Abstract
Context: In children, bone mineral content (BMC) and bone mineral density (BMD) measurements by dual-energy x-ray absorptiometry (DXA) are affected by height status. No consensus exists on how to adjust BMC or BMD (BMC/BMD) measurements for short or tall stature.
Objective: The aim of this study was to compare various methods to adjust BMC/BMD for height in healthy children.
Design: Data from the Bone Mineral Density in Childhood Study (BMDCS) were used to develop adjustment methods that were validated using an independent cross-sectional sample of healthy children from the Reference Data Project (RDP).
Setting: We conducted the study in five clinical centers in the United States.
Participants: We included 1546 BMDCS and 650 RDP participants (7 to 17 yr of age, 50% female).
Intervention: No interventions were used.
Main Outcome Measures: We measured spine and whole body (WB) BMC and BMD Z-scores for age (BMC/BMDage), height age (BMC/BMDheight age), height (BMCheight), bone mineral apparent density (BMADage), and height-for-age Z-score (HAZ) (BMC/BMDhaz).
Results: Spine and WB BMC/BMDageZ and BMADageZ were positively (P < 0.005; r = 0.11 to 0.64) associated with HAZ. Spine BMDhaz and BMChazZ were not associated with HAZ; WB BMChazZ was modestly associated with HAZ (r = 0.14; P = 0.0003). All other adjustment methods were negatively associated with HAZ (P < 0.005; r = −0.20 to −0.34). The deviation between adjusted and BMC/BMDage Z-scores was associated with age for most measures (P < 0.005) except for BMC/BMDhaz.
Conclusions: Most methods to adjust BMC/BMD Z-scores for height were biased by age and/or HAZ. Adjustments using HAZ were least biased relative to HAZ and age and can be used to evaluate the effect of short or tall stature on BMC/BMD Z-scores.
DXA bone Z-scores adjusted for height-for-age Z-score (HAZ) yielded the least-biased approach for estimating the effect of short (or tall) stature on measures of bone mineral density.
During childhood and adolescence, body size and maturation are major determinants of bone mineral content (BMC) and bone mineral density (BMD) as measured by dual-energy x-ray absorptiometry (DXA). As the skeleton grows and expands, BMC (measured in grams) increases exponentially. Although DXA measures of areal BMD (measured in grams per square centimeter) incorporate bone size and are less influenced by body size than BMC, the bone size adjustment is incomplete. The two-dimensional image does not incorporate the depth of bone; therefore, smaller bones of comparable volumetric BMD (measured in grams per cubic centimeter) appear to have lower areal BMD (1). Accordingly, controversy exists regarding the use of areal BMD in children, and BMC has been proposed as the preferred measure (2,3,4).
Like height and weight, the age-related increases in BMC and BMD are nonlinear, and variability also increases with age. Consequently, pediatric BMD and BMC results are expressed as Z-scores (sd scores) (5), so that an individual child’s test results can be appropriately compared with those of his/her same-age peers. However, many children at-risk for inadequate bone accrual, such as those with disorders involving inflammation, malabsorption, or immobilization, also have faltering linear growth and delayed sexual maturation. Consequently, a low BMD or BMC Z-score in the context of short stature or delayed maturation is difficult to interpret, raising the question of the degree to which the low bone status can be attributed to smaller bone size relative to age.
Recently, the International Society of Clinical Densitometry (ISCD) convened a panel to address the clinical use of DXA in children, recommending that “in children with linear growth or maturational delay, spine and total body (less head) BMC and areal BMD results should be adjusted for absolute height or height age, or compared with pediatric reference data that provide age-, sex-, and height-specific Z-scores” (5). Presently, there are no reference data for determining height-specific Z-scores for BMC or BMD, nor are there established guidelines on how to adjust BMC/BMD results for absolute height. A commonly used technique in clinical practice is to substitute bone age or “height age” (the age at which a child’s height is the median height-for-age on the growth chart) for chronological age as a means of adjusting for short stature. Of particular concern with the use of the height age approach is that children who are short-for-age will be compared with children of similar height who are younger and at an earlier stage of sexual maturation. A similar problem may occur using height-specific Z-scores because they do not take age into account. Bone mineral apparent density (BMAD) has also been used as a size-adjusted measure of DXA BMD (6). An alternative approach that would simultaneously consider both height and age involves adjusting for height-for-age Z-score (HAZ). The appropriateness of these approaches has not been assessed.
Establishing criteria for evaluating the validity of adjustment approaches is a significant challenge. In the absence of a gold standard for determining bone mineral “status” in children, the choice of an adjustment technique can be based on carefully conceived relative criteria. We propose that ideal size-adjustment techniques will result in similar Z-score distributions among healthy children who are of short, tall, and average stature. The ideal technique will not exhibit bias with respect to height (i.e. will have a zero correlation with HAZ) and age (i.e. will have the same effect at all ages). Comparisons in healthy children are important to identify potential biases in various techniques to account for short stature in children with chronic diseases, who may have additional factors affecting their bone accretion.
The goals of this study were: 1) to quantify the magnitude of the effect of height status on DXA BMC/BMD Z-scores; and 2) to compare adjustments to BMC/BMD Z-scores based upon height age, height-specific reference data, BMAD, and HAZ in otherwise healthy children. Specific attention was given to short-for-age or tall-for-age subgroups to determine whether adjustment techniques resulted in bone Z-score distributions similar to those of children of average stature.
Subjects and Methods
Study populations
The first data source was the Bone Mineral Density in Childhood Study (BMDCS), described previously (7). Briefly, healthy children from all major racial and ethnic groups were recruited from July 2002 to November 2003 from five regional clinical centers in the United States: Children’s Hospital of Los Angeles (Los Angeles, CA), Cincinnati Children’s Hospital Medical Center (Cincinnati, OH), Creighton University (Omaha, NE), Children’s Hospital of Philadelphia (CHOP) (Philadelphia, PA), and Columbia University (New York, NY). The inclusion and exclusion criteria were used to select a sample of children in good health with normal physical development and free of previous or current medical conditions that might affect bone acquisition. Subjects were evaluated annually; the data presented here are based on the first three visits.
The second data source was a cross-sectional sample of healthy children recruited as part of the Reference Data Project on Skeletal Development (RDP) at CHOP, using entry criteria similar to BMDCS. DXA scans were obtained on the same scanner model, but a different device from the one used in the BMDCS. Thus, these data represent an independent validation sample to compare various height adjustment methods for spine and whole body (WB) DXA measurements in healthy children and explore potential biases.
For both studies, written informed consent was obtained from the participant’s parent or guardian, and assent was obtained from the participants. Participants 18 yr of age or older provided written consent. The protocol was approved by the Institutional Review Boards of each Clinical Center.
Bone densitometry
For the BMDCS, DXA scans of the WB, posteroanterior lumbar spine (L1–L4, fast array mode), nondominant forearm, and left proximal femur (fast array) were obtained using bone densitometers (QDR4500A, QDR4500W, and Delphi A models; Hologic, Inc., Bedford, MA) as described previously (7). All scans were analyzed centrally by the DXA Core Laboratory (University of California, San Francisco, CA) using Hologic software release 12.3.
For the RDP Study, posteroanterior lumbar spine (array mode) and WB scans were obtained on a Delphi A model, following the same positioning and analysis techniques. Scans were analyzed using Hologic software release 12.4 and reviewed by a single investigator (B.S.Z.) for quality assurance. CHOP maintains two identical DXA models at different locations, one used for the RDP study and the other used for the BMDCS. Cross-calibration of these DXA devices using WB phantoms and human volunteers revealed differences in WB BMC and BMD measurements. A multiplicative correction factor was applied to the WB results for the RDP study to rectify the calibration difference.
Growth, maturation, and demographic measures
Height and weight were measured with participants dressed in lightweight clothing, without shoes. For the BMDCS, pubertal stage was determined by physical examination performed by a physician or nurse practitioner with expertise in pediatric endocrinology and categorized using the criteria of Tanner (8). For the RDP study, Tanner stage was assessed by a validated self-evaluation questionnaire (9,10).
Ethnicity (Hispanic/Latino vs. Non-Hispanic/Latino) and race information was elicited by questionnaire using National Institutes of Health classifications. For these analyses, race was categorized as Black vs. all other races.
Statistical analysis
Calculation of Z-scores
Sex-specific HAZ, weight-for-age, and body mass index-for-age Z-scores were calculated using the Centers for Disease Control and Prevention (CDC) 2000 growth charts (11).
Sex- and race-specific BMC and BMD-for-age Z-scores were calculated using the BMDCS reference data (7) (BMCageZ and BMDageZ). In addition, Z-scores were calculated substituting height age for chronological age, thereby generating BMCheight ageZ and BMDheight ageZ. Height age was determined as the age at which the child’s height was the median value for height from the CDC growth chart. Children whose height was greater than the median value for height in young adults (age 20) were not assigned a height age.
Creation of additional BMDC reference curves
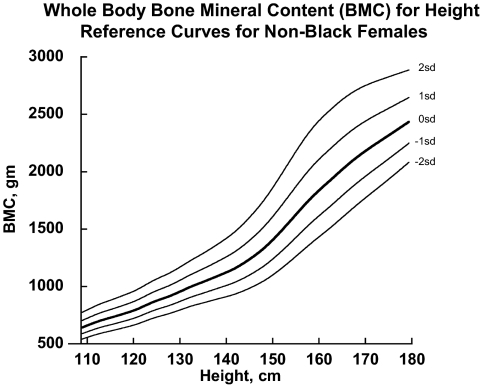
Additional sex- and race-specific reference curves for spine- and WB BMC-for-height (BMCheightZ) and BMAD-for-age (BMADage) (6) were generated using the same source data and techniques used to create the BMDCS reference data (7). BMAD was calculated as BMC/bone area1.5 for the spine and BMC/height for the WB (6). The curves were created with the LMS method (12) using the LMS Chartmaker software as illustrated in Fig. 1 (13). The LMS values resulting from the creation of these curves were used to calculate Z-scores for BMCheight and BMADage.
Figure 1.
Example of BMC for height reference curves for generating BMCheight Z-scores. Reference lines from −2 sd to +2 sd from the median are shown.
HAZ predicted bone Z-score model development and adjustment for HAZ
The relationships between HAZ and bone Z-scores (BMCageZ, BMDageZ) for spine, WB, hip, and forearm were determined by random effects generalized least squares regression. The source data were the same as those used to create the published BMDCS reference curves described above, accounting for the multiple observations per subject. Sex and race differences in the relationship between HAZ and bone Z-scores were complex, so separate analyses were performed for each sex and for Black vs. non-Black samples. Interaction and higher-order polynomial terms were evaluated to determine whether they improved the model fit. Residual plots were inspected to confirm the absence of age trends in the fit of the models.
The resulting models provided prediction equations to calculate HAZ-predicted bone Z-scores. The HAZ-adjusted BMC or BMD Z-scores (BMChazZ and BMDhazZ) were calculated as:
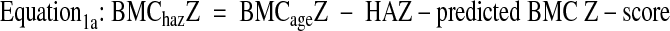 |
Equation1b:BMDhazZ = BMDageZ− HAZ-predicted BMD Z-score
Using this approach, if a subject’s bone Z-score is appropriate for his/her height status, then the adjusted Z-score is zero. If the bone Z-score is greater than expected given the height status, the HAZ-adjusted Z-score is positive, and if the bone Z-score is less than expected given the height status, then the HAZ-adjusted Z-score is negative.a
Statistical analyses
The published BMC and BMD reference data are limited to ages 6 to 16 yr for girls and 6 to 17 yr for boys, so results were restricted to these age ranges. Descriptive statistics were calculated for the outcome measures for both study samples and/or for the subgroups described below.
t-Tests were used to determine whether the mean adjusted Z-scores in the RDP sample were significantly different from zero. Subjects were divided into short (HAZ < −1), average (−1 ≤ HAZ ≤ 1), and tall (HAZ > 1) groups. One-way ANOVA was used to test for significant differences in bone Z-scores across HAZ groups. The absence of statistically significant differences between groups was used as one criterion for evaluating adjustment methods because an appropriate adjustment technique would result in similar Z-score distributions for short, average, and tall children. Pearson correlations were examined to determine whether Z-score adjustment methods were significantly associated with HAZ (i.e. not independent of height status).
Bias in adjustment methods was further explored by testing for age trends, i.e. an unbiased adjustment technique would result in the same pattern of adjustment across all ages. All height adjustment methods were compared with BMCage and BMDage Z-scores, respectively, by calculating the difference between the Z-scores. For example, the difference between spine BMCheight ageZ and spine BMCageZ was computed for further analysis. Graphical inspection, correlation, and regression analyses were used to test for age trends in these Z-score differences.
All statistical analyses were performed in STATA 10.0 (StataCorp, College Station, TX), and a P value of 0.05 was considered statistically significant.
Results
Description of the study samples
BMDCS
Of the original 1554 subjects recruited at baseline, 93% (n = 1438) returned for the third study visit. However, because the published reference data for calculating BMC and BMD-for-age Z-scores does not encompass the entire age range, the data presented here are restricted to the 3945 observations on 1546 subjects (7 to 16 yr of age for girls and 7 to 17 yr of age for boys) for whom bone Z-scores could be calculated. This subsample was 50% female and 24% Black. Thirty-five percent of the sample were prepubertal (Tanner stage 1) at the time of the study visit, 38% were peripubertal (Tanner stages 2 through 4), and 27% had completed puberty (Tanner stage 5).
RDP study
A total of 850 subjects were recruited. Of these, 650 were in the age range for which the BMDCS bone Z-scores could be calculated. The subsample was 45% Black and 50% female; 33% were prepubertal (Tanner stage 1), 56% were peripubertal (Tanner stages 2 through 4), and 10% had completed puberty (Tanner stage 5).
Descriptive statistics for each sample are presented in Table 1. The RDP sample was significantly (P < 0.05) younger, taller, and heavier than the BMDCS, but the magnitude of the differences was small.
Table 1.
Descriptive statistics
| Variable | BMDCS cohort (n = 1546)
|
RDP sample (n = 650)
|
||||||
|---|---|---|---|---|---|---|---|---|
| Mean | sd | Minimum | Maximum | Mean | sd | Minimum | Maximum | |
| Age (yr) | 11.8 | 2.7 | 7.0 | 17.0 | 11.4 | 2.7 | 7.0 | 17.0 |
| Height age (yr)a | 11.5 | 2.7 | 5.6 | 19.4 | 11.4 | 2.8 | 5.6 | 19.4 |
| Height Z-score | 0.2 | 0.8 | −2.6 | 2.7 | 0.3 | 0.9 | −2.3 | 3.2 |
| Weight Z-score | 0.3 | 0.8 | −2.2 | 2.4 | 0.4 | 1.0 | −2.3 | 3.5 |
| BMI Z-score | 0.3 | 0.9 | −2.7 | 2.3 | 0.4 | 1.0 | −3.3 | 3.0 |
n = 1420 for the Bone Mineral Density in Childhood Study (BMDCS) cohort and n = 593 for the Reference Data Project (RDP) sample.
Comparison of height adjustment methods
HAZ adjustment of bone Z-scores
Prediction equations for the calculation of HAZ adjusted bone Z-scores using the BMDCS data (Table 2) demonstrated that the relationship between HAZ and bone Z-scores was complex. For many measurement sites, the interaction term for age and HAZ was significant, signaling that the effect of HAZ on bone Z-score varied as a function of age. In some cases, a higher-order polynomial term (age2 and the interaction of age2 and HAZ) improved the fit of the model. In general, the explained variance (overall R2) for these models was greater for BMC than for BMD measures, greater for females than males, and greater for non-Black than Black children. The effect of HAZ on hip and forearm outcomes was small, with R2 values ranging from 0.02 to 0.13.
Table 2.
Prediction equations for BMC and BMD Z-scores based on HAZ
| Prediction equation | R2 | |
|---|---|---|
| Spine BMC for age | ||
| Males | ||
| Non-Black | −0.013∗Age + 0.833∗HAZ + 0.086 | 0.35 |
| Black | 0.035∗Age + 0.213∗HAZ + 0.037∗Age∗HAZ − 0.636 | 0.30 |
| Females | ||
| Non-Black | −0.024∗Age + 0.562∗HAZ + 0.019∗Age∗HAZ + 0.204 | 0.37 |
| Black | 0.419∗HAZ − 0.078 | 0.25 |
| Spine BMD for age | ||
| Males | ||
| Non-Black | −0.016∗Age + 0.228∗HAZ + 0.024∗Age∗HAZ + 0.148 | 0.10 |
| Black | 0.025∗Age + 0.430∗HAZ − 0.438 | 0.11 |
| Females | ||
| Non-Black | −0.032∗Age + 0.325∗HAZ + 0.017∗AGE∗HAZ + 0.322 | 0.16 |
| Black | 0.337∗HAZ − 0.067 | 0.05 |
| WB BMC for age | ||
| Males | ||
| Non-Black | −0.021∗Age + 0.631∗HAZ + 0.013∗Age∗HAZ + 0.183 | 0.40 |
| Black | −0.187∗Age + 1.497∗HAZ + 0.010∗Age2 − 0.204∗Age∗HAZ + 0.010∗Age2∗HAZ + 0.646 | 0.36 |
| Females | ||
| Non-Black | −0.027∗Age + 0.572∗HAZ + 0.015∗Age∗HAZ + 0.244 | 0.44 |
| Black | −0.159∗Age + 1.146∗HAZ + 0.008∗Age2 − 0.149∗Age∗HAZ + 0.008∗Age2∗HAZ + 0.567 | 0.35 |
| WB BMD for age | ||
| Males | ||
| Non-Black | −0.031∗Age + 0.411∗ HAZ + 0.343 | 0.11 |
| Black | −0.028∗Age + 1.193∗HAZ + 0.002∗Age2 − 0.216∗Age∗HAZ + 0.011∗Age2∗HAZ − 0.067 | 0.08 |
| Females | ||
| Non-Black | −0.052∗Age + 0.533∗HAZ + 0.548 | 0.17 |
| Black | 0.225∗HAZ − 0.052 | 0.14 |
| Hip neck BMD for age | ||
| Males | ||
| Non-Black | −0.018∗Age + 0.361∗HAZ + 0.189 | 0.06 |
| Black | 0.034∗Age + 0.279∗HAZ − 0.506 | 0.05 |
| Females | ||
| Non-Black | −0.021∗Age + 0.440∗HAZ + 0.202 | 0.12 |
| Black | Not significant | Ns |
| Total hip BMD for age | ||
| Males | ||
| Non-Black | −0.028∗Age + 0.009∗HAZ + 0.033∗Age∗HAZ + 0.292 | 0.04 |
| Black | −0.092∗Age + 1.446∗HAZ + 0.005∗Age2 − 0.239∗Age∗HAZ + 0.011∗Age2∗HAZ + 0.277 | 0.04 |
| Females | ||
| Non-Black | −0.264∗Age +−0.454∗HAZ + ∗0.010∗Age2 + 0.150∗Age∗HAZ − 0.005∗Age2∗HAZ + 1.586 | 0.11 |
| Black | 0.022∗Age − 0.290∗HAZ + 0.043∗Age∗HAZ − 0.285 | 0.02 |
| Forearm BMD for age | ||
| Males | ||
| Non-Black | 0.370∗HAZ − 0.028 | 0.06 |
| Black | 0.045∗Age + 0.291∗HAZ − 0.636 | 0.07 |
| Females | ||
| Non-Black | 0.462∗HAZ − 0.042 | 0.13 |
| Black | 0.031∗Age + 0.308∗HAZ − 0.422 | 0.11 |
Age in years is used in all calculations.
Comparison of bone Z-scores
Bone Z-scores using chronological age, height age, HAZ adjustment equations, BMADage, and BMCheight were calculated for the RDP sample. The means (± sd) for spine BMCageZ and BMDageZ (Table 3) were not significantly different from 0.0 ± 1.0, indicating similar Z-score distributions for the RDP and BMDCS samples. WB BMCageZ was significantly greater than zero for the RDP sample. Of the various height adjustment methods, mean spine BMADage and WB BMCheight ageZ were not significantly different from zero. All others had mean values that differed significantly from the expected values (Z-score mean of zero).
Table 3.
Unadjusted and adjusted BMC and BMD Z-scores for the RDP cohort
| All
|
Corr (R) with HAZa | ||
|---|---|---|---|
| Mean | sd | ||
| Spine BMC | |||
| BMCage | −0.05 | 1.08 | 0.60 |
| BMCheight age | −0.31 | 0.91 | −0.26 |
| BMCheight | −0.19 | 0.87 | −0.20 |
| BMChaz | −0.10 | 0.87 | NS |
| Spine BMD | |||
| BMDage | −0.05 | 1.04 | 0.36 |
| BMDheight age | −0.22 | 1.04 | −0.28 |
| BMADage | −0.02 | 0.98 | 0.11 |
| BMDhaz | −0.08 | 0.98 | NS |
| WB BMC | |||
| BMCage | 0.28 | 1.07 | 0.64 |
| BMCheight age | −0.01 | 0.86 | −0.34 |
| BMCheight | −0.17 | 1.06 | −0.32 |
| BMADage | 0.32 | 1.07 | 0.41 |
| BMChaz | 0.21 | 0.86 | 0.14 |
NS, Not significant.
Pearson correlation coefficients for the entire cohort for each bone Z-score and HAZ.
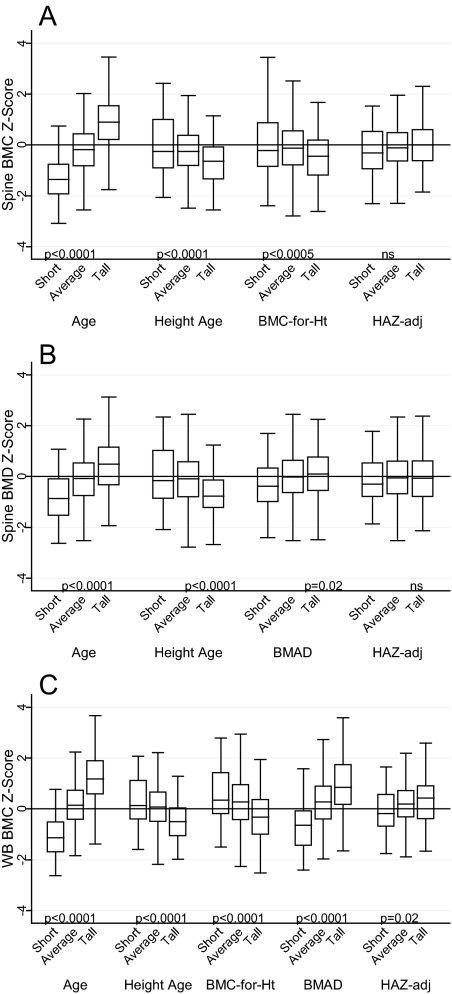
Unadjusted BMCageZ and BMDageZ were significantly and positively associated with HAZ (Table 3). That is, children with lower height relative to age had lower BMC and BMD relative to age. Spine BMDhaz and BMChazZ were not significantly associated with HAZ; WB BMChazZ was modestly associated with HAZ (r = 0.14). All other adjustment methods were significantly and negatively associated with HAZ. Spine and WB BMADageZ were positively associated with HAZ. There were highly significant differences between short, average, and tall children in bone Z-scores, with the exception of spine BMChaz and BMDhazZ (Fig. 2).
Figure 2.
A, Spine BMC Z-scores for age, compared with spine BMC Z-scores calculated using height age, BMC-for-height, and HAZ-adjusted Z-score (HAZadj) for short (HAZ < −1), average (−1 ≤ HAZ ≤ 1), and tall (HAZ > 1) children. B, Spine BMD Z-scores for age, compared with spine BMD Z-scores calculated using height age, BMAD, and HAZadj for short, average, and tall children. C, WB BMC Z-scores for age, compared with WB BMC Z-scores calculated using height age, BMC-for-height, BMAD, and HAZadj for short, average, and tall children. An unbiased adjustment method will have a similar Z-score distribution among short, average, and tall children.
Differences between adjusted and unadjusted Z-scores were significantly (P ≤ 0.05) correlated with age for most measures, ranging from r = −0.07 for WB BMADage to 0.24 for spine and WB BMCheight ageZ. The HAZ adjustment method did not result in differences that were significantly associated with age. This was explored further in children with short and tall stature, as shown in Fig. 3. Nearly all adjustment methods resulted in a greater change in Z-score among older children. For the HAZ adjustment method, the age-related increase was far more modest than for the other adjustment methods.
Figure 3.
Difference between BMC/BMD for age Z-scores (BMC/BMDage Z-scores) and adjusted Z-scores (e.g. BMC for age − BMC for height Z-score) relative to age for short children (A) and tall children (B). An unbiased adjustment method will have a similar effect at all ages.
Discussion
Previous bone mineral acquisition in childhood studies demonstrated the importance of linear growth and sexual maturation on bone outcomes (14,15,16,17,18,19,20,21,22). However, guidelines for incorporating body size into clinical interpretation of DXA-based bone outcomes have not been established, and there has been limited consideration of how to validate size-related adjustments of bone outcomes in the absence of a gold standard for bone health in childhood.
Size-related artifacts in the DXA measures of BMD have been recognized for some time (4). A widely used correction for size-related artifacts is BMAD (6,23). However, BMAD was not specifically designed to address the growth-related size dependencies. Molgaard et al. (24) were among the first to propose a scheme for using growth status in the clinical interpretation of DXA measurements. They proposed a three-stage approach using height-for-age, bone area-for-height, and BMC-for-bone area to correspond to “short” bones, “narrow” bones, and “light” bones as possible sources of bone deficits. This technique took advantage of unique sex- and age-specific distributions of height and BMC but did not combine them into a single measure. The clinical utility of this approach was never validated. Moreover, with the advent of DXA software and hardware innovations, the source data for this approach have become obsolete.
Other approaches have since been suggested. Leonard et al. (3) showed that WB bone area-for-height and BMC-for-height Z-scores were the best indicators of peripheral quantitative computed tomography measures of bone dimensions and strength; BMC-for-bone area and BMC-for-lean-mass Z-scores were poor indicators of bone strength. However, they did not evaluate the effects of age or short or tall stature-for-age. Correction for lean body mass has also been proposed (25,26) because it correlates highly with BMC/BMD and is an indicator of the mechanical forces to which bones are exposed. These approaches require additional assessments (i.e. body composition, puberty stage) and complex calculations, and they do not offer a validated approach for interpretation of DXA measurements in the context of short (or tall) stature.
The present study evaluated the specific issue of how to best assess the effect of short (or tall) stature on BMC/BMD Z-score in clinical practice, with the premise that an appropriate correction is one that would result in similar distributions of adjusted BMC or BMD Z-scores among short, average, and tall children. We used a large independent sample of healthy children free of chronic diseases, for whom short (or tall) stature is likely to reflect genetic potential for growth rather than disease processes. This is a particularly important feature of this validation approach because an appropriate adjustment method should result in a bias-free distribution of adjusted Z-scores—an assumption that is not feasible when trying to validate an adjustment method in samples of children with chronic disease, where disease severity adversely affects growth and may get progressively worse with age.
We examined adjustment methods using HAZ, height age, height-specific reference data, and BMAD in short, average, and tall children to explore potential biases. The methods that used height-age and height-specific reference data yielded Z-scores that were not independent of height status overall, but among short children the average Z-scores were close to zero. However, further inspection revealed that the differences between adjusted and unadjusted Z-scores were age dependent, such that the use of height age and height-specific reference data had a much greater effect at older ages than at younger ages, likely due to the fact that older short children were compared with younger, less physically mature children. Consequently, height-age and height-specific reference data inaccurately represent the effects of short stature on bone outcomes in older children.
BMAD was adopted to reduce the size-related effects of DXA areal BMD measurements. However, the analyses demonstrated that BMAD does not fully remove the effects of height status on areal BMD. The HAZ adjustment method was the only approach for which the effects of short (or tall) stature were not significant and the age effect was most modest. HAZ can easily be calculated using the EpiInfo software provided to the public at no cost from the Centers for Disease Control and Prevention (27) and the HAZ adjustment involves simple calculations using the equations provided. Therefore, this technique can be readily applied in clinical practice, overcoming one of the major obstacles to applying the ISCD pediatric guidelines.
This study had several limitations. The validation sample consisted mainly of Black and Caucasian children due to the study catchment area, and ethnic differences were not evaluated due to the small sample size that would have resulted from further subdividing the group of short children by race. In addition, short stature was defined as HAZ less than −1; there were no children with HAZ less than −2.0 in the BMDCS at baseline, and none below −2.5 in the validation sample. Some children with chronic diseases for whom this approach is intended, will have height deficits that are more severe than the children represented in this sample. Also, the HAZ adjustment is based on Hologic systems, is not applicable to equipment from other manufacturers, and does not account for other factors that might influence BMC and BMD such as body composition.
The strengths of this study far outweigh its limitations. A large national sample was used to develop the reference data and various adjustment methods. A large independent sample was used to validate these methods. Several kinds of comparisons were used to demonstrate that the HAZ adjustment method most closely met the criteria for a valid adjustment method. In the peripubertal years, children who have early or delayed puberty are often tall-for-age or short-for-age, so in part, the HAZ adjustment method captures some of the effect of pubertal timing. The HAZ adjustment technique was not validated for hip and forearm DXA outcomes, but the analysis showed that height status had a very modest effect on hip and forearm Z-scores. The clinical utility of these outcomes remains to be determined.
In sum, this study demonstrated that DXA bone Z-scores adjusted for HAZ yielded the least biased approach for estimating the effect of short (or tall) stature on measures of BMD. Short children are likely to have lower bone Z-scores, but the effect of short stature varied as a function of age. The difference between the unadjusted and HAZ-adjusted Z-score informs the clinician of the degree to which a low Z-score may be attributable to short stature, which may be useful in determining when a child with decreased BMD requires treatment. Further validation of this approach using other bone outcomes such as fracture is needed to fully evaluate the validity of this approach.
Acknowledgments
This paper would not have been possible without the generous contribution of the children and their families who participated in the Bone Mineral Density in Childhood Study and the Reference Project on Skeletal Development in Childhood. The authors also appreciate the generous contribution of the following research staff who assisted with this project: Susan Kaup, Adriana Natoli, Chantal Dilzer, Margarita Gomelsky, Samantha Ferrante, Gail Jackson, Kunthear Van, Christine Cinaglia, Heather Mitchell, and Donna Paulhamus.
Footnotes
This work was supported by the National Institute of Child Health and Human Development contracts NO1-HD-1-3228, -3329, -3330, -3331, -3332, and -3333; the General Clinical Research Center (5-MO1-RR-000240); the Clinical and Translational Research Center of The Children’s Hospital of Philadelphia/University of Pennsylvania (UL1-RR024134); and the Research Institute of The Children’s Hospital of Philadelphia.
Disclosure Summary: All authors are supported by the National Institute of Child Health and Human Development contracts that supported this research. There are no further disclosures.
Example: for a 14.6 yr old Caucasian boy with a spine BMDageZ of −2.20 and HAZ of −1.80, his predicted HAZ-adjusted spine BMD Z would be: (14.6 * −0.016) + (−1.80 * 0.228) + (14.6 * −1.80 * 0.024) + 0.148 = −1.13. His spine BMDhazZ would be −2.20 − (−1.13) = −1.07. Therefore, a large portion of his low BMD for age may be attributable to his short stature.
First Published Online January 26, 2010
Abbreviations: BMAD, Bone mineral apparent density; BMC, bone mineral content; BMD, bone mineral density; DXA, dual-energy x-ray absorptiometry; HAZ, height-for-age Z-score; WB, whole body.
References
- Leonard MB, Zemel BS 2002 Current concepts in pediatric bone disease. Pediatr Clin North Am 49:143–173 [DOI] [PubMed] [Google Scholar]
- Heaney RP 2003 Bone mineral content, not bone mineral density, is the correct bone measure for growth studies. Am J Clin Nutr 78:350–351; author reply 351–352 [DOI] [PubMed] [Google Scholar]
- Leonard MB, Shults J, Elliott DM, Stallings VA, Zemel BS 2004 Interpretation of whole body dual energy x-ray absorptiometry measures in children: comparison with peripheral quantitative computed tomography. Bone 34:1044–1052 [DOI] [PubMed] [Google Scholar]
- Prentice A, Parsons TJ, Cole TJ 1994 Uncritical use of bone mineral density in absorptiometry may lead to size-related artifacts in the identification of bone mineral determinants. Am J Clin Nutr 60:837–842 [DOI] [PubMed] [Google Scholar]
- Gordon CM, Bachrach LK, Carpenter TO, Crabtree N, El-Hajj Fuleihan G, Kutilek S, Lorenc RS, Tosi LL, Ward KA, Ward LM, Kalkwarf HJ 2008 Dual energy x-ray absorptiometry interpretation and reporting in children and adolescents: the 2007 ISCD Pediatric Official Positions. J Clin Densitom 11:43–58 [DOI] [PubMed] [Google Scholar]
- Bachrach LK, Hastie T, Wang MC, Narasimhan B, Marcus R 1999 Bone mineral acquisition in healthy Asian, Hispanic, black, and Caucasian youth: a longitudinal study. J Clin Endocrinol Metab 84:4702–4712 [DOI] [PubMed] [Google Scholar]
- Kalkwarf HJ, Zemel BS, Gilsanz V, Lappe JM, Horlick M, Oberfield S, Mahboubi S, Fan B, Frederick MM, Winer K, Shepherd JA 2007 The bone mineral density in childhood study: bone mineral content and density according to age, sex and race. J Clin Endocrinol Metab 92:2087–2099 [DOI] [PubMed] [Google Scholar]
- Tanner JM 1962 Growth at adolescence. 2nd ed. Oxford, UK: Blackwell Scientific Publication [Google Scholar]
- Morris NM, Udry JR 1980 Validation of a self-administered instrument to assess stage of adolescent development. J Youth Adolesc 9:271–280 [DOI] [PubMed] [Google Scholar]
- Schall JI, Semeao EJ, Stallings VA, Zemel BS 2002 Self-assessment of sexual maturity status in children with Crohn’s disease. J Pediatr 141:223–229 [DOI] [PubMed] [Google Scholar]
- Kuczmarski R, Ogden CL, Grummer-Strawn LM June 8, 2000 CDC growth charts—United States. Advance data from vital and health statistics. Hyattsville, MD: National Center for Health Statistics [Google Scholar]
- Cole TJ, Green PJ 1992 Smoothing reference centile curves: the LMS method and penalized likelihood. Stat Med 11:1305–1319 [DOI] [PubMed] [Google Scholar]
- Cole T, Pan H 2002 LMS program version 1.16. Compiled 15 April 2002. 1.16 ed. http://homepage.mac.com/tjcole/FileSharing1.html [Google Scholar]
- Bailey DA, Faulkner KG, McKay HA, Drinkwater DT, Mirwald RL 1996 Bone mineral acquisition during the adolescent growth spurt. J Bone Miner Res 11:S465 [Google Scholar]
- del Rio L, Carrascosa A, Pons F, Gusinyé M, Yeste D, Domenech FM 1994 Bone mineral density of the lumbar spine in white Mediterranean Spanish children and adolescents: changes related to age, sex, and puberty. Pediatr Res 35:362–366 [DOI] [PubMed] [Google Scholar]
- Gilsanz V, Roe TF, Mora S, Costin G, Goodman WG 1991 Changes in vertebral bone density in black girls and white girls during childhood and puberty. N Engl J Med 325:1597–1600 [DOI] [PubMed] [Google Scholar]
- Heaney RP, Dowell MS, Hale CA, Bendich A 2003 Calcium absorption varies within the reference range for serum 25-hydroxyvitamin D. J Am Coll Nutr 22:142–146 [DOI] [PubMed] [Google Scholar]
- Katzman DK, Bachrach LK, Carter DR, Marcus R 1991 Clinical and anthropometric correlates of bone mineral acquisition in healthy adolescent girls. J Clin Endocrinol Metab 73:1332–1339 [DOI] [PubMed] [Google Scholar]
- Lettgen B, Neudorf U, Hosse R, Peters S, Reiners C 1996 [Bone density in children and adolescents with rheumatic diseases. Preliminary results of selective measurement of trabecular and cortical bone using peripheral computerized tomography]. Klin Padiatr 208:114–117 [DOI] [PubMed] [Google Scholar]
- McKay HA, Bailey DA, Mirwald RL, Davison KS, Faulkner RA 1998 Peak bone mineral accrual and age at menarche in adolescent girls: a 6-year longitudinal study. J Pediatr 133:682–687 [DOI] [PubMed] [Google Scholar]
- Mølgaard C, Thomsen BL, Michaelsen KF 1998 Influence of weight, age and puberty on bone size and bone mineral content in healthy children and adolescents. Acta Paediatr 87:494–499 [DOI] [PubMed] [Google Scholar]
- Slemenda CW, Reister TK, Hui SL, Miller JZ, Christian JC, Johnston Jr CC 1994 Influences on skeletal mineralization in children and adolescents: evidence for varying effects of sexual maturation and physical activity. J Pediatr 125:201–207 [DOI] [PubMed] [Google Scholar]
- Carter DR, Bouxsein ML, Marcus R 1992 New approaches for interpreting projected bone densitometry data. J Bone Miner Res 7:137–145 [DOI] [PubMed] [Google Scholar]
- Mølgaard C, Thomsen BL, Prentice A, Cole TJ, Michaelsen KF 1997 Whole body bone mineral content in healthy children and adolescents. Arch Dis Child 76:9–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree NJ, Kibirige MS, Fordham JN, Banks LM, Muntoni F, Chinn D, Boivin CM, Shaw NJ 2004 The relationship between lean body mass and bone mineral content in paediatric health and disease. Bone 35:965–972 [DOI] [PubMed] [Google Scholar]
- Högler W, Briody J, Woodhead HJ, Chan A, Cowell CT 2003 Importance of lean mass in the interpretation of total body densitometry in children and adolescents. J Pediatr 143:81–88 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention 2008 Epi Info version 3.5.1 (http://www.cdc.gov/epiinfo/). Atlanta: CDC [Google Scholar]