Abstract
Object
Cerebral cortex electrophysiology is poorly sampled using standard, low spatial resolution clinical intracranial electrodes. Adding microelectrode arrays to the standard clinical macroelectrode arrays increases the spatial resolution and may ultimately improve the clinical utility of intracranial electroencephalography (iEEG). However, the safety of hybrid electrode systems containing standard clinical macroelectrode and microelectrode arrays is not yet known. The authors report on their preliminary experience in 24 patients who underwent implantation of hybrid electrodes.
Methods
In this study, 24 consecutive patients underwent long-term iEEG monitoring with implanted hybrid depth and subdural grid and strip electrodes; both clinical macroelectrodes and research microelectrodes were used. The patients included 18 women and 6 men with an average age of 35 ± 12 years (range 21–65). The mean hospital stay was 11 ± 4 days (range 5–20), with mean duration of implantation 7.0 ± 3.2 days (range 3–15). Data from the 198 consecutive craniotomies for standard clinical subdural grid insertion (prior to surgery in the 24 patients described here) were used for comparison to investigate the relative risk of complications.
Results
Focal seizure identification and subsequent resection was performed in 20 patients. One patient underwent a subsequent operation after neurological deterioration secondary to cerebral swelling and a 5-mm subdural hematoma. There were no infections. The overall complication rate was 4.2% (only 1 patient had a complication), which did not significantly differ from the complication rate previously reported by the authors of 6.6% when standard subdural and depth intracranial electrodes were used. There were no deaths or permanent neurological deficits related to electrode implantation.
Conclusions
The authors demonstrate the use of hybrid subdural strip and grid electrodes containing high-density microwire arrays and standard clinical macroelectrodes. Hybrid electrodes provide high spatial resolution electrophysiology of the neocortex that is impossible with standard clinical iEEG. In this initial study in 24 patients, the complication rate is acceptable, and there does not appear to be increased risk associated with the use of hybrid electrodes compared with standard subdural and depth iEEG electrodes. More research is required to show whether hybrid electrode recordings will improve localization of epileptic foci and tracking the generation of neocortical seizures.
Keywords: complication, electroencephalography, epilepsy surgery, high-frequency oscillation, microelectrode, subdural hybrid electrode
The success of epilepsy surgery—often the only chance for freedom from seizures in patients with focal epilepsy—is dependent on the surgeon's ability to localize not only the seizure onset zone, but also the epileptogenic zone. We are currently dependent on iEEG to define epileptogenic tissue in both lesional and nonlesional partial epilepsy. Despite advances in animal research that are challenging current paradigms about seizure generation and cortical electrophysiology, clinical iEEG continues to utilize narrow bandwidth recordings from widely spaced macroelectrodes; these probably do not have the resolution necessary to probe the spatiotemporal scales involved in the generation of epileptic activity.6,15,16
Standard clinical intracranial monitoring is at present performed with widely spaced macroelectrodes (5–10 mm) measuring ∼ 1–10 mm2 in diameter. To obtain better spatial resolution we designed hybrid clinical electrodes composed of standard clinical macroelectrodes combined with microwire arrays. The closely spaced (0.5–1 mm) microwire electrode (40 μm diameter) arrays provide high-resolution spatial sampling and are capable of probing the fine structure of cerebral cortex EEG. Furthermore, in using wide-bandwidth recording techniques (from DC to 1000 Hz) combined with high-resolution subdural grids and strips, we are able to probe the physiological range of the human iEEG.
The hybrid subdural grid and strip electrodes are composed of standard macroelectrode configurations with high-resolution microwire arrays interposed between macroelectrodes. These microwires are trimmed so that they are essentially flush with the silastic substrate of the grid and strip, and therefore do not violate the pial plane (Figs. 1 and 2). In this study, we present Phase I data on safety issues related to complications associated with hybrid depth, subdural grid and strip electrodes containing both clinical macroelectrodes and high-resolution microwire arrays. The hybrid subdural and depth electrodes are currently used under an investigational device exemption.
Fig. 1.
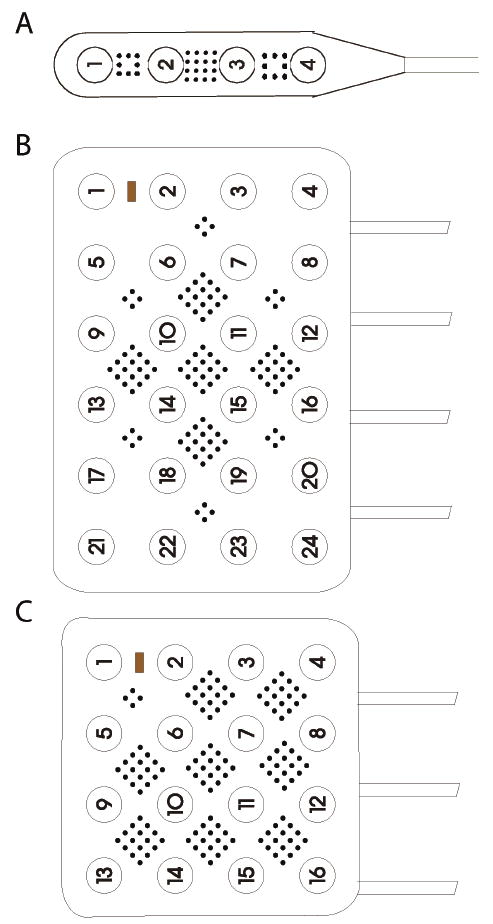
Schematics of a 1 × 4 contact strip with 32 microwires (A), a hybrid 4 × 6 grid with 104 microwires (B), and a hybrid 4 × 4 grid with 112 microwires (C). Microwires are 40-μm diameter, platinum-iridium wire spaced 1-mm apart. Clinical macrocontacts are 4-mm diameter and spaced 10-mm center-to-center.
Fig. 2.

Depictions of the hybrid grid for intracranial monitoring. A. Low magnification view of hybrid 4 × 4 grid prior to implantation. B. High magnification view of the grid subunit. Note how 4 macroelectrodes surround a diamond-shaped and centered 4 × 4 grid of microwires. C. Low magnification view of implanted grid in a patient. D. High magnification view of grid subunit over human cortex. note the improved spatial resolution of microwires compared to macroelectrodes.
Methods
Patient Population and Data Collection
All patients were older than 21 years of age and gave informed consent for this Mayo Clinic Institutional Review Board–approved study. The purpose of this prospective study was to gather data to establish the safety and efficacy of hybrid electrode arrays. Twenty-four consecutive patients underwent extraoperative, long-term, video iEEG monitoring at the Mayo Clinic with hybrid electrodes.
The safety of the device and complications of its use were retrospectively reviewed by one of the authors (J.J.V.G.). Data pertaining to demographics, complications, and implantation were analyzed. Twenty-three patients were referred for invasive monitoring because of medically resistant, partial epilepsy. An additional patient was referred for implantation prior to motor cortex stimulation for intractable facial pain. Before admission for invasive monitoring, all patients with epilepsy had undergone noninvasive techniques to localize the epileptogenic region; these techniques included outpatient EEG, inpatient prolonged video EEG monitoring, structural MR neuroimaging, SISCOM functional neuroimaging, and neuropsychological testing. Magnetic resonance imaging was performed in all patients with 1.5-T or, later in the study, 3-T magnets according to the epilepsy protocol techniques described by Jack et al.8 These imaging studies and the patients' clinical histories and scalp EEG recordings of habitual seizures were presented at our multidisciplinary epilepsy surgical conference, with neurologists, neuroradiologists, and neurosurgeons present to discuss surgical options and approach. Invasive monitoring was indicated to determine the seizure onset zone, which was not well-localized on other noninvasive tests. The patient who underwent electrode implantation for intractable facial pain was discussed at a multidisciplinary brain stimulation conference after evaluation by a team including a neurologist, a neurosurgeon, a psychiatrist, and an oral surgeon specializing in atypical facial pain. Preoperative functional MR imaging defined the facial motor cortex as the target for subdural electrode implantation for direct brain stimulation to treat the intractable facial pain.
Surgical Technique
Electrode insertion was performed with the patient in a state of general anesthesia; craniotomy (including bur-hole craniotomy) and dural opening were performed in all cases. The placement of the grids, strips, or depth electrodes was guided by noninvasive testing, according to the recommendations of the attendants at the epilepsy surgery conference, or in the case of atypical facial pain, to encompass the facial motor cortex. All hybrid grids, strips, and depth electrodes were purchased from Adtech Medical Instrument Cooperation. Postoperatively, the patients were monitored in the intensive care unit. Postoperative spiral CT scanning was performed to confirm electrode locations and rule out occult hemorrhage in all cases. Prophylactic antibiotic therapy, either cefazolin or vancomycin, was administered intravenously while the grids were in place, and continued for 3 doses after they had been removed. Head dressings were applied postoperatively and left in place throughout the monitoring period. Anticonvulsant medications were tapered as appropriate. After an appropriate period of evaluation, the patients returned to the operative theater for resection of the epileptic focus or explantation of the electrodes. The patient with atypical facial pain returned to the intensive care unit postoperatively and underwent intermittent cortical stimulation with patient-blinded assessments of pain. Because the pain was found to be reduced, the patient returned to surgery for pulse generator-driven subdural strip implantation.
Hybrid Electrodes and Data Acquisition
The subdural strip and grid electrodes (AdTech, Inc.) are composed of microwire arrays and standard clinical macroelectrodes. The microwire arrays contain 40-μm-diameter platinum-iridium wire spaced 0.5–1 mm apart. The subdural microwires contact the pial surface, but do not penetrate into the neocortex (Figs. 1 and 2). The microscale local field potentials recorded from the microwires are probably generated by excitatory and inhibitory synaptic currents within the dense distal synaptic arborization and connections of cortical layers 1 and 2. Clinical macrocontacts are 4-mm-diameter platinum-iridium discs spaced 5–10 mm apart, center-to-center.
All data were acquired in parallel with a 128-channel clinical (XLTEK Inc.) and a 256-channel research system (Neuralynx Inc.). Custom cabling allows wide-bandwidth recordings from all electrodes with the Neuralynx system. Most commercial EEG acquisition systems currently in use cannot record from microwires because of the relatively low amplifier input impedance. Raw continuous wide-bandwidth iEEG, sampled at 32 kHz, and video segments of seizures are transmitted directly to a 70-terabyte online data storage warehouse. The 1–2 terabyte/day files are compressed offline (lossless compression) and archived using a custom file format with further file decimation for storage. As part of our standard clinical iEEG protocol, scalp EEG data (with the international 10–20 system) are simultaneously acquired and archived with the iEEG data in all patients. Surgical steel sutures (Ethicon Inc.) are placed in the vertex region of the scalp to serve as the ground and reference electrodes for the micro- and macroelectrode recordings. Clinical raw output data are shown in Fig. 3.
Fig. 3.
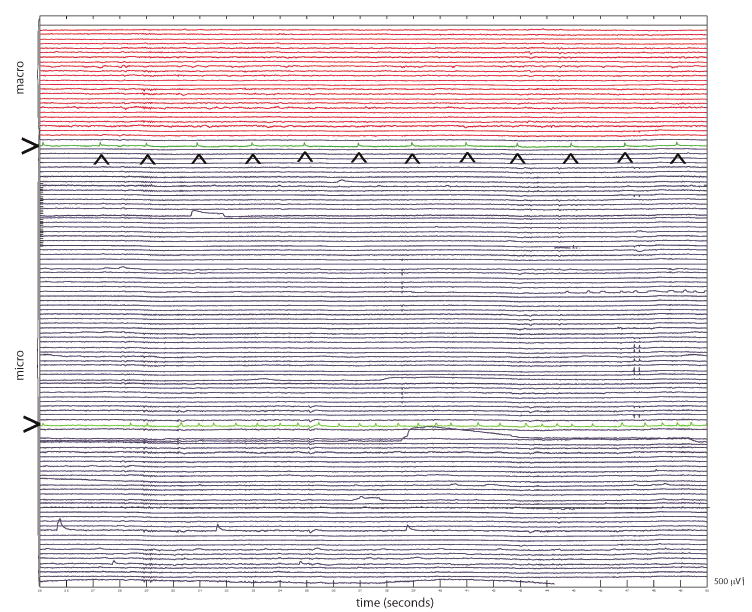
Intracranial EEG recording from frontal lobe neocortex in a patient with intractable partial epilepsy. The top 24 channels (red) are iEEG recorded from the clinical macroelectrodes. The bottom 104 channels are from the microwire electrodes. Note the asynchronous periodic epileptiform activity (individual spikes are marked by the arrowheads) that is not detected by the adjacent macroelectrodes recordings (green color-coded; marked with >). The microscale local field potential activity that is not captured by standard intracranial EEG recordings is highlighted.
Statistical Analysis
Commercially available software (JMP 6.0, SAS Institute Inc.) was used for statistical analysis. Data obtained in 198 consecutive craniotomies for subdural grid insertion were used for comparison with the 24 cases we report here. The 24 patients in the present study are different from these 198 patients.
Results
Table 1 displays the demographic and electrode-placement data in all 24 patients. On average, 56.7 ± 27.4 macroelectrodes were placed (range 16–96). No grids were placed in 6 patients, 1 grid in 12 patients, and 2 grids in 6 patients. These iEEG paradigms resulted in seizure foci identification in 20 of the 23 patients referred for medically refractory partial epilepsy, and led to a subsequent resection in the same number. No patient refused surgery after electrode implantation. The 3 cases of nonresectable foci were caused by multifocal seizures and not electrode implantation over eloquent brain tissue. Only 1 patient required further implantation of strip electrodes, and there were no grid adjustments. Previous focal or lobar resections had been performed in 6 of 23 patients. During iEEG monitoring, continuous vital signs monitoring revealed 2 patients with a temperature > 38.4°C. Postoperative laboratory examinations revealed leukocytosis in 11 of 24 patients, and hyponatremia in 1. Eleven patients received perioperative steroids for brain relaxation. Magnetic resonance imaging revealed lesions in 10 patients, and the remaining epilepsy patients had focal lesions. The patient who had grid placement for motor cortex stimulation had a normal MRI.
TABLE 1. Summary of data obtained in patients with regular and hybrid electrodes*.
| Characteristic | Patients w/Regular Electrodes† | Patients w/Hybrid Electrodes |
|---|---|---|
| age in years (range) | 28 ± 14 (1–73) | 35 ± 12 (21–65) |
| sex ratio (M/F) | 104:94 | 6:18 |
| nonlesional epilepsy | 69 (48%) | 14 (58%) |
| periop data | ||
| mean LOS in days | 12 ± 5 (5–28) | 11 ± 4 (5–20) |
| mean no of days of iEEG monitoring (range) | 8 ± 4 (2–24) | 7 ± 3 (3–15) |
| no. w/ fever (≥38.4°C) | 39 (20%) | 2 (8%) |
| no. w/ leukocytosis (>10.5 WBC) | 82 (41%) | 11 (46%) |
| no. w/ corticosteroids | 87 (44%) | 11 (46%) |
| implanted side | ||
| rt | 101 (51%) | 8 (33%) |
| lt | 87 (44%) | 8 (33%) |
| both | 10 (5%) | 8 (33%) |
| implanted lobe | ||
| frontal | 143 (72%) | 17 (71%) |
| neocortical temporal | 155 (78%) | 16 (67%) |
| mesial temporal | 57 (29%) | 15 (63%) |
| parietal | 97 (49%) | 3 (13%) |
| occipital | 25 (13%) | 1 (4 %) |
| interhemispheric | 51 (26%) | 2 (8 %) |
| implants used | ||
| mean no. of cables per patient | 10 ± 5 | 8 ± 3 |
| mean no. of electrodes per patient | 63 ± 23 | 57 ± 27 |
Data are given as means ± standard deviations except as noted.
Abbreviations: LOS = length of stay; periop = perioperative; WBC = white blood cell count.
Data from the study by Van Gompel et al., 2008, of 198 patients with epilepsy who underwent implantation of standard electrodes.
The hybrid electrode recordings demonstrated microscale local field potential activity that was not captured by standard macroelectrode iEEG recordings. Figure 3 shows a representative iEEG recording obtained in frontal lobe neocortex in a patient with intractable partial epilepsy. The recording shows clear, multifocal epileptiform discharges recorded from the microelectrode arrays that were not detected by the adjacent macroelectrode recordings.
Of the group of 23 patients with partial epilepsy, 1 patient, a 37-year-old woman with nocturnal secondarily generalized seizures, required an operation because of neurological deterioration. No lesion was found on MR images, video-EEG showed left frontotemporal onset, and left temporal hyperperfusion was demonstrated on SISCOM imaging. The following electrodes were implanted in the patient: left frontal grid (6 × 6), 2 left frontal strips (1 × 8), 2 left temporal strips (1 × 4), and 2 left temporal depths directed into the amygdala and anterior hippocampus (1 × 4). The implantation in this patient was uncomplicated, and the patient underwent follow-up for 5 days. During this period, she had 5 seizures which were localized to the left temporal lobe. Between the Day 4 and 5 after implantation, her condition deteriorated, and she had a reduced level of consciousness and aphasia. A head CT scan obtained at that time was compared with the scan from postoperative Day 1, and showed a midline shift of 5 mm with evidence of generalized cerebral edema, and a 5-mm-thick fluid collection under the frontal grid. On postimplantation Day 5, the grids were explanted due to her condition and because it was felt that her iEEG results were diagnostic of a mesial and neocortical temporal onset seizures. The fluid collection was confirmed to be subdural blood, but did not appear to have sufficient mass to explain her symptoms. Within 24 hours of electrode explantation, her neurological examination improved to baseline. The patient returned 3 months later for a left anterior temporal lobectomy with amydalohippocampectomy, and at the 18-month follow-up examination was seizure-free.
There were no infections in any patient after a minimum follow-up period of 3 months. The average follow-up period was 15 months (range 3–34 months). A small subdural hematoma developed in 1 patient, as discussed above. The overall complication rate in all patients with any type of hybrid electrode (hybrid grids, strips, or depths) was 4.2% (1 patient). Of the 18 patients who received hybrid grid electrodes, there was 1 complication (as described above) resulting in a 5.6% complication rate in this group. There were no complications in patients who received depth or subdural strip implantation. There were no permanent neurological deficits or deaths related to the implantation of electrodes.
A grid was implanted in 1 patient for 3 days of motor cortex stimulation, and good results were achieved. The patient reported significant improvements in facial pain at 3 months postoperatively. Electrodes were placed in 23 patients for seizures, and 20 of these subsequently underwent resection; this amounts to a 87% focal identification rate with subsequent resection. Repeated or initial lobectomy was performed in 13 patients, 8 underwent lesionectomy, 2 had grid explantation and received a vagus nerve stimulator. The pathological findings were consistent with gliosis in 12 patients, cortical dysplasia in 4, mesial temporal sclerosis in 3, and low-grade glioma in 2.
Discussion
Over the last 20 years there has been a rejuvenation of interest in resection for the treatment of epilepsy. The results have been impressive, ranging from ∼ 50% seizure-free outcomes in nonlesional cases to nearly 90% seizure-free outcomes in some lesional focal epilepsies (cavernomas and mesial temporal sclerosis).18 This renewed interest has been guided primarily by our increased ability to localize the seizure onset zone with improved imaging techniques such as MR imaging, new functional imaging studies, and electrode implantation for invasive EEG. Although imaging modalities continue to improve with new coils and higher field strengths, intracranial EEG has remained relatively technologically stagnant with the continued use of relatively narrow bandwidth recordings (0.1–100 Hz) and large, widely spaced electrodes with limited spatial resolution. The use of hybrid electrodes described in the present study extends the spatial resolution of EEG, and may ultimately prove clinically useful for identifying epileptogenic areas of the brain. In fact, the use of hybrid depth electrodes containing microwires has already proven useful for identifying electrophysiology in human hippocampus, such as ripples (80–250 Hz) and fast ripples (250–1000 Hz).1–4,10,11 These results are promising, but the safety of hybrid electrode arrays has not been reported previously.
Our overall complication rate was 4.2% (1 complication); there were no permanent neurological deficits and no deaths. This finding is not statistically different from our experience with standard intracranial electrode implantations.13 In our previous experience with standard clinical subdural grid and strip electrodes, in 198 consecutive patients undergoing implantation of standard grid electrodes for epilepsy, there were 13 complications (6.6%) including 5 infections and 6 hematomas.13 Three patients (1.5%) suffered permanent deficits related to implantation, and there were no deaths. In our patients implanted with simply depth or strip electrodes, there were no complications, a finding consistent with the low rates reported in the literature.12,17 Among our 18 patients with hybrid grid electrodes there was 1 complication (5.6%), a lower complication rate than that reported in our larger series of standard grid implantations. Major complication rates reported in the literature on subdural grid implantations range from 4 to 13%.5,14 Hamer and colleagues7 report on the largest series of iEEG with a pure grid population, report a slightly lower incidence of hematoma, 2.5%, but certainly a higher incidence of infection at 12.1% which may be due to different techniques. In their comparative series, Lee et al.9 reported a 7.8% rate of delayed subdural hematoma, 3.9% rate of infection, 2% rate of epidural hematoma, and a 2% rate of delayed cerebral swelling. Therefore, in terms of reported literature, there appears to be no evidence of significantly increased rates of death or complications associated with the use of hybrid subdural strip and grid electrode technology.
Conclusions
In the present study, we have demonstrated the use of hybrid subdural strip and grid electrodes containing high-density microwire arrays and standard clinical macroelectrodes. The hybrid electrodes provide high spatial resolution recording of the neocortex that is not possible with standard macroelectrodes. Subdural microwire recordings are very stabile during prolonged monitoring (2–10 days), and probe the fine scale spatial structure of human neocortical iEEG. Our preliminary results indicate that there is no significant increase in death or complications associated with the use of hybrid electrode implantation. Therefore, investigation and use of this device should continue so that its clinical applicability can be defined. More research is required to show whether hybrid electrode recordings will improve localization of epileptic brain foci and tracking of neocortical seizure generation.
Abbreviations used in this paper
- iEEG
intracranial electroencephalography
- SISCOM
subtraction ictal SPECT coregistered to MR imaging
Footnotes
Disclaimer: The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
References
- 1.Bragin A, Claeys P, Vonck K, Van Roost D, Wilson C, Boon P, et al. Analysis of initial slow waves (ISWs) at the seizure onset in patients with drug resistant temporal lobe epilepsy. Epilepsia. 2007;48:1883–1894. doi: 10.1111/j.1528-1167.2007.01149.x. [DOI] [PubMed] [Google Scholar]
- 2.Bragin A, Engel J, Jr, Wilson CL, Fried I, Buzsaki G. High-frequency oscillations in human brain. Hippocampus. 1999;9:137–142. doi: 10.1002/(SICI)1098-1063(1999)9:2<137::AID-HIPO5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 3.Bragin A, Wilson CL, Almajano J, Mody I, Engel J., Jr High-frequency oscillations after status epilepticus: epileptogenesis and seizure genesis. Epilepsia. 2004;45:1017–1023. doi: 10.1111/j.0013-9580.2004.17004.x. [DOI] [PubMed] [Google Scholar]
- 4.Bragin A, Wilson CL, Staba RJ, Reddick M, Fried I, Engel J., Jr Interictal high-frequency oscillations (80–500 Hz) in the human epileptic brain: entorhinal cortex. Ann Neurol. 2002;52:407–415. doi: 10.1002/ana.10291. [DOI] [PubMed] [Google Scholar]
- 5.Burneo JG, Steven DA, McLachlan RS, Parrent AG. Morbidity associated with the use of intracranial electrodes for epilepsy surgery. Can J Neurol Sc. 2006;33:223–227. doi: 10.1017/s0317167100005023. [DOI] [PubMed] [Google Scholar]
- 6.Gardner AB, Worrell GA, Marsh E, Dlugos D, Litt B. Human and automated detection of high-frequency oscillations in clinical intracranial EEG recordings. Clin Neurophysiol. 2007;118:1134–1143. doi: 10.1016/j.clinph.2006.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamer HM, Morris HH, Mascha EJ, Karafa MT, Bingaman WE, Bej MD, et al. Complications of invasive video-EEG monitoring with subdural grid electrodes. Neurology. 2002;58:97–103. doi: 10.1212/wnl.58.1.97. [DOI] [PubMed] [Google Scholar]
- 8.Jack CR., Jr Magnetic resonance imaging in epilepsy. Mayo Clin Proc. 1996;71:695–711. doi: 10.1016/S0025-6196(11)63008-5. [DOI] [PubMed] [Google Scholar]
- 9.Lee WS, Lee JK, Lee SA, Kang JK, Ko TS. Complications and results of subdural grid electrode implantation in epilepsy surgery. Sur Neurol. 2000;54:346–351. doi: 10.1016/s0090-3019(00)00324-4. [DOI] [PubMed] [Google Scholar]
- 10.Staba RJ, Wilson CL, Bragin A, Fried I, Engel J., Jr Quantitative analysis of high-frequency oscillations (80–500 Hz) recorded in human epileptic hippocampus and entorhinal cortex. J Neurophysiol. 2002;88:1743–1752. doi: 10.1152/jn.2002.88.4.1743. [DOI] [PubMed] [Google Scholar]
- 11.Staba RJ, Wilson CL, Bragin A, Jhung D, Fried I, Engel J., Jr High-frequency oscillations recorded in human medial temporal lobe during sleep. Ann Neurol. 2004;56:108–115. doi: 10.1002/ana.20164. [DOI] [PubMed] [Google Scholar]
- 12.Swartz BE, Rich JR, Dwan PS, DeSalles A, Kaufman MH, Walsh GO, et al. The safety and efficacy of chronically implanted subdural electrodes: a prospective study. Surg Neurol. 1996;46:87–93. doi: 10.1016/0090-3019(96)00083-3. [DOI] [PubMed] [Google Scholar]
- 13.Van Gompel JJ, Worrell GA, Bell ML, Patrick TA, Cascino GD, Raffel C, et al. Intracranial EEG with subdural grid electrodes: technique, complications, and outcomes. Neurosurgery. 2008 doi: 10.1227/01.NEU.0000324996.37228.F8. in press. [DOI] [PubMed] [Google Scholar]
- 14.Wiggins GC, Elisevich K, Smith BJ. Morbidity and infection in combined subdural grid and strip electrode investigation for intractable epilepsy. Epilepsy. 1999;37:73–80. doi: 10.1016/s0920-1211(99)00037-6. [DOI] [PubMed] [Google Scholar]
- 15.Worrell GA, Gardner AB, Stead SM, Hu S, Goerss S, Cascino GJ, et al. High-frequency oscillations in human temporal lobe: simultaneous microwire and clinical macroelectrode recordings. Brain. 2008;131:928–937. doi: 10.1093/brain/awn006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Worrell GA, Parish L, Cranstoun SD, Jonas R, Baltuch G, Litt B. High-frequency oscillations and seizure generation in neocortical epilepsy. Brain. 2004;127:1496–1506. doi: 10.1093/brain/awh149. [DOI] [PubMed] [Google Scholar]
- 17.Wyler AR, Ojemann GA, Lettich E, Ward AA., Jr Subdural strip electrodes for localizing epileptogenic foci. J Neurosurg. 1984;60:1195–1200. doi: 10.3171/jns.1984.60.6.1195. [DOI] [PubMed] [Google Scholar]
- 18.Zevgaridis D, van Velthoven V, Ebeling U, Reulen HJ. Seizure control following surgery in supratentorial cavernous malformations: a retrospective study in 77 patients. Acta Neurochir (Wien) 1996;138:672–677. doi: 10.1007/BF01411470. [DOI] [PubMed] [Google Scholar]


