistically significant, probably due to the limited sample size [F(1,14) = 3.31, p > .05].
The Controlled Oral Word Association Test (COWAT) and Trail Making Test (TNT), were not significantly different between left and right temporal lobe epilepsy patients before or after surgery.
Preoperative factors
Seven noninvasive preoperative factors were prospectively selected and evaluated as potential predictors of outcome (Table 2). None achieved significance, as the Bonferroni correction required p ≤ 0.007 for significance. However, the Bonferroni correction is likely overly severe (Altman, 1991), and a trend toward better outcome was present when scalp EEG contained no extratemporal or contralateral discharges (p = 0.04) and when SISCOM was localized to the resection (p = 0.04).
MRI re-review was performed at the multidisciplinary epilepsy surgical conference prior to surgery. Subtle MRI abnormalities were often appreciated in the cohort that had not been appreciated in the initial neuroradiology report. Patients felt to have probable MTS or focal lesions on re-review were excluded from the study. However, 11 patients had subtle concordant abnormalities on MRI re-review of uncertain significance (see Fig. 2). These patients trended toward better outcomes (p = 0.03). The abnormalities seen were: (1) subtle hippocampal atrophy without abnormal FLAIR signal (three patients), (2) subtle hippocampal FLAIR signal without atrophy (seven patients), and (3) amygdala enlargement (one patient).
Figure 2.
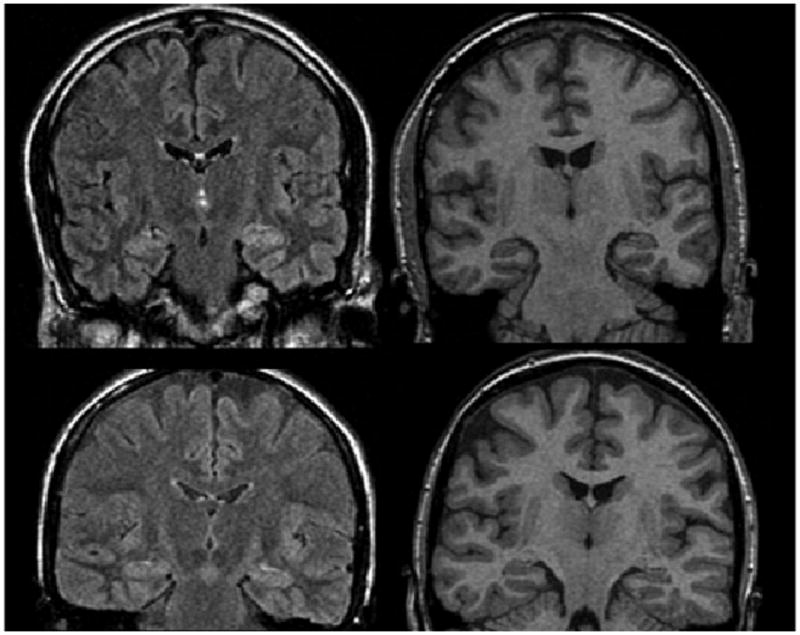
Examples of subtle abnormalities appreciated on MRI re-review. Upper panel shows FLAIR (left) and T1-SPGR (right) images of subtle left hippocampal signal abnormality without atrophy. Lower panel shows FLAIR (left) and T1-SPGR (right) images of subtle left hippocampal atrophy without signal abnormality.
Epilepsia © ILAE
Each patient had their habitual seizures recorded with video scalp EEG. The EEG seizure onset type [I, II, or III (Ebersole & Pacia, 1996)] was not associated with outcome class. However, this result is confounded by the fact that the majority of patients had type I ictal EEG pattern.
Invasive studies
Twenty-two patients had intracarotid amobarbital testing [Wada test, (Trenerry & Loring, 2005)] to lateralize language, and to help quantify the risk of verbal memory decline with surgery (Rausch et al., 2003, Stroup et al., 2003). Patients with left temporal epilepsy were all found to be left hemisphere dominant for language and at risk for clinically significant verbal memory decline after surgery. Only nine patients (22.5% of 40 patients) had chronic intracranial EEG monitoring. These patients had noninvasive preoperative test that were discordant and did not clearly lateralize the side of seizure origin. These patients had scalp EEG recorded seizures potentially arising from brain regions outside the planned anterior temporal lobe resection (eight patients with independent bitemporal onset seizures, and one patient with posterior neocortical onset seizures). Of these patients, only three became seizure-free after surgery.
Thirty-seven patients (93%) had intraoperative electrocorticography using subdural strips over the superior and inferior temporal gyri, and depth electrodes placed orthogonally through the middle temporal gyrus into the amygdala and hippocampus to help guide the extent of resection. Resections were less extensive when performed on the dominant hemisphere (average 33 mm) compared to the nondominant hemisphere (average 42 mm). However, outcomes did not differ significantly between dominant and nondominant resections.
Pathologic tissue examination demonstrated gliosis in resected tissue of most patients (80%) (Table 4), and MTS was identified in only 7 of 40 patients (18%). One patient was shown to have cortical dysplasia (Taylor type 2 with balloon cells).
Discussion
Our results demonstrate that epilepsy surgery can be effective for carefully selected patients with temporal lobe epilepsy and a nonlesional MRI. In our cohort, 60% became free of disabling seizures following anterior temporal lobectomy. Another 15% of patients had a substantial reduction in seizure frequency (Engel class II outcome).
Our study shows that identification of good candidates for temporal lobectomy is possible even when MRI does not show an obvious epileptogenic lesion. The cohort consisted largely of good surgical candidates based on noninvasive studies, aside from the lack of structural abnormalities on neuroimaging. This is consistent with the small number of patients with chronic intracranial monitoring (25%) despite the nonlesional MRI. Other noninvasive tests help to identify these patients by virtue of the concordance of the test results. Most of our patients had interictal discharges exclusively or predominantly involving the region of resection, well-localized ictal scalp EEG patterns, and SISCOM and/or PET studies showing abnormalities concordant to the surgery. In addition, most patients had type I ictal scalp EEG patterns, which has been associated with good outcomes in patients with temporal lobe epilepsy (Holmes et al., 2000; Sylaja et al., 2004). Therefore, lower rates of surgical success in other studies could relate to patient selection, type of surgery performed, and technologic limitations, such as the lack of functional neuroimaging (Sylaja et al., 2004).
The only preoperative tests that were associated with Engel class I outcome were interictal scalp EEG, and SISCOM hyperperfusion concordant with the temporal lobe resection. Note, however, PET was not available at our institution until 2003 and relatively few patients in this study had PET imaging. PET imaging has been reported previously to be associated with seizure-free outcome (Theodore et al., 1997; Carne et al., 2004).
Some of the patients in this study had pathologically confirmed MTS. One might speculate that subtle MTS, perhaps not appreciated by the pathologist, could explain the surgical success seen in the cohort. If so, in the future, better neuroimaging techniques and volumetric analysis of the mesial temporal lobes could potentially distinguish patients with a higher chance of favorable surgical outcome. On the other hand, MTS was only occasionally present on pathologic review (7 of 40 patients, 18%). The majority of pathologic specimens contained merely gliosis. Of those with gliosis, 19 of 32 were seizure-free (59%). Therefore, as others have also suggested (Holmes et al., 2000; Cohen-Gadol et al., 2005) temporal lobe epilepsy with a nonlesional modern MRI may largely represent a pathophysiologically distinct but still surgically remediable entity.
Moreover, we found that the subtle hippocampal alterations appreciated on MRI re-review rarely correlated with pathologic MTS. Only 3 of the 10 patients with subtle indeterminate hippocampal alterations appreciated on MRI re-review had pathologic evidence of MTS. The subtle MRI alterations of the hippocampus or amygdala did tend to correlate with better outcomes, however. These abnormalities may indicate subtle structural alterations or seizure-related edema (Cohen-Gadol et al., 2004; Briellmann et al., 2005).
The MRI re-review findings underscore the critical role of a multidisciplinary epilepsy surgical conference in the presurgical evaluation of patients with epilepsy. Several patients were excluded from this study after probable MTS was appreciated in the conference setting, after the MRI had initially been interpreted as normal by a neuroradiologist. Moreover, subtle alterations were appreciated in 28% of patients after repeated scrutiny by neuroradiologists, neurologists, and neurosurgeons with expertise in epilepsy. The MRI re-review was performed after the phase I epilepsy monitoring unit evaluation, and, therefore, was likely influenced by clinical history, electrophysiology data, seizure semiology, and functional neuroimaging results (SISCOM, PET, and MRS).
The pre- and postoperative neuropsychological tests demonstrate that patients with right and left temporal lobe epilepsy are neuropsychologically different, with left temporal patients tending to have lower verbal abilities compared to right temporal patients at presentation, and decline after surgery (Rausch et al., 2003). Patients with left temporal lobe epilepsy had deficits on measures of semantic fluency and confrontation naming. Delayed verbal memory also declined after surgery, but the data for this observation are limited. Other abilities that might be considered as lexical fluency and rapid executive function were not different between patients with left and right temporal lobe epilepsy, and seemed stable across evaluations.
Conclusion
In conclusion, a nonlesional MRI does not preclude successful epilepsy surgery for temporal lobe epilepsy. The goal of this study was to investigate whether noninvasive diagnostic studies could identify patients likely to achieve seizure-free outcomes after temporal lobectomy. We find that in the setting of favorable electrophysiologic and functional neuroimaging findings, excellent outcomes are possible in many patients. Subtle MRI alterations of the mesial temporal lobe, strictly concordant interictal discharges, and concordant SISCOM localization may correlate with favorable surgical outcomes. As neuroimaging evolves, with the advent of more sensitive MRI modalities and increasing magnet strength, subtle abnormalities may become more readily apparent. Further research will be needed to determine if our observations remain relevant.
These outcome results demonstrate that patients who fail epilepsy surgery (not Engel class I) generally do so within the first few months of surgery. In addition, despite the reasonable success rate of epilepsy surgery in this patient group, only one patient had discontinued anticonvulsant medications. Despite medically intractable seizures originating from the dominant temporal lobe many patients continue to perform within the normal range on verbal memory tests. The neuropsychological outcome of patients with nonlesional temporal lobe epilepsy strongly depends on the lateralization of seizures and surgery, and is consistent with previous reports (Trenerry et al., 1993, 1996; Rausch et al., 2003; Stroup et al., 2003). The most clinically significant consequence is the decline in verbal memory for patients undergoing dominant temporal lobectomy. Patients undergoing dominant temporal lobectomy should be counseled about the likelihood of clinically significant verbal memory decline (Ivnik et al., 1988).
A significant limitation of the current study is the uncertainty of seizure onset localization, that is, medial temporal versus neocortical temporal. Because only one patient had a grid covering neocortex, and all the patients had resections that included anterior temporal neocortex, amygdala, and hippocampus, it is not possible to differentiate neocortical and mesial temporal lobe epilepsy surgery outcomes in this study.
Acknowledgments
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Footnotes
Disclosure: The authors have nothing to disclose.
References
- Altman DG. Practical statistics for medical research. Chapman and Hall; London England: 1991. [Google Scholar]
- Berkovic SF, McIntosh AM, Kalnins RM, Jackson GD, Fabinyi GC, Brazenor GA, Bladin PF, Hopper JL. Preoperative MRI predicts outcome of temporal lobectomy: an actuarial analysis. Neurology. 1995;45:1358–1363. doi: 10.1212/wnl.45.7.1358. [DOI] [PubMed] [Google Scholar]
- Briellmann RS, Wellard RM, Jackson GD. Seizure-associated Abnormalities in Epilepsy: Evidence from MR Imaging. Epilepsia. 2005;46:760–766. doi: 10.1111/j.1528-1167.2005.47604.x. [DOI] [PubMed] [Google Scholar]
- Carne RP, O'Brien TJ, Kilpatrick CJ, MacGregor LR, Hicks RJ, Murphy MA, Bowden SC, Kaye AH, Cook MJ. MRI-negative PET-positive temporal lobe epilepsy: a distinct surgically remediable syndrome. Brain. 2004;127(Pt10):2276–2285. doi: 10.1093/brain/awh257. [DOI] [PubMed] [Google Scholar]
- Cascino GD. Surgical treatment for epilepsy. Epilepsy Res. 2004;60:179–186. doi: 10.1016/j.eplepsyres.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Cohen-Gadol AA, Britton JW, Worrell GA, Meyer FB. Transient cortical abnormalities on magnetic resonance imaging after status epilepticus: case report. Surg Neurol. 2004;61:479–482. doi: 10.1016/S0090-3019(03)00540-8. discussion 482. [DOI] [PubMed] [Google Scholar]
- Cohen-Gadol AA, Bradley CC, Williamson A, Kim JH, Westerveld M, Duckrow RB, Spencer DD. Normal magnetic resonance imaging and medial temporal lobe epilepsy: the clinical syndrome of paradoxical temporal lobe epilepsy. J Neurosurg. 2005;102:902–909. doi: 10.3171/jns.2005.102.5.0902. [DOI] [PubMed] [Google Scholar]
- Cohen-Gadol AA, Wilhelmi BG, Collignon F, White JB, Britton JW, Cambier DM, Christianson TJ, Marsh WR, Meyer FB, Cascino GD. Long-term outcome of epilepsy surgery among 399 patients with non-lesional seizure foci including mesial temporal lobe sclerosis. J Neurosurg. 2006;104:513–524. doi: 10.3171/jns.2006.104.4.513. [DOI] [PubMed] [Google Scholar]
- Ebersole JS, Pacia SV. Localization of temporal lobe foci by ictal EEG patterns. Epilepsia. 1996;37:386–399. doi: 10.1111/j.1528-1157.1996.tb00577.x. [DOI] [PubMed] [Google Scholar]
- Hennessy MJ, Elwes RD, Rabe-Hesketh S, Binnie CD, Polkey CE. Prognostic factors in the surgical treatment of medically intractable epilepsy associated with mesial temporal sclerosis. Acta Neurol Scand. 2001;103:344–350. doi: 10.1034/j.1600-0404.2001.103006344.x. [DOI] [PubMed] [Google Scholar]
- Holmes MD, Born DE, Kutsy RL, Wilensky AJ, Ojemann GA, Ojemann LM. Outcome after surgery in patients with refractory temporal lobe epilepsy and normal MRI. Seizure. 2000;9:407–411. doi: 10.1053/seiz.2000.0423. [DOI] [PubMed] [Google Scholar]
- Ivnik RJ, Sharbrough FW, Laws ER., Jr Anterior temporal lobectomy for the control of partial complex seizures: information for counseling patients. Mayo Clinic Proc. 1988;63:783–793. doi: 10.1016/s0025-6196(12)62358-1. [DOI] [PubMed] [Google Scholar]
- Jack CR., Jr Magnetic resonance imaging in epilepsy. Mayo Clin Proc. 1996;71:695–711. doi: 10.1016/S0025-6196(11)63008-5. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr, Rydberg CH, Krecke KN, Trenerry MR, Parisi JE, Rydberg JN, Cascino GD, Riederer SJ. Mesial temporal sclerosis: diagnosis with fluid-attenuated inversion-recovery versus spin-echo MR imaging. Radiology. 1996;199:367–373. doi: 10.1148/radiology.199.2.8668780. see comment. [DOI] [PubMed] [Google Scholar]
- Jeha LE, Najm IM, Bingaman WE, Khandwala F, Widdess-Walsh P, Morris HH, Dinner DS, Nair D, Foldvary-Schaeffer N, Prayson RA, Comair Y, O'Brien R, Bulacio J, Gupta A, Luders HO. Predictors of outcome after temporal lobectomy for the treatment of intractable epilepsy. Neurology. 2006;66:1938–1940. doi: 10.1212/01.wnl.0000219810.71010.9b. [DOI] [PubMed] [Google Scholar]
- McIntosh AM, Kalnins RM, Mitchell LA, Fabinyi GC, Briellmann RS, Berkovic SF. Temporal lobectomy: long-term seizure outcome, late recurrence and risks for seizure recurrence. Brain. 2004;127:2018–2030. doi: 10.1093/brain/awh221. [DOI] [PubMed] [Google Scholar]
- O'Brien TJ, So EL, Mullan BP, Hauser MF, Brinkmann BH, Bohnen NI, Hanson D, Cascino GD, Jack CR, Jr, Sharbrough FW. Subtraction ictal SPECT co-registered to MRI improves clinical usefulness of SPECT in localizing the surgical seizure focus. Neurology. 1998;50:445–454. doi: 10.1212/wnl.50.2.445. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan K, So EL, Silbert PL, Jack CR, Jr, Cascino GD, Sharbrough FW, O'Brien PC. Predictors of outcome of anterior temporal lobectomy for intractable epilepsy: a multivariate study. Neurology. 1998;51:465–471. doi: 10.1212/wnl.51.2.465. [DOI] [PubMed] [Google Scholar]
- Rausch R, Kraemer S, Pietras CJ, Le M, Vickrey BG, Passaro EA. Early and late cognitive changes following temporal lobe surgery for epilepsy. Neurology. 2003;60:951–959. doi: 10.1212/01.wnl.0000048203.23766.a1. [DOI] [PubMed] [Google Scholar]
- Sander JW, Hart YM, Johnson AL, Shorvon SD. National General Practice Study of Epilepsy: newly diagnosed epileptic seizures in a general population. Lancet. 1990;336:1267–1271. doi: 10.1016/0140-6736(90)92959-l. [DOI] [PubMed] [Google Scholar]
- Semah F, Picot MC, Adam C, Broglin D, Arzimanoglou A, Bazin B, Cavalcanti D, Baulac M. Is the underlying cause of epilepsy a major prognostic factor for recurrence? Neurology. 1998;51:1256–1262. doi: 10.1212/wnl.51.5.1256. [DOI] [PubMed] [Google Scholar]
- Sharbrough F, Chatrian GE, Lesser RP, Luders H, Nuwer M, Pictor TM. America EEG Society: guidelines for standard electrode position nomenclature. J Clin Neurophysiol. 1991;8:200–202. [PubMed] [Google Scholar]
- Stroup E, Langfitt J, Berg M, McDermott M, Pilcher W, Como P. Predicting verbal memory decline following anterior temporal lobectomy. Neurology. 2003;60:1266–1273. doi: 10.1212/01.wnl.0000058765.33878.0d. [DOI] [PubMed] [Google Scholar]
- Sylaja PN, Radhakrishnan K, Kesavadas C, Sarma PS. Seizure outcome after anterior temporal lobectomy and its predictors in patients with apparent temporal lobe epilepsy and normal MRI. Epilepsia. 2004;45:803–808. doi: 10.1111/j.0013-9580.2004.48503.x. [DOI] [PubMed] [Google Scholar]
- Theodore WH, Sato S, Kufta CV, Gaillard WD, Kelley K. FDG-positron emission tomography and invasive EEG: seizure focus detection and surgical outcome. Epilepsia. 1997;38:81–86. doi: 10.1111/j.1528-1157.1997.tb01081.x. [DOI] [PubMed] [Google Scholar]
- Trenerry MR, Jack CR, Jr, Ivnik RJ, Sharbrough FW, Cascino GD, Hirsc-horn KA, Marsh WR, Meyer WR, Meyer FB. MRI Hippocampal volumes and memory function before and after temporal lobectomy. Neurology. 1993;43:1800–1805. doi: 10.1212/wnl.43.9.1800. [DOI] [PubMed] [Google Scholar]
- Trenerry MR, Jack CR, Jr, Cascino GD, Sharbrough FW, So EL. Bilateral magnetic resonance imaging-determined hippocampal atrophy and verbal memory before and after temporal lobectomy. Epilepsia. 1996;37:526–533. doi: 10.1111/j.1528-1157.1996.tb00604.x. [DOI] [PubMed] [Google Scholar]
- Trenerry MR, Loring DW. The Intracarotid amobarbital procedure. In: Wyllie E, Gupta A, Lachhwani DK, editors. The treatment of epilepsy: principles & practice. 4. Lippincott Williams & Wilkins; Philadelphia: 2005. pp. 1000–1005. [Google Scholar]
- Wiebe S, Blume WT, Girvin JP, Eliasziw M, Effectiveness, Efficiency of Surgery for Temporal Lobe Epilepsy Study G A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345:311–318. doi: 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]
- Wieser HG, Blume WT, Fish D, Goldensohn E, Hufnagel A, King D, Sperling MR, Luders H. ILAE Commission Report. Proposal for a new classification of outcome with respect to epileptic seizures following epilepsy surgery. Epilepsia. 2001;42:282–286. [PubMed] [Google Scholar]


