Abstract
Context: Regional fat is increasingly recognized as a determinant of bone mineral density (BMD), an association that may be mediated by adipokines, such as adiponectin and leptin, and inflammatory fat products. Chronic inflammation is deleterious to bone, and visceral adipose tissue (VAT) predicts inflammatory markers such as soluble intercellular adhesion molecule-1 and E-selectin, whereas sc adipose tissue (SAT) and VAT predict IL-6 in adolescents.
Objective: Our objective was to determine associations of regional fat mass and adipokines with BMD. We hypothesized that girls with greater VAT relative to SAT would have lower bone density mediated by inflammatory cytokines, adiponectin, and leptin.
Design: This was a cross-sectional study.
Setting: The study was conducted at a clinical research center.
Subjects: Subjects included 30 girls (15 obese, 15 normal weight) 12–18 yr old, matched for maturity (bone age), race, and ethnicity.
Outcome Measures: We assessed regional fat (SAT, VAT) using magnetic resonance imaging, total fat, and BMD using dual-energy x-ray absorptiometry. Fasting leptin, adiponectin, IL-6, soluble intercellular adhesion molecule-1, and E-selectin were obtained.
Results: Mean body mass index sd score was 3.7 ± 1.5 in obese subjects and 0.1 ± 0.4 kg/m2 in controls. VAT was a negative predictor of spine BMD and bone mineral apparent density, whole-body BMD and bone mineral content/height in obese girls and whole-body BMD and bone mineral content/height for the group as a whole after controlling for SAT, as was the ratio of VAT to SAT. In a regression model that included VAT/SAT, adipokines, and cytokines, E-selectin and adiponectin were negative predictors of BMD and leptin a positive predictor.
Conclusion: VAT is an independent inverse determinant of bone density in obesity. This association may be mediated by adipokines and a chronic inflammatory state.
Visceral fat negatively predicts bone density in adolescent girls; this association may be mediated by adipokines and inflammatory fat products.
There is a growing understanding of the impact of fat mass on bone density, and recent studies have reported differential effects of regional fat mass on bone in both animals and humans (1,2,3,4). Importantly, the impact of specific fat compartments on insulin sensitivity and lipid levels is well known, with visceral adipose tissue (VAT) imparting greater risk of insulin resistance and hyperlipidemia than sc adipose tissue (SAT) (5,6), and it is possible that VAT and SAT also differ in their impact on bone. A positive association of SAT with bone density has been consistently reported (1,2); however, data are conflicting regarding the association of VAT and bone, with studies reporting positive, negative, or no associations (1,2,3,4). Most studies examined these associations in adult men and women (1,4,7,8), sometimes using surrogates for VAT such as waist circumference or waist to hip ratio (7,8), although one recent report indicated that SAT was a positive and VAT a negative predictor of bone structural parameters in young adults (2). However, studies have not consistently examined the impact of VAT after controlling for SAT or the impact of the relative proportion of VAT to SAT (VAT/SAT) on bone.
Importantly, there is also a significant knowledge deficit regarding chemical mediators of the associations between regional fat and bone. Potential mediators such as inflammatory cytokines and hormones secreted by adipocytes or vascular endothelium within fat have not been examined in this context in a pediatric population. These include inflammatory cytokines such as IL-6, TNF-α, adhesion molecules such as E-selectin and soluble intercellular adhesion molecule-(sICAM)-1, and hormones such as adiponectin and leptin. Inflammatory cytokines stimulate osteoclastic activity (9), and a stimulatory effect of adiponectin on osteoclast differentiation and action [through activation of the receptor activator of nuclear factor-κB (RANK)-RANK ligand pathway] has been reported (10). Although the leptin knockout mouse has high trabecular bone density (11), cortical bone is impacted negatively, and these changes are reversed with leptin administration; studies in humans reported positive associations between leptin and bone (12,13). Importantly, data indicate differential expression and secretion of these inflammatory and other adipokines by regional fat depots. We previously reported that VAT is an independent predictor of E-selectin and sICAM1, whereas SAT predicts IL-6 (14), and both SAT and VAT predict adiponectin levels (6). Leptin is best predicted by SAT.
The adolescent years are characterized by marked increases in bone mass accrual rates and gender-specific changes in body composition. It is unclear whether reported associations between regional fat and bone in older people apply to this younger population. Potential mediators of associations between regional fat and bone have also not been reported. We examined associations of VAT and SAT, and the relative proportion of VAT to SAT with bone density measures in adolescent obese and normal-weight girls and determined whether these associations are influenced by inflammatory cytokines (IL-6, sICAM1, and E-selectin), adiponectin, and leptin. We hypothesized a negative association between VAT and the ratio of VAT to SAT with bone density measures in adolescent girls mediated by inflammatory cytokines and adiponectin.
Subjects and Methods
Subject selection
Forty-seven adolescent girls 12–18 yr old, 17 obese and 30 normal weight, were screened for this study. Fifteen obese subjects met eligibility criteria and were matched to 15 normal-weight controls for race, ethnicity, and bone age (within 2 yr). Telephone prescreens were used to minimize the number of normal-weight subjects screened. Bone age was used as an indicator of maturity and was assessed using methods of Greulich and Pyle (15). Earlier onset of puberty is reported in overweight girls (16), and hormonal, body composition, and bone density changes in adolescents are more closely associated with pubertal stage and bone age than with chronological age. We therefore used bone age, rather than chronological age, to match obese subjects to controls. We previously reported baseline characteristics but not bone density measures or their associations with regional fat mass and inflammatory markers for these subjects (6).
All obese girls by definition had a body mass index (BMI) greater than the 95th percentile for age. Normal-weight girls were required to have a BMI between the 15th and 85th percentiles. Menarchal status did not differ between the groups; two normal-weight and three overweight girls were premenarchal. Subjects from all racial and ethnic backgrounds were recruited through mass mailings to primary care providers, advertisements in community newspapers, and research listings within the Partners HealthCare network. Each study group included 12 Caucasians, two African-Americans, and one subject with a multiple racial background. Study groups were also matched for ethnic background with 13 non-Hispanic and two Hispanic subjects each. The Institutional Review Board of Partners HealthCare system approved the study, and informed assent and consent were obtained from subjects and parents.
Anthropometric measurements
Subjects were weighed to the nearest 0.1 kg in a hospital gown on an electronic scale at the Clinical Research Center of Massachusetts General Hospital. Height was measured to the nearest 0.1 cm using a single stadiometer and an average of three measurements was taken.
Experimental protocol
Subject inclusion criteria included a normal TSH and fasting glucose of less than 126 mg/dl (to rule out girls with thyroid dysfunction and diabetes, respectively, both of which can impact bone metabolism), and hematocrit greater than 30% (to avoid drawing blood from girls with significant anemia). Exclusion criteria included pregnancy, use of medications that could affect bone mass (such as estrogens, progestins, or glucocorticoids), a weight loss or gain of more than 2 kg within the 3 months preceding the study, diabetes mellitus, and thyroid disorders. Eligible subjects were admitted to the Clinical Research Center of Massachusetts General Hospital in the fasting state and in the early follicular phase of their menstrual cycles (17). Fasting levels of leptin, adiponectin, E-selectin, sICAM, and IL-6 were obtained. Body composition was determined using magnetic resonance imaging (Signa 1.5 Tesla, General Electric Medical Systems, Milwaukee, WI) and dual-energy x-ray absorptiometry (DXA; QDR 4500; Hologic, Waltham, MA). Magnetic resonance imaging assessments were performed in the fasting state and included measurements of SAT and VAT at the lumbar 4–5 level (6). We used DXA to assess bone density at the spine, hip, and whole body, and Z-scores [sd scores (SDS) for age and gender] were reported using Hologic databases. To adjust for body size, we used measurements of bone mineral apparent density (BMAD) for the spine, which we calculated using the following formula: [(bone mineral content for lumbar vertebrae 2–4)/(bone area for lumbar 2–4)1.5] (18). We also calculated bone mineral content (BMC) to height for the whole body as another height adjusted measure.
Biochemical analyses
We used RIA to assess adiponectin [Linco Diagnostics, St. Charles, MO; coefficient of variation (CV) 6.4–8.4% and lowest detectable concentration 0.001 ng/ml] and leptin (Linco Diagnostics; CV 3.4–8.3%, sensitivity 0.5 ng/ml). ELISA was used to measure E-selectin (R&D Systems, Minneapolis, MN; CV 5.2–6.6% and sensitivity 0.01 ng/ml), sICAM-1 (R&D Systems; CV 6.0–10.1% and sensitivity 0.35 ng/ml), and IL-6 (R&D Systems; sensitivity 0.094 pg/ml, CV 6.5–9.6%). All samples were stored at −80 C until analysis in duplicate.
Statistical analysis
JMP statistical program version 4 (SAS Institute, Cary, NC) was used for statistical analyses. Results are reported as mean ± sd. Statistical significance was defined as P < 0.05. We used a matched design in this study to limit variations in bone density from predictors known to impact bone density such as race, ethnicity, and maturity (therefore of little interest) and to instead focus on variations in bone density related to predictors of interest, such as regional body composition measures. Because the groups were markedly differentiated by their BMI, they were still significantly different in terms of many parameters known to impact bone density (other than those used for matching), including BMI and body composition. Matched-pair analysis was used for analyzing the differences between means for the two groups.
To determine whether SAT and VAT predicted measures of bone density after controlling for each other, we first performed stepwise regression modeling with VAT and SAT entered into the model. We expected VAT to be negatively and SAT to be positively associated with bone density in this regression model. As a next step, we calculated the ratio of VAT to SAT for our subjects as an indicator of the relative proportion of VAT to SAT. Importantly, both VAT and SAT increase with increasing weight and BMI, and there were strong, positive, and linear associations of VAT with SAT (r = 0.83, P < 0.0001), VAT and SAT with BMI-SDS (r = 0.82 and 0.96, respectively, P < 0.0001 for both), and VAT and SAT with fat mass (r = 0.78 and 0.98, P < 0.0001 for both). Given these linear associations, the ratio of VAT to SAT was considered a valid parameter to use in the study as an indicator of the amount of VAT relative to the amount of SAT. Finally, to determine whether adipokines (leptin, adiponectin) and inflammatory fat products (IL-6, sICAM, and E-selectin) mediate the association of regional fat with bone density, we performed stepwise regression modeling with VAT to SAT entered into the model along with adipokines and inflammatory fat products of interest. The first model included VAT to SAT, E-selectin (as representative of inflammatory fat products), and adiponectin. We then also added leptin and finally lean mass to the regression model. We expected mediators of the association of VAT to SAT with bone to replace VAT to SAT as independent predictors of bone density. P = 0.10 was used to enter and leave the model.
We first included both groups in regression analyses to determine predictors of bone density across a range of BMI and body composition and then also examined these predictors within obese girls alone to determine whether associations changed when examined within a group with high levels of SAT and VAT. For the group as a whole, we also refitted the regression model by the generalized estimating equations approach to take into account interdependence of values (given the matched groups) (19). Data derived from this analysis for the most part did not differ from those derived from stepwise regression modeling, and the statistical significance remained about the same. For the purpose of this paper, data from stepwise regression modeling are reported followed by information derived from the generalized estimating equations approach.
In addition, although we report Z-scores for bone density measures, we used absolute bone density for correlation analysis and regression modeling. This is because our groups were already matched for maturity, race, and bone age, parameters that are important determinants of bone density Z-scores. Typically, Z-score assessment using available databases takes into account chronological age rather than bone age, which in healthy children, should not differ markedly from bone age. However, using Z-score measurements derived from databases based on chronological age could lead to overestimation of bone density status in obese girls who tend to be more mature than normal-weight counterparts. For this reason and because our groups were matched for maturity, our regression analyses were based on absolute measures of bone density rather than bone density Z-scores.
Results
Clinical characteristics
Clinical characteristics of our subjects are summarized in Table 1. Obese girls did not differ from normal-weight adolescent girls for bone age and pubertal stage per study design but were younger than normal-weight controls for chronological age. Obesity is associated with earlier age of onset of puberty, and this accounts for the younger chronological age in obese girls after matching for bone age and pubertal stage. As expected, obese girls had higher BMI, fat mass, and lean mass as well as regional fat (SAT and VAT) than controls. All inflammatory markers including the adhesion molecules, sICAM1 and E-selectin, were higher in obese adolescent girls. Absolute measures of bone mineral density (BMD) and BMAD did not differ between the groups. However, Z-scores for lumbar BMD, BMAD, hip BMD, whole-body BMD, and BMC to height were higher in obese compared with normal-weight girls. Because DXA reported Z-scores compare one’s bone density to the mean for chronological age (and not bone age) and gender, higher Z-scores in obese girls were likely a consequence of their younger chronological age compared with normal-weight controls. We therefore use absolute measures of bone density in our regression analyses.
Table 1.
Clinical characteristics and bone density measures in 15 obese and 15 normal-weight adolescent girls
| Obese (n = 15) | Controls (n = 15) | P (matched pairs) | |
|---|---|---|---|
| Age (yr) | 14.0 ± 1.9 | 15.9 ± 1.7 | 0.0008 |
| Bone age (yr) | 15.1 ± 1.9 | 15.8 ± 1.8 | NS |
| Tanner stage (breasts) | 4.3 ± 1.0 | 4.5 ± 1.1 | NS |
| BMI (kg/m2) | 34.4 ± 7.1 | 21.7 ± 1.9 | <0.0001 |
| BMI-SDS | 3.7 ± 1.5 | 0.1 ± 0.4 | <0.0001 |
| Height-SDS | 0.6 ± 0.7 | 0.0 ± 0.9 | NS |
| Total lean mass (kg) | 53.4 ± 9.1 | 40.1 ± 4.3 | 0.0001 |
| Total fat mass (kg) | 39.3 ± 13.6 | 16.5 ± 3.4 | <0.0001 |
| Bone density measures | |||
| Lumbar BMD (g/cm2) | 1.00 ± 0.13 | 0.97 ± 0.10 | NS |
| Lumbar BMD Z-score | 0.49 ± 0.52 | −0.42 ± 0.91 | 0.008 |
| Lumbar BMAD (g/cm3) | 0.16 ± 0.02 | 0.15 ± 0.01 | NS |
| Hip BMD (g/cm2) | 1.04 ± 0.11 | 0.99 ± 0.10 | NS |
| Hip BMD Z-score | 1.24 ± 0.64 | 0.32 ± 0.89 | 0.02 |
| Total BMD (g/cm2) | 1.03 ± 0.10 | 1.01 ± 0.07 | NS |
| Total BMD Z-score | 0.73 ± 0.74 | −0.30 ± 0.93 | 0.004 |
| Total BMC/height (g/cm) | 13.11 ± 1.91 | 11.81 ± 1.15 | 0.02 |
| Regional fat mass | |||
| SAT (cm2) | 449.4 ± 174.9 | 151.3 ± 54.1 | <0.0001 |
| VAT (cm2) | 46.8 ± 8.7 | 20.8 ± 8.2 | 0.0009 |
| VAT to SAT ratio | 0.11 ± 0.04 | 0.14 ± 0.06 | 0.05 |
| Adipokines and inflammatory products of fat | |||
| Adiponectin (ng/ml) | 8.34 ± 2.21 | 9.81 ± 2.93 | NS |
| VAT/adiponectin (cm2/ng · ml) | 6.1 ± 3.6 | 2.4 ± 1.3 | 0.003 |
| Leptin (ng/ml) | 42.8 ± 18.2 | 10.0 ± 4.1 | <0.0001 |
| IL-6 | 2.22 ± 1.88 | 1.08 ± 0.63 | 0.02 |
| sICAM1 (mg/ml) | 279.0 ± 79.6 | 176.6 ± 51.9 | 0.003 |
| E-selectin | 61.5 ± 25.9 | 38.1 ± 17.0 | 0.004 |
NS, Not significant, even after controlling for height or height SDS.
Associations between bone density measures and regional fat mass
After controlling for SAT, we noted that VAT was a negative predictor of lumbar BMAD, whole-body BMD, and BMC to height for the group as a whole and lumbar BMD, BMAD, whole-body BMD, and BMC to height in obese girls (Table 2 shows data from regression modeling with SAT and VAT entered into the model). These associations persisted even after controlling for height SDS. Refitting the regression model by the generalized estimating equations approach did not change our results except that VAT was no longer a predictor of whole-body BMD for the group as a whole. In addition, although we primarily report associations with absolute measures of bone density in this study for reasons discussed in Statistical analysis, we did find that VAT was a negative predictor of hip and whole-body BMD Z-scores for obese subjects.
Table 2.
Visceral fat inversely predicted lumbar spine and whole-body bone density measures after controlling for sc fat in obese adolescents and all subjects
| Parameter | Parameter estimate | F ratio | P | R2 | Total variability explained by model | |
|---|---|---|---|---|---|---|
| Lumbar BMD | ||||||
| Obese | Intercept | 0.887 | ||||
| SAT | 0.0007 | 12.1 | 0.006 | 0.30 | ||
| VAT | −0.0043 | 5.4 | 0.04 | 0.25 | 0.55 | |
| All subjects | Intercept | 0.909 | ||||
| SAT | 0.0002 | 5.4 | 0.03 | 0.18 | 0.18 | |
| Lumbar BMAD | ||||||
| Obese | Intercept | 0.137 | ||||
| SAT | 0.0001 | 16.5 | 0.002 | 0.41 | ||
| VAT | −0.0006 | 5.8 | 0.04 | 0.21 | 0.62 | |
| All subjects | Intercept | 0.145 | ||||
| SAT | 0.00008 | 10.6 | 0.003 | 0.26 | ||
| VAT | −0.00045 | 3.3 | 0.08 | 0.09 | 0.35 | |
| Total BMD | ||||||
| Obese | Intercept | 0.975 | ||||
| SAT | 0.0006 | 17.9 | 0.002 | 0.24 | ||
| VAT | −0.004 | 11.7 | 0.007 | 0.41 | 0.65 | |
| All subjects | Intercept | 1.002 | ||||
| SAT | 0.0004 | 8.8 | 0.007 | 0.14 | ||
| VAT | −0.0028 | 4.5 | 0.04 | 0.13 | 0.27 | |
| Total BMC to height | ||||||
| Obese | Intercept | 11.504 | ||||
| SAT | 0.0121 | 20.3 | 0.001 | 0.32 | ||
| VAT | −0.083 | 10.9 | 0.008 | 0.35 | 0.67 | |
| All subjects | Intercept | 11.393 | ||||
| SAT | 0.0101 | 21.9 | 0.0001 | 0.38 | ||
| VAT | −0.586 | 7.1 | 0.01 | 0.14 | 0.52 | |
Because VAT correlated negatively and SAT positively with bone density measures, we anticipated that girls with the highest VAT and lowest SAT would have the lowest measures of bone density. We therefore used the ratio of VAT to SAT to characterize subjects based on relative proportions of VAT vs. SAT. Girls with greater visceral compared with sc fat would be expected to have a higher VAT to SAT ratio than those with lower visceral compared with sc fat. When we divided the obese girls into two groups based on VAT to SAT above or below the median, we noted that those with VAT to SAT ratio above the median had lower bone density measures at multiple sites compared with girls with VAT to SAT ratio below the median (Table 3), despite the fact that BMI, BMI SDS, fat mass, and percent body fat did not differ.
Table 3.
Bone density measures in obese adolescent girls with VAT to SAT ratio above the median vs. those with VAT to SAT ratio below the median
| VAT to SAT ratio ≥ median (n = 8) | VAT to SAT ratio < median (n = 7) | P | |
|---|---|---|---|
| Bone density measures | |||
| Lumbar BMD (g/cm2) | 0.92 ± 0.09 | 1.08 ± 0.11 | 0.02a |
| Lumbar BMD Z-score | 0.23 ± 0.52 | 0.70 ± 0.38 | 0.09 |
| Lumbar BMAD (g/cm3) | 0.17 ± 0.02 | 0.15 ± 0.01 | 0.02 |
| Hip BMD (g/cm2) | 0.97 ± 0.07 | 1.07 ± 0.07 | 0.03 |
| Hip BMD Z-score | 0.88 ± 0.14 | 1.22 ± 0.26 | 0.02 |
| Total BMD (g/cm2) | 0.97 ± 0.06 | 1.10 ± 0.09 | 0.01a |
| Total BMD Z-score | 0.32 ± 0.29 | 1.17 ± 0.85 | 0.05 |
| Total BMC to height (g/cm) | 11.70 ± 0.95 | 14.64 ± 1.65 | 0.005 |
Significant, even after controlling for height or height SDS.
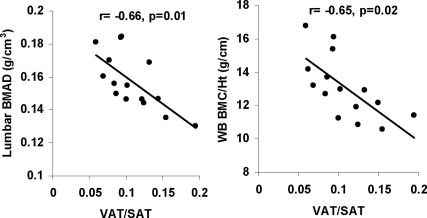
The ratio of VAT to SAT correlated inversely with height adjusted measures of bone density in the group as a whole and with lumbar BMD and BMAD and whole-body BMD and BMC to height in obese adolescent girls (r = −0.67, −0.66, −0.61, and −0.65, P = 0.01, 0.01, 0.03, and 0.02, respectively) (Fig. 1). Again these associations remained significant even after controlling for height SDS. Additionally, VAT to SAT ratio was a negative predictor of lumbar and hip BMD Z-scores for obese subjects. Girls with VAT to SAT ratio above the median also had higher E-selectin levels than those with VAT to SAT ratio below the median (79.3 ± 24.1 vs. 42.3 ± 13.8 ng/ml, P = 0.007). In contrast, levels of sICAM-1, IL-6, leptin, adiponectin, and lean mass did not differ between these groups (data not shown).
Figure 1.
Relationship between the ratio of VAT to SAT and height-adjusted measures of bone density for obese girls. VAT to SAT ratio correlated inversely with lumbar BMAD (r = −0.66, P = 0.01) and whole-body BMC to height (r = −0.65, P = 0.02).
We then performed stepwise regression modeling to determine independent predictors of bone density measures. When we included VAT to SAT ratio, E-selectin, and adiponectin in the regression model (Table 4), we observed that E-selectin alone or with adiponectin and VAT to SAT ratio inversely predicted the various measures of bone density in the group as a whole and within obese girls. When VAT and SAT were used in the model separately (rather than the ratio), E-selectin remained a very strong inverse predictor of bone density. Refitting the regression model by the generalized estimating equations approach did not change our results except that both E-selectin and adiponectin were inverse predictors of hip BMD for the group as a whole.
Table 4.
E-selectin and adiponectin were significant independent and negative predictors of bone density in a regression model (VAT to SAT ratio, adiponectin and E-selectin included in the model)
| Parameter | Parameter estimate | F ratio | P | R2 | Total variability explained by model | |
|---|---|---|---|---|---|---|
| Lumbar BMD | ||||||
| Obese | Intercept | 1.370 | ||||
| E-selectin | −0.004 | 29.5 | 0.0003 | 0.70 | ||
| Adiponectin | −0.017 | 4.6 | 0.06 | 0.10 | 0.80 | |
| All subjects | Intercept | 1.33 | ||||
| E-selectin | −0.002 | 9.0 | 0.006 | 0.20 | ||
| Adiponectin | −0.016 | 5.5 | 0.03 | 0.10 | ||
| VAT to SAT ratio | −0.718 | 5.1 | 0.03 | 0.13 | 0.43 | |
| Lumbar BMAD | ||||||
| Obese | Intercept | 0.212 | ||||
| E-selectin | −0.0005 | 17.1 | 0.002 | 0.58 | ||
| Adiponectin | −0.003 | 5.4 | 0.04 | 0.15 | 0.73 | |
| All subjects | Intercept | 0.208 | ||||
| E-selectin | −0.0002 | 4.7 | 0.04 | 0.11 | ||
| Adiponectin | −0.003 | 9.5 | 0.005 | 0.18 | ||
| VAT to SAT ratio | −0.128 | 8.0 | 0.009 | 0.16 | 0.46 | |
| Hip BMD | ||||||
| Obese | Intercept | 1.171 | ||||
| E-selectin | −0.003 | 18.6 | 0.001 | 0.63 | 0.63 | |
| All subjects | Intercept | 1.081 | ||||
| E-selectin | −0.002 | 5.7 | 0.02 | 0.19 | 0.19 | |
| Total BMD | ||||||
| Obese | Intercept | 1.207 | ||||
| E-selectin | −0.003 | 18.1 | 0.001 | 0.62 | 0.62 | |
| All subjects | Intercept | 1.099 | ||||
| E-selectin | −0.002 | 7.9 | 0.009 | 0.23 | 0.23 | |
| Total BMC to height | ||||||
| Obese | Intercept | 16.765 | ||||
| E-selectin | −0.060 | 22.1 | 0.0006 | 0.67 | 0.67 | |
| All subjects | Intercept | 17.099 | ||||
| E-selectin | −0.024 | 4.8 | 0.04 | 0.09 | ||
| Adiponectin | −0.186 | 3.2 | 0.09 | 0.08 | ||
| VAT to SAT ratio | −13.779 | 7.7 | 0.01 | 0.20 | 0.37 | |
Of interest, addition of leptin to the regression model (VAT to SAT ratio, E-selectin, adiponectin, and leptin) altered these results only minimally, and E-selectin remained the most important predictor of most bone density measures in obese and normal-weight controls. In the group as a whole, with E-selectin as a negative predictor, leptin positively predicted lumbar BMD, whole-body BMD, and whole-body BMC to height (35, 41, and 48% of variance explained by these two covariates), whereas adiponectin, E-selectin, and VAT to SAT ratio remained negative predictors of lumbar BMAD (46% of variance) and E-selectin predicted hip BMD (19% of variance). With the generalized estimating equations approach, the only difference was that leptin did not predict lumbar BMD for the group as a whole.
When lean mass was added to the model, it replaced adiponectin or leptin as predictors of bone density for most sites, whereas E-selectin remained a significant independent predictor of bone density measures. For the group as a whole, E-selectin and lean mass explained 40, 31, and 55% of the variability of lumbar, hip, and whole BMD and 71% of the variability in whole-body BMC to height. E-selectin, adiponectin, and lean mass explained 48% of the variability in lumbar BMAD when VAT to SAT ratio was part of the model (with E-selectin, leptin, adiponectin, and lean mass). When VAT and SAT were considered separately, E-selectin and SAT predicted lumbar BMD (49% variance explained), E-selectin and VAT predicted hip BMD (32% of variance), and predictors of lumbar BMAD, whole-body BMD and BMC to height were unchanged. With the generalized estimating equations approach lumbar BMD and BMAD and whole-body BMD were predicted inversely by adiponectin and positively by lean mass, hip BMD was predicted inversely by E-selectin, and whole-body BMC to height was predicted inversely by E-selectin and positively by lean mass.
Overall, E-selectin, adiponectin, and VAT were negative predictors of bone density measures, and lean mass, leptin, and SAT were positive predictors of bone density.
Discussion
Our data indicate that visceral fat is a negative predictor of bone density measures in obese girls. We also show that circulating levels of specific adipokines may depend on the relative proportion of visceral vs. sc fat and that adipokines such as E-selectin and adiponectin may mediate the inverse associations of VAT with bone density.
In our study, SAT and VAT had reciprocal associations with bone density measures, with SAT demonstrating positive associations and VAT demonstrating inverse associations (after controlling for SAT). Our data are consistent with studies in young adults that indicate similar associations of bone density with SAT and VAT (2) but differ from another study that reported a positive association of waist circumference, a surrogate for VAT, and bone (8). However, in the latter case, the authors did not control for sc fat in their analysis of associations of waist circumference and bone density. Of importance, in our study, obese girls with the highest VAT and the lowest SAT (thus the highest VAT to SAT ratio) had the lowest bone density measures. Additionally, the associations of VAT and VAT to SAT ratio with bone density measures were primarily evident in the obese girls, and associations were weaker when the group was considered as a whole. Given that the ratio of VAT to SAT trended higher in the normal-weight girls, we speculate that a critical amount of VAT may be necessary before we see the deleterious effects of VAT or the ratio of VAT to SAT on bone. However, a study in healthy young adults also reported an inverse association between VAT and bone structural parameters (2).
Adipose tissue secretes various inflammatory cytokines and hormones, from either adipocytes or endothelial cells in blood vessels in fat. Of note, site-specific fat depots have a differential impact on the secretion of inflammatory cytokines and adipokines. For example, we previously reported in obese and normal-weight girls that VAT is an important determinant of levels of sICAM1, E-selectin, and TNF-α receptors 1 and 2, and studies by others confirmed these findings (20,21). Both SAT and VAT have been related to IL-6 levels (14,22). Inflammatory cytokines such as TNF-α and IL-6 are known to activate osteoclast differentiation and activation and inhibit osteoclast apoptosis, and increased secretion of these cytokines from visceral fat may cause a decrease in bone density from increased bone resorption (9). However, levels of these cytokines did not differ in girls with high vs. low VAT to SAT ratios. In contrast, girls with higher VAT to SAT had higher levels of E-selectin than those with lower VAT to SAT ratio, suggesting that increased E-selectin secretion in subjects with a higher proportion of VAT to SAT may negatively impact bone density. Although the source of E-selectin secretion from fat is unclear, this is typically secreted by endothelial cells in blood vessels, and therefore, blood vessels in adipose tissue are a possible source of this adhesion molecule. One recent paper, however, indicated that E-selectin may be secreted by visceral adipocytes (rather than endothelial cells), given that expression of E-selectin was 8-fold greater in visceral adipocytes than in VAT as a whole (20). Little is known about the impact of E-selectin on bone and possible pathways linking the two are yet to be identified; however, based on our data, further investigation of this question is warranted.
Clues to the possible link between adhesion molecules and bone come from studies of Perut et al. (23) and Schweitzer et al. (24). In a proinflammatory environment, endothelial cells are induced to secrete high levels of adhesion molecules such as E-selectin and sICAM1. E-selectin is necessary for modifying the rolling of CD34+ cells on bone marrow endothelial cells, whereas ICAM1 contributes to firm adhesion of these cells on endothelium in the presence of E-selectin interaction. Also, ICAM1 binds to the integrin, lymphocyte function-associated antigen-1, expressed by hematopoietic cells and preosteoclasts (which share the same progenitor cell), and may stimulate osteoclast differentiation. Finally, increased expression of adhesion molecules in VAT may lead to recruitment and activation of macrophages (20), which may also activate preosteoclasts through the secretion of cytokines such as TNF-α.
In addition to inflammatory cytokines, adipocytes secrete adiponectin and leptin, both of which have an impact on bone metabolism. Adiponectin receptors are expressed on osteoclasts, and adiponectin can increase RANK ligand and decrease osteoprotegerin expression, thus increasing osteoclast activity (10). Consistent with this model, inverse associations of adiponectin and bone density have been reported in studies in adults (12,13,25,26) and also in children (27). In this study, we similarly found that adiponectin was inversely associated with bone density measures, particularly for the spine. Of note and somewhat surprisingly, adiponectin levels were not significantly lower in obese girls compared with controls in our study, and it is possible that a difference between the groups would have been evident if we had measured high-molecular-weight adiponectin instead of total adiponectin (because the high molecular weight form is more strongly associated with insulin sensitivity than is total adiponectin) (28). Of importance, leptin is yet another adipokine that has an impact on bone. The leptin-deficient ob/ob mouse has increased trabecular but decreased cortical bone, both of which normalize with leptin replacement (11,29). In humans, mostly positive associations are reported between leptin and bone density (12,13), and high leptin levels in obesity would be expected to be associated with high bone density. Consistent with these studies, leptin positively predicted lumbar BMD, whole-body BMD, and whole-body BMC to height in our subjects after controlling for various covariates. In addition, adiponectin and leptin replaced VAT and SAT as predictors of some bone density measures in our regression model, suggesting that these hormones may also mediate the impact of regional fat on bone.
Therefore, whereas increased secretion of E-selectin by VAT and adiponectin by VAT and SAT would be deleterious to bone, increased secretion of leptin from SAT would be beneficial to cortical bone. The relative proportions of VAT and SAT would determine concentrations of E-selectin, leptin, and adiponectin in the circulation and the subsequent effects on bone density.
Limitations of our study include its associative nature; therefore, causality cannot be determined. In addition, our small sample size prevented us from performing regression modeling with more covariates entered into the model, and our data need to be confirmed in a larger group of subjects. However, these are important preliminary data for a more comprehensive study examining the bone-fat connection. Another consideration is that DXA reports of bone density may be affected by body composition and that DXA may overestimate bone density in individuals with increased fat mass and underestimate bone density in individuals with decreased fat mass. Despite these concerns, DXA remains the standard of care for assessing bone density (30), and data indicate that DXA reports of bone density are overall corroborated by computed tomography (CT) measures of bone density and microarchitecture (31,32). For example, adult women with anorexia nervosa (who have significantly decreased fat mass) have low bone density as assessed by DXA, and CT studies indicate that these women have truly low cortical and trabecular bone density and impaired bone microarchitecture (33,34). In addition, in healthy young people, an inverse association has been reported between VAT and parameters of bone microarchitecture assessed by CT scan (2), similar to our data indicating an inverse association of VAT with bone density measures as assessed by DXA.
To conclude, we demonstrate that obese girls with higher visceral compared with sc fat are likely to have lower bone density than those with more sc than visceral fat. These associations of fat and bone are possibly mediated by adhesion molecules, such as E-selectin, and adipokines, such as adiponectin and leptin. Mechanisms whereby E-selectin may impact bone metabolism merits further study.
Acknowledgments
We thank Ellen Anderson and her Bionutrition team as well as the skilled nursing staff of the Clinical Research Center for their help in completing this study. Most of all, we thank our subjects, who made this study possible.
Footnotes
This work was supported by grants National Institutes of Health Grants 5P30DK4620-15 and M01-RR-01066.
Disclosure Summary: The authors have no conflict of interest to declare.
First Published Online January 15, 2010
Abbreviations: BMAD, Bone mineral apparent density; BMC, bone mineral content; BMD, bone mineral density; BMI, body mass index; CT, computed tomography; CV, coefficient of variation; DXA, dual-energy x-ray absorptiometry; RANK, receptor activator of nuclear factor-κB; SAT, sc adipose tissue; SDS, sd score; sICAM, soluble intercellular adhesion molecule; VAT, visceral adipose tissue.
References
- Dolan SE, Carpenter S, Grinspoon S 2007 Effects of weight, body composition, and testosterone on bone mineral density in HIV-infected women. J Acquir Immune Defic Syndr 45:161–167 [DOI] [PubMed] [Google Scholar]
- Gilsanz V, Chalfant J, Mo AO, Lee DC, Dorey FJ, Mittelman SD 2009 Reciprocal relations of subcutaneous and visceral fat to bone structure and strength. J Clin Endocrinol Metab 94:3387–3393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lac G, Cavalie H, Ebal E, Michaux O 2008 Effects of a high fat diet on bone of growing rats. Correlations between visceral fat, adiponectin and bone mass density. Lipids Health Dis 7:16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Kanazawa I, Yamamoto M, Kurioka S, Yamauchi M, Yano S, Sugimoto T 2009 Associations between components of the metabolic syndrome versus bone mineral density and vertebral fractures in patients with type 2 diabetes. Bone 45:174–179 [DOI] [PubMed] [Google Scholar]
- Cali AM, Caprio S 2009 Ectopic fat deposition and the metabolic syndrome in obese children and adolescents. Horm Res 71(Suppl 1):2–7 [DOI] [PubMed] [Google Scholar]
- Misra M, Bredella MA, Tsai P, Mendes N, Miller KK, Klibanski A 2008 Lower growth hormone and higher cortisol are associated with greater visceral adiposity, intramyocellular lipids, and insulin resistance in overweight girls. Am J Physiol Endocrinol Metab 295:E385–E392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CJ, Oh KW, Rhee EJ, Kim KH, Jo SK, Jung CH, Won JC, Park CY, Lee WY, Park SW, Kim SW 2008 Relationship between body composition and bone mineral density in perimenopausal Korean women. Clin Endocrinol (Oxf) 71:18–26 [DOI] [PubMed] [Google Scholar]
- Ağbaht K, Gürlek A, Karakaya J, Bayraktar M 2009 Circulating adiponectin represents a biomarker of the association between adiposity and bone mineral density. Endocrine 35:371–379 [DOI] [PubMed] [Google Scholar]
- Riggs BL, Khosla S, Melton 3rd LJ 2002 Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev 23:279–302 [DOI] [PubMed] [Google Scholar]
- Luo XH, Guo LJ, Xie H, Yuan LQ, Wu XP, Zhou HD, Liao EY 2006 Adiponectin stimulates RANKL and inhibits OPG expression in human osteoblasts through the MAPK signaling pathway. J Bone Miner Res 21:1648–1656 [DOI] [PubMed] [Google Scholar]
- Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, Shen J, Vinson C, Rueger JM, Karsenty G 2000 Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell 100:197–207 [DOI] [PubMed] [Google Scholar]
- Jürimäe J, Jürimäe T, Leppik A, Kums T 2008 The influence of ghrelin, adiponectin, and leptin on bone mineral density in healthy postmenopausal women. J Bone Miner Metab 26:618–623 [DOI] [PubMed] [Google Scholar]
- Zoico E, Zamboni M, Di Francesco V, Mazzali G, Fantin F, De Pergola G, Zivelonghi A, Adami S, Bosello O 2008 Relation between adiponectin and bone mineral density in elderly post-menopausal women: role of body composition, leptin, insulin resistance, and dehydroepiandrosterone sulfate. J Endocrinol Invest 31:297–302 [DOI] [PubMed] [Google Scholar]
- Russell M, Bredella M, Tsai P, Mendes N, Miller KK, Klibanski A, Misra M 2009 Relative growth hormone deficiency and cortisol excess are associated with increased cardiovascular risk markers in obese adolescent girls. J Clin Endocrinol Metab 94:2864–2871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greulich W, Pyle S 1959 Radiographic atlas of skeletal development of the hand and wrist. 2nd ed. Stanford, CA: Stanford University Press [Google Scholar]
- Lee JM, Appugliese D, Kaciroti N, Corwyn RF, Bradley RH, Lumeng JC 2007 Weight status in young girls and the onset of puberty. Pediatrics 119:e624–e630 [DOI] [PubMed] [Google Scholar]
- Faria AC, Bekenstein LW, Booth Jr RA, Vaccaro VA, Asplin CM, Veldhuis JD, Thorner MO, Evans WS 1992 Pulsatile growth hormone release in normal women during the menstrual cycle. Clin Endocrinol (Oxf) 36:591–596 [DOI] [PubMed] [Google Scholar]
- Katzman DK, Bachrach LK, Carter DR, Marcus R 1991 Clinical and anthropometric correlates of bone mineral acquisition in healthy adolescent girls. J Clin Endocrinol Metab 73:1332–1339 [DOI] [PubMed] [Google Scholar]
- Zeger SL, Liange KY, Albert PS 1988 Models for longitudinal data: a generalized estimating equations approach. Biometrics 44:1049–1060 [PubMed] [Google Scholar]
- Dolinková M, Dostálová I, Lacinová Z, Michalský D, Haluzíková D, Mráz M, Kasalický M, Haluzík M 2008 The endocrine profile of subcutaneous and visceral adipose tissue of obese patients. Mol Cell Endocrinol 291:63–70 [DOI] [PubMed] [Google Scholar]
- Sam S, Haffner S, Davidson MH, D'Agostino Sr RB, Feinstein S, Kondos G, Perez A, Mazzone T 2009 Relation of abdominal fat depots to systemic markers of inflammation in type 2 diabetes. Diabetes Care 32:932–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pou KM, Massaro JM, Hoffmann U, Vasan RS, Maurovich-Horvat P, Larson MG, Keaney Jr JF, Meigs JB, Lipinska I, Kathiresan S, Murabito JM, O'Donnell CJ, Benjamin EJ, Fox CS 2007 Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study. Circulation 116:1234–1241 [DOI] [PubMed] [Google Scholar]
- Perut F, Cenni E, Unger RE, Kirkpatrick CJ, Giunti A, Baldini N 2009 Immunogenic properties of renal cell carcinoma and the pathogenesis of osteolytic bone metastases. Int J Oncol 34:1387–1393 [PubMed] [Google Scholar]
- Schweitzer KM, Vicart P, Delouis C, Paulin D, Dräger AM, Langenhuijsen MM, Weksler BB 1997 Characterization of a newly established human bone marrow endothelial cell line: distinct adhesive properties for hematopoietic progenitors compared with human umbilical vein endothelial cells. Lab Invest 76:25–36 [PubMed] [Google Scholar]
- Jürimäe J, Rembel K, Jürimäe T, Rehand M 2005 Adiponectin is associated with bone mineral density in perimenopausal women. Horm Metab Res 37:297–302 [DOI] [PubMed] [Google Scholar]
- Lenchik L, Register TC, Hsu FC, Lohman K, Nicklas BJ, Freedman BI, Langefeld CD, Carr JJ, Bowden DW 2003 Adiponectin as a novel determinant of bone mineral density and visceral fat. Bone 33:646–651 [DOI] [PubMed] [Google Scholar]
- Misra M, Miller KK, Cord J, Prabhakaran R, Herzog DB, Goldstein M, Katzman DK, Klibanski A 2007 Relationships between serum adipokines, insulin levels, and bone density in girls with anorexia nervosa. J Clin Endocrinol Metab 92:2046–2052 [DOI] [PubMed] [Google Scholar]
- Hara K, Horikoshi M, Yamauchi T, Yago H, Miyazaki O, Ebinuma H, Imai Y, Nagai R, Kadowaki T 2006 Measurement of the high-molecular weight form of adiponectin in plasma is useful for the prediction of insulin resistance and metabolic syndrome. Diabetes Care 29:1357–1362 [DOI] [PubMed] [Google Scholar]
- Karsenty G 2006 Convergence between bone and energy homeostases: leptin regulation of bone mass. Cell Metab 4:341–348 [DOI] [PubMed] [Google Scholar]
- Bianchi ML, Baim S, Bishop NJ, Gordon CM, Hans DB, Langman CB, Leonard MB, Kalkwarf HJ 15 July 2009 Official positions of the International Society for Clinical Densitometry (ISCD) on DXA evaluation in children and adolescents. Pediatr Nephrol doi: 10.1007/s00467-009-129-z [DOI] [PubMed] [Google Scholar]
- Nishiyama KK, Macdonald HM, Buie HR, Hanley DA, Boyd SK 19 Oct 2009 Postmenopausal women with osteopenia have higher cortical porosity and thinner cortices at the distal radius and tibia than women with normal aBMD: an in vivo HR-pQCT study. J Bone Miner Res doi: 10.1359/jbmr.091020 [DOI] [PubMed] [Google Scholar]
- Wren TA, Liu X, Pitukcheewanont P, Gilsanz V 2005 Bone acquisition in healthy children and adolescents: comparisons of dual-energy x-ray absorptiometry and computed tomography measures. J Clin Endocrinol Metab 90:1925–1928 [DOI] [PubMed] [Google Scholar]
- Lawson EA, Miller KK, Bredella MA, Phan C, Misra M, Meenaghan E, Rosenblum L, Donoho D, Gupta R, Klibanski A 9 Sept 2009 Hormone predictors of abnormal bone microarchitecture in women with anorexia nervosa. Bone doi: 10.1016/j.bone.2009.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milos G, Spindler A, Rüegsegger P, Seifert B, Mühlebach S, Uebelhart D, Häuselmann HJ 2005 Cortical and trabecular bone density and structure in anorexia nervosa. Osteoporos Int 16:783–790 [DOI] [PubMed] [Google Scholar]