Abstract
Context: Inverse associations are reported between circulating 25-hydroxyvitamin D and visceral adiposity. The effects of vitamin D levels on atherosclerosis are unknown.
Objective: The objective of this study was to test for relationships between vitamin D, adiposity, bone density, and atherosclerosis in African-Americans.
Design: Circulating 25-hydroxyvitamin D, 1,25 dihydroxyvitamin D, intact PTH, C-reactive protein and computed tomography-derived calcified atherosclerotic plaque (CP), bone density, and fat volumes were measured.
Setting: Examinations were performed at a single outpatient general clinical research center visit.
Subjects: Three hundred forty African-Americans with type 2 diabetes were evaluated. Mean ± SD age was 55.6 ± 9.6 yr, diabetes duration 10.6 ± 8.3 yr, glomerular filtration rate 1.6 ± 0.5 ml/sec, body mass index 35.6 ± 8.7 kg/m2, and 25-hydroxyvitamin D concentration 50.4 ± 30.5 nmol/liter.
Main Outcome Measure: Biomarkers were tested for association with pericardial, visceral, im, and sc adipose tissues; thoracic and lumbar vertebral bone density; and aorta, coronary, and carotid artery CP.
Results: Adjusting for age, gender, body mass index, glycosylated hemoglobin, and glomerular filtration rate, 25-hydroxyvitamin D was negatively associated with visceral adiposity (P = 0.009) and positively associated with carotid artery CP and aorta CP (P = 0.013 and 0.014, respectively) but not with coronary artery CP or bone density.
Conclusions: We confirmed an inverse association between vitamin D and visceral adiposity in African-Americans with diabetes. In addition, positive associations exist between 25-hydroxyvitamin D and aorta and carotid artery CP in African-Americans. The effects of supplementing vitamin D to raise the serum 25-hydroxyvitamin D level on atherosclerosis in African-Americans are unknown. Prospective trials are needed to determine the cardiovascular effects of supplemental vitamin D in this ethnic group.
Positive associations exist between 25-hydroxyvitamin D concentration and calcified atherosclerotic plaque in African Americans with type 2 diabetes.
Marked ethnic differences exist in bone metabolism and development of calcified atherosclerotic plaque (CP). Relative to European-Americans, African-Americans have lower rates of osteoporosis (despite ingesting less dietary calcium), form fewer calcium-containing kidney stones and manifest skeletal resistance to PTH (1,2,3). Systemic differences in regulation of calcium and phosphorus appear to be involved (4). Related phenomena may include the markedly lower amounts of calcified CP in African-Americans, despite the presence of more severe conventional cardiovascular disease risk factors (5,6,7,8,9). Together these observations suggest biologically mediated ethnic differences in the regulation of bone and vascular health.
Vitamin D is critically important for absorption of dietary calcium and phosphorus in the gastrointestinal tract to maintain bone health. Vitamin D receptors are also present on heart, stomach, liver, brain, skin, pancreatic islets (β cells), thyroid, parathyroid, adrenal gland, and immune cells (10), and low vitamin D levels have been associated with diabetes (11,12), inflammation (13), hypertension (14,15,16,17), and subclinical atherosclerosis (18). Low levels of vitamin D are far more common in African-Americans than European-Americans (19,20,21), and supplementation is typically recommended in those with 25-hydroxyvitamin D levels less than 74.9 nmol/liter (30 ng/ml) (22). Low levels of 25-hydroxyvitamin D are associated with increased mortality in predominantly non-African-derived populations (23).
Inverse relationships between bone density and CP have been observed in predominantly European-derived populations (24,25,26,27,28,29). Inverse relationships have also been observed between visceral adiposity and vitamin D in Europeans (30) and more recently in African-Americans and Hispanics (31,32). It remains unclear whether circulating vitamin D levels relate to bone density or CP in African-derived populations. The African American-Diabetes Heart Study is exploring the inherited and environmental causes of CP in African-Americans with longstanding type 2 diabetes mellitus and preserved kidney function. We measured circulating forms of vitamin D, highly sensitive C-reactive protein (hsCRP) and intact PTH as well as determined regional adipose tissue volumes, CP, and thoracic and lumbar vertebral bone density using computed tomography (CT). Relationships between organ-specific adipose tissue and vascular CP with vitamin D, CRP, and intact PTH were assessed.
Subjects and Methods
Study populations
African-Americans with type 2 diabetes recruited in the African American-Diabetes Heart Study formed the study population. Diabetes was diagnosed after the age of 30 yr in the absence of historical evidence of ketoacidosis. Subjects who underwent prior coronary artery bypass surgery or carotid endarterectomy had that vascular bed excluded from analysis because it was felt that the CP in the relevant arteries would be impacted by these procedures. Participants with prior myocardial infarction or stroke were included. The study was approved by the Institutional Review Board at the Wake Forest University School of Medicine, and all participants provided written informed consent.
Examinations were conducted in the General Clinical Research Center of the Wake Forest University School of Medicine and included interviews for medical history, current medications and health behaviors, measurements of body size, resting blood pressure, 12-lead electrocardiogram, fasting blood draw, and spot urine collection. Laboratory assays included urine albumin and creatinine, total cholesterol, low-density lipoprotein, high-density lipoprotein, triglycerides, hemoglobin A1c (HbA1c), hsCRP, blood urea nitrogen, serum glucose, creatinine, albumin, calcium, phosphorus, 25-hydroxyvitamin D, 1,25 dihydroxyvitamin D, and intact PTH. The vitamin D assays were developed and performance characteristics determined by Quest Diagnostics Nichols Institute (San Juan Capistrano, CA). The 25-hydroxyvitamin D assay was performed using liquid chromatography tandem mass spectroscopy and a radioreceptor assay was used for 1,25 dihydroxyvitamin D. Intra- and interassay coefficients of variation for 25-hydroxyvitamin D are: (low control = 49.9–62.4 nmol/liter; medium control = 112.3–137.3 nmol/liter; high control = 237.1–262.1 nmol/liter; acceptance ≤15%), interassay, low = 9.7% (D2), 13.5% (D3); medium = 11.6% (D2), 10.7% (D3); high = 8.8% (D2), 8.4% (D3); and intraassay, low = 9.0% (D2), 11.2% (D3); medium = 6.9% (D2), 7.5% (D3); high = 8.1% (D2), 8.5% (D3). Intra- and interassay coefficients of variation for 1,25 dihydroxyvitamin D are: (low control = 65–91 pmol/liter; medium control = 143–169 pmol/liter; high control = 286–338 pmol/liter); results were: interassay, low = 19% (D2), 13% (D3); medium = 17% (D2), 10% (D3); high = 11% (D2), 6% (D3) and intraassay, low = 15% (D2), 7% (D3); medium = 13% (D2), 10% (D3); and high = 11% (D2), 7% (D3). Modification of Diet in Renal Disease estimated glomerular filtration rates (GFRs) were computed (33). History of cardiovascular disease was provided by participant self-report.
Vascular imaging
Calcified atherosclerotic plaque was measured in the coronary, carotid, and infrarenal abdominal aorta arteries with single and multidetector CT systems using a standard electrocardiogram-gated CT scanning protocol based on those currently implemented in the National Heart, Lung, and Blood Institute’s Multi-Ethnic Study of Atherosclerosis (MESA) (34). The calcium mass score (SmartScore; General Electric Healthcare, Waukesha, WI), accounting for the volume and density of CP on a pixel-by-pixel basis and highly correlated with the standard Agatston score using a 90 Hounsfield unit threshold, 0.5 mm2 minimum lesion size (two adjacent pixels) was used for comparability between vascular territories.
Adipose tissue imaging
Pericardial adipose tissue (PAT) and visceral adipose tissue (VAT) were measured from volumetric CT acquisitions to reduce variability related to slice location using the Volume Analysis software (Advantage Windows Workstation, GE Healthcare) and a threshold of −190 to −30 as the definitions of fat containing tissue. PAT is the combined adipose tissue superficial (paracardial) and deep (epicardial) to the pericardium; however, the pericardium extends superiorly to encase the great vessels and inferiorly borders the diaphragm (35). Our methods for measuring PAT segments a volume for measurement that covers 45 mm in length along the z-axis (cephalocaudad) of the individual based on origin of the left main coronary such that it extends 15 mm above and 30 mm below. This PAT volume includes the majority of the coronary arteries and myocardium and excludes PAT located superiorly around the aorta and pulmonary arteries and adjacent to the abdomen, as reported (36).
In the abdomen, VAT, sc adipose tissue (SAT), and intermuscular adipose tissue (IMAT) were measured on abdominal CT scans with technical factors: helical mode, 120 kVp, 250 mA, 4 × 2.5 mm collimation, standard reconstruction kernel, and a display field of view of 500 mm. The landmark for analysis was the first lumbar disk above the lumbar-sacrum junction, most commonly designated as L4-L5. A volume 15 mm in z-axis length of the abdomen was segmented for the sc, abdominal wall, and intraabdominal compartments. VAT was defined as the fat containing pixels located within the abdominal cavity, SAT was defined as the fat containing pixels between the skin surface and lean tissue of the abdominal wall, and IMAT was measured within the abdominal wall and paraspinal muscles. Studies in human cadavers revealed that the area measured by CT is an accurate estimate of VAT, SAT, and IMAT volume.
Bone imaging
Quantitative CT for volumetric trabecular bone mineral density (BMD; milligrams per cubic centimeter) of the thoracic and lumbar vertebrae were measured using images obtained for CP in the coronary and abdominal aorta, including an external calibration phantom as in previous reports. Detailed descriptions have been published (37).
Statistical methods
Generalized linear models were fitted to test for associations between circulating 25-hydroxyvitamin D, 1,25 dihydroxyvitamin D, hsCRP, and intact PTH treated separately as predictors with PAT, VAT, IMAT, and SAT; thoracic and lumbar vertebral BMD; and aorta, coronary, and carotid artery CP (38). The Box-Cox method was applied to identify the appropriate transformation of each outcome variable that would best approximate the distributional assumptions of conditional normality and homogeneity of variance of the residuals (39). The natural log of (coronary CP+1), (carotid CP+1), (aorta CP+1), (IMAT+1), (PAT+1), (urine albumin to creatinine ratio+1), and (thoracic BMD+1) as well as the square root of (VAT) and (lumbar BMD) were analyzed. There was no need to transform GFR and SAT. Before these transformations, observed values of aorta, carotid, and coronary CP exceeding the 95th percentile were winsorized at their 95th percentile. Analyses were run without adjustment, adjusting for age and gender and adjusting for age, gender, body mass index, GFR, and HBA1c. Because the adjustments did not affect the parameter estimates significantly, we show only results obtained using the fully adjusted model. Standard regression diagnostics for collinearity and influence were computed for each model reported.
Results
The study population consisted of 340 unrelated African-Americans with type 2 diabetes mellitus. Demographic characteristics of the 140 men and 200 women are listed in Table 1. Two participants had undergone carotid endarterectomy and 17 coronary artery bypass surgery; these 19 vascular beds were excluded from analysis. Table 2 contains all laboratory results. Participants had mean ± sd 25-hydroxyvitamin D 50.4 ± 30.5 nmol/liter; 1,25 dihydroxyvitamin D 125.3 ± 46.3 pmol/liter; intact PTH 60.3 ± 32.8 ng/liter; serum calcium 2.4 ± 0.1 mmol/liter; phosphorus 1.1 ± 0.2 mmol/liter; and Modification of Diet in Renal Disease equation estimated GFR 1.6 ± 0.5 ml/sec. Among participants, 80 (23.5%) reported taking a multivitamin and 13 (3.8%) took vitamin D and/or calcium supplements.
Table 1.
Demographic characteristics of African American-Diabetes Heart Study participants
| Variable | Female (n = 200) | Male (n = 140) | All (n = 340) | |||
|---|---|---|---|---|---|---|
| Age (yr) | ||||||
| Mean sd | 55.1 | 9.1 | 56.3 | 10.4 | 55.6 | 9.6 |
| Median | 54.0 | 55.0 | 55.0 | |||
| Diabetes duration (yr) | ||||||
| Mean sd | 10.2 | 7.2 | 11.1 | 9.6 | 10.6 | 8.3 |
| Median | 8.0 | 9.0 | 8.0 | |||
| Body mass index (kg/m2) | ||||||
| Mean sd | 37.9 | 9.2 | 32.5 | 7.0 | 35.6 | 8.7 |
| Median | 36.7 | 31.1 | 33.8 | |||
| Systolic BP (mm Hg) | ||||||
| Mean sd | 133.3 | 20.2 | 132.9 | 17.8 | 133.1 | 19.2 |
| Median | 130.0 | 132.0 | 132.0 | |||
| Diastolic BP (mm Hg) | ||||||
| Mean sd | 76.1 | 11.8 | 78.3 | 10.9 | 77.0 | 11.5 |
| Median | 76.0 | 78.5 | 77.0 | |||
| Lipid medications | ||||||
| n (%) | 112 (57.4%) | 72 (52.2%) | 184 (55.2%) | |||
| Smoking | ||||||
| Never (%) | 100 (50%) | 46 (32.4%) | 146 (42.7%) | |||
| Former (%) | 58 (29%) | 60 (42.3%) | 118 (34.5%) | |||
| Current (%) | 42 (21%) | 36 (25.3%) | 22 (22.8%) | |||
BP, Blood pressure.
Table 2.
Laboratory characteristics, by gender
| Variable | Male
|
Female
|
Combined
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | sd | Median | Mean | sd | Median | Mean | sd | Median | |
| Coronary CP mass score | 907.0 | 1717.0 | 136.25a | 461.0 | 1295.0 | 20.3 | 642.3 | 1495.0 | 40.8 |
| Coronary CP score greater than 0% | 109 (79.6%) | 147 (77.4%) | 256 (78.3) | ||||||
| Carotid CP mass score | 268.4 | 786.9 | 12b | 137.8 | 398.5 | 0.0 | 192.3 | 594.7 | 3.0 |
| Carotid CP score greater than 0% | 84 (61%) | 93 (49.0%) | 177 (54.1%) | ||||||
| Aorta CP mass score | 7561.6 | 13720.7 | 1343.0 | 4881.6 | 8421.7 | 724.0 | 6007.9 | 11025.5 | 1084.5 |
| Aorta CP score greater than 0% | 111 (81.0%) | 152 (80.4%) | 263 (80.7%) | ||||||
| GFR (ml/sec) | 1.6 | 0.5 | 1.5 | 1.6 | 0.5 | 1.2 | 1.6 | 0.5 | 1.2 |
| Urine albumin: creatinine (mg/mmol) | 20.3 | 64.5 | 2.9 | 20.4 | 75.4 | 1.6 | 20.4 | 70.9 | 2.0 |
| Serum creatinine (μ mol/liter) | 83.9 | 22.9 | 76.3a | 68.6 | 15.3 | 61.0 | 68.6 | 22.9 | 68.6 |
| C-reactive protein (mg/liter) | 9.1 | 13.4 | 3.8a | 15.3 | 26.4 | 6.9 | 12.7 | 22.1 | 5.7 |
| Fasting blood sugar (mmol/liter) | 9.0 | 3.9 | 8.0 | 8.2 | 3.4 | 7.3 | 8.5 | 3.6 | 7.5 |
| HDL cholesterol (mmol/liter) | 1.1 | 0.3 | 1.1a | 1.3 | 0.3 | 1.2 | 1.2 | 0.3 | 1.2 |
| LDL cholesterol (mmol/liter) | 2.7 | 1.0 | 2.6 | 2.9 | 1.0 | 2.6 | 2.8 | 1.0 | 2.6 |
| Triglycerides (mmol/liter) | 1.6 | 2.3 | 1.2 | 1.4 | 1.1 | 1.1 | 1.5 | 1.7 | 1.1 |
| HbA1c, proportion of total | 0.1 | 0.0 | 0.1 | 0.1 | 0.0 | 0.1 | 0.1 | 0.0 | 0.1 |
| Visceral adipose (cm3 per 15 mm) | 175.6 | 83.7 | 164.2 | 179.4 | 66.0 | 170.5 | 177.8 | 73.9 | 168.5 |
| Pericardial adipose (cm3 per 45 mm) | 21.0 | 860.4 | 87.8 | 86.6 | 34.5 | 82.9 | 59.1 | 557.6 | 83.3 |
| Subcutaneous adipose (cm3 per 15 mm) | 341.8 | 162.6 | 323.3 | 512.3 | 172.8 | 490.8 | 440.8 | 188.3 | 423.0 |
| Intermuscular adipose (cm3 per 15 mm) | 9.4 | 6.4 | 7.9 | 11.7 | 8.8 | 9.4 | 10.8 | 8.0 | 8.6 |
| Lumbar BMD (mg/cm3) | 174.4 | 44.6 | 174.6 | 184.4 | 50.0 | 183.8 | 180.1 | 47.9 | 177.6 |
| Thoracic BMD (mg/cm3) | 197.6 | 48.7 | 197.4 | 212.3 | 55.4 | 207.4 | 206.2 | 53.1 | 204.7 |
| 25-Hydroxyvitamin D (nmol/liter)c | 49.2 | 26.7 | 44.9 | 51.4 | 32.7 | 39.9 | 50.4 | 30.5 | 43.7 |
| 1,25 dihydroxyvitamin D (pmol/liter)c | 119.9 | 45.0 | 111.8 | 129.0 | 46.8 | 124.8 | 125.3 | 46.3 | 119.6 |
| Intact PTH (ng/liter)c | 55.7 | 34.8 | 49.0 | 63.6 | 29.2 | 53.5 | 60.3 | 32.8 | 52.0 |
| Serum calcium (mmol/liter) | 2.4 | 0.1 | 2.4 | 2.4 | 0.1 | 2.4 | 2.4 | 0.1 | 2.4 |
| Serum phosphorus (mmol/liter) | 1.1 | 0.2 | 1.1a | 1.2 | 0.2 | 1.2 | 1.1 | 0.2 | 1.2 |
HDL, High-density lipoprotein; LDL, low-density lipoprotein.
P < 0.01 for gender comparison;
P < 0.05 for gender comparison;
normal ranges for 25-hydroxyvitamin D greater than 75 nmol/liter; 1,25 dihydroxyvitamin D = 47–187 pmols/liter; intact PTH = 15–72 ng/liter.
Table 3 contains the results of analyses comparing relationships between vitamin D levels, intact PTH, and hsCRP vs. organ-specific adipose tissue volume. In the fully adjusted model (age, gender, body mass index, HbA1c, and GFR adjusted), a negative association was observed between 25-hydroxyvitamin D and VAT (P = 0.009); similar relationships were seen in men and women (P = 0.046 and 0.039, respectively, data not shown). In addition, nonsignificant trends toward a negative relationship between 25-hydroxyvitamin D with SAT (P = 0.153) and IMAT (P = 0.083) were observed. Serum hsCRP was positively associated with IMAT (P = 0.015).
Table 3.
Vitamin D, intact PTH, CRP, and organ-specific adipose tissue volume
| Tissue | Parameter estimate | se | P value |
|---|---|---|---|
| 25-Hydroxyvitamin D | |||
| Visceral | −0.031 | 0.012 | 0.009 |
| Pericardial | −0.002 | 0.002 | 0.195 |
| Subcutaneous | −0.894 | 0.624 | 0.153 |
| Intermuscular | −0.005 | 0.003 | 0.083 |
| 1,25 Dihydroxyvitamin D | |||
| Visceral | −0.007 | 0.008 | 0.425 |
| Pericardial | 0.0002 | 0.001 | 0.862 |
| Subcutaneous | 0.301 | 0.425 | 0.48 |
| Intermuscular | 0.0004 | 0.002 | 0.829 |
| Intact PTH | |||
| Visceral | 0.008 | 0.004 | 0.077 |
| Pericardial | 0.0004 | 0.001 | 0.523 |
| Subcutaneous | 0.238 | 0.227 | 0.297 |
| Intermuscular | 0.001 | 0.001 | 0.464 |
| C-reactive protein | |||
| Visceral | 0.053 | 0.066 | 0.431 |
| Pericardial | 0.016 | 0.011 | 0.135 |
| Subcutaneous | 4.089 | 3.442 | 0.236 |
| Intermuscular | 0.036 | 0.015 | 0.015 |
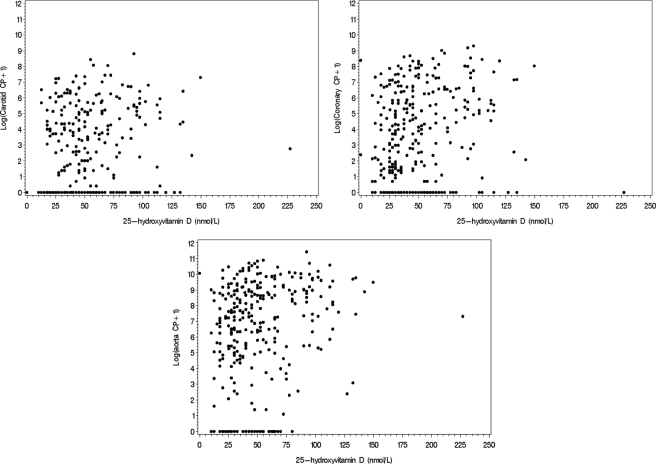
Table 4 contains results of association analyses between vitamin D, intact PTH, and CRP with vertebral bone density and aorta, coronary and carotid artery CP. 25-Hydroxyvitamin D was positively associated with both carotid artery CP and aorta CP (P = 0.013 and 0.014, respectively) but not bone density or coronary artery CP. These associations are presented graphically in Fig. 1. A negative association was detected between 1,25 dihydroxyvitamin D and lumbar bone density (P = 0.047), and a trend was observed for negative association between 1,25 dihydroxyvitamin D and thoracic bone density (P = 0.083).
Table 4.
Vitamin D, PTH, and CRP associations with bone density and calcified plaque
| Parameter estimate | se | P value | |
|---|---|---|---|
| 25-Hydroxyvitamin D | |||
| Lumbar BMD | −0.006 | 0.008 | 0.439 |
| Thoracic BMD | −0.213 | 0.233 | 0.362 |
| Coronary CP | 0.016 | 0.013 | 0.204 |
| Carotid CP | 0.028 | 0.011 | 0.013 |
| Aorta CP | 0.036 | 0.015 | 0.014 |
| 1,25 Dihydroxyvitamin D | |||
| Lumbar BMD | −0.010 | 0.005 | 0.047 |
| Thoracic BMD | −0.281 | 0.162 | 0.083 |
| Coronary CP | −0.013 | 0.009 | 0.144 |
| Carotid CP | −0.005 | 0.008 | 0.551 |
| Aorta CP | −0.002 | 0.010 | 0.819 |
| Intact PTH | |||
| Lumbar BMD | −0.003 | 0.003 | 0.290 |
| Thoracic BMD | −0.024 | 0.086 | 0.776 |
| Coronary CP | −0.0002 | 0.005 | 0.972 |
| Carotid CP | −0.005 | 0.004 | 0.263 |
| Aorta CP | −0.001 | 0.005 | 0.835 |
| C-reactive protein | |||
| Lumbar BMD | −0.002 | 0.460 | 0.970 |
| Thoracic BMD | −1.668 | 1.334 | 0.212 |
| Coronary CP | −0.0007 | 0.074 | 0.993 |
| Carotid CP | 0.05 | 0.068 | 0.461 |
| Aorta CP | 0.046 | 0.085 | 0.591 |
Figure 1.
Relationships between log (calcified atherosclerotic plaque + 1) and 25-hydroxyvitamin D.
Discussion
The present study evaluated African-Americans with type 2 diabetes for association between circulating vitamin D and quantitative measures of CP in three vascular beds, four organ-specific adipose tissue depots, and vertebral BMD in the thoracic and lumbar spine. We confirm the recently reported negative association between VAT and 25-hydroxyvitamin D in African-Americans (31,32) and observed similar trends for SAT and IMAT. An important novel positive association was detected between serum 25-hydroxyvitamin D concentrations and both carotid artery CP and infrarenal aorta CP in our sample of persons with diabetes. It is unknown whether positive associations exist between vitamin D and CP in African-Americans lacking diabetes; however, there is no a priori reason to expect differences in this relationship based on the presence of diabetes.
Whereas calcified atherosclerotic plaque documents the presence of subclinical atherosclerosis and coronary artery CP is predictive of future cardiovascular events in European-Americans, Asian-Americans, and African-Americans (40), our study suggests that the relationship between 25-hydroxyvitamin D and subclinical atherosclerosis in African-Americans is unique. Higher levels of 25-hydroxyvitamin D seem to be positively associated with aorta and carotid CP in African-Americans but not with coronary CP. These results contradict what is observed in individuals of European descent. Studies in Amish, European-American (Health Professionals Follow-Up Study), and Italian participants reveal that 25-hydroxyvitamin D concentrations are inversely associated with subclinical atherosclerosis as measured by CP or carotid intima-media thickness (41,42,43,44). In the MESA study [comprising European-Americans, Asian (Chinese)-Americans, African-Americans, and Hispanic-Americans] and in the Amish, lower 25-hydroxyvitamin D concentrations were not associated with prevalent coronary CP, but lower vitamin D levels were associated with increasing risk for incident coronary CP in MESA participants after adjusting for age, gender, and ethnicity (41,44). We observed a significant positive association between 25-hydroxyvitamin D and both aorta CP and carotid artery CP in the present analyses in African-Americans. Additionally, we detected no evidence of association between hsCRP and CP, VAT, PAT, SAT, or bone density in this African-American sample.
These results led us to reconsider the role of 25-hydroxyvitamin D replacement in African-Americans with vitamin D insufficiency. Current practice encourages vitamin D replacement in those with low levels because this is presumed to be protective from (and serve as a treatment for) osteopenia and osteoporosis. Vitamin D supplementation has also been postulated to favor cardiovascular health (45). In theory, vitamin D-induced prevention of osteopenia should also be associated with lower levels of CP. However, the inverse relationship between bone mineralization and CP appeared to be weaker in African-Americans than European-Americans, assuming preserved kidney function, although relatively small numbers of African-American subjects were evaluated (46). The effects of vitamin D supplementation on development and progression of CP remain unknown (47), and it would not be unexpected to see differential effects of supplementation based on ethnicity (48).
In a study including 208 postmenopausal African-American women with 25-hydroxyvitamin D levels less than 49.9 nmol/liter (<20 ng/ml), Aloia et al. (49) performed a randomized controlled trial comparing vitamin D plus calcium supplementation to calcium supplementation alone. In these calcium-replete women, no effects of vitamin D supplementation were seen on the rate of bone loss or bone turnover after 3 yr, nor were differences detected in urinary calcium excretion, PTH levels, or rates of nephrolithiasis. Supplemental vitamin D and calcium in Women’s Health Initiative participants (9.1% African-American) improved hip BMD and increased kidney stones, without significant effects on hip fracture or total or cardiovascular mortality (50). A follow-up Women’s Health Initiative report demonstrated nonsignificant reductions in total mortality among postmenopausal women receiving supplemental vitamin D (51). A metaanalysis evaluating 57,311 frail elderly postmenopausal women in 18 randomized controlled trials also detected significant reductions in total mortality with supplemental vitamin D, although the impact of ethnicity, baseline vitamin D level, and vitamin D dosage were not assessed (23). Ethnic differences in the relationship between vitamin D levels and atherosclerosis remain important to determine because epidemiological studies revealed consistent ethnic differences in CP (5,6,7,8,9) and rates of myocardial infarction in those with equal access to medical care (52,53,54). African-Americans were at lower risk than European-Americans, despite presence of more severe cardiovascular disease risk factors.
An important limitation of this and other studies with CT-derived measures of CP are their cross-sectional nature. In the future, it will be important to quantify vitamin D levels and their associations with adiposity and CT-derived CP in longitudinal studies containing large numbers of African-American participants.
In conclusion, VAT and 25-hydroxyvitamin D are significantly and negatively associated in African-Americans with diabetes. In contrast, significant positive associations are observed between 25-hydroxyvitamin D and both carotid artery CP and aorta CP. The direct relationship between vitamin D and quantity of CP in African-Americans may differ from that in European-derived populations, in which lower vitamin D levels appear to be associated with excess risks for atherosclerotic cardiovascular disease and osteoporosis. Until long-term safety studies are performed, the effect of supplementing vitamin D on atherosclerosis in African-Americans with vitamin D deficiency remains unknown. In addition, the normal range for serum concentrations of 25-hydroxyvitamin D may differ based on ethnicity and needs to be determined in the African-American population.
Acknowledgments
The investigators acknowledge the cooperation of our participants; study recruiters; Ms. Carrie Smith; Ms. Cassandra Bethea; and CT analysts, Ms. Susan Pillsbury, Blair Gordy, and Melanie Wilder.
Footnotes
This work was supported in part by Grant M01 RR07122 from the General Clinical Research Center of the Wake Forest University School of Medicine; National Institute of Diabetes and Digestive and Kidney Diseases Grant RO1 DK071891 (to B.I.F.); National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant RO1 AR048797 (to J.J.C.); and National Heart, Lung, and Blood Institute Grant R01 HL67348 (to D.W.B.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online January 8, 2010
Abbreviations: BMD, Bone mineral density; CP, atherosclerotic plaque; CT, computed tomography; GFR, glomerular filtration rate; HbA1c, hemoglobin A1c; hsCRP, highly sensitive C-reactive protein; IMAT, intermuscular adipose tissue; MESA, Multi-Ethnic Study of Atherosclerosis; PAT, pericardial adipose tissue; SAT, sc adipose tissue; VAT, visceral adipose tissue.
References
- Luckey MM, Wallenstein S, Lapinski R, Meier DE 1996 A prospective study of bone loss in African-American and white women—a clinical research center study. J Clin Endocrinol Metab 81:2948–2956 [DOI] [PubMed] [Google Scholar]
- Acheson LS 2005 Bone density and the risk of fractures: should treatment thresholds vary by race? JAMA 293:2151–2154 [DOI] [PubMed] [Google Scholar]
- Stamatelou KK, Francis ME, Jones CA, Nyberg LM, Curhan GC 2003 Time trends in reported prevalence of kidney stones in the United States: 1976–1994. Kidney Int 63:1817–1823 [DOI] [PubMed] [Google Scholar]
- Aloia JF, Patel M, Dimaano R, Li-Ng M, Talwar SA, Mikhail M, Pollack S, Yeh JK 2008 Vitamin D intake to attain a desired serum 25-hydroxyvitamin D concentration. Am J Clin Nutr 87:1952–1958 [DOI] [PubMed] [Google Scholar]
- Bild DE, Detrano R, Peterson D, Guerci A, Liu K, Shahar E, Ouyang P, Jackson S, Saad MF 2005 Ethnic differences in coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 111:1313–1320 [DOI] [PubMed] [Google Scholar]
- Newman AB, Naydeck BL, Whittle J, Sutton-Tyrrell K, Edmundowicz D, Kuller LH 2002 Racial differences in coronary artery calcification in older adults. Arterioscler Thromb Vasc Biol 22:424–430 [DOI] [PubMed] [Google Scholar]
- Freedman BI, Hsu FC, Langefeld CD, Rich SS, Herrington DM, Carr JJ, Xu J, Bowden DW, Wagenknecht LE 2005 The impact of ethnicity and sex on subclinical cardiovascular disease: the Diabetes Heart Study. Diabetologia 48:2511–2518 [DOI] [PubMed] [Google Scholar]
- Budoff MJ, Nasir K, Mao S, Tseng PH, Chau A, Liu ST, Flores F, Blumenthal RS 2006 Ethnic differences of the presence and severity of coronary atherosclerosis. Atherosclerosis 187:343–350 [DOI] [PubMed] [Google Scholar]
- Carnethon MR, Bertoni AG, Shea S, Greenland P, Ni H, Jacobs Jr DR, Saad M, Liu K 2005 Racial/ethnic differences in subclinical atherosclerosis among adults with diabetes: the Multiethnic Study of Atherosclerosis. Diabetes Care 28:2768–2770 [DOI] [PubMed] [Google Scholar]
- Bikle DD 1992 Clinical counterpoint: vitamin D: new actions, new analogs, new therapeutic potential. Endocr Rev 13:765–784 [DOI] [PubMed] [Google Scholar]
- Hyppönen E, Power C 2006 Vitamin D status and glucose homeostasis in the 1958 British birth cohort: the role of obesity. Diabetes Care 29:2244–2246 [DOI] [PubMed] [Google Scholar]
- Pittas AG, Lau J, Hu FB, Dawson-Hughes B 2007 The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab 92:2017–2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarcin O, Yavuz DG, Ozben B, Telli A, Ogunc OA, Yuksel M, Toprak A, Yazici D, Sancak S, Deyneli O, Akalin S 2009 Effect of vitamin D deficiency and replacement on endothelial function in asymptomatic subjects. J Clin Endocrinol Metab 94:4023–4030 [DOI] [PubMed] [Google Scholar]
- Forman JP, Bischoff-Ferrari HA, Willett WC, Stampfer MJ, Curhan GC 2005 Vitamin D intake and risk of incident hypertension: results from three large prospective cohort studies. Hypertension 46:676–682 [DOI] [PubMed] [Google Scholar]
- Scragg R, Sowers M, Bell C 2007 Serum 25-hydroxyvitamin D, ethnicity, and blood pressure in the Third National Health and Nutrition Examination Survey. Am J Hypertens 20:713–719 [DOI] [PubMed] [Google Scholar]
- Schmitz KJ, Skinner HG, Bautista LE, Fingerlin TE, Langefeld CD, Hicks PJ, Haffner SM, Bryer-Ash M, Wagenknecht LE, Bowden DW, Norris JM, Engelman CD 2009 Association of 25-hydroxyvitamin D with blood pressure in predominantly 25-hydroxyvitamin D deficient Hispanic and African Americans. Am J Hypertens 22:867–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins D, Wolf M, Pan D, Zadshir A, Tareen N, Thadhani R, Felsenfeld A, Levine B, Mehrotra R, Norris K 2007 Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med 167:1159–1165 [DOI] [PubMed] [Google Scholar]
- Michos ED, Melamed ML 2008 Vitamin D and cardiovascular disease risk. Curr Opin Clin Nutr Metab Care 11:7–12 [DOI] [PubMed] [Google Scholar]
- Harris SS, Soteriades E, Coolidge JA, Mudgal S, Dawson-Hughes B 2000 Vitamin D insufficiency and hyperparathyroidism in a low income, multiracial, elderly population. J Clin Endocrinol Metab 85:4125–4130 [DOI] [PubMed] [Google Scholar]
- Looker AC, Dawson-Hughes B, Calvo MS, Gunter EW, Sahyoun NR 2002 Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone 30:771–777 [DOI] [PubMed] [Google Scholar]
- Harris SS, Dawson-Hughes B 1998 Seasonal changes in plasma 25-hydroxyvitamin D concentrations of young American black and white women. Am J Clin Nutr 67:1232–1236 [DOI] [PubMed] [Google Scholar]
- Holick MF 2006 Vitamin D. In: Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ, eds. Modern nutrition in health and disease. Philadelphia: Lippincott Williams, Wilkins 329–345 [Google Scholar]
- Autier P, Gandini S 2007 Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med 167:1730–1737 [DOI] [PubMed] [Google Scholar]
- Anderson JB, Barnett E, Nordin BE 1964 The relation between osteoporosis and aortic calcification. Br J Radiol 37:910–912 [DOI] [PubMed] [Google Scholar]
- Silverman SL, Delmas PD, Kulkarni PM, Stock JL, Wong M, Plouffe Jr L 2004 Comparison of fracture, cardiovascular event, and breast cancer rates at 3 years in postmenopausal women with osteoporosis. J Am Geriatr Soc 52:1543–1548 [DOI] [PubMed] [Google Scholar]
- Tankó LB, Christiansen C, Cox DA, Geiger MJ, McNabb MA, Cummings SR 2005 Relationship between osteoporosis and cardiovascular disease in postmenopausal women. J Bone Miner Res 20:1912–1920 [DOI] [PubMed] [Google Scholar]
- Browner WS, Pressman AR, Nevitt MC, Cauley JA, Cummings SR 1993 Association between low bone density and stroke in elderly women. The study of osteoporotic fractures. Stroke 24:940–946 [DOI] [PubMed] [Google Scholar]
- Kiel DP, Kauppila LI, Cupples LA, Hannan MT, O'Donnell CJ, Wilson PW 2001 Bone loss and the progression of abdominal aortic calcification over a 25 year period: the Framingham Heart Study. Calcif Tissue Int 68:271–276 [DOI] [PubMed] [Google Scholar]
- Schulz E, Arfai K, Liu X, Sayre J, Gilsanz V 2004 Aortic calcification and the risk of osteoporosis and fractures. J Clin Endocrinol Metab 89:4246–4253 [DOI] [PubMed] [Google Scholar]
- Vilarrasa N, Maravall J, Estepa A, Sánchez R, Masdevall C, Navarro MA, Alia P, Soler J, Gómez JM 2007 Low 25-hydroxyvitamin D concentrations in obese women: their clinical significance and relationship with anthropometric and body composition variables. J Endocrinol Invest 30:653–658 [DOI] [PubMed] [Google Scholar]
- Young KA, Engelman CD, Langefeld CD, Hairston KG, Haffner SM, Bryer-Ash M, Norris JM 2009 Association of plasma vitamin D levels with adiposity in Hispanic and African Americans. J Clin Endocrinol Metab 94:3306–3313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valina-Toth AL, Lai Z, Yoo W, Bou-Samra A, Gadegbeku CA, Flack JM 2009 Relationship of vitamin D and parathyroid hormone to obesity and body composition in African Americans. Clin Endocrinol (Oxf) doi:10.1111/j.1365-2265.2009.03676.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D 1999 A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130:461–470 [DOI] [PubMed] [Google Scholar]
- Detrano RC, Anderson M, Nelson J, Wong ND, Carr JJ, McNitt-Gray M, Bild DE 2005 Coronary calcium measurements: effect of CT scanner type and calcium measure on rescan reproducibility—MESA study. Radiology 236:477–484 [DOI] [PubMed] [Google Scholar]
- Wheeler GL, Shi R, Beck SR, Langefeld CD, Lenchik L, Wagenknecht LE, Freedman BI, Rich SS, Bowden DW, Chen MY, Carr JJ 2005 Pericardial and visceral adipose tissues measured volumetrically with computed tomography are highly associated in type 2 diabetic families. Invest Radiol 40:97–101 [DOI] [PubMed] [Google Scholar]
- Ding J, Kritchevsky SB, Harris TB, Burke GL, Detrano RC, Szklo M, Jeffrey CJ, Multi-Ethnic Study of Atherosclerosis 2008 The association of pericardial fat with calcified coronary plaque. Obesity (Silver Spring) 16:1914–1919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman BI, Bowden DW, Ziegler JT, Langefeld CD, Lehtinen AB, Rudock ME, Lenchik L, Hruska KA, Register TC, Carr JJ 2009 Bone morphogenetic protein 7 (BMP7) gene polymorphisms are associated with inverse relationships between vascular calcification and BMD: the Diabetes Heart Study. J Bone Miner Res 24:1719–1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeger SL, Liang KY 1986 Longitudinal data analysis for discrete and continuous outcomes. Biometrics 42:121–130 [PubMed] [Google Scholar]
- Box GEP, Cox DR 1964 An analysis of transformations. J R Stat Soc Series B Stat Methodol 26:211–246 [Google Scholar]
- Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O'Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA 2008 Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med 358:1336–1345 [DOI] [PubMed] [Google Scholar]
- Michos ED, Streeten EA, Ryan KA, Rampersaud E, Peyser PA, Bielak LF, Shuldiner AR, Mitchell BD, Post W 2009 Serum 25-hydroxyvitamin D levels are not associated with subclinical vascular disease or C-reactive protein in the old order Amish. Calcif Tissue Int 84:195–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannucci E, Liu Y, Hollis BW, Rimm EB 2008 25-Hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med 168:1174–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Targher G, Bertolini L, Padovani R, Zenari L, Scala L, Cigolini M, Arcaro G 2006 Serum 25-hydroxyvitamin D3 concentrations and carotid artery intima-media thickness among type 2 diabetic patients. Clin Endocrinol (Oxf) 65:593–597 [DOI] [PubMed] [Google Scholar]
- de Boer IH, Kestenbaum B, Shoben AB, Michos ED, Sarnak MJ, Siscovick DS 2009 25-Hydroxyvitamin D levels inversely associate with risk for developing coronary artery calcification. J Am Soc Nephrol 20:1805–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, O'Keefe JH, Bell D, Hensrud DD, Holick MF 2008 Vitamin D deficiency an important, common, and easily treatable cardiovascular risk factor? J Am Coll Cardiol 52:1949–1956 [DOI] [PubMed] [Google Scholar]
- Carr JJ, Register TC, Hsu FC, Lohman K, Lenchik L, Bowden DW, Langefeld CD, Xu J, Rich SS, Wagenknecht LE, Freedman BI 2008 Calcified atherosclerotic plaque and bone mineral density in type 2 diabetes: the diabetes heart study. Bone 42:43–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zittermann A, Schleithoff SS, Koerfer R 2007 Vitamin D and vascular calcification. Curr Opin Lipidol 18:41–46 [DOI] [PubMed] [Google Scholar]
- Papadimitropoulos E, Wells G, Shea B, Gillespie W, Weaver B, Zytaruk N, Cranney A, Adachi J, Tugwell P, Josse R, Greenwood C, Guyatt G 2002 Meta-analyses of therapies for postmenopausal osteoporosis. VIII: meta-analysis of the efficacy of vitamin D treatment in preventing osteoporosis in postmenopausal women. Endocr Rev 23:560–569 [DOI] [PubMed] [Google Scholar]
- Aloia JF, Talwar SA, Pollack S, Yeh J 2005 A randomized controlled trial of vitamin D3 supplementation in African American women. Arch Intern Med 165:1618–1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson RD, LaCroix AZ, Gass M, Wallace RB, Robbins J, Lewis CE, Bassford T, Beresford SA, Black HR, Blanchette P, Bonds DE, Brunner RL, Brzyski RG, Caan B, Cauley JA, Chlebowski RT, Cummings SR, Granek I, Hays J, Heiss G, Hendrix SL, Howard BV, Hsia J, Hubbell FA, Johnson KC, Judd H, Kotchen JM, Kuller LH, Langer RD, Lasser NL, Limacher MC, Ludlam S, Manson JE, Margolis KL, McGowan J, Ockene JK, O'Sullivan MJ, Phillips L, Prentice RL, Sarto GE, Stefanick ML, Van Horn L, Wactawski-Wende J, Whitlock E, Anderson GL, Assaf AR, Barad D 2006 Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med 354:669–683 [DOI] [PubMed] [Google Scholar]
- LaCroix AZ, Kotchen J, Anderson G, Brzyski R, Cauley JA, Cummings SR, Gass M, Johnson KC, Ko M, Larson J, Manson JE, Stefanick ML, Wactawski-Wende J 2009 Calcium plus vitamin D supplementation and mortality in postmenopausal women: the Women’s Health Initiative calcium-vitamin D randomized controlled trial. J Gerontol A Biol Sci Med Sci 64:559–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young BA, Maynard C, Boyko EJ 2003 Racial differences in diabetic nephropathy, cardiovascular disease, and mortality in a national population of veterans. Diabetes Care 26:2392–2399 [DOI] [PubMed] [Google Scholar]
- Karter AJ, Ferrara A, Liu JY, Moffet HH, Ackerson LM, Selby JV 2002 Ethnic disparities in diabetic complications in an insured population. JAMA 287:2519–2527 [DOI] [PubMed] [Google Scholar]
- Young BA, Rudser K, Kestenbaum B, Seliger SL, Andress D, Boyko EJ 2006 Racial and ethnic differences in incident myocardial infarction in end-stage renal disease patients: the USRDS. Kidney Int 69:1691–1698 [DOI] [PubMed] [Google Scholar]