Abstract
Context: Although numerous studies have explored the relation of IGF-I with cancer incidence, few have investigated the association between IGF-I and cancer mortality.
Objective: This study examined the association of serum IGF-I levels with cancer mortality in older community-dwelling men.
Design, Setting, and Participants: We conducted a prospective, population-based study of 633 men aged 50 yr and older (mean = 73) who attended a 1988–1991 research clinic visit when blood was obtained for measurement of IGF-I. Participants were followed for vital status through July 2006.
Main Outcome Measure: All-cancer mortality was assessed.
Results: Median IGF-I was 96 ng/ml. During the 18-yr follow-up, 368 deaths occurred; 74 (20%) were due to cancer. Cox regression analyses showed a significant quadratic association between IGF-I and all-cancer mortality (P = 0.039). Higher levels of IGF-I were associated with progressively higher risk of cancer death after adjusting for age, IGF-binding protein-1, adiposity, exercise, current smoking, and previous cancer. The adjusted risk of cancer death was statistically significant for IGF-I levels above 120 ng/ml, with magnitudes of effect ranging from 1.61 [95% confidence interval (CI) = 1.28–2.02] to 2.61 (95% CI = 1.46–4.64). For the 46% of men with IGF-I above 100 ng/ml, the risk of fatal cancer was 1.82 (95% CI = 1.11–2.96) compared to the risk with lower levels.
Conclusions: Higher serum IGF-I in older men is associated with increased risk of cancer death, independent of age, adiposity, lifestyle, and cancer history. These results suggest caution in the use of IGF-I-enhancing therapies to slow the adverse effects of aging.
Higher serum IGF-I in older men is associated with increased risk of cancer death, independent of age, adiposity, lifestyle, and cancer history.
Cancer constitutes a significant and growing public health problem in the United States with an estimated 1.44 million new cases and 560,000 deaths from cancer in 2007 (1). It is anticipated that cancer will soon overtake heart disease as the leading cause of death. Given the association of age with cancer as well as the aging of the population, the magnitude of this problem is projected to increase substantially in coming decades (1).
In searching for risk factors for cancer incidence, attention has been increasingly focused on the role of IGFs, including IGF-I. Structurally similar to insulin, IGFs are growth-stimulatory peptides that affect the growth and development of diverse tissues. IGFs interact with several binding proteins [IGF-binding proteins (IGFBPs)] that, in turn, regulate serum IGF levels. IGF-I mediates the effects of GH and participates in the regulation of mitogenesis, cellular differentiation, and apoptosis (2,3,4,5) and may also play a role in carcinogenesis (6,7). Results from a metaanalysis suggest that higher serum IGF-I levels are associated with increased risk of several common cancers including breast, colorectal, and prostate (8). It has been proposed that increased IGF-I levels or bioactivity may influence the promotion and progression of transformed cells by exerting mitogenic and antiapoptotic effects.
Numerous studies have examined the relation of IGF-I and GH with cancer incidence (8,9,10,11,12,13,14,15,16,17), but few have investigated the associations with cancer mortality, some focusing on site-specific cancers (e.g. deaths due to carcinoma of the pancreas, lung) (18,19). To our knowledge, only two population-based studies have looked at serum IGF-I and all-cancer mortality. Saydah and colleagues (20) examined data from the National Health and Nutrition Examination Survey (NHANES) III Mortality Study and found no difference in 12-yr cancer mortality in adults 20 yr and older when comparing the lowest to the highest quartile of baseline IGF-I. In contrast, recent findings from the Study of Health in Pomerania (SHIP) suggest an increased 9-yr risk of cancer death for men with extremely low IGF-I levels (<10th percentile) (21). The objective of the present study was to examine the association of serum IGF-I levels with 18-yr risk of cancer mortality in older community-dwelling individuals.
Subjects and Methods
Participants
The Rancho Bernardo Study is a population-based study of middle-class Caucasian individuals residing in a southern California community who were enrolled in a study of heart disease risk factors from 1972–1974 (22). Between 1988 and 1991, 80% (n = 1727) of the surviving community-dwelling men and women participated in a follow-up clinic visit. Of these individuals, 1538 (638 men and 900 women) had sufficient stored serum for measurement of IGF-I. Excluded from these analyses were eight participants who were under the age of 50 yr, nine because of insulin use, and one woman who was premenopausal. An additional 335 women who were using oral estrogens at the time of the blood sample draw were excluded because first-pass hepatic effects of estrogen reduce IGF-I levels to a variable degree (23). The remaining 633 men and 552 postmenopausal, non-estrogen-using women are the focus of this study. The University of California-San Diego Institutional Review Board approved this study; all participants provided written informed consent before participation.
Procedures
During the 1988–1991 clinic visit, a standardized self-administered questionnaire was used to assess personal history of cigarette smoking (never/former/current), alcohol consumption (number of drinks per day during the past 30 d), physical exercise three or more times per week (yes/no), and medical history including history of cancer. In the clinic, height and weight were measured with participants wearing light clothing and no shoes. Waist circumference (centimeters) was measured at the bending point and hip circumference (centimeters) was measured at the maximum girth enabling us to use the waist-to-hip ratio. Body mass index [weight (kilograms)/height (meters) squared] and waist-to-hip ratio were used as estimates of overall and central adiposity, respectively.
Nonfasting blood samples were obtained by venipuncture between 0800 and 1300 h; serum was separated and frozen at −70 C. IGF-I and IGFBP-1 levels were measured on twice-thawed samples 8–11 yr later (between 2000 and 2001) in the Maine Center for Osteoporosis Research and Education Laboratory under the direction of Dr. Clifford J. Rosen. Serum IGF-I was determined by RIA using a commercial kit (Nichols Institute Diagnostics, San Clemente, CA) modified to optimize sensitivity and specificity; samples were diluted 1:105 and were pretreated by acid-ethanol cryoprecipitation to remove IGFBPs. The assay sensitivity was 6.3 ng/ml; intra- and interassay coefficients of variation were 3.3 and 11.4%, respectively. IGFBP-1 levels were measured by immunoradiometric assay (Diagnostics Systems Laboratories, Inc., Webster, TX) with a sensitivity of 0.33 ng/ml and intra- and interassay coefficients of variation of 3.9 and 13.5%, respectively.
Updated information on cancer history was obtained from standard interview questionnaires administered at clinic visits and biannually by mailed questionnaires. Individuals who gave a date of onset of cancer before the date of assessment of IGF-I were considered to have a history of cancer.
Vital status was assessed annually by mailed questionnaires through July 2006. Follow-up time was defined as the interval between a participant’s 1988–1991 clinic visit and either the date of death or date of last contact. The maximum length of follow-up was 18 yr (median 10.8 yr for men, 12.4 yr for women). Vital status was known for each of the 1185 participants. Death certificates were obtained for 88% of decedents and were coded for the underlying cause of death by a certified nosologist using the ninth revision of the International Classification of Disease. Cancer mortality included deaths assigned codes 140–208.
Statistical analyses
Descriptive statistics were age adjusted using generalized linear models. Values for IGF-I and IGFBP-1 were log-transformed to decrease skewness; geometric means are reported for age-adjusted values. Univariate analyses were performed to compare those with missing to those with complete data. Partial correlations were used to assess age-adjusted associations between IGF-I levels and participant characteristics.
Adjusted Cox proportional hazards models (24) were used to examine the association of IGF-I with the risk of cancer mortality after adjustment for age, IGFBP-1, body mass index, waist-to-hip ratio, alcohol consumption, exercise, current smoking, and previous cancer. Preliminary analyses established the optimal functional form for the main exposure variable (25). Both cubic and quadratic associations were initially assessed; the quadratic term for IGF-I was found to be statistically significant at the α = 0.05 level. Accordingly, IGF-I was modeled as a continuous, quadratic variable and was centered to prevent collinearity with the quadratic term in the proportional hazards model. To gain additional insight into the nonlinear relation between IGF-I levels and cancer mortality, we examined hazard ratios (HRs) using contrast statements in SAS PHREG for 1-sd increments in IGF-I levels. All multivariable models included a continuous variable for age; other variables were included in the final model if they were significant at the α = 0.10 level in bivariate analyses, changed the risk estimate by more than 15%, or improved the precision of the risk estimate. Verification of the proportional hazards assumption was established per examination of the time-dependent covariate, which was not statistically significant. A test of interaction of IGF-I by sex was significant; therefore, separate analyses for men and women were conducted. The limited number of cancer events (n = 34) in postmenopausal, non-estrogen-using women was insufficient to allow meaningful interpretation; thus, results are presented only for men.
Two-sided P values <0.05 were considered statistically significant. Data were analyzed using SAS (version 9.1; SAS Institute, Inc., Cary, NC).
Results
During the 18-yr follow-up, there were 368 deaths among men, 74 (20%) due to cancer. The most common types were prostate (n = 18), lung (n = 17), and colorectal (n = 12) carcinomas, together comprising nearly two thirds of cancer deaths in men.
Among these 633 men, age ranged from 51–98 yr, with a mean of 73 ± 9 yr. Median (interquartile range) of IGF-I and IGFBP-1 levels were 96 (69–124) and 21 (10–41) ng/ml, respectively. Median IGF-I levels were higher in men who later died of cancer (109 vs. 95 ng/ml; P = 0.02). The distributions of age and age-adjusted IGF-I, IGFBP-1, body mass index (BMI) and other covariates are shown in Table 1. A total of 95 men (15%) reported a history of cancer, 18 of whom later died of cancer.
Table 1.
Age and age-adjusted characteristics of men, Rancho Bernardo, CA, 1988–1991
| Characteristic | Men (n = 633) |
|---|---|
| IGF-I (ng/ml) | 88.1 ± 1.0 |
| IGFBP-1 (ng/ml) | 19.2 ± 1.0 |
| Age (yr) | 73.4 ± 0.3 |
| BMI (kg/m2) | 26.0 ± 0.1 |
| Waist-to-hip ratio | 0.94 ± 0.002 |
| Alcohol consumption (≥2 drinks/d) | 265 (41.4) |
| Exercise (≥3 times/wk) | 471 (74.1) |
| Smoking status | |
| Never | 195 (31.5) |
| Former | 379 (59.9) |
| Current | 56 (8.6) |
| Previous cancera | 95 (15.2) |
Results are presented as mean ± se or n (%). IGF-I and IGFBP-1 were log-transformed (geometric means are reported).
Excludes nonmelanoma skin cancer.
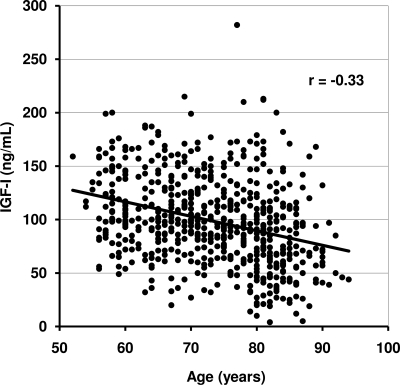
Table 2 shows age and age-adjusted correlations of IGF-I levels with participant characteristics. Serum IGF-I levels were inversely associated with age (r = −0.33; P < 0.001) and IGFBP-1 (r = −0.26; P < 0.001) and positively associated with waist-to-hip ratio (P < 0.05). IGF-I was not significantly associated with BMI, alcohol intake, exercise, current smoking, or previous cancer. Figure 1 illustrates the decrease in IGF-I levels for older participants; the association of IGF-I levels with age did not vary for those with and without a subsequent cancer death (P = 0.74).
Table 2.
Age and age-adjusted associations between IGF-I and characteristics of men aged at least 50 yr, Rancho Bernardo, CA, 1988–1991
| Characteristic | Men (n = 633)
|
|
|---|---|---|
| r | P value | |
| Age (yr) | −0.33 | <0.001 |
| IGFBP-1 (ng/ml) | −0.26 | <0.001 |
| BMI (kg/m2) | 0.06 | 0.342 |
| Waist-to-hip ratio | 0.09 | 0.036 |
| Alcohol (≥2 drinks/d) | 0.02 | 0.836 |
| Exercise (≥3 times/wk) | −0.05 | 0.130 |
| Current smoker | −0.03 | 0.413 |
| Previous cancera | 0.03 | 0.556 |
Note that r indicates partial correlations for age-adjusted associations with IGF-I (log-transformed).
Excludes nonmelanoma skin cancer.
Figure 1.
Correlation of IGF-I levels with age.
Age-adjusted HRs for risk of cancer mortality by levels of IGF-I, IGFBP-1, and anthropometric and lifestyle covariates are shown in Table 3. A significant quadratic (nonlinear) association was found between IGF-I levels and cancer mortality (P < 0.05). Consistent with the interpretation of a continuous variable, we report the risks for 1-unit (i.e. 1-sd) increments. Both current smoking and reported history of cancer were also significantly associated with an increased risk of cancer mortality [HR = 2.39; 95% confidence interval (CI) = 1.20–4.77, and HR = 1.95; 95% CI = 1.14–3.34, respectively). BMI, waist-to-hip ratio, alcohol consumption, and exercise (at least three times/wk) were not significantly associated with cancer mortality risk.
Table 3.
Age-adjusted HRs for risk of cancer mortality, Rancho Bernardo, CA, 1988–2006
| Characteristic | Men (n = 633)
|
||
|---|---|---|---|
| HR | 95% CI | P value | |
| IGF-I (1-sd increments) | 0.048 | ||
| <40 | 0.81 | 0.43–1.50 | |
| 40–79 | 1.01 | 0.66–1.53 | |
| 80–119 | 1.26 | 0.99–1.61 | |
| 120–159 | 1.58 | 1.27–1.96 | |
| 160–199 | 1.98 | 1.38–2.84 | |
| 200–239 | 2.48 | 1.41–4.34 | |
| IGFBP-1 (ng/ml) | 0.90 | 0.71–1.14 | 0.368 |
| BMI (kg/m2) | 0.98 | 0.91–1.06 | 0.701 |
| Waist-to-hip ratio | 0.98 | 0.93–1.03 | 0.466 |
| Alcohol (≥2 drinks/d) | 1.17 | 0.73–1.87 | 0.516 |
| Exercise (≥3 times/wk) | 0.64 | 0.38–1.06 | 0.082 |
| Current smoker | 2.39 | 1.20–4.77 | 0.014 |
| Previous cancera | 1.95 | 1.14–3.34 | 0.015 |
IGFBP-1 was log-transformed. The association of IGF-I was modeled as a continuous (rather than categorical) predictor of cancer mortality. IGF-I was centered to prevent collinearity with the quadratic term.
Excludes nonmelanoma skin cancer.
In multivariate analyses (Table 4), higher levels of IGF-I remained significantly associated with progressively higher risk of cancer death after further adjusting for IGFBP-1, BMI, waist-to-hip ratio, physical exercise (at least three times/wk), current smoking, and previous cancer. Table 4 demonstrates how the HR changes for different concentrations of IGF-I, even though the unit difference is always 40 ng/ml, consistent with the nonlinear relation between IGF-I levels and risk of cancer mortality. The adjusted risk of cancer death was statistically significant for IGF-I levels above 120 ng/ml, with magnitudes of effect ranging from 1.61 (120–159 ng/ml) to 2.61 among men with the highest IGF-I levels (200–239 ng/ml). The latter should be interpreted with caution given the small number of men with IGF-I levels above 200 ng/ml (n = 7) as reflected in the wider confidence interval (HR = 2.61; 95% CI = 1.46–4.64). We further examined the multivariate association by dichotomizing IGF-I at the approximate median value of 100 ng/ml. For the 46% of men to IGF-I at or above 100 ng/ml, the adjusted risk of cancer death was 1.82 (95% CI = 1.11–2.96) compared with those with lower levels. Of the 74 cancer deaths in men, 58% occurred in those with IGF-I levels of 100 ng/ml or higher. Exclusion of deaths that occurred during the first 2 yr (to minimize associations due to occult disease) yielded similar results, as did exclusion of men with a history of diabetes. IGFBP-1 levels were not independently associated with cancer mortality in age-adjusted (Table 3) or multivariate analyses (data not shown). Furthermore, IGF-I levels were not associated with all-cause or noncancer mortality in these men (data not shown).
Table 4.
Multivariable-adjusted HRs for risk of cancer mortality, Rancho Bernardo, CA, 1988–2006
| Characteristic | Men (n = 633)
|
|||
|---|---|---|---|---|
| Cancer deaths/participants (%) | HR | 95% CI | P value | |
| IGF-I (1-sd increments) | 0.039 | |||
| <40 | 3/40 (7.5) | 0.79 | 0.41–1.49 | |
| 40–79 | 13/167 (7.8) | 1.00 | 0.65–1.53 | |
| 80–119 | 32/253 (12.6) | 1.27 | 0.98–1.64 | |
| 120–159 | 14/125 (11.2) | 1.61 | 1.28–2.02 | |
| 160–199 | 8/41 (19.5) | 2.05 | 1.41–2.98 | |
| 200–239 | 4/7 (57.1) | 2.61 | 1.46–4.64 | |
HRs were adjusted for IGFBP-I, age, BMI, waist-to-hip ratio, exercise (at least three times/wk), current smoking, and previous cancer. The association of IGF-I was modeled as a continuous predictor of cancer mortality. IGF-I was centered to prevent collinearity with the quadratic term, and P value reflects the significance of the quadratic term.
Discussion
The IGF system is complex, and much remains to be learned regarding both the determinants of serum IGF-I levels and their consequences. IGF-I levels peak at puberty and decline with increasing age. Absolute levels vary widely across individuals, and it has been proposed that approximately 50% of the variability may be explained by genetics (26,27). Levels may also be influenced by use of augmentive therapies (recombinant IGF-I, developed to treat diabetes) and possible long-term use of GH given to patients with the idea that it has antiaging effects but, at the same time, can increase IGF-I levels. Regardless of their determinants, this study suggests that serum IGF-I may constitute an important, readily available marker of the risk of cancer progression and mortality. These associations were independent of the insulin-dependent IGFBP-1 and were not explained by age, adiposity, current smoking, physical activity, or history of cancer. To our knowledge, this is the first study to report an association of high circulating IGF-I levels with increased risk of cancer mortality among older, community-dwelling men.
Results of this study are in accord with those of Swerdlow and colleagues (28) who reported that GH-deficient patients treated with human GH (presumably resulting in higher IGF-I levels) at young ages had significantly increased risks of mortality from cancer (standardized mortality ratio = 2.8). In the present study, risks of cancer mortality increased in a nonlinear association with IGF-I levels; risks were 60% higher from 120–159 ng/ml, 100% higher from 160–199 ng/ml, and 160% higher from 200–239 ng/ml.
Results of the present study differ from those of Saydah and colleagues (20), who reported no association of IGF-I with cancer mortality when comparing lowest to highest quartiles of baseline IGF-I in more than 6000 participants from the NHANES III study. However, the NHANES analyses were not stratified by sex (a test for potential interaction by sex was not significant at the α = 0.05 level), and they studied a wide age range (ages 20 yr and older). In our analyses, the association between IGF-I levels and risk of cancer death varied by sex (P < 0.05), necessitating separate analyses for men and women. In addition, the NHANES study was based on a much younger population (mean age = 43 yr) with a relatively short length of follow-up (median = 9 yr). In the present report, Rancho Bernardo men were 73 yr old on average, and the follow-up was 18 yr. Perhaps the difference in results for these two studies suggests that the association of elevated IGF-I with cancer mortality is evident only at advanced age, a time when IGF-I levels are usually relatively low.
In the only other large population-based study to examine IGF-I and cancer mortality, SHIP, the risk of cancer mortality was almost 2-fold higher in men with IGF-I levels in the lowest decile compared with men with higher levels (21). In contrast to the SHIP study, our strongest associations for an increased risk were observed in the higher IGF-I levels. A sensitivity analysis for ages 50 yr and older was performed in the SHIP study, with no change in results observed. However, we would expect their proportion of deaths in this age range to be less than those we observed because the mean age and length of follow-up in the SHIP study was only 51 and 8.5 yr, respectively. Therefore, even though the two study populations were similar in regard to race and waist circumference, the difference in age distribution may partly explain the differences in study findings. Why low IGF-I would predict cancer death in younger men, whereas high IGF-I increases risk in older men is not clear. Additional population-based studies are needed to confirm these divergent associations.
It is biologically plausible that higher IGF-I levels are associated with cancer mortality in that IGF-I has been found to stimulate replication and inhibit apoptosis of previously transformed cells (29). Evidence from an in vivo study of prostate cancer (30) suggests that IGF-I effects are mediated by the androgen receptor, which binds circulating IGF-I, resulting in disease progression and metastatic spread. Thus, IGF-I may play an important role in not only carcinogenesis but tumor progression as well, and this may be especially true for hormone-dependent cancers.
Several strengths and limitations of this study were considered. Our IGF-I levels were comparable to those of other study populations similar in age and body size to the men in the present report (31). This study is strengthened by its prospective design and relatively large sample size. However, there were an insufficient number of cancer deaths to examine individual cancers in men or to evaluate the influence of IGF-I on cancer death in women. Because of stored sample volume limitations, IGFBP-3, the most abundant IGFBP, was not measured. Only one measure of IGF-I was available, which may have introduced some misclassification bias. However, a single nonfasting IGF-I measure has been shown to effectively characterize individual levels over at least 1 yr (32). Furthermore, misclassification bias would be expected to lead to an underestimate of the true association. No information was available about tumor characteristics (e.g. size, stage, and degree of differentiation), treatments received, and recurrences; therefore, the effect of these characteristics on the association of IGF-I and cancer mortality is unknown. Competing cause of death is always an issue in studies of older adults. In this study, IGF-I levels were not associated with all-cause or noncancer mortality in Rancho Bernardo men, supporting the specificity of the IGF-I cancer mortality association reported here.
The findings from this study suggest that higher serum IGF-I in older men is associated with increased risk of cancer death, independent of age, adiposity, and lifestyle. If confirmed in other populations, these results suggest that use of IGF-I-enhancing therapies to slow the adverse effects of aging should be reexamined and that serum IGF-I may have potential importance as a biomarker for prognostic testing.
Footnotes
This work was supported by Grant DK007386-28 from the National Institute of Diabetes and Digestive and Kidney Diseases and National Institute of Aging Grants AG028507 and AG018339.
Disclosure Summary: The authors have nothing to disclose.
First Published Online January 15, 2010
Abbreviations: BMI, Body mass index; CI, confidence interval; HR, hazard ratio; IGFBP, IGF-binding protein; NHANES, National Health and Nutrition Examination Survey; SHIP, Study of Health in Pomerania.
References
- American Cancer Society 2007 Cancer Facts and Figures. Atlanta: American Cancer Society [Google Scholar]
- Moschos SJ, Mantzoros CS 2002 The role of the IGF system in cancer: from basic to clinical studies and clinical applications. Oncology 63:317–332 [DOI] [PubMed] [Google Scholar]
- Pollak M 2000 Insulin-like growth factor physiology and cancer risk. Eur J Cancer 36:1224–1228 [DOI] [PubMed] [Google Scholar]
- Djavan B, Waldert M, Seitz C, Marberger M 2001 Insulin-like growth factors and prostate cancer. World J Urol 19:225–233 [DOI] [PubMed] [Google Scholar]
- Dunn SE, Kari FW, French J, Leininger JR, Travlos G, Wilson R, Barrett JC 1997 Dietary restriction reduces insulin-like growth factor I levels, which modulates apoptosis, cell proliferation, and tumor progression in p53-deficient mice. Cancer Res 57:4667–4672 [PubMed] [Google Scholar]
- Wu Y, Cui K, Miyoshi K, Hennighausen L, Green JE, Setser J, LeRoith D, Yakar S 2003 Reduced circulating insulin-like growth factor I levels delay the onset of chemically and genetically induced mammary tumors. Cancer Res 63:4384–4388 [PubMed] [Google Scholar]
- Wu Y, Yakar S, Zhao L, Hennighausen L, LeRoith D 2002 Circulating insulin-like growth factor-I levels regulate colon cancer growth and metastasis. Cancer Res 62:1030–1035 [PubMed] [Google Scholar]
- Renehan AG, Zwahlen M, Minder C, O'Dwyer ST, Shalet SM, Egger M 2004 Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet 363:1346–1353 [DOI] [PubMed] [Google Scholar]
- Schernhammer ES, Holly JM, Hunter DJ, Pollak MN, Hankinson SE 2006 Insulin-like growth factor-I, its binding proteins (IGFBP-1 and IGFBP-3), and growth hormone and breast cancer risk in The Nurses Health Study II. Endocr Relat Cancer 13:583–592 [DOI] [PubMed] [Google Scholar]
- Yu H, Jin F, Shu XO, Li BD, Dai Q, Cheng JR, Berkel HJ, Zheng W 2002 Insulin-like growth factors and breast cancer risk in Chinese women. Cancer Epidemiol Biomarkers Prev 11:705–712 [PubMed] [Google Scholar]
- Chan JM, Stampfer MJ, Giovannucci E, Gann PH, Ma J, Wilkinson P, Hennekens CH, Pollak M 1998 Plasma insulin-like growth factor-I and prostate cancer risk: a prospective study. Science 279:563–566 [DOI] [PubMed] [Google Scholar]
- Ma J, Pollak MN, Giovannucci E, Chan JM, Tao Y, Hennekens CH, Stampfer MJ 1999 Prospective study of colorectal cancer risk in men and plasma levels of insulin-like growth factor (IGF)-I and IGF-binding protein-3. J Natl Cancer Inst 91:620–625 [DOI] [PubMed] [Google Scholar]
- Giovannucci E, Pollak MN, Platz EA, Willett WC, Stampfer MJ, Majeed N, Colditz GA, Speizer FE, Hankinson SE 2000 A prospective study of plasma insulin-like growth factor-1 and binding protein-3 and risk of colorectal neoplasia in women. Cancer Epidemiol Biomarkers Prev 9:345–349 [PubMed] [Google Scholar]
- Yu H, Spitz MR, Mistry J, Gu J, Hong WK, Wu X 1999 Plasma levels of insulin-like growth factor-I and lung cancer risk: a case-control analysis. J Natl Cancer Inst 91:151–156 [DOI] [PubMed] [Google Scholar]
- Lukanova A, Lundin E, Toniolo P, Micheli A, Akhmedkhanov A, Rinaldi S, Muti P, Lenner P, Biessy C, Krogh V, Zeleniuch-Jacquotte A, Berrino F, Hallmans G, Riboli E, Kaaks R 2002 Circulating levels of insulin-like growth factor-I and risk of ovarian cancer. Int J Cancer 101:549–554 [DOI] [PubMed] [Google Scholar]
- Stolzenberg-Solomon RZ, Limburg P, Pollak M, Taylor PR, Virtamo J, Albanes D 2004 Insulin-like growth factor (IGF)-1, IGF-binding protein-3, and pancreatic cancer in male smokers. Cancer Epidemiol Biomarkers Prev 13:438–444 [PubMed] [Google Scholar]
- Lönn S, Inskip PD, Pollak MN, Weinstein SJ, Virtamo J, Albanes D 2007 Glioma risk in relation to serum levels of insulin-like growth factors. Cancer Epidemiol Biomarkers Prev 16:844–846 [DOI] [PubMed] [Google Scholar]
- Lin Y, Tamakoshi A, Kikuchi S, Yagyu K, Obata Y, Ishibashi T, Kawamura T, Inaba Y, Kurosawa M, Motohashi Y, Ohno Y 2004 Serum insulin-like growth factor-I, insulin-like growth factor binding protein-3, and the risk of pancreatic cancer death. Int J Cancer 110:584–588 [DOI] [PubMed] [Google Scholar]
- Wakai K, Ito Y, Suzuki K, Tamakoshi A, Seki N, Ando M, Ozasa K, Watanabe Y, Kondo T, Nishino Y, Ohno Y, Group JS 2002 Serum insulin-like growth factors, insulin-like growth factor-binding protein-3, and risk of lung cancer death: a case-control study nested in the Japan Collaborative Cohort (JACC) Study. Jpn J Cancer Res 93:1279–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saydah S, Graubard B, Ballard-Barbash R, Berrigan D 2007 Insulin-like growth factors and subsequent risk of mortality in the United States. Am J Epidemiol 166:518–526 [DOI] [PubMed] [Google Scholar]
- Friedrich N, Haring R, Nauck M, Lüdemann J, Rosskopf D, Spilcke-Liss E, Felix SB, Dörr M, Brabant G, Völzke H, Wallaschofski H 2009 Mortality and serum insulin-like growth factor (IGF)-I and IGF binding protein 3 concentrations. J Clin Endocrinol Metab 94:1732–1739 [DOI] [PubMed] [Google Scholar]
- Criqui MH, Barrett-Connor E, Austin M 1978 Differences between respondents and non-respondents in a population-based cardiovascular disease study. Am J Epidemiol 108:367–372 [DOI] [PubMed] [Google Scholar]
- Sonnet E, Lacut K, Roudaut N, Mottier D, Kerlan V, Oger E 2007 Effects of the route of oestrogen administration on IGF-1 and IGFBP-3 in healthy postmenopausal women: results from a randomized placebo-controlled study. Clin Endocrinol (Oxf) 66:626–631 [DOI] [PubMed] [Google Scholar]
- Cox D 1972 Regression models and life tables with discussion. J R Stat Soc (B) 34:187–220 [Google Scholar]
- Woodward M 2005 Epidemiology: study design and data analysis. 2nd ed. Boca Raton, FL: Chapman, Hall/CRC [Google Scholar]
- Harrela M, Koistinen H, Kaprio J, Lehtovirta M, Tuomilehto J, Eriksson J, Toivanen L, Koskenvuo M, Leinonen P, Koistinen R, Seppälä M 1996 Genetic and environmental components of interindividual variation in circulating levels of IGF-I, IGF-II, IGFBP-1, and IGFBP-3. J Clin Invest 98:2612–2615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Y, Pedersen NL, Brismar K, Hall K, de Faire U 1996 Quantitative genetic analyses of insulin-like growth factor I (IGF-I), IGF-binding protein-1, and insulin levels in middle-aged and elderly twins. J Clin Endocrinol Metab 81:1791–1797 [DOI] [PubMed] [Google Scholar]
- Swerdlow AJ, Higgins CD, Adlard P, Preece MA 2002 Risk of cancer in patients treated with human pituitary growth hormone in the UK, 1959–85: a cohort study. Lancet 360:273–277 [DOI] [PubMed] [Google Scholar]
- Smith GD, Gunnell D, Holly J 2000 Cancer and insulin-like growth factor-I. A potential mechanism linking the environment with cancer risk. BMJ 321:847–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culig Z, Hobisch A, Cronauer MV, Radmayr C, Trapman J, Hittmair A, Bartsch G, Klocker H 1994 Androgen receptor activation in prostatic tumor cell lines by insulin-like growth factor-I, keratinocyte growth factor, and epidermal growth factor. Cancer Res 54:5474–5478 [PubMed] [Google Scholar]
- Harrela M, Koistinen R, Tuomilehto J, Nissinen A, Seppälä M 2000 Low serum insulin-like growth factor-binding protein-1 is associated with an unfavourable cardiovascular risk profile in elderly men. Ann Med 32:424–428 [DOI] [PubMed] [Google Scholar]
- Kaaks R, Toniolo P, Akhmedkhanov A, Lukanova A, Biessy C, Dechaud H, Rinaldi S, Zeleniuch-Jacquotte A, Shore RE, Riboli E 2000 Serum C-peptide, insulin-like growth factor (IGF)-I, IGF-binding proteins, and colorectal cancer risk in women. J Natl Cancer Inst 92:1592–1600 [DOI] [PubMed] [Google Scholar]