Abstract
Background: Children with Prader-Willi syndrome (PWS) have decreased muscle mass, hypotonia, and impaired linear growth. Recombinant human GH (hGH) treatment reportedly improves body composition and physical function in children with PWS, but these studies lack long-term control data. To assess the impact of hGH therapy begun early in life on the natural history of PWS, we compared height, body composition, and strength in similar-age children with PWS naïve to hGH with those treated with hGH for 6 yr.
Objectives: Forty-eight children with PWS were studied: 21 subjects (aged 6–9 yr) treated with hGH for 6 yr (beginning at 4–32 months, mean 13 ± 6 months) were compared with 27 children of similar age (5–9 yr) prior to treatment with hGH. Percent body fat, lean body mass, carbohydrate/lipid metabolism, and motor strength were compared using analysis of covariance.
Results: PWS children treated with hGH demonstrated lower body fat (mean, 36.1 ± 2.1 vs. 44.6 ± 1.8%, P < 0.01), greater height (131 ± 2 vs. 114 ± 2 cm; P < 0.001), greater motor strength [increased standing broad jump 22.9 ± 2.1 vs. 14.6 ± 1.9 in. (P < 0.001) and sit-ups 12.4 ± 0.9 vs. 7.1 ± 0.7 in 30 sec (P < 0.001)], increased high-density lipoprotein cholesterol (58.9 ± 2.6 vs. 44.9 ± 2.3 mg/dl, P < 0.001), decreased low-density lipoprotein (100 ± 8 vs. 131 ± 7 mg/dl, P < 0.01), and no difference in fasting glucose or insulin.
Conclusions: hGH treatment in children with PWS, begun prior to 2 yr of age, improves body composition, motor function, height, and lipid profiles. The magnitude of these effects suggests that long-term hGH therapy favorably alters the natural history of PWS to an extent that exceeds risks and justifies consideration for initiation during infancy.
Long-term GH therapy alters the natural history of Prader-Willi syndrome to an extent that compares favorably with possible risks.
Prader-Willi Syndrome (PWS), initially described by Prader, Willi, and Labhart in 1956, is characterized by obesity, hypotonia, delayed motor skill acquisition, short stature, mental retardation, hypothalamic dysfunction, and hypogonadism (1). The genetic abnormality has been located on chromosome 15 (q11-13), a deletion of the paternal allele or presence of maternal disomy, PWS was the first human disorder associated with imprinting (2). Other central defining features in children with PWS include abnormal body composition with increased fat mass and decreased lean body mass (3). Infants with PWS typically demonstrate poor weight gain and hypotonia that precede hyperphagia and obesity. However, even at this young age, percent body fat measurements are increased (4).
In hopes of alleviating abnormalities in linear growth and body composition and because most children with PWS show evidence for subnormal GH secretion, recombinant human GH (hGH) therapy for children with PWS has been investigated. Several studies show that hGH instituted during childhood for up to 4 yr improves but does not normalize body composition, energy expenditure, and strength and agility in children with PWS (5,6,7,8). These findings led us to investigate whether earlier early institution of hGH therapy might: 1) prevent development of such severe body composition abnormalities and therefore lead to more normal body composition later in childhood and 2) improved hypotonia and motor milestone achievement during the first 2 yr of life, leading to greater strength and agility in childhood. In a randomized, controlled trial of hGH treatment of infants and toddlers with PWS, body composition and motor development improved significantly compared with nontreated infants and toddlers with PWS (9,10).
However, none of the studies reporting beneficial effects of hGH therapy for young children with PWS have included a control group for longer than 12 months; consequently, assertions about the degree to which long-term hGH therapy changes the natural history of PWS without hGH treatment remain speculative. Because hGH therapy is now Food and Drug Administration approved and widely prescribed for children with PWS, successful recruitment of subjects to a long-term prospective study comparing GH treatment with nontreatment is unlikely to occur. In addition, several cases of sudden unexpected death temporally associated with institution of hGH treatment in children with PWS have been reported. Whereas a causative relationship between exposure to hGH and sudden death remains uncertain, and the number of cases reported in excess of expected deaths remains small, these occurrences have prompted the inclusion of cautionary language in the drug prescribing information and altered the risk/benefit analysis for hGH therapy in a way distinctly different from other hGH treatment indications. Thus, it is crucial for physicians counseling families about GH therapy for their child with PWS to have confidence in long-term critical analysis of the value of such treatment.
Subjects from a study of early GH treatment of children with PWS (5) are now similar in age to those recruited for a previous randomized, controlled study of GH treatment for school-age children with PWS, (9). This afforded a special opportunity to compare the effects of long-term GH treatment on body composition, physical function, linear growth, and lipid metabolism in two age-matched groups of children with PWS, one of which was GH naïve and the other in whom treatment with GH was initiated before age 2 yr. A comparison of these two groups could provide the best illustration to date of the degree to which long-term GH therapy changed the natural history of growth, body composition, and physical function in children with PWS.
Patients and Methods
Two distinct cohorts of infants and children with PWS were recruited with a total of 48 subjects. Written informed consent was obtained from all patients and parents, and the study was approved by the university Human Subject Committees. All patients had a diagnosis of PWS confirmed by high-resolution cytogenetics, fluorescent in situ hybridization, and/or methylation studies. The early treatment cohort consists of 21 infants with PWS who were recruited at the ages of 4–20 months for initiation of GH therapy, previously reported, considered the treatment group (9). Sixty-two percent of these subjects had deletion of chromosome 15q11-13, 37% had uniparental disomy, and one patient was diagnosed by an abnormal methylation test. These subjects received GH therapy at 1 mg/m2/d for 6 yr (current ages 6–9 yr). The GH-naive cohort consists of 27 children with confirmed PWS aged 5–9 yr who had never received GH therapy. Sixty-eight percent of these subjects had deletion of chromosome 15q11-13, and 31% had uniparental disomy. In addition to confirmation of the PWS diagnosis, all subjects had physical examinations that confirmed prepubertal status. The cohort of 27 children with PWS naïve to hGH were drawn from a larger cohort of 50 children to create a cohort based on the best matched for age and gender with the 21 subjects in the hGH-treated cohort. No patients had evidence for hypothyroidism or adrenal insufficiency.
Length/height measurements were obtained using an appropriate Harpenden stadiometer, (Holtain, UK). Stat GrowthCharts software (version 2.01, Holtain, UK) was used to determine weight, height, and weight-for-height z-scores. Fasting levels of insulin, lipid profile, and free T4, and IGF-I were obtained (Quest Diagnostics, San Juan Capistrano, CA).
All patients had percent body fat and fat-free mass measured by dual-energy x-ray absorptiometry (Lunar Prodigy, version 3.6; GE Medical Systems, Madison, WI). Motor strength testing was performed by a modified Bruininks-Oseretsky test (11). This included broad jump, agility run, sit-ups, and upper arm strength assessments. The evaluator of motor strength testing was blinded to the treatment status of each child.
Statistical methods
Demographic variables were summarized as proportions and percentages (categorical variables) or means and ses (continuous variables). To account for possible confounding effects of age and gender, an analysis of covariance (ANCOVA) model was used to perform age and gender adjusted comparisons of outcome measures between the two cohorts. Age- and gender-adjusted least squares means (±se) were computed and reported for all body composition, motor function, and fasting lipid profile measures. Analyses were performed by the complete-case-analysis principle, i.e. no data imputation was performed. Model assumptions of the ANCOVA model were assessed graphically. All computations were performed with SAS software (version 9.1; SAS Institute, Cary, NC). All statistical tests were two sided, and P < 0.05 was used to indicate statistical significance.
Results
Patient characteristics and study results are summarized in Tables 1 and 2.
Table 1.
Demographics
| hGH-naïve cohort (n = 27) | Early hGH treatment cohort (n = 21) | |
|---|---|---|
| Age (yr, mean ± se) | 8.2 ± 0.33 (range 5–9) | 6.7 ± 0.21 (range 6–9) |
| BMI | 23.7 ± 1.1 (range 16.0–39.9) | 21.9 ± 1.2 (range 14.4–32.5) |
| Gender | ||
| Female | 27 (45%) | 27 (47%) |
| Male | 33 (55%) | 30 (53%) |
| Genotype | ||
| Deletion of chromosome 15q11-13 | 13 (62%) | 18 (68%) |
| Uniparental disomy | 8 (37%) | 8 (31%) |
There were no significant differences detected in basic clinical characteristics (age, gender, BMI, genotype) between the two cohorts. BMI, Body mass index.
Table 2.
Least squares means (±se) adjusted (for age and gender) of body composition, motor function, and lipid profile parameters for the two cohorts
| GH-naïve cohort (n = 27) Mean ± sea | Early-treatment cohort (n = 21) Mean ± sea | P valueb | |
|---|---|---|---|
| Percent body fat | 44.6 ± 1.8 | 36.1 ± 2.1 | 0.006 |
| Fat-free mass (kg) | 16.7 ± 0.9 | 24.1 ± 1.1 | <0.0001 |
| Height (cm) | 114.5 ± 1.8 | 131.4 ± 2.1 | <0.0001 |
| Height z-score | −1.6 ± 0.3 | 1.2 ± 0.2 | <0.0001 |
| Weight (kg) | 32 ± 2.3 | 38 ± 2.6 | 0.062 |
| BMI | 23.7 ± 1.1 | 21.9 ± 1.2 | 0.33 |
| Standing broad jump (in.) | 14.6 ± 2.0 | 22.9 ± 2.1 | 0.012 |
| Sit-ups | 7.1 ± 0.7 | 12.4 ± 1.0 | 0.0003 |
| 20-yd agility run (sec) | 11.6 ± 1.1 | 8.9 ± 1.3 | 0.17 |
| Weight-lift repetitions | 63.9 ± 6.6 | 49.6 ± 5.7 | 0.09 |
| IGF-I (ng/ml) | 112 ± 18 | 346 ± 20 | 0.001 |
| IGF-I SDS | −1.45 ± 0.30 | 1.39 ± 0.34 | 0.0001 |
| HDL cholesterol (mg/dl) | 44.9 ± 2.3 | 58.9 ± 2.6 | 0.0005 |
| LDL cholesterol (mg/dl) | 131.3 ± 7.1 | 100.2 ± 8.0 | 0.0099 |
| Total cholesterol (mg/dl) | 189.9 ± 7.3 | 177.3 ± 8.2 | 0.29 |
| Triglycerides (mg/dl) | 68.4 ± 10.6 | 94.2 ± 11.9 | 0.14 |
| Fasting insulin | 7.1 ± 1.3 | 10.2 ± 1.5 | 0.14 |
| HOMA-IR | 1.4 ± 0.3 | 2.1 ± 0.3 | 0.1 |
BMI, Body mass index; SDS, sd score; HOMA-IR, homeostasis model assessment index of insulin resistance.
Least squares mean, adjusted for age and gender.
Based on two-sided F test.
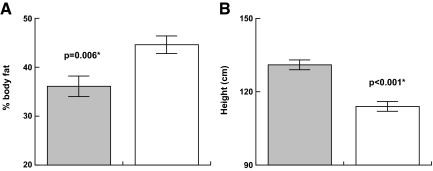
Body composition (see Fig. 1)
Figure 1.
Effect of GH on anthropometrics in PWS [GH treated (shaded) vs. untreated]. A, Percent body fat. B, height.
Percent body fat and muscle mass (lean body mass) were assessed by dual-energy x-ray absorptiometry. Lower percent body fat was evident in early hGH treatment children with PWS when compared with the hGH-naïve PWS subjects (adjusted least squares means of 36.1 ± 2.1 vs. 44.6 ± 1.8%; P = 0.006). Fat-free mass (muscle mass) was greater in the children with PWS who received early hGH treatment-treatment group compared with the hGH-naïve PWS subjects (24.1 ± 1.1 vs.16.7 ± 0.9 kg; P < 0.001).
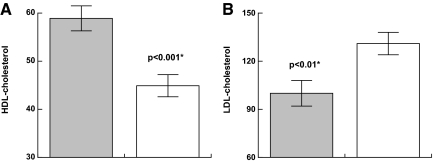
Carbohydrate and lipid metabolism (see Fig. 2)
Figure 2.
Effect of GH on lipids in PWS [GH treated (shaded) vs. untreated] A, HDL. B, LDL.
Carbohydrate and lipid metabolism were evaluated using fasting morning blood samples. Children with PWS who received early hGH treatment were compared with the GH-naïve PWS children and demonstrated statistically significant lower total cholesterol: least squares adjusted means; 177 ± 8.2 vs. 190 ± 7.3 mg/dl; higher high-density lipoprotein (HDL): 59 ± 2.6 vs. 45 ± 2.2 mg/dl (P = 0.0005); lower low-density lipoprotein (LDL) 100 ± 8 vs. 131 ± 7 mg/dl (P = 0.009); and unchanged triglycerides 68 ± 11 vs. 94 ± 12 mg/dl (P = 0.1). Glucose was not significantly different between the two groups. Fasting insulin was also was not significantly different between the two groups, 10 ± 1 vs. 7 ± 1 mIU/ml (P = 0.1).
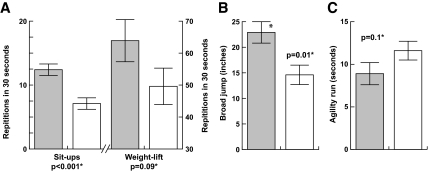
Motor strength (see Fig. 3)
Figure 3.
Effect of GH on strength in PWS [GH treated (shaded) vs. untreated]. A, Repetitions in 30 sec. B, Broad jump. C, Agility. *, Age- and gender-matched analysis using ANCOVA.
A modified Bruininks-Oseretsky test was used to test strength and agility, with four subtests for different muscle groups of the body. Children with PWS who received early hGH treatment demonstrated improved functional motor strength of increased standing broad jump with an adjusted least squares mean of 22.9 ± 2.1 vs. 14.6 ± 1.9 in. (P = 0.01) and sit-ups of 12.4 ± 0.9 vs. 7.1 ± 0.7 (P < 0.001). Clear trends were seen in the two other areas of the Bruininks-Oseretsky testing, including improved agility run (8.9 ± 1.3 vs. 11.6 ± 1.1 sec; P = 0.1) and weight-lift repetitions (63.9 ± 6.6 vs. 49.6 ± 5.7; P = 0.09), although these did not reach statistical significance.
Other observations and adverse events
IGF-I values increased with hGH treatment, although these remained within normal ranges (112 ± 18 to 346 ± 20 ng/ml; −1.45 ± 0.30 to 1.39 ± 0.34 sd score). No adverse events were noted during this study. Specifically, no reports of headaches, abnormal blood glucose levels, or reports of abnormal sleep-related breathing disorders were noted, nor were any significant differences in the development of scoliosis observed between early-hGH-treatment and hGH-naïve patients.
Discussion
This analysis of similar-aged children with PWS, one group treated with hGH for 6 yr and the other naïve to hGH therapy, offers a unique assessment of the degree to which early-in-life hGH treatment alters the clinical course of this disorder. It also extends and tests the validity of findings of previous studies of hGH therapy in children under age 3 yr with PWS, none of which had a control group for longer than 12 months (12,13,14,15,16).
Infants and toddlers with PWS commonly demonstrate hypotonia, associated with poor suck, feeding, compromised respiratory function, early failure to thrive, and delay in attainment of developmental motor skills. Early abnormalities in body composition in PWS are present before the onset of characteristic hyperphagia and progressive obesity (with increased percent body fat and decreased muscle mass) and diminished GH secretion in PWS is well documented (17,18,19). Impaired physical function in PWS during childhood is most often related to these body composition abnormalities and hypotonia, rather than to linear growth deficits. Prior studies of treated children followed longitudinally (using subjects as their own controls) have shown that prolonged treatment with hGH improves but does not normalize physical function and body composition in PWS (2,6,16,20,21,22,23). The analysis herein of an early-in-life GH treatment cohort confirms and extends the findings of our and others’ prior shorter-term reports (10,16). Importantly, whereas no adverse effects of hGH therapy occurred in our patients and in other recent long-term hGH treatment studies, (23), case reports prompted hGH product labeling to include a possible temporal association between institution of hGH therapy and increased risk for respiratory failure and death in children with PWS (24,25).
Consequently, to more clearly justify hGH therapy in light of a potential rare but serious risk, it is important to critically assess whether such treatment changes the natural history or the long-term course of children with PWS in not only a statistically significant but also a clinically significant and meaningful way that exceeds potential risks. A limitation of this study is that it is a nonrandomized study, which could be subject to unobserved, confounding factors for which we cannot account for in the analysis. With growth failure due to PWS now an approved indication for hGH therapy in the United States and Europe, the successful completion of a long-term prospective, randomized, controlled trial would be at least very difficult and possibly deemed unethical. By performing identical tests of body composition, physical function, and lipid and carbohydrate metabolism in two groups of similar-aged children with PWS, one of which was treated with hGH since infancy, this study offers some important insights that would be sought in such a trial.
Does hGH therapy affect the natural history of PWS in a beneficial and meaningful way? The findings of this study suggest that the answer to the question is yes. When compared with school-aged children with PWS who had not been treated with GH, the early-in-life GH treatment children showed, on average: 1) 8.5% (absolute) reduction in body fat (19% relative reduction); 2) nearly a doubling in broad jump and sit-up performance; 3) 14 mg/dl higher HDL cholesterol levels and 31 mg/dl lower LDL cholesterol levels; and 4) height increased by 16 cm.
These differences in body fat percentage, motor strength, and height compared with peers appear of sufficient magnitude to validate commonly heard (but difficult to test) parental reports of improved movement and agility, engagement in physical activities, and quality of life as a result of GH therapy.
Conclusions
Multiple studies have reported beneficial effects of hGH therapy in children with PWS, but the degree to which these effects alter the natural history of the disorder remain uncertain due to lack of long-term control data. This study, which compares similar-aged children with PWS who had received or not received long-term hGH therapy, provides the strongest evidence to date that hGH therapy begun early in life beneficially and significantly alters the natural history of PWS by reducing body fat, improving muscle strength and physical function, and improving lipid profiles without adverse effects. Thus, physicians and families can favorably weigh the sustained long-term value of hGH treatment in PWS against potential risks.
Acknowledgments
The authors thank all of the participating children and families for their enthusiastic cooperation as well as the invaluable help of our study coordinator, Heidi Luebke, M.S.
Footnotes
Disclosure Summary: The authors have declared no conflict of interest and nothing to disclose.
First Published Online January 8, 2010
Abbreviations: ANCOVA, Analysis of covariance; HDL, high-density lipoprotein; hGH, human GH; LDL, low-density lipoprotein; PWS, Prader-Willi syndrome.
References
- Prader A, Labhart A, Willi H 1956 Ein syndrom von adipositas, kleinwuchs, kryptorchismus and oligophrenie. Schweiz Med Wochenschr 86:1260–1261 [Google Scholar]
- Goldstone AP, Holland AJ, Hauffa BP, Hokken-Koelega AC, Tauber M 2008 Recommendations for the diagnosis and management of Prader-Willi syndrome. J Clin Endocrinol Metab 93:4183–4197 [DOI] [PubMed] [Google Scholar]
- Brambilla P, Bosio L, Manzoni P, Pietrobelli A, Beccaria L, Chiumello G 1997 Peculiar body composition in patients with Prader-Labhart-Willi syndrome. Am J Clin Nutr 65:1369–1374 [DOI] [PubMed] [Google Scholar]
- Bekx MT, Carrel AL, Shriver TC, Li Z, Allen DB 2003 Decreased energy expenditure is caused by abnormal body composition in infants with Prader-Willi syndrome. J Pediatr 143:372–376 [DOI] [PubMed] [Google Scholar]
- Carrel AL, Myers SE, Whitman BY, Allen DB 1999 Growth hormone improves body composition, fat utilization, physical strength and agility, and growth in Prader-Willi syndrome: a controlled study. J Pediatr 134:215–221 [DOI] [PubMed] [Google Scholar]
- Carrel AL, Myers SE, Whitman BY, Allen DB 2002 Benefits of long-term GH therapy in Prader-Willi syndrome: a 4-year study. J Clin Endocrinol Metab 87:1581–1585 [DOI] [PubMed] [Google Scholar]
- Haqq AM, Stadler DD, Jackson RH, Rosenfeld RG, Purnell JQ, LaFranchi SH 2003 Effects of growth hormone on pulmonary function, sleep quality, behavior, cognition, growth velocity, body composition, and resting energy expenditure in Prader-Willi syndrome. J Clin Endocrinol Metab 88:2206–2212 [DOI] [PubMed] [Google Scholar]
- Eiholzer U, Gisin R, Weinmann C, Kriemler S, Steinert H, Torresani T, Zachmann M, Prader A 1998 Treatment with human growth hormone in patients with Prader-Labhart-Willi syndrome reduces body fat and increases muscle mass and physical performance. Eur J Pediatr 157:368–377 [DOI] [PubMed] [Google Scholar]
- Carrel AL, Moerchen V, Myers SE, Bekx MT, Whitman BY, Allen DB 2004 Growth hormone improves mobility and body composition in infants and toddlers with Prader-Willi syndrome. J Pediatr 145:744–749 [DOI] [PubMed] [Google Scholar]
- Eiholzer U, L'allemand D, Schlumpf M, Rousson V, Gasser T, Fusch C 2004 Growth hormone and body composition in children younger than 2 years with Prader-Willi syndrome. J Pediatr 144:753–758 [DOI] [PubMed] [Google Scholar]
- Bruininks RH 1978 Bruininks-Oseretsky test of motor proficiency. Circle Pines, MN: American Guidance Service [Google Scholar]
- Bell J, Parker KL, Swinford RD, Hoffman AR, Maneatis T, Lippe B 2009 Long-term safety of recombinant human growth hormone in children. J Clin Endocrinol Metab doi: 10.1210/jc.2009-0178 [DOI] [PubMed] [Google Scholar]
- de Lind van Wijngaarden RF, Siemensma EP, Festen DA, Otten BJ, van Mil EG, Rotteveel J, Odink RJ, Bindels-de Heus GC, van Leeuwen M, Haring DA, Bocca G, Houdijk EC, Hoorweg-Nijman JJ, Vreuls RC, Jira PE, van Trotsenburg AS, Bakker B, Schroor EJ, Pilon JW, Wit JM, Drop SL, Hokken-Koelega AC 2009 Efficacy and safety of long-term continuous growth hormone treatment in children with Prader-Willi syndrome. J Clin Endocrinol Metab 94:4205–4215 [DOI] [PubMed] [Google Scholar]
- Tauber M, Hokken-Koelega AC, Hauffa BP, Goldstone AP 2009 About the benefits of growth hormone treatment in children with Prader-Willi syndrome. J Pediatr 154:778; author reply 779 [DOI] [PubMed] [Google Scholar]
- Fillion M, Deal C, Van Vliet G 2009 Retrospective study of the potential benefits and adverse events during growth hormone treatment in children with Prader-Willi. J Pediatr 154:230–233 [DOI] [PubMed] [Google Scholar]
- Lindgren AC, Lindberg A 2008 Growth hormone treatment completely normalizes adult height and improves body composition in Prader-Willi syndrome: experience from KIGS. Horm Res 70:182–187 [DOI] [PubMed] [Google Scholar]
- Angulo M, Castro-Magana M, Uy J 1991 Pituitary evaluation and growth hormone treatment in Prader-Willi syndrome. J Pediatr Endocrinol 4:167–171 [Google Scholar]
- Costeff H, Holm VA, Ruvalcaba R, Shaver J 1990 Growth hormone secretion in Prader-Willi syndrome. Acta Paediatr Scand 79:1059–1062 [DOI] [PubMed] [Google Scholar]
- Corrias A, Bellone J, Beccaria L, Bosio L, Trifirò G, Livieri C, Ragusa L, Salvatoni A, Andreo M, Ciampalini P, Tonini G, Crinò A 2000 GH/IGF-I axis in Prader-Willi syndrome: evaluation of IGF-I levels and of the somatotroph responsiveness to various provocative stimuli. J Endocrinol Invest 23:84–89 [DOI] [PubMed] [Google Scholar]
- Festen DA, de Lind van Wijngaarden R, van Eekelen M, Otten BJ, JM W, Duivenvoorden HJ, Hokken-Koelega A 2008 Randomized controlled GH trial: effects on anthropometry, body composition and body proportions in a large group of children with Prader-Willi syndrome. Clin Endocrinol (Oxf) 69:443–451 [DOI] [PubMed] [Google Scholar]
- Lindgren AC, Hagenäs L, Ritzén EM 1999 Growth hormone treatment of children with Prader-Willi syndrome: effects on glucose and insulin homeostasis. Swedish National Growth Hormone Advisory Group. Horm Res 51:157–161 [DOI] [PubMed] [Google Scholar]
- Eiholzer U, Nordmann Y, l'Allemand D, Schlumpf M, Schmid S, Kromeyer-Hauschild K 2003 Improving body composition and physical activity in Prader-Willi syndrome. J Pediatr 142:73–78 [DOI] [PubMed] [Google Scholar]
- de Lind van Wijngaarden RF, de Klerk LW, Festen DA, Duivenvoorden HJ, Otten BJ, Hokken-Koelega AC 2009 Randomized controlled trial to investigate the effects of growth hormone treatment on scoliosis in children with Prader-Willi syndrome. J Clin Endocrinol Metab 94:1274–1280 [DOI] [PubMed] [Google Scholar]
- Bakker B, Maneatis T, Lippe B 2007 Sudden death in Prader-Willi syndrome: brief review of five additional cases. Concerning the article by U. Eiholzer et al.: deaths in children with Prader-Willi syndrome. A contribution to the debate about the safety of growth hormone treatment in children with PWS (Horm Res 2005;63:33–39). Horm Res 67:203–204 [DOI] [PubMed] [Google Scholar]
- Eiholzer U 2005 Deaths in children with Prader-Willi syndrome. A contribution to the debate about the safety of growth hormone treatment in children with PWS. Horm Res 63:33–39 [DOI] [PubMed] [Google Scholar]