Abstract
A candidate early precursor to pelvic serous cancer – the “p53 signature” – is commonly found in the benign mucosa of the distal fallopian tube and harbors p53 mutations and evidence of DNA damage. We examined tubes from women with pre-existing (germ-line) mutations in p53 (Li Fraumeni Syndrome or LFS) for evidence of this precursor. Fallopian tubes from two cases of LFS were immunostained for p53, Ki-67 (proliferation) and H2AX (DNA damage response) and analyzed for p53 mutations by laser capture micro-dissection (LCM) and p53 genomic sequencing (exons 2-11). A common single nucleotide repeat (snp) in exon 3 (rs 1042522) and deletion sequencing chromatograms in exon 4 were examined in combination to estimate LOH in both LFS tubes and advanced serous carcinomas from the general population. LFS tubal epithelium contained abundant (10-20 per section) p53 signatures with evidence of DNA damage and low proliferative activity. Six of 11 LFS microdissected p53 signatures (55%) and 15 of 21 serous carcinomas (71%) revealed LOH at p53 locus relative to background epithelium. The LFS model confirms prior observations that the distal fallopian tube is particularly prone to focal epithelial p53 gene inactivation - p53 mutation and LOH- in the absence of malignancy or increased epithelial proliferation. The fact that the LFS is not associated with ovarian cancers is consistent with the concept that loss of p53 function must be accompanied by at least one more genotoxic event (BRCA1/2 functional inactivation) to produce the malignant phenotype. This is in keeping with a general model of carcinogenesis, in which different and often independent risk factors operate at multiple points in the serous carcinogenic spectrum.
Introduction
Pelvic serous carcinoma (“ovarian cancer”) typically presents in an advanced stage, with a brief interval from the onset of symptoms to the discovery of widespread disease. Because of its rapid evolution and propensity for growth on ovarian and peritoneal surfaces, serous cancer was presumed by many to originate from the ovarian surface epithelium or OSE.1 However, serous ovarian cancers are rarely identified early in their development and classifications of precursor lesions in the OSE have not been uniform.2 3 4 5 Recent studies on the pathogenesis of serous ovarian carcinoma have highlighted the distal fallopian tube as a potential site of origin for many of these pelvic tumors. Several authors have documented non invasive (intraepithelial) serous carcinomas in the distal tubal mucosa of prophylactic salpingectomies of women with BRCA1 or BRCA2 mutations (BRCA+), as well as women with presumed ovarian and peritoneal serous carcmomas.6 7 8 9 10 11 12 Coupled with prior observations in the fallopian tube, these findings have raised the possibility that a serous carcinogenic sequence exists in the distal fallopian tube, and have generated a new paradigm for both early detection and prevention of ovarian cancer. 13 Moreover, a potential non-malignant precursor to serous carcinoma – termed the p53-signature – has been recently described.14 This entity possesses several characteristics in common with serous carcinomas of the fallopian tube, including: 1) typical location in the distal fallopian tube (fimbria), 2) intense p53 immunostaining, 3) the secretory cell phenotype ( BCL-2 and HMFG2+), 4) a DNA damage response signified by immunohistochemical localization of H2AX, 5) presence of ovarian cancer-related p53 mutations, 6) physical proximity to and shared p53 mutations with carcinoma , and 7) similar epidemiologic risk factors including low parity and low body mass index.14 15 16 17
Although the p53 signatures is occasionally seen in continuity with early (intraepithelial) serous carcinoma of the tube, they are typically found in normal appearing epithelium of the tubal fimbria and the sequence of genetic events leading to their formation and their progression to malignancy is unclear.12 14 An important variable in their development is the distal fallopian tube but even in this site, the number of p53 signatures rarely exceeds 2-3 per case.
Li Fraumeni syndrome (LFS) is a rare familial disorder characterized by an inherited (germ-line) mutation in p53. Individuals with LFS appear prone to a range of malignancies at a rate estimated as high as 50-fold over the general population, which may be further increased by exposure to ionizing radiation. 18 19 These patients are at increased risk for breast cancer, but the link between ovarian cancer and LFS is not established. Nevertheless, as LFS is characterized by germ-line p53 mutations, we explored the possibility that this variable would influence the risk of development of p53 signatures in the distal fallopian tubes of these individuals.
Materials and methods
Case material
This study was approved by the Institutional Review Board at Brigham and Women's Hospital. Two patients with LFS from which normal appearing fallopian tubes had been removed during surgical procedures were identified and slides retrieved from the pathology files at Brigham and Women's Hospital and Massachusetts General Hospital. Additional archived pelvic serous carcinomas in the files at Brigham and Women's Hospital were included. The tissues analyzed included the following: 1) fallopian tubes from two cases of LFS; 2) 21 serous ovarian and tubal carcinomas from the general population. Frequencies of p53 signatures identified in (1) were compared to those for other populations described previously.14 20
Immunophenotyping
Sections from the LFS tubes were analyzed for a marker of proliferation (MIB-1 corresponding to Ki-67; M7240, DAKO, Carpintaria, CA) and p53 (OP43, Oncogene Science, Cambridge, MA), each at 1:100 dilution. Nuclear MIB-1 staining index was estimated as a function of the number of p53 positive cells in a given field. 14
H2AX was used as a marker for DNA damage. Punctuate staining by this marker is associated with γ-H2AX, the phosphorylated form of the core histone γ-H2AX that localizes to the vicinity of - and is recognized as a marker for - double-stranded DNA breakage (Upstate Cell Signaling Solution, Charlottesville, VA; monoclonal JBW301, 1:200). H2AX is phosphorylated at a unique serine residue (S139) in its C-terminus at sites of DNA double strand breaks.16 This staining was only performed on the fallopian tubes from the patients with LFS.
An antibody to bcl-2, an inhibitor of apoptosis, which has been demonstrated to selectively highlight the cytoplasm of secretory epithelial cells in the fallopian tube, was also used.21
Antigen-antibody complexes were localized with the Envision technique using peroxidase and diamino-benzidine as the reporter, as previously described. 14
P53 mutation analysis
Selected tissue blocks containing p53 signature-positive and negative loci from patients with LFS were serially sectioned and the preservation of the p53 epithelial signature confirmed by immunostaining. Laser capture microdissection (LCM) was used to isolate p53 signature and control somatic DNA from selected cases using the PALM microbeam instrument. Samples were incubated at 62 degrees C. with proteinase K for 48 hours followed by the addition of Chelex (Biorad, Burlingame, CA) and boiling for 10 minutes. Genomic DNA was amplified by polymerase chain reaction (PCR), using tailed primers designed to amplify exons 2, 3, 5-9 and 11 of p53. A secondary amplification was performed using primers specific to the tail sequence used in the primary amplification. PCR products were then sequenced from both strands using tail primers. Data was analyzed using the Mutation Surveyor program (Soft Genetics, State College, PA). Candidate mutations found by the software were compared to a reference database for cancer-associated p53 mutations (Universal mutation database, http://ww.umd.be:2072/IFAMTP53A.shtml.
To asses possible LOH for the p53 locus, sequencing chromatograms in exon 5 containing the patient specific LFS mutation were examined. Allele loss at a heterozygous common single nucleotide repeat (snp) in exon 4 (rs1042522) was also examined to determine LOH, and the two results were combined to estimate heterozygosity.
Results
Fallopian tubes from women with LFS display a markedly increased frequency of p53 positive epithelia, particularly in the distal fallopian tube
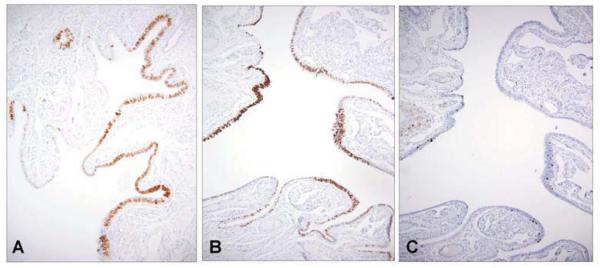
(Figures 1A and 1B). Numerous linear segments of p53 positive epithelium were identified, including as many as 20 in each section of the fimbria. The frequency of p53 positive epithelium in the more proximal tube was reduced relative to the fimbria but was elevated in all sections. In the endometrium of LFS women, p53 was negative in the epithelia with the exception of a single normal appearing gland in one section (not shown).
1.
p53 signatures in a distal fallopian tube segment from two patients with Li-Fraumeni syndrome (LFS) (A&B) are characterized by strong linear nuclear p53 localization in secretory cells. The number of cells demonstrating nuclear DNA proliferation is low (C).
In contrast to the p53 positive epithelia, the adjacent epithelium demonstrated variable but weaker p53 staining (Figure 1 A&B) confined to the secretory cells. This pattern of immunostaining is similar normal mucosa of the general population (not shown). In addition, the number of p53 positive nuclei in the LFS tubes varied, with numerous foci containing less than 12 consecutive p53 positive nuclei, including many single cells, indicating p53 positive cells that apparently had not undergone cell division following the acquisition of p53 positivity. Overall, the proliferative index (MIB-1) was low (Figure 1C).
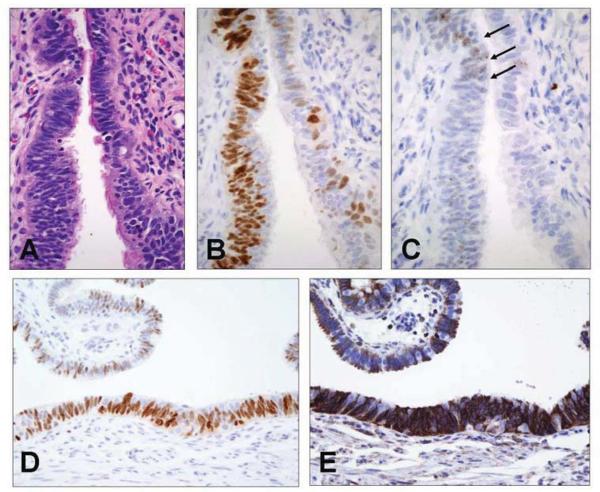
P53 positive foci in fimbria of the patients with LFS exhibit similar DNA damage response to p53 signatures (Figure 2)
2.
A p53 signature at higher power demonstrates normal morphology (A) and strong p53 nuclear staining (B). Punctate nuclear staining with an antibody to H2AX is characteristic of localization of γ-H2AX (C, arrows). Another p53 signature (D) stains strongly for bcl-2, a secretory cell marker (E).
Figure 2B depicts a p53 positive focus of cells immunostained with H2AX (Figure 2C).14 Small numbers of nuclei contained punctuate immunopositivity consistent with intra-nuclear localization of γ-H2AX (arrows), similar to prior descriptions of p53 signatures in other populations.14 Single, p53 positive cells also contained evidence of DNA damage via H2AX staining (not shown).
P53 positive foci in LFS are identical in cell type to p53 signatures
Immunostains for bcl-2 revealed strong cytoplasmic staining of the p53-H2AX signatures in LFS, consistent with their origin in secretory epithelial cells (Figures 2D and 2E). The generally low proliferative index of these p53 positive foci was similar to other studies of p53 signatures (Figure 1C).13 14 20
P53 signatures associated with LFS and pelvic serous carcinomas display high frequencies of LOH at a second p53 allele
P53 sequence analysis from all 11 p53-H2AX signatures revealed a single germ-line mutation (482_487delCCATCT). Further sequence analysis designed to identify LOH revealed the following; heterozygosity in 5 and loss of the wild type allele in 6 relative to the control tissue, for a frequency of 55% (Table 1 and Figure 3). Of 21 consecutive pelvic serous carcinomas analyzed, 15 (71%) harbored both a point mutation and evidence of LOH (Table 1). The difference in frequencies of LOH in the two groups was not significant (p = 0.33; chi square).
Table 1.
p53 mutation and LOH analysis of Li-Fraumeni syndrome (LFS) associated p53 signatures and tumor tissues (T) from pelvic serous carcinoma (PSC)
| Sample | Mutation | cDNA Δ | AA Δ | LOH |
|---|---|---|---|---|
| LFS1 | 482_487delCCATCT | 482_487 | NA | |
| LFS2 | 482_487delCCATCT | 482_487 | NA | |
| LFS3 | 482_487delCCATCT | 482_487 | NA | |
| LFS4 | 482_487delCCATCT | 482_487 | NA | LOH |
| LFS5 | 482_487delCCATCT | 482_487 | NA | |
| LFS6 | 482_487delCCATCT | 482_487 | NA | |
| LFS7 | 482_487delCCATCT | 482_487 | NA | LOH |
| LFS8 | 482_487delCCATCT | 482_487 | NA | LOH |
| LFS9 | 482_487delCCATCT | 482_487 | NA | LOH |
| LFS10 | 482_487delCCATCT | 482_487 | NA | LOH |
| LFS11 | 482_487delCCATCT | 482_487 | NA | LOH |
| PSC11T | c.488A>G,p.Y163YC | 488A>G | Y163C | LOH |
| PSC12Ta | c.683_686delACTG | 683del4 | NA | LOH |
| PSC12Tb | c.683_686delACTG | 683del4 | NA | LOH |
| PSC13T | 48DelCCA | 48del3 | R248W | LOH |
| PSC14T | c.742C>CT,p.R248RW | 742C>T | NONE | |
| PSC15T | c.578A>G,p.H193R | 578A>G | H193R | LOH |
| PSC16T | 439delG | 439delG | NA | LOH |
| PSC17T | c.949delC | 949delC | NA | LOH |
| PSC18T | c.988delC | 988delC | NA | LOH |
| PSC20T | c.747G>T,p.R249S | 747G>T | R249S | |
| PSC21T | c.818G>GA,p.R273RH | 818G>A | R273H | |
| PSC22T | c.659A>G,p.Y220C | 659A>G | Y220C | LOH |
| PSC23T | c.406C>CG,p.Q136QE | 406C>G | Q136E | |
| PSC24T | c.772_79elGAAGACTCCAGGTCAGGAGCC | 772del20 | NA | LOH |
| PSC25T | c.524G>GA,p.R175RH | 524G>A | R175H | |
| PSC26T | c.844C>G,p.R282G | 844C>G | R282G | LOH |
| PSC27T | IVS560-3T>TG/c.596G>GA,p.G199GE | IVS560- 3T>G |
NA | |
| PSC28T | c.637C>CT,p.R213RX | 637C>T | R213X | |
| PSC29T | c.711G>A,p.M237I | 711G>A | M237I | LOH |
| PSC30T | c.838A>G | 838A>G | R280G | LOH |
| PSC31T | c.438G>A | 438G>A | W146X | LOH |
| PSC32T | c.734G>C | 734G>C | G245A | LOH |
Δ, change; LOH, mutation and loss of heterozygosity of the wild-type allele.
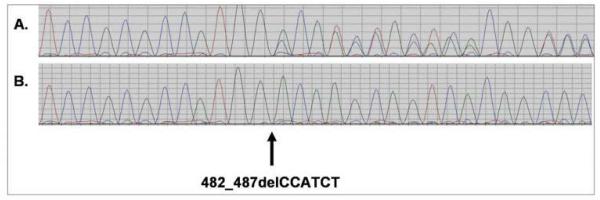
3.
Comparison of deletion chromatograms from control epithelia (no LOH, upper) and a p53 signature (LOH, lower). The sequence chromatogram of the heterozygous LFS deletion 482_487delCCATCT is shown in panel A. The position of the deletion is marked by the arrow and both wild type and mutant alleles are clearly present (as demonstrated by the presence of sequence from both alleles superimposed after cDNA position 481). The sequence chromatogram from sample LFS10 is shown in panel B. Only the mutant allele is present demonstrating loss of heterozygosity of the wild type allele.
Discussion
Mutations in the tumor suppressor gene p53 are present in over 90% of pelvic serous cancers, which are aggressive neoplasms that are typically discovered after spread to the peritoneum has occurred.22 The late stage of tumor presentation has fostered the impression that serous carcinogenesis evolves via rapid transitions from benign to malignant epithelium in the ovarian cortex or surface epithelium.1 However, the past few years has witnessed a greater attention to the distal fallopian tube, where not only non-invasive (intraepithelial) serous carcinomas but a serous cancer precursor candidate, termed the p53 signature, has been described. 6-12, 14 The p53 signature, like tubal intraepithelial carcinoma, involves secretory epithelial cells, contains p53 mutations, evidence of DNA damage, location in the distal fallopian tube, continuity with intraepithelial carcinoma, and association with decreased parity.5 14-17 With the increased awareness that the distal tube can give rise to both non-invasive (intraepithelial) carcinomas and a credible precursor to serous cancer (p53 signatures), irrespective of genetic risk, the field of ovarian cancer investigation has been expanded to include the distal fallopian tube as an important site of tumor origin.
Because p53 signatures have been associated with p53 mutations, and presumably arise via DNA damage, we addressed the hypothesis that in patients with germ-line mutations in p53, p53 signatures might be increased in frequency. This study shows, for the first time, that the distal fallopian tubes of women with LFS are exquisitely prone to developing p53 signatures identical to those described in women without LFS, including women with BRCA1/2 mutations and the general population.14 20 Relative to the endometrium and proximal fallopian tube, the frequency increases markedly in the region of the fimbria, where the number of p53 signatures exceeds by an order of magnitude the frequency observed in women without germline p53 mutations. Although fallopian tubes from only two cases of LFS were available for this study the high number of p53 signatures is in dramatically contrast to our prior findings in over 300 fallopian tubes from women with benign and malignant conditions, with and without increased (BRCA) genetic risk. 15 In these latter groups, the number of p53 signatures encountered following extensive sectioning and immunostaining of an entire distal fallopian tube rarely exceeds three. The intense staining for p53 seen in the p53 signatures of LFS is in sharp contrast to the background epithelium of tubes from these women, suggesting that a constitutive point mutation in the p53 gene will not result in delayed degradation (and accumulation) of p53 protein.23 Most of the foci sampled contained LOH of the second allele, albeit in lower frequency than observed in the serous carcinomas. This could be a function of the small DNA samples obtained by LCM and potential difficulties in generating gene product that is representative of the biologic process.
P53 mutations are integral to pelvic serous cancers and the role of germline or acquired BRCA1/2 mutations - followed by gene inactivation- on serous cancer development, is irrefutable. From 70-80% of ovarian tumors from BRCA1/2 mutation carriers exhibit LOH of the wild type allele.24 A similar frequency of BRCA1/2 LOH is seen in sporadic serous carcinomas.25 Prior functional inactivation of p53 appears critical to cell survival when LOH occurs in the wild-type BRCA allele26 27 and supports the models put forth by Knudson and Weinberg, in which two or more genetic events are required to generate the malignant phenotype.28 29 The existence of both p53 signatures and smaller numbers of p53 positive nuclei, including single strongly p53-positive cells, suggests that loss of p53 function in the context of the genotoxic injury is followed by variable turnover of the p53 positive cells. Whether this diversity in the number of p53 positive cells in a p53 signature reflects relative inhibition of cell expansion following the genotoxic insult, or epithelial cell targets with inherently different growth potentials, is unknown. The answer to this question might shed light on cell survival and “stemness” within the secretory cell population of the fallopian tube, similar to other models.30
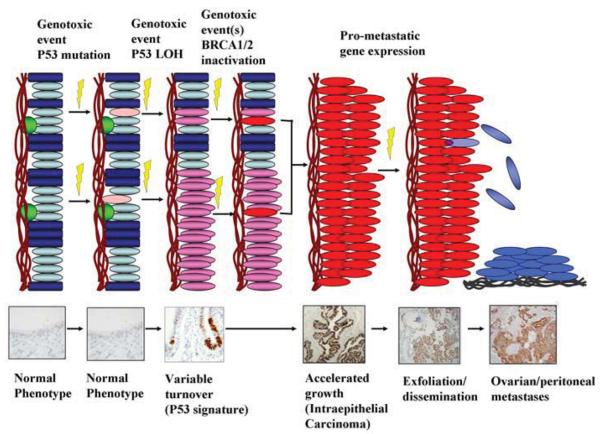
Figure 4 depicts a model for a serous carcinogenic sequence that takes into account sequential inactivation of both p53 and BRCA1/2. These events are triggered by DNA damage under mechanisms that remain to be elucidated and likely require the participation of additional oncogenic or tumor suppressor pathways (D. Dinulescu, personal communication). An intriguing hypothesis that is supported by both the LFS and BRCA1/2 model is that loss of p53 function in the context of a sub-morbid genotoxic insult is followed in many instances by continued - albeit gradual- cell turnover, leading to the p53 signature; a second event(s), inactivation of BRCA, would be necessary for the transition to a proliferative and potentially malignant lesion (intraepithelial carcinoma). A striking feature of this model is the apparent lack of a connection between the first (p53 inactivation) and second (BRCA1/2 inactivation) steps in this process. P53 signatures are common and highly abundant in tubes from the general population and women with LFS respectively, neither of which is particularly prone to ovarian cancer. This leaves the mechanism of BRCA1/2 inactivation a critical question, particularly in women who do not harbor inherited mutations in the gene. While this model will require refinement, it is becoming increasingly clear that the initial events in the pathogenesis of pelvic serous cancer often occur in the distal fallopian tube, involve inactivation of the p53 locus and could predate malignancy by years. If this model is validated, it will establish the existence of an extended time span between a microscopically and genetically discernible precursor and the onset of serous malignancy. This interval could be relevant to not only our perceptions of the timing of serous cancer development, but to its site of origin and, ultimately, prevention.
4.
A model for serous carcinogenesis in the distal fallopian tube, genotoxic stress imposed on the secretory cells (ovals) produces a p53 mutation followed by LOH of the non-mutated allele, with accumulation of p53 protein. Capacity of the affected cell for expansion is variable. Functional loss of BRCA1/2 via one or more mechanisms is a necessary component of progression to malignancy and is increased in women with inherited heterozygous BRCA1/2 mutations.
Acknowledgements
This work was supported by grants from the NCI (P50CA10500 [SPORE]: D. Cramer, PI), NCI 1R21CA124688-01A1 (CP Crum, PI), The Columbia Hospital For Women Research Foundation (CP Crum, PI), the Francis Ward Paine and TSA Pemberton Funds from the Division of Women's and Perinatal Pathology, Brigham and Women's Hospital, and a gift in memory of Elizabeth Ford Smith.
Footnotes
Originality, author participation and conflict of interest
This manuscript has not been concurrently submitted or published elsewhere. All authors have reviewed and approved this manuscript for publication. There are no conflicts of interest by any of the participating authors.
References
- 1.Shan W, Liu J. Epithelial ovarian cancer: focus on genetics and animal models. Cell Cycle. 2009;8:731–5. doi: 10.4161/cc.8.5.7848. [DOI] [PubMed] [Google Scholar]
- 2.Brewer MA, Mitchell MF, Bast RC. Prevention of ovarian cancer. In Vivo. 1999;13:99–106. [PubMed] [Google Scholar]
- 3.Bell DA, Scully RE. Early de novo ovarian carcinoma. A study of fourteen cases. Cancer. 1994;73:1859–64. doi: 10.1002/1097-0142(19940401)73:7<1859::aid-cncr2820730714>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 4.Dubeau L. The cell of origin of ovarian epithelial tumours. Lancet Oncol. 2008;9:1191–7. doi: 10.1016/S1470-2045(08)70308-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piek JM, Kenemans P, Verheijen RH. Intraperitoneal serous adenocarcinoma: a critical appraisal of three hypotheses on its cause. Am J Obstet Gynecol. 2004;191:718–32. doi: 10.1016/j.ajog.2004.02.067. [DOI] [PubMed] [Google Scholar]
- 6.Finch A, Shaw P, Rosen B, Murphy J, Narod SA, Colgan TJ. Clinical and pathologic findings of prophylactic salpingo-oophorectomies in 159 BRCA1 and BRCA2 carriers. Gynecol. Oncol. 2006;100:58–64. doi: 10.1016/j.ygyno.2005.06.065. [DOI] [PubMed] [Google Scholar]
- 7.Powell CB, Kenley E, Chen LM, Crawford B, McLennan J, Zaloudek C, et al. Risk-reducing salpingooophorectomy in BRCA mutation carriers: role of serial sectioning in the detection of occult malignancy. J. Clin. Oncol. 2005;23:127–132. doi: 10.1200/JCO.2005.04.109. [DOI] [PubMed] [Google Scholar]
- 8.Cass I, Holschneider C, Datta N, Barbuto D, Walts AE, Karlan BY. BRCA-mutation-associated fallopian tube carcinoma: a distinct clinical phenotype? Obstet. Gynecol. 2005 Dec.106(6):1327–1334. doi: 10.1097/01.AOG.0000187892.78392.3f. [DOI] [PubMed] [Google Scholar]
- 9.Leeper K, Garcia R, Swisher E, Goff B, Greer B, Paley P. Pathologic findings in prophylactic oophorectomy specimens in high-risk women. Gynecol. Oncol. 2002 Oct.87(1):52–56. doi: 10.1006/gyno.2002.6779. [DOI] [PubMed] [Google Scholar]
- 10.Callahan MJ, Crum CP, Medeiros F, Kindelberger DW, Elvin JA, Garber JE, et al. Primary fallopian tube malignancies in BRCA-positive women undergoing surgery for ovarian cancer risk reduction. J. Clin. Oncol. 2007 Sep 1;25(25):3985–3990. doi: 10.1200/JCO.2007.12.2622. [DOI] [PubMed] [Google Scholar]
- 11.Kindelberger DW, Lee Y, Miron A, Hirsch MS, Feltmate C, Medeiros F, et al. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: evidence for a causal relationship. Am. J. Surg. Pathol. 2007 Feb.31(2):161–169. doi: 10.1097/01.pas.0000213335.40358.47. [DOI] [PubMed] [Google Scholar]
- 12.Carlson JW, Miron A, Jarboe EA, Parast MM, Hirsch MS, Lee Y, et al. Serous tubal intraepithelial carcinoma: its potential role in primary peritoneal serous carcinoma and serous cancer prevention. J. Clin. Oncol. 2008 Sep 1;26(25):4160–4165. doi: 10.1200/JCO.2008.16.4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jarboe E, Folkins A, Nucci MR, Kindelberger D, Drapkin R, Miron A, et al. Serous carcinogenesis in the fallopian tube: a descriptive classification. Int. J. Gynecol. Pathol. 2008 Jan.27(1):1–9. doi: 10.1097/pgp.0b013e31814b191f. [DOI] [PubMed] [Google Scholar]
- 14.Lee Y, Miron A, Drapkin R, Nucci MR, Medeiros F, Saleemuddin A, et al. A candidate precursor to serous carcinoma that originates in the distal fallopian tube. J. Pathol. 2007 Jan.211(1):26–35. doi: 10.1002/path.2091. [DOI] [PubMed] [Google Scholar]
- 15.Saleemuddin A, Folkins AK, Garrett L, Garber J, Muto MG, Crum CP, et al. Risk factors for a serous cancer precursor (“p53 signature”) in women with inherited BRCA mutations. Gynecol. Oncol. 2008 Aug 20; doi: 10.1016/j.ygyno.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thiriet C, Hayes JJ. Chromatin in need of a fix: phosphorylation of H2AX connects chromatin to DNA repair. Mol Cell. 2005;18:617–22. doi: 10.1016/j.molcel.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 17.Piek JM, van Diest PJ, Zweemer RP, Jansen JW, Poort-Keesom RJ, Menko FH, Gille JJ, Jongsma AP, Pals G, Kenemans P, Verheijen RH. Dysplastic changes in prophylactically removed Fallopian tubes of women predisposed to developing ovarian cancer. J Pathol. 2001;195:451–6. doi: 10.1002/path.1000. [DOI] [PubMed] [Google Scholar]
- 18.Kleinerman RA. Radiation-sensitive genetically susceptible pediatric sub-populations. Pediatr Radiol. 2009 Feb;39(Suppl 1):S27–31. doi: 10.1007/s00247-008-1015-6. Epub 2008 Dec 16. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evans SC, Lozano G. The Li-Fraumeni syndrome: an inherited susceptibility to cancer. Mol Med Today. 1997 Sep;3(9):390–5. doi: 10.1016/S1357-4310(97)01105-2. [DOI] [PubMed] [Google Scholar]
- 20.Folkins AK, Jarboe EA, Saleemuddin A, Lee Y, Callahan MJ, Drapkin R, Garber JE, Muto MG, Tworoger S, Crum CP. A candidate precursor to pelvic serous cancer (p53 signature) and its prevalence in ovaries and fallopian tubes from women with BRCA mutations. Gynecol Oncol. 2008;109:168–73. doi: 10.1016/j.ygyno.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piek JM, van Diest PJ, Verheijen RH, Kenemans P. Cell cycle-related proteins p21 and bcl-2: markers of differentiation in the human fallopian tube. Histopathology. 2001;38:481–2. doi: 10.1046/j.1365-2559.2001.1163c.x. [DOI] [PubMed] [Google Scholar]
- 22.Köbel M, Huntsman D, Gilks CB. Critical molecular abnormalities in high-grade serous carcinoma of the ovary. Expert Rev Mol Med. 2008;10:e22. doi: 10.1017/S146239940800077X. [DOI] [PubMed] [Google Scholar]
- 23.Terzian T, Suh YA, Iwakuma T, Post SM, Neumann M, Lang GA, Van Pelt CS, Lozano G. The inherent instability of mutant p53 is alleviated by Mdm2 or p16INK4a loss. Genes Dev. 2008;22:1337–44. doi: 10.1101/gad.1662908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dworkin AM, Spearman AD, Tseng SY, Sweet K, Toland AE. Methylation not a frequent “second hit” in tumors with germline BRCA mutations. Fam Cancer. 2009 Apr 2; doi: 10.1007/s10689-009-9240-1. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 25.Gras E, Cortes J, Diez O, Alonso C, Matias-Guiu X, Baiget M, Prat J. Loss of heterozygosity on chromosome 13q12-q14, BRCA-2 mutations and lack of BRCA-2 promoter hypermethylation in sporadic epithelial ovarian tumors. Cancer. 2001;92:787–95. doi: 10.1002/1097-0142(20010815)92:4<787::aid-cncr1384>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 26.Holstege H, Joosse SA, van Oostrom CT, Nederlof PM, de Vries A, Jonkers J. High incidence of protein-truncating TP53 mutations in BRCA1-related breast cancer. Cancer Res. 2009 Apr 15;69(8):3625–33. doi: 10.1158/0008-5472.CAN-08-3426. [DOI] [PubMed] [Google Scholar]
- 27.Press JZ, De Luca A, Boyd N, Young S, Troussard A, Ridge Y, Kaurah P, Kalloger SE, Blood KA, Smith M, Spellman PT, Wang Y, Miller DM, Horsman D, Faham M, Gilks CB, Gray J, Huntsman DG. Ovarian carcinomas with genetic and epigenetic BRCA1 loss have distinct molecular abnormalities. BMC Cancer. 2008 Jan 22;8:17. doi: 10.1186/1471-2407-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knudson AG. Two genetic hits (more or less) to cancer. Nat Rev Cancer. 2001;1:157–62. doi: 10.1038/35101031. [DOI] [PubMed] [Google Scholar]
- 29.Hahn WC, Weinberg RA. Rules for making human tumor cells. N Engl J Med. 2002;347:1593–603. doi: 10.1056/NEJMra021902. Erratum in: N Engl J Med. 2003;348:674. [DOI] [PubMed] [Google Scholar]
- 30.Senoo M, Pinto F, Crum CP, McKeon F. p63 Is essential for the proliferative potential of stem cells in stratified epithelia. Cell. 2007;129:523–36. doi: 10.1016/j.cell.2007.02.045. [DOI] [PubMed] [Google Scholar]