Abstract
The important role that surface chemical analysis methods can and should play in the characterization of nanoparticles is described. The types of information that can be obtained from analysis of nanoparticles using Auger electron spectroscopy (AES); X-ray photoelectron spectroscopy (XPS); time of flight secondary ion mass spectrometry (TOF-SIMS); low energy ion scattering (LEIS); and scanning probe microscopy (SPM), including scanning tunneling microscopy (STM) and atomic force microscopy (AFM), are briefly summarized. Examples describing the characterization of engineered nanoparticles are provided. Specific analysis considerations and issues associated with using surface analysis methods for the characterization of nanoparticles are discussed and summarized, along with the impact that shape instability, environmentally induced changes, deliberate and accidental coating, etc., have on nanoparticle properties.
Introduction
The increasing use of engineered nanoparticles in research and product development in application areas related to medicine, sensing, environmental and consumer products engenders a growing need to understand their properties and behaviors as they are synthesized, applied, and evolve (or age) in a particular environment, process, or application. There is also an increasing awareness of the need to understand the health, safety, and environmental impacts of nanoparticles in both their synthesized form and as they evolve through application or environmental interaction. Although novel and unusual properties of nanoparticles and other nanostructured materials excite scientists, technologists, and, often, the general public, the sometimes surprising properties of many of these materials raise analysis and characterization issues that can also be unexpected by analysts, scientists, and production engineers [1-3].
It is increasingly recognized that reports on the properties of nanoparticles and other nanostructured materials are sometimes based on inadequate characterization. Consequently, the validity of some of the conclusions may be questionable [2,4]. The major objectives of this paper are to identify the subset of important information that can be obtained about nanoparticles using tools under the general heading of surface chemical analysis and to examine some of the issues and challenges faced when performing such analyses.
It is widely recognized that as particle size decreases to the nanometer scale, there are a variety of reasons, including quantum confinement effects, that cause their physical and chemical properties to differ from those associated with their bulk form. Equally important and widely acknowledged, but seemingly less understood, is recognition that a large portion of the atoms in nanoparticles are at or near the surface of the particles. Grainger and Castner [2] point out that over the past 40 or more years, surface scientists have obtained detailed knowledge about the behavior of surfaces, including the important role of deliberate and accidental surface layers, which has lead to the development of a set of tools that can be used to understand and characterize surfaces. They further argue that the same rigor that has been applied to surface studies is needed (but, with few exceptions, is usually not used) to understand and control the properties of nanoparticles. They called this nanosurface analysis. For example, nanosurface analysis is extensively used to characterize supported nanoparticle catalysts, but virtually unused to characterize unsupported nanoparticles in biomedical applications. In a different report, Karakoti et al. [5] noted that the importance of nanoparticle surface chemistry, especially as applied to toxicity, has been (surprisingly) underemphasized.
A March 2006 article in Small Times magazine described a workshop designed to identify roadblocks to nanobiotechnology commercialization.[4] In this article, several experts opined that many of the important physical characteristics needed to understand the physical and chemical properties of nanoparticles are unreported in research reports and apparently often unmeasured. This was found to be especially true in areas related to assessments of particle toxicity. The article further notes that the changes these particles undergo when placed in various environments for storage or use are especially important and usually unknown. In many cases, nanoparticles are coated with surfactants or contaminants, and these are often not well characterized and, many times, not even adequately identified. Based on these and other articles, it appears two aspects of nanoparticle properties are not always fully appreciated. These include the importance that the nanoparticle surface composition and structure play on their properties and performance and how significantly these properties can change with time and environmental exposure.
Because of the increasing importance of nanoparticle characterization, working groups of the International Bureau of Weights of Measures (BIPM), Consultative Committee for Amount of Substance: metrology in chemistry (CCQM), and International Organization for Standards (ISO) Technical Committee (TC) 229 on Nanotechnology are focusing considerable attention on nanomaterials characterization. Specific working groups are focused on the characterization of nanoparticles for Environmental Health and Safety (EHS) issues and for toxicology studies. Surface characterization is a subset of several analysis needs, and the surface characterization needs of ISO TC 229 are being addressed in a technical report (TR14187) being prepared by the ISO TC 201 Committee on Surface Chemical Analysis. This article introduces some of the topics and issues expected to be included in ISO TR14187. A Working Party on Manufactured Nanomaterials (WPMN) of the Organization for Economic Co-operation and Development (OECD) has established a list of physical-chemical properties and material characterization needs for nanostructured materials [6]. Among the 16 properties on this list are characteristics related to surface chemistry and surface charge. Although there are many different types of information needs for nanoparticles and other nanostructured materials, ISO TC229 Joint Working Group (JWG) 3 has tentatively focused on approximately seven of the measurement criteria that emphasize the importance of surface chemistry. These criteria include properties of surface layers and surface contamination, the chemical state or enrichment of species on the surface or at interfaces, and information about surface functionality.
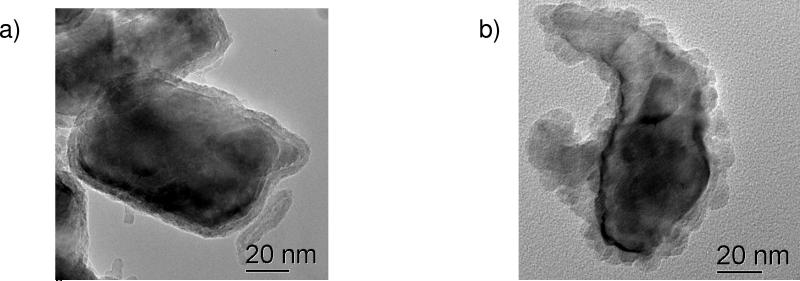
Three examples of nanoparticles where surface chemical analysis tools have been important for understanding particle structure and behavior are shown in Figure 1. These examples relate to nanoparticle synthesis (LuPO4 in apoferritin), environmental-induced changes in the nanoparticles (iron nanoparticles), and confirmation of the desired property changes in chemically functionalized nanoparticles (functionalized carbon nanotubes [CNTs]). The LuPO4 particles formed inside an apoferritin template have potential application in cancer radioimmunotherapy and radioimmunoimaging [7]. One analysis need for these particles was to confirm the presence of the Lu in the phosphate form inside the aproferritin shell. This was accomplished using XPS. Zero valent iron nanoparticles have been used to reduce environmental contaminants such as CCl4. In nanoparticle form, they sometimes exhibit beneficial reaction pathways relative to micron-sized particles and can also be delivered by injection into contaminated wells. We have found that iron nanoparticles with a metal-core/oxide-shell structure are more reactive than particles with iron only in +2 or +3 states. However, not all metal-core/oxide-shell iron particles produce the same chemical pathways. It has been found that “bulk” tools, such as X-ray diffraction (XRD), and high-resolution methods, such as transmission electron microscopy (TEM), combined with surface methods, including XPS, are all important for detailed characterization of these particles [8-9]. In recent work, the impact of natural organic coatings formed on the particles in solution has been studied. XPS, as well as optical methods, again have been useful for characterizing these coatings [10]. CNTs in many different forms are used for a variety of different technologies. For many applications, including sensor technologies, it is beneficial to functionalize their surface. In the example in Figure 1, a succinimidyl ester is adsorbed onto the CNT surface [11]. Amine groups on a protein can react with the anchored succinimidyl ester to immobilize proteins on the surface. Possible applications of this approach include the creation of biosensors and the possibility of using such interactions to guide self assembly of CNTs. For this example, XPS, in combination with high-resolution electron microscopy, provides important information about the presence and nature of the molecules attached to the surface [11-12].
Figure 1.
Three examples of surfaces layers or attached molecules that play an important role in the synthesis or properties of nanoparticles and for which surface analysis has provided useful information: a) LuPO4 particles which were formed inside an apoferritin template [7], b) Iron metal-core oxide-shell nanoparticles which can reduce environmental contaminants [9] and c) carbon nanotubes functionalized by sorption of a succinimidyl ester [11].
As the importance of nanoparticle surfaces is recognized, it would seem logical that tools developed to extract information about surfaces could and should be applied to nanomaterials. At least two different issues appear to limit both the attempted applications of these methods and the impact of these tools in nanoparticle characterization. First, many of the tools appear to lack the necessary degree of spatial resolution in three dimensions needed to analyze individual nanoparticles (or the variations of composition within the particles). Consequently, some researchers may not consider application of the tools even though they can often provide essential information that cannot be obtained by other means. Second, the tools are sometimes applied to nanostructured materials without considering a range of analysis challenges or issues that these materials present. This can lead to inconsistent or even wrong conclusions.
Grainger and Castner [2] and Baer et al. [1,13] have pointed out several important characteristics of appropriate nanoparticle analysis including: analysis during and immediately after synthesis, understanding the interactions (and time dependence) of particles when placed in the application environment (biological, solution, catalysis), and the necessity of multi-technique analysis (as noted in the examples from Figure 1). Although this paper focuses primarily on the application of surface analysis tools, such tools provide only an important (and often ignored) subset of the information needed to understand and control the behavior of nanoparticles. This paper addresses two important issues. First, the types of information that can be gained from application of surface analysis methods, and, second, some of the technical challenges faced when applying surface analysis tools (and often other tools) to nanoparticle characterization.
Surface Chemical Analysis Tools and Example Applications
Although many different surface-analysis techniques can be useful for the characterization of nanoparticle surfaces, this paper will focus on information that can be obtained from electron spectroscopies (Auger electron spectroscopy [AES] and X-ray photoelectron spectroscopy [XPS]); ion-based methods (secondary ion mass spectrometry [TOF-SIMS] and low energy ion scattering [LEIS]); and scanning probe microscopy (SPM), including atomic force microscopy (AFM) and scanning tunneling microscopy (STM). A considerable amount of effort has been devoted toward the development of guides and standards for AES, XPS, SIMS, and SPM methods since they are primary surface analytical methods. This includes work by Standards Committees ASTM E42 on Surface Analysis and ISO TC201on Surface Chemical Analysis. The second ion-based method, LEIS, has been added to this list because of its extreme surface sensitivity that can provide useful information for nanoparticle analysis. It can be argued that SPM-based methods, which allow visualization of nanostructures on surfaces, have been a significant driver for the development of nanotechnology. Finally, literature reports suggest that XPS has evolved into the most widely used surface spectroscopy [14-15]. Detailed discussions of these methods are available from many sources [16-18]. The primary objective of the short descriptions that follow is to highlight features particularly useful for nanoparticle analysis. Although these are generally labeled surface analysis methods, each technique has nanometer resolution in at least one dimension.
Table 1 lists some types of information that can be extracted from nanoparticles using surface analysis tools along with several characteristics of the methods. It is important to recognize that the different surface analysis techniques can provide complementary and comprehensive information as highlighted in a bubble chart developed at the UK National Physical Laboratory (NPL) [19-20]. This chart summarizes the types of information that can be provided by each of several different analysis methods. The analysis methods in the NPL chart go beyond those discussed here, and many may be useful for nanoparticle characterization. The types of information that can be obtained include topography, elemental composition, molecular and chemical state, and structure.
Table I.
| Electron Spectroscopies |
Information available | Probe |
Detected |
Lateral Resolution |
Information Depth |
Depth Resolution |
|---|---|---|---|---|---|---|
| Auger electron spectroscopy (AES) | • Surface composition of individual large nanoparticles or distribution of smaller nanoparticles (depending on spatial resulution of specific instrument). • Enrichment or depletion of elements at surface • Presence and/or thickness of coatings and/or contaminants |
electrons (~ 3 to 20 kV) | Auger electrons | ≈ 10 nm | ≈ 10 nm | ≈ 2 nm |
| X-ray photoelectron spectroscopy (XPS) | • Analysis of a collection of particles deposited on a substrate or other support. • Surface composition and chemical state • Presence and nature of functional groups on the surface • Enrichment or depletion of elements at surface • Presence and/or thickness of coatings or contaminats • Nanoparticle Size (when smaller than ~ 10 nm, can sometimes determine average particle size when too small to be detectred by other methods or in complex matrix.) • Electrical properties of nanopartlcles and coatings |
X-rays | Photoelectrons | ≈ 2 μm | ≈ 10 nm | ≈ 2 nm |
| Incident Ion Methods | ||||||
| Secondary Ion Mass Spectrometery (SIMS) | • Usually analysis of a collection of particles or larger individual particles deposited on a supporting substrate. • Presence of surface coatings or contamiinants on collections of nanoparticles • Functional groups on surface |
Ions (~ 3 - 20 kV) | sputtered ions | ≈ 50 nm (inorganic) > 200 nm (organic) |
≈ 1 nm | ≈ 1 nm (inorganic) ≈ 10 nm (organic) |
| Low energy ion scattering (LEIS) | • Presence of ultra thin coating or contamination • Effects of size |
Ions (~ 2 to 10 kV) | elastically scattered ions | ≈ 100 μm | ≈ 10 nm | ≈ 0.2 nm |
| Scanning Probe Microscopies | |||||
|---|---|---|---|---|---|
| Scanning Tunneling Microscopy (STM) | • Electrical characteristics of individual nanoparticles • Nanoparticle formation and/or size distribution of particles deposited or grown on a surface |
stylus | tunneling current | ≈ 1 nm | ≈ 10 nm |
| Atomic force microscopy (AFM) | • Shape, texture and roughness of individual particles and their distribution for an assembly of particles • When particle structure is known, can provide information about crystalligraphic orientation |
stylus | force or displacement | ≈ 1 nm | ≈ 10 nm |
The following paragraphs summarize the essence of five surface analysis methods and provide a few examples of their use. These examples offer a sample, but not a comprehensive picture, of what can be done. Applications are limited only by the ingenuity of the research team involved. It will be apparent that application of these tools is most often useful when they are applied in combination with other tools providing complementary information (i.e., multi-technique analysis). We have found, for example, from studies on 50 nm iron metal-core/oxide-shell nanoparticles that XPS, TEM, and XRD are standard measurements that should be applied to particles from most experimental tests. Because of environmental and time (or processing induced) changes in the particles, we make many measurements after exposure to different environments and after any significant time of particle storage. Whenever possible, we apply tools that work in the environment of interest (in situ measurements) and attempt to compare results for consistency. When materials are removed from solution for ex situ analysis, we usually carefully dry them [21] and handle them without exposure to air before analysis.
Electron Spectroscopies
Both AES and XPS involve the detection of electrons emitted from samples with kinetic energies typically below 2000 keV. Although a wider range of energies are available when synchrotron X-ray sources are used for XPS, most of the discussion will focus on laboratory-based sources. Much of the value of these methods is their surface sensitivity that arises from the short distances that electrons travel at these energies without undergoing inelastic scattering and energy loss [13-14,22]. Therefore, the electrons detected in Auger or photoelectron peaks are from the outer few nanometers of the material, as indicated in Table 1. Catalysis was one of the first areas where the combination of “bulk” analysis methods with these surface analysis methods allowed information about the enrichment or depletion of elements on the surface to be determined [23-25]. Because electrons that emerge from the material that have lost energy appear in the background region of the spectra [26], it is possible to use these methods to provide depth, enrichment, or layering information within the XPS and AES analysis volume. Consequently, these two methods can be used with multiple approaches to obtain important information about layering or coatings on particle and nanoparticle surfaces [27].
Although both X-ray and electron excitations produce Auger electrons, AES is usually associated with the use of incident electrons to generate Auger electrons. These incident electrons typically range in energy from 2 to 20 keV. As might be expected, X-rays (often Mg or Al Kα) are the incident radiation in XPS. This technique is also referred to as Electron Spectroscopy for Chemical Analysis (ESCA)—coined by Kai Siegbahn, who was awarded a Nobel Prize for his development of this technique. Because an electron beam can be focused to less than 10 nanometers in size, it is possible to analyze individual nanoparticles with AES. However, issues related to electron penetration and scattering can result in worse resolution than expected [28] based on beam size alone. Although XPS does not have the spatial resolution to analyze individual nanoparticles (with the possible exception of a few special synchrotron-based systems with a highly focused and bright source of X-rays), it is often possible to analyze collections of particles (in a single layer or, effectively, in powder form) and to obtain useful information [13,29,27].
Both AES and XPS can be extremely important tools for determining the presence, composition, and thickness of coatings on nanoparticles, as well as surface enrichment and depletion at particle surfaces. To the surprise of many, XPS can sometimes be used to determine particle sizes when conditions are not appropriate for analysis by other methods [13,25,30]. The size, shape, and layered structure of nanoparticles influence XPS data in several different ways including [13]:
- ■ Peak intensities and relative peak intensities of
- peaks for different elements
- different peaks for the same element
- same peaks excited with different X-ray energies
- ■ Peak energies
- binding energies of peaks
- value of the Auger parameter
■ Background signals from electrons that have lost energy
Depending on what is known about the specimen and the analysis objective, each of these influences can be used to extract useful information about nanostructured samples [8,30].
Although electron spectroscopies may not be as used in every situation where they would be appropriate, they are increasingly employed (especially XPS) for nanoparticle characterization in a variety of ways. It is valuable to identify specific analysis objectives based on the type of research involved [31-32]. Understanding the analysis objectives assists both the data collection and the nature of the data processing. This can significantly improve the quality and value of the resulting information. One simple example of how solution composition alters the surface of a nanoparticle is provided before summarizing several other examples from the literature. The topics covered include: i) contamination, particle coatings, and oxidation; ii) particle size; iii) particle location; iv) surface acidity; and v) electrical properties of particles.
i) Intended or unintended coatings, as previously noted, can have a significant impact on a variety of nanoparticle properties. The influence of natural organic material (NOM) on the reactivity of iron metal-core/oxide-shell nanoparticles in deionized (DI) water serves as an example of both the formation of coatings and their impact. Johnson et al. [10] demonstrated that when NOM was added to water, the nanoparticles were less likely to be retained in a soil column. Previous reactivity and microscopy work has shown how the particles change properties in solution as a function of time [33]. We have started an examination of the influence of waterborne NOM on the rate of particle change. Although the studies involve many parts, we have used XPS to examine changes in the particle surface chemistry, TEM to examine morphology changes, and XRD to examine the overall oxidation of the particles as a function of time [1,33].
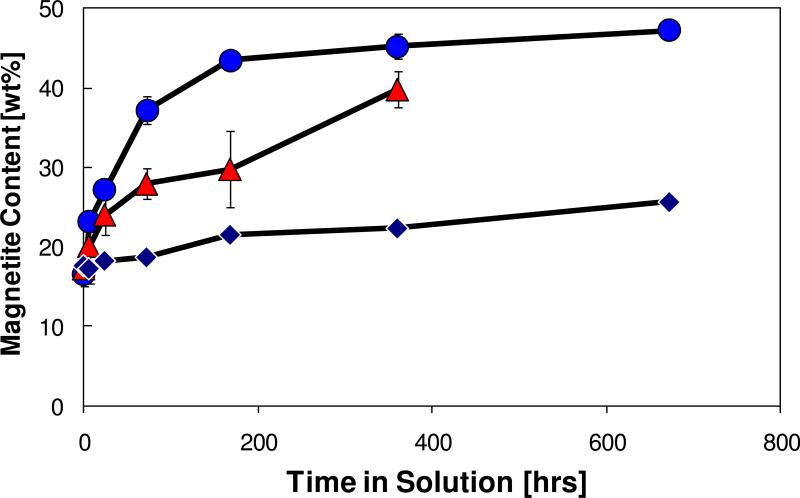
An overview of these measurements is provided in Figures 2–4. The TEM images [Figure 2] show the types of changes that occur in solution over a day. The oxide shell changes from highly ordered to something more nodular. The XPS measurements [Figure 3] show that the Fe near the surface starts as Fe+3, but a Fe+2 component is formed after exposure to the solution. The XPS results also show that the nature of the surface C and O are altered by both water exposure and again by the presence of NOM. Carbon is a ubiquitous feature of materials exposed to the atmosphere, but the addition of NOM thickens the carbon layer on the particles, as well as increasing the O to Fe ratio [Table 2]. Note that it is possible using the approach of Smith [34] or that of Shard et al. [29] to estimate the thickness of a carbon overlayer on the particles. Although there are additional complications because of O in the NOM, the Smith approach has been used to estimate carbon layer thickness in Table 2. The presence of the NOM increases the thickness of the overlayer on the nanoparticle surfaces, although it is too small to be directly observed in the TEM [10] (even if the effects of electron beam damage on the carbon overlayer could be eliminated). The O 1s photoelectron peak after water exposure shows an increase in a shoulder around 531.5 eV, consistent with an increase in OH- species attached to the surface. The increase in the peak in the same region after exposure to the NOM solution is likely to also include oxygen species present in the NOM. The time-dependent XRD measurements [Figure 4] imply that the presence of NOM on the particle surface slows oxidation of the nanoparticles. This simple example clearly shows how changes in the environment of nanoparticles and coatings on their surface impact the behavior of nanoparticles.
Figure 2.
TEM images of iron metal-core oxide-shell nanoparticles a) before exposure to water and b) after exposure to DI water for 24 hours.
Figure 4.
The weight % magnetite for metal-core/oxide shell nanoparticles measured as a function of the time the particles have been in solution as determined by XRD measurements. The increase of the fraction of magnetite is an indication of particle corrosion. The particles exposed to pure DI water (•) oxidize more quickly than particles exposed to DI water to which NOM has been added at 20 mg/L (▲) or 200 mg/L (◆).
Figure 3.
XPS spectra from iron metal-core oxide-shell nanoparticles as received, after 24 hours of exposure to DI water and after 24 hours to Di water containing 20 mg/L natural organic material (NOM). The Fe 2p photoelectron peaks are consistent with an initial oxide shell of mostly Fe+3. After solution exposure the decrease in the ~ 719 eV satellite and some peak broadening suggest the appearance of an Fe+2 component. More apparent are an increase C=O bonds at ~289 eV in the C 1s photoelectron peaks for water and NOM exposure and the significant increase in ~ 531.5 peak feature for O 1s due to OH- and other O in the NOM.
| Initial | H2O | H2O+NOM | |
|---|---|---|---|
| Fe [at%] | 30 | 16 | 10 |
| O [at%] | 42 | 53 | 47 |
| C [at%] | 28 | 31 | 43 |
| Approximate C thickness [nm] | 0.87 | 0.98 | 1.5 |
Many different examples of the use of electron spectroscopy to monitor the presence of contamination, the effectiveness of cleaning processes, or the extent of oxidation have been reported in the literature. The examples highlighted here demonstrate the range of systems that can be examined. XPS and near edge X-ray absorption fine structure (NEXAFS) were used to examine the ability to remove the coatings formed on Rh nanoparticles synthesized in a polyvinylpyrrolidone (PVP) containing solution. The presence of a contamination layer was identified by XPS using standard analysis methods. Of equal importance was the ability to monitor the removal of a contamination layer [35]. In another study AES, due to its higher spatial resolution, was used to monitor contamination removal on a Si substrate supporting an array of nanoparticles. The oxidation state of Si and the presence of O on Pt nanoparticles were also examined [36]. Additional analysis tools used in this study included AFM, scanning electron microscopy (SEM), and XPS.
When particle shape is known, it is possible to obtain quantitative information about the thickness of a contamination layer (or a particle coating) using XPS [29,37]. For this application, the XPS is data modeled assuming a core shell structure of the particles. Using this approach, the oxidation rate for the Si nanoparticles could be measured and compared to that of silicon wafers [37].
ii) The size of nanoparticles will affect the relative strength of the signal intensities of the photoelectron peaks. This allows XPS data to be used to obtain information about particle size. As discussed in a recent review [13], a variety of approaches, initially developed for small metal catalyst particles, have been developed to extract this information [25, 38-39]. For example, the ratio of photoelectron intensities (from spherical particles of Cu or other metals) having different escape depths can be used to approximate the average particle size [40].
Background signals and photoelectron binding energies of nanoparticles can also be used to determine sizes of spherical particles. The use of energy loss electrons (usually considered the background signal) for Au particles on a polystyrene substrate [41] provides one specific example. The particle sizes determined by XPS compared well to those determined by electron spectroscopy. Many studies have shown that metal clusters and nanoparticles, particularly when they are supported on nonmetal supports, have XPS binding energies that differ from those of bulk materials. The binding energy (BE) shifts vary with sufficient regularity that Gonzalez-Elipe et al.[42] proposed BE peak position could be a useful way to determine particle size. Nosova et al. [43] determined the relations among the BE, particle size, and turnover number for hydrogenation of vinylacetylene by Pt particles on a variety of supports.
In each of these approaches, an average particle size is determined, and the particle shape is assumed. Whenever possible, it may be useful to verify the actual shape by electron microscopy (TEM or SEM) or, possibly, SPM.
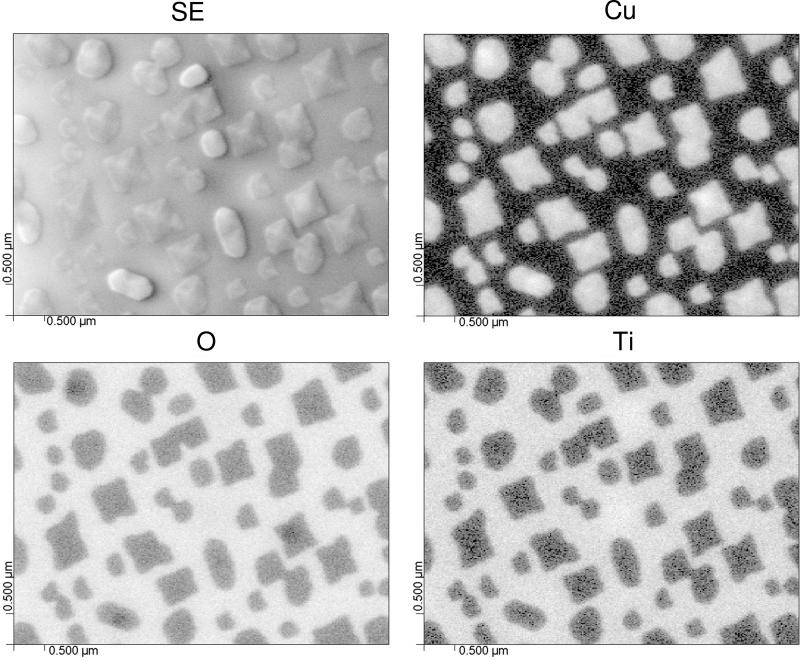
iii) The resolution available with the electron beam allows AES to be used to collect information about individual nanoparticles or nanoparticle arrays. Liang et al. [44] have been interested in the formation of Cu2O nanodots for possible chemical or photochemical applications [45]. AES was used to examine the nature of the nanodots formed on a SrTiO2 substrate after deposition using oxygen plasma assisted molecular beam epitaxy. A secondary electron image of the nanodots, along with AES maps for Cu, Ti, and O, are shown in Figure 5. One objective of the AES analysis was to determine if a Cu2O wetting layer was observed between the Cu2O nanodots. Such a wetting layer was not observed [44]. The secondary electron (SE) image and AES maps indicate that nanodots can be formed with differing shapes. This process has been examined as a function of the amount of material deposited, and AFM measurements will be shown in a later section.
Figure 5.
Secondary electron (SE) image and scanning Auger microscopy elemental maps for Cu, O and Ti from Cu2O nanodots grown on a SrTiO2 substrate [44]. The lighter regions are enriched in the elements of interest. The Cu and Ti maps show high contrast because they are present in some areas and absent in others. The O contrast is less because it is present in both the substrate and the nanodots.
AES combined with SEM and TEM has also been used to examine the location of a composite of organic-inorganic nanoparticles (COIN) on leukemia cells. The combined analysis approach provided reliable high-resolution information about the nanoparticles and their binding to cell surface antigens [46]. In this example, AES proved to be useful, despite a coating applied to minimize charging on the biological surface. AES has also been used to measure the concentrations of Au nanoparticles grown within a polyelectrolyte matrix. The need here was to identify the location and amount of gold retained on the polyelectrolyte brush surface. TEM, AFM, and X-ray reflectivity (XRR) measurements completed the tool set to obtain the detailed understanding of this complex nanostructured material [46].
iv) Determining the nature and distribution of active sites on nanostructured surfaces is an important challenge with relevance to catalysis and, possibly, particle toxicity. XPS has been used to identify and quantify the presence and distribution of Brønsted and Lewis acid sites for ZSM-5 zeolites [47]. The method involved the deconvolution of N 1s XPS features for pyridine chemisorbed on the zeolite. Three different N states were identified and assigned to Lewis sites and weak and strong Brønsted acid sites. Comparison of the XPS data with IR spectroscopic data suggested that XPS could be used to both identify and quantify the nature of surface acidity.
v) In addition to the composition and chemistry, it has been possible to use XPS in combination with sample substrate biasing and controlled electron flood gun voltage to obtain electrical information from films and particles [48-49]. By biasing a collection of nanoparticles while conducting XPS, it is possible to learn aspects of the electrical properties of Au/silica (core/shell) nanoparticles, particularly for particles embedded in a layer [49].
Ion-Based Surface Analysis Methods
Ion beams can be used in a variety of ways to obtain information about the nature of nanoparticles. One of the primary uses of TOF-SIMS is to extract molecular information about the functional groups and, possibly, molecular orientation of molecular coatings on particles surfaces. During SIMS measurements primary ion beams of Ga+, Ar+, O2+, Cs+, C60+, Au+, Bi+, or other atomic, molecular, or cluster ions with energies between 3 and 20 keV strike the sample surface and result in the ejection of secondary ions. To extract surface molecular information, TOF-SIMS is used in a “static” mode that involves a low density and low total dose of ions such that the surface damage and alteration is minimized. Both atomic and molecular secondary ions are used to extract the surface information [50].
Also known as Ion Scattering Spectrometry (ISS), LEIS is a well-established, but not widely used method. However, recent developments have made it particularly useful because of the high sensitivity to the outermost atomic layers of a sample [51]. In LEIS, a low energy ion beam (typically an inert gas ion) is scattered off of the surface. The amount of energy lost by the ion during this scattering process is used to determine the identity of the elements present in the outermost surface of the material under analysis. Here, low energy means ion energies less than a few thousand electron volts (which is low relative to the million volts used in Rutherford backscattering spectroscopy). The high surface sensitivity (near surface depth resolution) makes LEIS a particularly useful tool for surface enrichment measurements of catalysts or a wide variety of other materials.
Like the electron spectroscopies, SIMS is useful for obtaining molecular information about surface layers, functional groups added to the surface, and contamination. Three differences between the electron spectroscopies and SIMS are the higher detection sensitivity of TOF-SIMS for some species, the complicated and nonlinear signal dependence of TOF-SIMS signals (which may complicate quantitative analysis), and the changes generated at the surface by ion sputtering. The high sensitivity of TOF-SIMS for some functional groups has been usefully applied in many ways. For example, it has been used to examine peptides conjugated to gold nanoparticles as part of a protein kinase assay [52] and to examine multilayer plasma deposited organic coatings on alumina nanoparticles [53]. For relatively large nanoparticles produced during welding, SIMS with sputter profiling has been used to examine the complex layers that forms on these particles [47]. It must be noted that the sputtering of nanoparticles and particles in general may be significantly different from that of thin films or bulk materials [1,54]. Therefore, sputter depth profiling is not likely the optimum approach for getting layer or depth information about nanoparticles.
In addition to understanding surface coatings or functional groups, SIMS has proven to be equally useful for examining the basic composition of nanoparticles, both as they are being processed inside the TOF-SIMS vacuum system and after they have been introduced into the vacuum system for analysis. In situ thermo-TOF-SIMS was used to examine the thermal decomposition of zinc acetate dehydrate during nanoparticle formation within a SIMS system [55]. SIMS, in combination with TEM and in-situ optical transmission spectroscopy, has been used to study the composition and plasmon resonance of unique ZrN nanoparticles produced by laser ablation/evaporation and adiabatic expansion from zirconium nitride powder targets [56]. TOF-SIMS has also been used to characterize the composition and oxidation state of spark-generated nanoparticles made from pairs of Ir-Ir and Ir-C electrodes. From detailed analysis of the SIMS data, it was also possible to obtain information about particle size [57].
LEIS seemed to be going out of favor until new instrumentation developments significantly enhanced the sensitivity of the method, making it useful for characterizing several nanoparticle properties. It can sometimes be used to determine information about surface layers that might be missed by other surface sensitive techniques. A particularly interesting example is the use of LEIS to examine the outer surfaces of poly(propylene imine) dendrimers. Although the different generations of dendrimer have the same overall C/N ratio, the outer C/N surface ratio changes as the dendrimers age. By combining the high surface sensitivity of LEIS with the “near” surface sensitivity of XPS, it was possible to show that dendrimer aging changes primarily the outermost atomic layers [58].
As with XPS, LEIS does not have the resolution to examine individual particles, but can be used to measure the average size of particles and to look at changes in particle size as a function of time. It has been used, for example, to determine metal segregation and the average cluster size of Pt/Rh/CeO2/γ-Al2O3 supported catalysts. The cluster size measurement has been applied to atomically dispersed metals in a nanostructured catalyst and can be used to determine cluster size for particles up to 10 nm [59].
Scanning Probe Microscopies
STM and AFM are powerful techniques that have enabled major advances in nanotechnology. The development of these “sharp” tip based probes has made it possible for almost any laboratory around the world to examine surfaces of many types of materials with spatial resolutions approaching 1 nm for electrical properties (STM) or sample topography (AFM). AFM can provide 3-D imaging/visualization of nanoparticles distributed on a flat surface. It can provide qualitative and/or quantitative information about physical properties of nanoparticles including size, morphology, surface texture, and roughness. However, the influence of the AFM tip size and shape on the acquired images must be properly accounted and corrected for. Collection of a variety of images can be used to extract information about particle size distributions and volumes [60]. AFM can be conducted in vacuum, ambient conditions, liquids, or other environments [61].
The application of SPM methods to nanoparticles takes many different forms [18,62]. Many, but by no means all, applications involve examining nanoparticles on flat surfaces where many different types of information can be extracted including size and size distribution, as well as shape (including facets). The shapes of the Cu2O nanodots formed on a SrTiO2 and previously described have been examined by AFM (Figure 6) [44-45]. The figure shows the size and shape of these nanodots that form on the substrate as a function of the total amount of Cu2O deposited on the substrate.
Figure 6.
Plot of the lateral size and height of Cu2O nanodots grown on SrTiO2. AFM inserts and drawings show how the shape of the nanodots changes as an increased amount of Cu2O is deposited onto the substrate [44].
Somewhat uncommon applications of SPM methods have proven to have high value and two examples are noted here. By attaching a nanoparticle to a scanning probe tip, it is possible to measure the interactions of individual nanoparticles with a flat surface (possibly chemically functionalized) or to other nanoparticles attached to a flat surface [63]. Such measurements provide information about interaction forces and enable these forces to be examined in different environments. In some circumstances, it is useful to determine the roughness of nanoparticle surfaces where many standard methods of measuring surface roughness cannot be applied. AFM in combination with TEM has been used to investigate the impact of synthesis processes and particle size on the surface roughness of ceria nanoparticles [64].
Characterization of Carbon Nanotubes (CNTs)
A wide range of tools have been applied to characterize and understand various forms of CNTs, including single-wall carbon nanotubes (SWCNTs or SWNTs) and multi-walled CNTs (MWCNTs), as they are synthesized, functionalized, and applied. No attempt will be made here to summarize all appropriate methods. Electron microscopy and optical methods have proven to be highly valuable [65]. However, in many circumstances, surface analysis tools, also in combination with a variety of other methods, have proven to be important for obtaining critical information. Examples include the use of surface tools to optimize CNT growth conditions, study the effects of doping on the electronic structure, and verify surface functionalization.
AES has been used to characterize the growth of vertically aligned CNTs on a Si substrate by plasma-enhanced chemical vapor deposition [66]. The spatial resolution of AES allowed examination of the head and body of the nanotubes. The authors observed that details of the C(KVV) Auger peaks can be used to examine the ordering of the sp2 bonded carbon. AES measurements at the head of the nanotubes showed the presence of the Ni catalyst, indicating that the Ni remained at the top of the nanotubes during growth. Detailed analysis of the substrate allowed the deposition to be optimized to eliminate amorphous carbon byproducts. A 5 nm interfacial layer was identified on the substrate that had been previously unidentified because previous studies using energy dispersive X-ray measurements did not have the surface sensitivity to see the layer.
Many studies of CNTs involve efforts to alter their surface properties by surface functionalization or to alter the overall properties of the nanotubes by doping the particles in some way. Surface tools can provide information about the presence of the atoms or molecules added to the CNTs and the extent of charge transfer between the CNTs and the added material. The noncovalent surface functionalization of SWCNTs using succinimidyl ester for attachment of proteins was previously described [11]. A review by Daniel et al. [65] summarizes the efforts to functionalize CNT surfaces with DNA and the approaches toward characterization. The purpose of DNA functionalization has been both to create surfaces useful for sensing and as a method to direct the assembly of nanotubes. XPS and gas chromatography-mass spectrometry (GC-MS) thermal analysis were used to examine some details of CNTs functionalized by solvent-free and aqueous-based arenediazonium. Both methods suggested that only the arcne group was retained on the CNTs [12] after the process.
Another approach to functionalization involves the sulfuric/nitric acid oxidation of CNTs or other graphene-containing materials. A systematic, time-dependent, surface-sensitive study used XPS, Fourier transform infrared (FTIR), and Raman spectroscopies to examine in real time the evolution of the functionalization of carbon fibers sonicated in a mixture of the concentrated acids [67]. The study showed that oxidation occurred after the acid attack created sites that were subsequently oxidized. Smith et al. [68] used XPS to characterize the surfaces oxidized by the acid process as part of a study of the impact of these surface oxides on the colloidal stability of MWCNTs.
Efforts to alter the electronic structure of CNTs have involved both adding atoms within the structure of the CNTs and by attaching molecules to the surface likely to be involved in charge transfer. Tetracyano-p-quinodimethane (TCNQ) was shown by core level and valence band XPS, in combination with NEXAFS, to deplete the π-band density of states of SWNTs because of transfer to the TCNQ [69]. Another method of doping to change the electronic structure has involved low energy implantation of N. XPS has been used to examine both the amount of N implanted and the electronic structure of the implanted SWCNTs [70-71].
Charge transfer between Au nanoclusters and as synthesized and plasma oxidized CNTs have been examined by TEM and XPS. Core-level and valence band measurements showed little charge transfer between the Au clusters and MWCNTs. The authors of this paper note that the uncleanliness of the surface, the presence of amorphous phases, and agglomeration of as grown CNTs appear to be constraints to CNT applications[72]
Considerations for Nanoparticle Analysis
General Considerations
Some general issues associated with nanoparticles are useful to consider when analyzing nanoparticles. We highlight here some of the challenges already briefly noted. Billinge and Levin [73] have described the challenges for three dimensional structural characterization of nanoparticles. The level of detail that scientists, engineers, and manufacturers would like for nanostructured materials exceeds that currently possible, particularly on a routine basis [1-3]. In particular, new methods that involve novel integration of information from more than one method and the application of theoretical analysis are needed [73]. The following list of challenges and opportunities outlines some of these generic needs.
A variety of studies have shown that nanoparticles can be unstable with respect to the environment [5,74-76]. The inherently lower level of stability for many types of nanostructured materials significantly increases the attention that analysts need to pay to the impacts that an analysis probe, environmental conditions, and time can have on the materials analyses [77,33]. For example, the sorption of water onto the surface of ZnS nanoparticles changes the particle structure [76]. The energies associated with many of the probes used for particle analysis equal or exceed those needed to transform the particles in a variety of ways [1,77]. We have observed significant time-dependent reactivity associated with Fe metal-core oxide-shell nanoparticles when placed in water [33]. These issues impact the care needed for sample handling, the time and nature of the analyses, and the need for comparison of results from a variety of methods.
The significant fraction of atoms or molecules associated with surfaces and interfaces increases the potential impact of surface impurities, surface enrichment or depletion, and surface contamination [2] on nanoparticle properties and complicates accurate measurements. Surface coatings and impurities can significantly alter many aspects of nanoparticle behavior. The deliberate application of coatings has been used to control particle shape during growth [78], and the use of coatings on nanoparticles can be used to control particle spacing and the resulting properties of nanoparticle composites [79]. Because of capillary and sorption effects, the high surface area present in collections of nanoparticles may retain solvent in circumstances that can surprise researchers [21, 80]. Even using surface tools, it can sometimes be difficult to characterize the nature of the actual nanoparticle surfaces.
Surface tools are highly valuable in measuring surface coatings and contamination layers, but it can be important to combine shape or other data obtained by complementary methods to increase the value of the information that can be obtained. Shard et al. [29], for example, show how XPS can be used to accurately determine coating thickness when particle size and shape are known.
Because the physical properties of nanoparticles can change with size and the environment, some of the assumptions made in the analysis (e.g., electron inelastic mean free paths) may be inaccurate. Samples are a polymorph of bulk materials [81], which may impact the accuracy of electron spectroscopy measurements on nanoparticles. Ion beams will damage nanoparticles at rates significantly higher than typical for bulk materials for thin films. Sputtering of nanoparticles can be 10 times faster than for “bulk” films [54,82]. Such behavior can impact SIMS measurements on nanoparticles. Some of these issues are identified as specific technique concerns in a following section.
For the reasons already stated, the preparation, processing, and mounting of nanoparticles requires particular care and attention. A measurement made without reference to sample history and previous processing will often provide misleading or incorrect information. These effects also place a high value on measurements that can be made without removing materials from the environment of interest. In situ (and real time) analysis methods provide important new levels of information useful for advancing nanotechnology [2-3].
Sample Mounting Issues
The heightened concerns about contamination, impurities, and environmental effects necessarily raise issues and concerns about the handling and mounting of specimens for analysis. For nanoparticles, the issues associated with sample mounting are similar to those associated with mounting any sample for surface analysis, and a variety of guides have been developed [83-84]. For the set of surface techniques discussed here, the nanoparticles will normally be supported on a substrate and examined either individually (SPM and, sometimes, AES and SIMS) or as a collection of particles. Confirming the nanoparticles are immobilized without introducing surface contamination may be achieved using a variety of methods. Examples include the use of vacuum compatible tape (after checking for contamination issues), growth on a substrate, solution deposition onto a substrate, or dipping a support into solution.
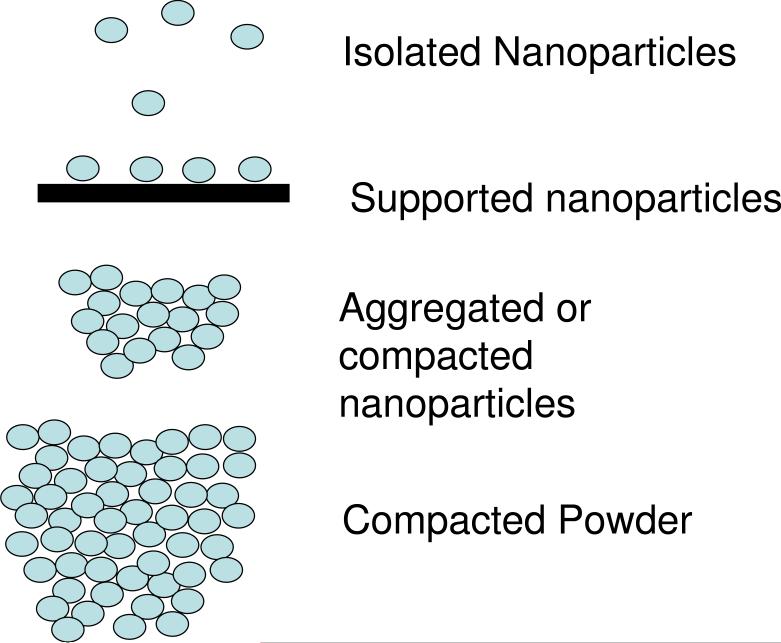
Different preparation and analysis methods produce a variety of particle distributions as shown in Figure 7. Some nanoparticle properties and measurement results can be impacted by agglomeration or aggregation or interactions of nanoparticles with the supporting substrate and/or with other nanoparticles (proximity effects [1,13]). Such effects include: i) buildup of charge during XPS measurements of metal clusters supported on insulating substrate [85-86], ii) coupling of plasmon modes in metal nanoparticles within close proximity [87-88], iii) coupling of quantum states [89], iv) impact of particle spacing on electronic and magnetic properties of composite [79,90], and v) effect of “buffer layers” on optical properties of silicon nanocrystal superlattices [91]
Figure 7.
Nanoparticles are applied and characterized with different degrees of isolation or aggregation. In some circumstances, the collections of nanoparticles have properties that differ from individual particles and particle separation distances or substrate interactions can be important. After reference [13].
Analysis Considerations for Specific Methods
In this section, we consider some of the nanoparticle analysis considerations relevant to the specific methods discussed in this paper. The topics are not comprehensive, but suggest both areas to be addressed and some of the solutions currently used to address them.
XPS characterization of nanoparticles
XPS has been used to analyze nanolayers almost since its inception. One of the major application areas has been the characterization of complex catalysts particles, which often included supported metal nanoparticles as the active components [92-93,25,94]. A wide variety of nanostructures are now routinely analyzed using XPS. While most XPS analysis is conducted assuming that the sample has a uniform flat surface layer, the shape and structure of nanoparticles can play an important role in accurate interpretation of XPS (and other) data of any nanostructured material [95]. As suggested by Figure 7, such materials do not present a uniform surface for analysis. Several analytical methods can be used to extract important information about nanoparticles and nanostructured materials by moving beyond the uniform layer analysis method. The appropriate approach also depends on the density and mounting of the particles. Often modeling of the experimental data is required to extract information not available from the standard simple analysis [13]. Examples are provided as follows.
Low density of substrate supported particles
Although the size of nanoparticles can be determine by both peak ratio and BE measurements, as noted earlier, the peak ratio method applies most readily when the particles are present at low density (not overlapping) on a surface [13, 25, 38-40]. An interesting feature of this approach is that the size of the particles can be determined even if they are too small to be observed by electron microscopy. However, if the particles are covered by contamination or buried within a substrate photoelectron peak ratios will be influenced by the covering layers and this method is not generally valid (or may require an overlayer correction) [25,34]. With the assumption or verification that the nanoparticles are primarily on the surface, the kinetic ratio method appears to apply equally well to rough as well as ideally flat surfaces.
In the ideal circumstance that nanoparticles are distributed as single layer on a flat surface, it is possible to use angle-resolved XPS to obtain information about particle size, size distribution, and separation [96]. It is also possible to examine the presence and even thickness of coatings [29], including corrosion or oxidation layers (these can be considered core shell type particles) [37]. For this type of analysis, sample preparation, the mounting method and the nature of the substrate are important because particles may interact either chemically or respond physically to the nature of the substrate. Also, it is desirable to minimize possible overlap of peaks between particles and the substrate.
Variable excitation energy XPS (ERXPS), available at synchrotrons, offers a relatively new and increasingly powerful alternative to obtaining depth information from powdered samples (such as nanoparticles) and other real surfaces that are not ideally smooth. Merzlikin et al. [97] have shown that ERXPS can be used to collect useful coating information even for very rough surfaces (including a collection of nanoparticles as described below). Other work by Merzlikin [98] directly showed the ability to use the ERXPS approach to examine core-shell nanoparticles.
Agglomerates of particles
It is frequently not possible to distribute particles at low density, and, in some circumstances, a larger collection of particles is needed to obtain sufficient signal. In a now classic study, Fulghum and Linton compared analysis methods to determine quantitatively the coverage of a specific surface adsorbate on the surfaces of particles in a collection of particles [99-100]. They showed that XPS can be used to obtain accurate surface coverage even on the surface of a powder sample. Castner and Campbell have also presented a detailed method for XPS analysis of nanoparticle agglomerates [27].
For nanoparticles, knowledge about the surface coating and/or contamination may be central to their application. The approach of Shard et al may be applied for both single layers of particles or agglomerates if the particle size is known [29]. Agglomerates of particles present what is effectively a very rough surface for analysis. The so called “magic” angle (analyzer geometry) presents another approach to data collection and analysis that can be used to estimate layer coverage, with appropriate consideration of the accuracy limits [27,101-102].
Size and curvature effects
The signal strength from a coating relative to that of the substrate (or particle core) can vary with particle size because of the electron path lengths and surface curvature [13,29,95]. Data collected on different sized Au nanoparticles coated with C16 COOH thiol molecules in a self assembled monolayer (SAM) shown in Figure 8 provide a demonstration of this effect. Although the SAM layer thickness is nearly constant, the ratio of the C signal from the SAM to the Au signal from the core particle changes as a function of particle size. As the particle gets smaller, the collection of particles contains an increasing percentage of SAM material. Surface curvature effects impact relative peak intensities of overlayers and substrates (cores) for all sizes of particles [13, 29], but when the particle size becomes smaller than the electron escape depth it also becomes possible to detect photoelectrons from both the top and bottom of the particles.
Figure 8.
The C to Au elemental composition ratio measured by XPS for SAM terminated Au nanoparticles as a function of particle size. As the particle size decreases the relative amount of C measured increases even when the coating thickness (a C16 COOH terminated SAM) remains constant. When the particle size is known, the substrate to coating signal intensity ratio can be used to determine coating thickness.
Binding energy and peak width changes
The size of particles (and interactions with the substrate) can impact the binding energy and peak width of photoelectron peaks, change the valence band peak shape, and alter the Auger parameter [30, 86]. These effects sometimes can be used to determine particle sizes, as noted above [25, 38-39]. The BE method for size determination may be less impacted by surface contamination than the peak ratio method and has been used as a measure of Au nanoparticle size for particles grown within polyaniline films [13]. However, not all size dependent BE shifts are confined to nanoparticles—the binding energy of SiO2 on Si varies with the film thickness due to interactions with the substrate. It can be important to remember that not all shifts in photoelectron binding energies associated with nanoparticles should be interpreted as chemical state changes.
AES analysis of nanoparticles
Many of the considerations described for XPS apply to AES as well because they relate to the impacts of particle size and distribution on the low energy electrons detected by the AES or XPS process. However, because AES involves electron excitation, issues of electron backscattering and transmission through a particle provide some additional areas to consider [28]. There is a general tendency of analysts to look at a secondary electron image and assume that all of the AES signals from a feature arise from the area where the electron beam strikes. However, Auger electrons will usually be produced and detected from both where to electron beam strikes the sample and some of the nearby surrounding region. This occurs because Auger electrons are produced not only from the region where an incident electron beam strikes, but from nearby areas that are excited by the primary beam as it penetrates into and is scattered by the sample. This is a well-known phenomenon, and there is an ISO standard for determining the analysis area and lateral resolution for AES and XPS analysis [103].
TOF-SIMS analysis of nanoparticles
TOF-SIMS analysis involves the bombardment of a substrate with ions that have energies on the order of tens of keV (which is comparable to the cohesive energy of a nm-sized cluster [104]) and typical penetration lengths (for monatomic ions on flat uniform substrates) up to tens of nm [105-106]. The affected area from a single collision is also on the order of one to tens of nm. Thus, the interaction area and energy of the primary ion is of the same order of magnitude as the size of a nanoparticle. Simulations indicate this may lead to a number of potential issues, challenges, and artifacts. The primary effect that is seen through both simulation [105-108] and experiment [109-110] is that the sputter yield/rate may increase several fold. Simulations show this to be due to recoil from the “back” and escape from the “sides” of the nanoparticle. It is also useful to recognize that with sputter time, the shape of particles will change.
The enhanced sputtering of nanoparticles has a potential impact on the information that can be obtained from organic or other coatings on these particles. The static limit is identified as the ion doses below which the molecular information is typically maintained, usually quoted as 1012–1013 ions/cm2. At these doses, on the order of 0.1–1% of the surface sites have been subject to direct impact. Because TOF-SIMS is often used to characterize functional groups associated with coatings on nanoparticle surfaces, it is desirable to stay well below the static limit and to know that this limit may be significantly different for nanoparticles relative to flat surfaces. To determine the static limit for nanoparticles, an experiment was devised whereby alcohol-functionalized titanium dioxide nanoparticles (TiO2 nps) were covalently attached to a silicon substrate covered with native oxide and an isocyanate self-assembled monolayer. The alcohol reacts with the isocyanate via standard urethane chemistry to yield a monolayer of TiO2 nps. In practice, this substrate also has a number of large aggregates (see Figure 9).
Figure 9.
Scanning electron micrographs of a TiO2 nanoparticle functionalized Si wafer showing large aggregates and small nanoparticles (left) after 1×1016 primary ion dose and a large aggregate (right) after 3×1016 primary ion dose. Note shadowing due to non-normal incidence of primary ions.
The substrate was sputtered using a PHI TRIFT II TOF-SIMS instrument with 15 kV Ga+ for a dose ranging from 5×1011 ions/cm2 to 3×1016 ions/cm2. Peak intensity data for 48Ti+, 48Ti16O+, and 8Ti16O2+ were recorded for doses up to 1×1016 ions/cm2 to measure differential sputtering of O, known to occur widely with metal oxides. These data are summarized in Figure 10. As is evident from the data, differential sputtering starts to occur for doses above 5×1012 ions/cm2 within the conventional measure for the static limit. Other data (not shown) indicate that the organic coating on the nanoparticles and substrate (whether from the SAM or adventitious carbon) shows some damage at a slightly lower ion dose, but still above 1×1012 ions/cm2. However, this analysis is complicated by the observation that the Ti and TiOx signals do not disappear when the individual nanoparticles are no longer seen in the SEM. This indicates that the secondary ion signals are arising primarily from the larger aggregates.
Figure 10.
Secondary ion signal as a function of ion dose for a TiO2 nanoparticle film containing isolated nanoparticles (~30-50 nm dia.) and large aggregates (~100-1000 nm). The damage threshold, indicated by the changing ratio of Ti/TiOx peak ratios, is still changing between 1012-1013 ions/cm2. The secondary ion signal arises primarily from the large aggregates.
This data indicates that aggregates of particles behave in a similar fashion as bulk materials (i.e., secondary ion (SI) signals are not affected by the nano-size character of the sample). However, in a sample with a mix of aggregates and individual nanoparticles, the signal arises primarily from the aggregates. This may be from poor coupling of the incoming primary ion to the nanoparticle, leading to little energy deposition in the nanoparticle. Substrate signals may dominate over SI signals arising from individual nanoparticles in a sparse monolayer. For cluster beams, this effect may be negligible. The sensitivity of the nanoparticles to perturbations leading to amorphization, loss of secondary structure, reduction, etc., may be influenced by the three-dimensional structure. Because of the angle dependence of sputter rates, sputtering can vary as much as 10 times for different portions of a nano-object [111].
Sample history and handling may also influence the SIMS analysis of nanostructured materials in a similar manner to other surface analysis methods. One other complicating factor is that the high electric fields used in the extraction region to accelerate the secondary ions into the spectrometer may induce “field-emission” of nanoparticles.
Scanning probe microscopy analysis of nanoparticle
Although a wide variety of information about nanoparticles supported by a flat substrate can be obtained using SPM methods, there are a variety of tip artifacts that can influence the accuracy of the measurements [112-113]. Chapter 3 of Braga and Ricci is devoted to recognizing and avoiding artifacts in AFM imaging [114]. International standards committees (ASTM E42 and ISO TC201) have subcommittees working on relevant standards and guides to these effects [115].
Summary and Conclusions
There is an increasing need for improved analysis of nanoparticles, including characterization of the nanoparticle surface. Surface chemical analysis methods can provide an important component of the needed information. These methods should be more generally (routinely) applied as part of a characterization suite of tools needed to fully characterize nanoparticles for many applications.
Challenges arise in the analysis of nanoparticles using many of the currently available techniques, including surface characterization methods. These issues are not universally recognized or accounted for and may include particle stability, environmentally-induced changes, contamination, and time-dependent behavior. Some technique-specific issues (or opportunities) related to the analysis of nanoparticles have been briefly discussed to indicate how nanoparticle analysis can differ from the characterization of thin films and other types of materials.
Adequate characterization of nanoparticles often involves characterization by a variety of complementary analysis methods. The impact of time and environment on particle properties implies the need for timely analysis, sample handling, in situ analysis, and reasonable understanding of the physical and chemical meaning of each type of measurement. Data consistency provides enhanced confidence in the measurement results. Differences among analysis methods can provide important information about effects of processing, sample handling, or assumptions made about the analysis results.
It is recognized that the tools discussed here are only a subset of those that can be applied to obtain important surface composition and chemical information from nanoparticles. Optical methods, including second harmonic generation (SHG) and sum frequency generation (SFG), can provide a wide variety of surface and interfacial information about nanoparticles [116] in differing environments.
Acknowledgements
This paper has evolved from research programs supported by the U.S. Department of Energy (DOE) and research conducted as part of the Environmental Molecular Sciences Laboratory (EMSL) User Program. It has benefited from interactions with colleagues from around the world and input from experts associated with ISO TC 201 Surface Chemical Analysis and ASTM Committee E42 on Surface Analysis. Aspects of the work have been supported by the DOE Offices of Basic Energy Sciences and Biological and Environmental Research. Portions of this work were conducted in EMSL, a DOE user facility operated by Pacific Northwest National Laboratory for the DOE Office of Biological and Environmental Research. We thank MH Engelhard for the XPS data on the iron nanoparticles. DGC and SDT thank NIH grants EB-002027 and GM-074511 for support and for funding some of the experimental work described in this paper. SDT thanks the NSF for an IGERT fellowship.
References
- 1.Baer DR, Amonette JE, Engelhard MH, Gaspar DJ, Karakoti AS, Kuchibhatla S, Nachimuthu P, Nurmi JT, Qiang Y, Sarathy V, Seal S, Sharma A, Tratnyek PG, Wang CM. Characterization challenges for nanomaterials. Surface and Interface Analysis. 2008;40:529–537. [Google Scholar]
- 2.Grainger DW, Castner DG. Nanobiomaterials and nanoanalysis: Opportunities for improving the science to benefit biomedical technologies. Advanced Materials. 2008;20:867–877. [Google Scholar]
- 3.Grassian VH. When Size Really Matters: Size-Dependent Properties and Surface Chemistry of Metal and Metal Oxide Nanoparticles in Gas and Liquid Phase Environments. Journal of Physical Chemistry C. 2008;112:18303–18313. [Google Scholar]
- 4.Stuart C. Particle Size Matters. Small Times. 2006:6. [Google Scholar]
- 5.Karakoti AS, Hench LL, Seal S. The potential toxicity of nanomaterials - The role of surfaces. Jom. 2006;58:77–82. [Google Scholar]
- 6.2008 Available from the World Wide Web. http://www.oecd.org/department/0,3355,en_2649_37015404_1_1_1_1_1,00.html.
- 7.Wu H, Engelhard MH, Wang J, Fisher DR, Lin Y. Synthesis of lutetium phosphate-apoferritin core-shell nanoparticles for potential applications in radioimmunoimaging and radioimmunotherapy of cancers. Journal of Materials Chemistry. 2008;18:1779–1783. [Google Scholar]
- 8.Baer D, Tratnyek P, Qiang Y, Amonette JE, Linehan JC, Sarathy V, Nurmi JT, Wang CM, Antony J. Synthesis, Characterization and Properties of Zero Valent Iron Nanoparticles. In: Fryxell G, Cao G, editors. Environmental Applications of Nanomaterials: Synthesis, Sorbents, and Sensors Imperial College Press; London: 2007. [Google Scholar]
- 9.Nurmi JT, Tratnyek PG, Sarathy V, Baer DR, Amonette JE, Pecher K, Wang CM, Linehan JC, Matson DW, Penn RL, Driessen MD. Characterization and properties of metallic iron nanoparticles: Spectroscopy, electrochemistry, and kinetics. Environmental Science & Technology. 2005;39:1221–1230. doi: 10.1021/es049190u. [DOI] [PubMed] [Google Scholar]
- 10.Johnson RL, Johnson GO, Nurmi JT, Tratnyek PG. Natural Organic Matter Enhanced Mobility of Nano Zerovalent Iron Environmental Science & Technology. 2009;43:5455–5460. doi: 10.1021/es900474f. [DOI] [PubMed] [Google Scholar]
- 11.Chen RJ, Zhang YG, Wang DW, Dai HJ. Noncovalent sidewall functionalization of single-walled carbon nanotubes for protein immobilization. Journal of the American Chemical Society. 2001;123:3838–3839. doi: 10.1021/ja010172b. [DOI] [PubMed] [Google Scholar]
- 12.Dyke CA, Stewart MP, Maya F, Tour JM. Diazonium-based functionalization of carbon nanotubes: XPS and GC-MS analysis and mechanistic implications. Synlett. 2004:155–160. [Google Scholar]
- 13.Baer DR, Engelhard MH. XPS Analysis of Nanostructured Materials and Biological Surfaces. Journal of Electron Spectroscopy and Related Phenomena. 2009 doi:10.1016/j.elspec.2009.09.003. [Google Scholar]
- 14.Powell CJ. Growth and trends in Auger-electron spectroscopy and x-ray photoelectron spectroscopy for surface analysis. Journal of Vacuum Science & Technology A. 2003;21:S42–S53. [Google Scholar]
- 15.Reniers F, Tewell C. New improvements in energy and spatial (x, y, z) resolution in AES and XPS applications. Journal of Electron Spectroscopy and Related Phenomena. 2005;142:1–25. [Google Scholar]
- 16.Brundle CR, Evans CA, Wilson S. Encyclopedia of Materials Characterization: Surfaces, Interfaces, Thin Films. Butterworth-Heinemann; Greenwich, CT: 1992. [Google Scholar]
- 17. Available from the World Wide Web: http://www.cea.com/
- 18.Scalf J, West P. [November 23, 2009];Part I: Introduction to Nanoparticle Characterization with AFM. 2006 Application Note - Pacific Nanotechnologies www.nanoparticles.org/pdf/scalf-west.pdf.
- 19.Gilmore IS, Seah MP, Johnstone JE. Quantification issues in ToF-SSIMS and AFM co-analysis in two-phase systems, exampled by a polymer blend. Surface and Interface Analysis. 2003;35:888. [Google Scholar]
- 20. Available from the World Wide Web: http://www.npl.co.uk/nanoanalysis.
- 21.Nurmi JT, Sarathy V, Tratnyek PTB, D. R, Amonette JE, Karkamkar A. Recovery of Iron/Iron Oxide Nanoparticles from Solution: Comparison of Methods and their Effects. Journal of Nanoparticles. 2009 In Press. [Google Scholar]
- 22.Jablonski A, Powell CJ. Information depth and the mean escape depth in Auger electron spectroscopy and X-ray photoelectron spectroscopy. Journal of Vacuum Science & Technology A. 2003;21:274–283. [Google Scholar]
- 23.Finster J, Lorenz P, Meisel A. Quantitative ESCA surface analysis applied to catalysts: Investigation of concentration gradients. Surface and Interface Analysis. 1979;1:179–184. [Google Scholar]
- 24.Srivastava S. ESCA Studies of Supported Catalysts. Applied Spectroscopy Reviews. 1988;24:81–97. [Google Scholar]
- 25.Venezia AM. X-ray photoelectron spectroscopy (XPS) for catalysts characterization. Catalysis Today. 2003;77:359–370. [Google Scholar]
- 26.Tougaard S. XPS for Quantitative Analysis of Surface Nano-structures. Microscopy and Microanalysis. 2005;11:676–677. [Google Scholar]
- 27.Frydman A, Schmal M, Castner DG, Campbell CT. A Method for Accurate Quantitative XPS Analysis of Multimetallic and Multiphase Catalysts on Support Particles. Journal of Catalysis. 1995;157:133–144. [Google Scholar]
- 28.Powell CJ. Effect of backscattered electrons on the analysis area in scanning Auger microscopy. Applied Surface Science. 2004;230:327–333. [Google Scholar]
- 29.Shard AG, J. W, J. SS. XPS topofactors: determining overlayer thickness on particles and fibres. Surface and Interface Analysis. 2009;41:541–548. [Google Scholar]
- 30.Yang DQ, Sacher E. [November 23, 2009];X-ray photoelectron spectroscopy characterization of Nanoparticles (NPs): I. Dimensional effects. 2008 http://www.scribd.com/doc/2194883/Nano-XPS-nanost-1.
- 31.Castle JE. Module to guide the expert use of x-ray photoelectron spectroscopy by corrosion scientists. Journal of Vacuum Science & Technology A. 2007;25:1–27. [Google Scholar]
- 32.Castle JE. Update on expert systems. Journal of Electron Spectroscopy and Related Phenomena. 2009 doi:10.1016/j.elspec.2009.07.005. [Google Scholar]
- 33.Sarathy V, Tratnyek PG, Nurmi JT, Baer DR, Amonette JE, Chun CL, Penn RL, Reardon EJ. Aging of iron nanoparticles in aqueous solution: Effects on structure and reactivity. Journal of Physical Chemistry C. 2008;112:2286–2293. [Google Scholar]
- 34.Smith GC. Evaluation of a simple correction for the hydrocarbon contamination layer in quantitative surface analysis by XPS. Journal of Electron Spectroscopy and Related Phenomena. 2005;148:21–28. [Google Scholar]
- 35.Ashida T, Miura K, Nomoto T, Yagi S, Sumida H, Kutluk G, Soda K, Namatame H, Taniguchi M. Synthesis and characterization of Rh(PVP) nanoparticles studied by XPS and NEXAFS. Surface Science. 2007;601:3898–3901. [Google Scholar]
- 36.Zhu J, Somorjai GA. Formation of Platinum Silicide on a Platinum Nanoparticle Array Model Catalyst Deposited on Silica during Chemical Reaction Nano Letters. 2001;1:8–13. [Google Scholar]
- 37.Yang DQ, Gillet JN, Meunier M, Sacher E. Room temperature oxidation kinetics of Si nanoparticles in air, determined by x-ray photoelectron spectroscopy. Journal of Applied Physics. 2005;97:6. [Google Scholar]
- 38.Kerkhof FPJM, Moulijn JA. Quantative-Analysis of XPS Intensities for Supported Catalysts. Journal of Physical Chemistry. 1979;83:1612–1619. [Google Scholar]
- 39.Kuipers HPCE, van Leuven HCE, Visser aWM. The Characaterization of Heterogeneous Catalysis by XPS Based on Geometrical-Probability. 1. Monometallic Catalysts. Surface and Interface Analysis. 1986;8:235–242. [Google Scholar]
- 40.Yang DQ, Meunier M, Sacher E. The estimation of the average dimensions of deposited clusters from XPS emission intensity ratios. Applied Surface Science. 2001;173:134–139. [Google Scholar]
- 41.Hajati S, Zaporojtchenko V, Faupel F, Tougaard S. Characterization of Au nano-cluster formation on and diffusion in polystyrene using XPS peak shape analysis. Surface Science. 2007;601:3261–3267. [Google Scholar]
- 42.Gonzalez-Elipe AR, Munuera G, Espinos JP. XPS Intensities and Binding-Energy Shifts as Metal Dispersion Parameters in NI/SIO2 Catalysts. Surface and Interface Analysis. 1990;16:375–379. [Google Scholar]
- 43.Nosova LV, Stenin MV, Nogin YN, Ryndin YA. EXAFS and XPS studies of the influence of metal particle size, nature of support and H2 and CO adsorption on the structure and electronic properties of palladium. Applied Surface Science. 1992;55:43–48. [Google Scholar]
- 44.Liang Y, Lea AS, McCready DE, Meethunkij P. Synthesis and Characterization of Self-Assembled Cu2O Nano-Dots. In: Baer DR, Clayton CR, Davis GD, Halada GP, editors. State-of-the-Art Application of Surface and Interface Analysis Methods to Environmental Material Interactions. Honor of James E. Castle's 65th The Electrochemical Society; The Electrochemical Society; Pennington, NJ: Washington DC: 2001. [Google Scholar]
- 45.Lyubinetsky I, Lea AS, Thevuthasan S, Baer DR. Formation of epitaxial oxide nanodots on oxide substrate: Cu2O on SrTiO3(100). Surface Science. 2005;589:120–128. [Google Scholar]
- 46.Koh AL, Shachaf CM, Elchuri S, Nolan GP, Sinclair R. Electron microscopy localization and characterization of functionalized composite organic-inorganic SERS nanoparticles on leukemia cells. Ultramicroscopy. 2008;109:111–121. doi: 10.1016/j.ultramic.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Borade R, Sayari A, Adnot A, Kaliaguine S. Characterization of acidity in ZSM-5 zeolites: an x-ray photoelectron and IR spectroscopy study. The Journal of Physical Chemistry. 2002;94:5989–5994. [Google Scholar]
- 48.Cohen H, Sarkar SK, Hodes G. Chemically Resolved Photovoltage Measurements in CdSe Nanoparticle Films. Journal of Physical Chemistry B. 2006;110:25508–25513. doi: 10.1021/jp0648590. [DOI] [PubMed] [Google Scholar]
- 49.Tunc I, Demirok UK, Suzer S, Correa-Duatre MA, Liz-Marzan LM. Charging/discharging of Au (core)/silica (shell) nanoparticles as revealed by XPS. Journal of Physical Chemistry B. 2005;109:2418242–184. doi: 10.1021/jp055614a. [DOI] [PubMed] [Google Scholar]
- 50.Ratner BD, Castner DG, Brison J, Barnes C, Daneshcvar R Static SIMS A Powerful Tool to Investigate Nanoparticles and Biology. 2009 http://www.semineedle.com/system/files/BuddyRatner_5-14-09.pdf?snc=5963. TeleSeminar. http://www.semineedle.com/system/files/BuddyRatner_5-14-09.pdf?snc=5963 (last accessed PDF on webpage)
- 51.Brongersma HH, Draxler M, de Ridder M, Bauer P. Surface composition analysis by low-energy ion scattering. Surface Science Reports. 2007;62:63–109. [Google Scholar]
- 52.Kim YP, Oh E, Oh YH, Moon DW, Lee TG, Kim HS. Protein kinase assay on peptide-conjugated gold nanoparticles by using secondary-ion mass spectrometric imaging. Angewandte Chemie-International Edition. 2007;46:6816–6819. doi: 10.1002/anie.200701418. [DOI] [PubMed] [Google Scholar]
- 53.Shi D, Zhou Y, Wang SX, Van Ooij WJ, Wang LM, Zhao JG. Multi-Layer Coating of Ultrathin Polymer Films on Nanoparticles of Alumina by a Plasma Treatment. Material Research Society Symposium. 2001;635:C4.28.1–C4.28.6. [Google Scholar]
- 54.Gaspar DJ, Laskin A, Wang W, Hunt SW, Finlayson-Pitts BJ. TOF-SIMS analysis of sea salt particles: imaging and depth profiling in the discovery of an unrecognized mechanism for pH buffering. Applied Surface Science. 2004;231:520–523. [Google Scholar]
- 55.Ghule AV, Ghule K, Chen CY, Chen WY, Tzing SH, Chang H, Ling YC. In situ thermo-TOF-SIMS study of thermal decomposition of zinc acetate dihydrate. Journal of Mass Spectrometry. 2004;39:1202–1208. [Google Scholar]
- 56.Reinholdt A, Detemple R, Stepanov AL, Weirich TE, Kreibig U. Novel nanoparticle matter: ZrN-nanoparticles. Applied Physics B-Lasers and Optics. 2003;77:681–686. [Google Scholar]
- 57.Szymczak W, Menzel N, Kreyling WG, Wittmaack K. TOF-SIMS characterisation of spark-generated nanoparticles made from pairs of Ir-Ir and Ir-C electrodes. International Journal of Mass Spectrometry. 2006;254:70–84. [Google Scholar]
- 58.Reijme MA, Maas AJH, Viitanen MM, van der Gon AWD, Brongersma HH, Bosman AW, Meijer EW. Intramolecular segregation in polymers and macromolecules studied by low-energy ion scattering. Surface Science. 2001;482:1235–1240. [Google Scholar]
- 59.Jansen WPA, Harmsen JMA, von der Gon AWD, Hoebink J, Schouten JC, Brongersma HH. Noble metal segregation and cluster size of Pt/Rh/CeO2/gamma-Al2O3 automotive three-way catalysts studied with low-energy ion scattering. Journal of Catalysis. 2001;204:420–427. [Google Scholar]
- 60.Scalf J, West P. [November 23, 2009];Part I: Introduction to Nanoparticle Characterization with AFM. www.nanoparticles.org/pdf/Scalf-West.pdf.
- 61.Serry FM. Environmental Controls for Scanning Probe Microscopy with Applications in NanoScience and Nanotechnology Development. Microscopy and Microanalysis. 2005;11:380–381. [Google Scholar]
- 62.McCarthy GS, Weiss PS. Scanning Probe Studies of Single Nanostructures. Chemical Reviews. 1999;99:1983–1990. doi: 10.1021/cr970110x. [DOI] [PubMed] [Google Scholar]
- 63.Vakarelski IU, Brown SC, Moudgil BM, Higashitani K. Nanoparticle-terminated scanning probe microscopy tips and surface samples. Advance Powder Technology. 2007;18:605–614. [Google Scholar]
- 64.Gupta S, Brouwer P, Bandyopadhyay S, Patil S, Briggs R, Jain J, Seal S. TEM/AFM investigation of size and surface properties of nanocrystalline ceria. Journal of Nanoscience and Nanotechnology. 2005;5:1101–1107. doi: 10.1166/jnn.2005.151. [DOI] [PubMed] [Google Scholar]
- 65.Daniel S, Rao TP, Rao KS, Rani SU, Naidu GRK, Lee HY, Kawai T. A review of DNA functionalized/grafted carbon nanotubes and their characterization. Sensors and Actuators B-Chemical. 2007;122:672–682. [Google Scholar]
- 66.Teo KBK, Chhowalla M, Amaratunga GAJ, Milne WI, Pirio G, Legagneux P, Wyczisk F, Olivier J, Pribat D. Characterization of plasma-enhanced chemical vapor deposition carbon nanotubes by Auger electron spectroscopy. Journal of Vacuum Science & Technology B. 2002;20:116–121. [Google Scholar]
- 67.Zhang G, Sun S, Yang D, Dodelet J-P, Sacher E. The surface analytical characterization of carbon fibers functionalized by H2SO4/HNO3 treatment. Carbon. 2008;46:196–205. [Google Scholar]
- 68.Smith B, Wepasnick K, Schrote KE, Cho HH, Ball WP, Fairbrother DH. Influence of Surface Oxides on the Colloidal Stability of Multi-Walled Carbon Nanotubes: A Structure-Property Relationship. Langmuir. 2009;25:9767–9776. doi: 10.1021/la901128k. [DOI] [PubMed] [Google Scholar]
- 69.Shiraishi M, Swaraj S, Takenobu T, Iwasa Y, Ata M, Unger WES. Spectroscopic characterization of single-walled carbon nanotubes carrier-doped by encapsulation of TCNQ. Physical Review B. 2005;71 Article Number 125419. [Google Scholar]
- 70.Xu F, Minniti M, Barone P, Sindona A, Bonanno A, Oliva A. Nitrogen doping of single walled carbon nanotubes by low energy N-2(+) ion implantation. Carbon. 2008;46:1489–1496. [Google Scholar]
- 71.Morant C, Andrey J, Prieto P, Mendiola D, Sanz JM, Elizalde E. XPS characterization of nitrogen-doped carbon nanotubes. Physica Status Solidi (a) 2006;203:1069–1075. [Google Scholar]
- 72.Felten A, Bittencourt C, Pireaux JJ. Gold clusters on oxygen plasma functionalized carbon nanotubes: XPS and TEM studies. Nanotechnology. 2006;17:1954–1959. [Google Scholar]
- 73.Billinge SJL, Levin I. The problem with determining atomic structure at the nanoscale. Science. 2007;316:561–565. doi: 10.1126/science.1135080. [DOI] [PubMed] [Google Scholar]
- 74.Chen W, Pan XL, Willinger MG, Su DS, Bao XH. Facile autoreduction of iron oxide/carbon nanotube encapsulates. Journal of the American Chemical Society. 2006;128:3136–3137. doi: 10.1021/ja056721l. [DOI] [PubMed] [Google Scholar]
- 75.Kuchibhatla S, Karakoti AS, Seal S. Hierarchical assembly of inorganic nanostructure building blocks to octahedral superstructures - a true template-free self-assembly. Nanotechnology. 2007:18. doi: 10.1088/0957-4484/18/7/075303. [DOI] [PubMed] [Google Scholar]
- 76.Zhang HZ, Gilbert B, Huang F, Banfield JF. Water-driven structure transformation in nanoparticles at room temperature. Nature. 2003;424:1025–1029. doi: 10.1038/nature01845. [DOI] [PubMed] [Google Scholar]
- 77.Phillips R, Quake SR. The Biological Frontier of Physics. Physics Today. 2006;59:38. [Google Scholar]
- 78.Scher EC, Manna L, Alivisatos AP. Shape control and applications of nanocrystals. Philosophical Transactions of the Royal Society of London Series A-Mathematical Physical and Engineering Sciences. 2003;361:241–255. doi: 10.1098/rsta.2002.1126. [DOI] [PubMed] [Google Scholar]
- 79.Frankamp BL, Boal AK, Tuominen MT, Rotello VM. Direct control of the magnetic interaction between iron oxide nanoparticles through dendrimer-mediated self-assembly. Journal of the American Chemical Society. 2005;127:9731–9735. doi: 10.1021/ja051351m. [DOI] [PubMed] [Google Scholar]
- 80.Gaspar DJ, Engelhard MH, Henry MC, Baer DR. Erosion rate variations during XPS sputter depth profiling of nanoporous films. Surface and Interface Analysis. 2005;37:417–423. [Google Scholar]
- 81.Baer DR, Burrows PE, El-Azab AA. Enhancing coating functionality using nanoscience and nanotechnology. Progress in Organic Coatings. 2003;47:342–356. [Google Scholar]
- 82.Baer DR, Engelhard MH, Gaspar DJ, Matson DW, Pecher KH, Williams JR, Wang CM. Challenges in Applying Surface Analysis Methods to Nanoparticles and Nanostructured Materials. Journal of Surface Analysis. 2005:12. [Google Scholar]
- 83.ASTM E 1078-02 - Standard Guide for Specimen Preparation and Mounting in Surface Analysis Annual Book of ASTM Standards. 2006.
- 84.ISO 18116:2005 . Surface Chemical Analysis—Guidelines for preparation and mounting of specimens for analysis, International Organization for Standardization. Geneva, Switzerland: 2005. [Google Scholar]
- 85.Dane A, Demirok UK, Aydinli A, Suzer S. X-ray photoelectron spectroscopic analysis of Si nanoclusters in SiO2 matrix. Journal of Physical Chemistry B. 2006;110:1137–1140. doi: 10.1021/jp0545748. [DOI] [PubMed] [Google Scholar]
- 86.Wertheim GK, Dicenzo SB. Cluster Growth and Core-Electron Binding-Energies in Supported Metal-Clusters. Physical Review B. 1988;37:844–847. doi: 10.1103/physrevb.37.844. [DOI] [PubMed] [Google Scholar]
- 87.Norman TJ, Grant CD, Magana D, Zhang JZ, Liu J, Cao DL, Bridges F, Van Buuren A. Near infrared optical absorption of gold nanoparticle aggregates. Journal of Physical Chemistry B. 2002;106:7005–7012. [Google Scholar]
- 88.Reinhard BM, Siu M, Agarwal H, Alivisatos AP, Liphardt J. Calibration of dynamic molecular rule based on plasmon coupling between gold nanoparticles. Nano Letters. 2005;5:2246–2252. doi: 10.1021/nl051592s. [DOI] [PubMed] [Google Scholar]
- 89.Bayer M, Hawrylak P, Hinzer K, Fafard S, Korkusinski M, Wasilewski ZR, Stern O, Forchel A. Coupling and entangling of quantum states in quantum dot molecules. Science. 2001;291:451–453. doi: 10.1126/science.291.5503.451. [DOI] [PubMed] [Google Scholar]
- 90.Schwartz DA, Norberg NS, Nguyen QP, Parker JM, Gamelin DR. Magnetic quantum dots: Synthesis, spectroscopy, and magnetism of CO2+- and Ni2+-doped ZnO nanocrystals. Journal of the American Chemical Society. 2003;125:13205–13218. doi: 10.1021/ja036811v. [DOI] [PubMed] [Google Scholar]
- 91.Glover M, Meldrum A. Effect of “buffer layers” on the optical properties of silicon nanocrystal superlattices. Optical Materials. 2005;27:977–982. [Google Scholar]
- 92.Unger WES, Gross T. Catalyst Characterization. In: Riviere JC, Myhra S, editors. Handbook of Surface and Interface Analysis: Methods for Problem Solving. Marcel Dekker, Inc.; New York: 1998. [Google Scholar]
- 93.Van Santen RA, Piet WNMvL, Moulijn JA, Averill BA. Catalysis: An Integrated Approach. Elsevier Science; Amsterdam, The Netherlands: 2002. [Google Scholar]
- 94.Venezia AM, Rossi A, Duca SD, Marorana A, Deganello G. Particle-Size and Metal-Support Interaction Effects in Pumice Supported Palladium Catalysts. Applied Catalysis A. 1995;125:113–128. [Google Scholar]
- 95. Available from the World Wide Web: X-Ray Photoelectron Spectroscopy Characterization of Nanoparticles (NPs) http://www.scribd.com/doc/2194883/Nano-XPS-nanost-1.
- 96.Piyakis KN, Yang DQ, Sacher E. The applicability of angle-resolved XPS to the characterization of clusters on surfaces. Surface Science. 2003;536:139–144. [Google Scholar]
- 97.Merzlikin SVNN, Tolkachev NNT, Strunskus T, Witte G, Glogowski T, Wöll C, Grünert W. Resolving the depth coordinate in photoelectron spectroscopy – Comparison of excitation energy variation vs. angular-resolved XPS for the analysis of a self-assembled monolayer model system. Surface Science. 2008;602:755–767. [Google Scholar]
- 98.Merzlikin S. [November 23, 2009];Depth Profiling by X-ray Photoelectron Spectroscopy. Faculty of Chemistry, Laboratory of Industrial Chemistry, Doctor of Natural Science. 2007 http://deposit.ddb.de/cgi-bin/dokserv?idn=987575090&dok_var=d1&dok_ext=pdf&filename=987575090.pdf.
- 99.Fulghum JE, Linton RW. Quantitation of Coverages on Rough Surfaces by XPS - An Overview. Surface and Interface Analysis. 1988;13:186–192. [Google Scholar]
- 100.Fulghum JE, Linton RW. Evaluation of XPS for the Quantitative-Determination of Surface Coverages - Fluoride Adsorption on Hydrous Ferric-Oxide Particles. Journal of Electron Spectroscopy and Related Phenomena. 1989;49:101–118. [Google Scholar]
- 101.Gunter PLJ, Gijzeman OLJ, Niemantsverdriet JW. Surface roughness effects in quantitative XPS: magic angle for determining overlayer thickness. Applied Surface Science. 1997;115:342–346. [Google Scholar]
- 102.Werner WSM. Magic-Angle for Surface-Roughness for Intensity Ratios in AES/XPS. Surface and Interface Analysis. 1995;23:696–704. [Google Scholar]
- 103.ISO 19319 . Surface chemical analysis - Auger electron spectroscopy and X-ray photoelectron spectroscopy - Determination of lateral resolution, analysis area, and sample area viewed by the analyser. International Organization for Standards; Geneva,Switzerland: 2003. [Google Scholar]
- 104.Qi WH. Modeling the relaxed cohesive energy of metallic nanoclusters. Materials Letters. 2006;60:1678–1681. [Google Scholar]
- 105.Postawa Z, Czerwinski B, Szewczyk M, Smiley EJ, Winograd N, Garrison BJ. Microscopic insights into the sputtering of Ag{111} induced by C-60 and Ga bombardment. Journal of Physical Chemistry B. 2004;108:7831–7838. [Google Scholar]
- 106.Shimizu R. Monte Carlo simulation studies in Japan on interaction of charged particles with solids during those early days in 1960s-1970s. Nuclear Instruments & Methods in Physics Research Section B-Beam Interactions with Materials and Atoms. 2005;232:117–124. [Google Scholar]
- 107.Jarvi TT, Pakarinen JA, Kuronen A, Nordlund K. Enhanced sputtering from nanoparticles and thin films: Size effects. EPL 82. 2008.
- 108.Jurac S, Johnson RE, Donn B. Monte Carlo calculations of the sputtering of grains: Enhanced sputtering of small grains. Astrophysical Journal. 1998;503:247–252. [Google Scholar]
- 109.Adriaensen L, Vangaever F, Gijbels R. Metal-assisted secondary ion mass spectrometry: Influence of Ag and Au deposition on molecular ion yields. Analytical Chemistry. 2004;76:6777–6785. doi: 10.1021/ac049108d. [DOI] [PubMed] [Google Scholar]
- 110.Marcus A, Winograd N. Metal nanoparticle deposition for TOF-SIMS signal enhancement of polymers. Analytical Chemistry. 2006;78:141–148. doi: 10.1021/ac0513921. [DOI] [PubMed] [Google Scholar]
- 111.Chen HH, Urquidez OA, Ichim S, Rodriguez LH, Brenner MP, Aziz MJ. Shocks in ion sputtering sharpen steep surface features. Science. 2005;310:294–297. doi: 10.1126/science.1117219. [DOI] [PubMed] [Google Scholar]
- 112.Starostina N, West P. In: AFM Metrology for Nanoparticle & Nanostructures Charqacterization: Visualization, Morphology Quantitation and Probe Artifacts. Hackley VA, Patri AK, Stein J, Moudgil BM, editors. Materials Research Society Materials Research Society; San Francisco: 2007. [Google Scholar]
- 113.Wong C, West PE, Olson KS, Mecartney ML, Starostina N. Tip dilation and AFM capabilities in the characterization of nanoparticles. Jom. 2007;59:12–16. [Google Scholar]
- 114.Braga PC, Ricci D. Atomic Force Microscopy. Humana Press; 2004. [Google Scholar]
- 115.ASTM E2382 - 04 Guide to Scanner and Tip Related Artifacts in Scanning Tunneling Microscopy and Atomic Force Microscopy Annual Book of ASTM Standards. 2004.
- 116.Eisenthal KB. Second Harmonic Spectroscopy of Aqueous Nano- and Microparticle Interfaces. Chemical reviews. 2006;106:1462–1477. doi: 10.1021/cr0403685. [DOI] [PubMed] [Google Scholar]