Abstract
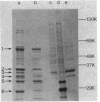
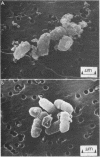
The effects of polymyxin B and polymyxin B nonapeptide (PMBN) on cell envelope integrity in Escherichia coli were compared. Both compounds caused loss of proteins from E. coli K-12 3300(pBR322), although PMBN released less protein than did polymyxin B. The origin of the released protein was determined both by polyacrylamide gel electrophoresis and by using specific enzyme markers (beta-lactamase in periplasm, beta-galactosidase in cytoplasm). The proteins released by both compounds were derived principally from the periplasm, accompanied in the case of polymyxin B by a low level of cytoplasmic proteins. Although polymyxin B and PMBN both caused release of periplasmic proteins, the individual proteins released by the compounds differed. The periplasmic fraction contained six principal polypeptides with molecular weights between 62,000 (polypeptide 1) and 29,000 (polypeptide 6). Polypeptide 6 was identified as the pBR322-encoded beta-lactamase, but the other proteins were not specifically identified. Polymyxin B caused considerable release of polypeptides 1, 2, and 5 with some release of polypeptides 4 and 6. PMBN released polypeptide 1 (trace), 3, 4, and 6 (trace). Scanning electron microscopy showed that polymyxin B and PMBN both caused surface damage in E. coli. However, polymyxin B produced greater morphological changes than PMBN.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bird A. E., Charsley C. H., Jennings K. R., Marshall A. C. High-performance liquid chromatographic assay of temocillin and epimerisation of its diastereoisomers. Analyst. 1984 Sep;109(9):1209–1212. doi: 10.1039/an9840901209. [DOI] [PubMed] [Google Scholar]
- Cerny G., Teuber M. Comparative polyacrylamide electrophoresis of periplasmic proteins released from gram-negative bacteria by polymyxin B. Arch Mikrobiol. 1972;82(4):361–370. doi: 10.1007/BF00424939. [DOI] [PubMed] [Google Scholar]
- Cerny G., Teuber M. Differential release of periplasmic versus cytoplasmic enzymes from Escherichia coli B by polymixin B. Arch Mikrobiol. 1971;78(2):166–179. doi: 10.1007/BF00424873. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Chopra I., Howe G. B., Ball P. R. Lysozyme-promoted association of protein I molecules in the outer membrane of Escherichia coli. J Bacteriol. 1977 Nov;132(2):411–418. doi: 10.1128/jb.132.2.411-418.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra I., Shales S. W. Comparison of the polypeptide composition of Escherichia coli outer membranes prepared by two methods. J Bacteriol. 1980 Oct;144(1):425–427. doi: 10.1128/jb.144.1.425-427.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles S. J., Chopra I. Biochemical and genetic characterization of the tet determinant of Bacillus plasmid pAB124. J Bacteriol. 1984 Apr;158(1):134–140. doi: 10.1128/jb.158.1.134-140.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallewell R. A., Sherratt D. J. Isolation and characterization of Co1E2 plasmid mutants unable to kill colicin-sensitive cells. Mol Gen Genet. 1976 Aug 2;146(3):239–245. doi: 10.1007/BF00701246. [DOI] [PubMed] [Google Scholar]
- Hedges R. W., Rodriguez-Lemoine V., Datta N. R factors from Serratia marcescens. J Gen Microbiol. 1975 Jan;86(1):88–92. doi: 10.1099/00221287-86-1-88. [DOI] [PubMed] [Google Scholar]
- HsuChen C. C., Feingold D. S. The mechanism of polymyxin B action and selectivity toward biologic membranes. Biochemistry. 1973 May 22;12(11):2105–2111. doi: 10.1021/bi00735a014. [DOI] [PubMed] [Google Scholar]
- Humphreys G. O., Willshaw G. A., Anderson E. S. A simple method for the preparation of large quantities of pure plasmid DNA. Biochim Biophys Acta. 1975 Apr 2;383(4):457–463. doi: 10.1016/0005-2787(75)90318-4. [DOI] [PubMed] [Google Scholar]
- Jacobson G. R., Takacs B. J., Rosenbusch J. P. Properties of a major protein released from Escherichia coli by osmotic shock. Biochemistry. 1976 Jun 1;15(11):2297–2303. doi: 10.1021/bi00656a008. [DOI] [PubMed] [Google Scholar]
- Klemperer R. M., Gilbert P., Meier A. M., Cozens R. M., Brown M. R. Influence of suspending media upon the susceptibility of Pseudomonas aeruginosa NCTC 6750 and its spheroplasts to polymyxin B. Antimicrob Agents Chemother. 1979 Feb;15(2):147–151. doi: 10.1128/aac.15.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopylova-Sviridova T. N., Soukovatitsin V. V., Fodor I. Synthesis of proteins coded by plasmid vectors of pCV series (Apr, Tcr) and their recombinant derivatives (pDm) in E. coli minicells. Gene. 1979 Oct;7(2):121–139. doi: 10.1016/0378-1119(79)90028-3. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Van Alphen L. Molecular architecture and functioning of the outer membrane of Escherichia coli and other gram-negative bacteria. Biochim Biophys Acta. 1983 Mar 21;737(1):51–115. doi: 10.1016/0304-4157(83)90014-x. [DOI] [PubMed] [Google Scholar]
- NEWTON B. A. Reversal of the antibacterial activity of polymyxin by divalent cations. Nature. 1953 Jul 25;172(4369):160–161. [PubMed] [Google Scholar]
- NEWTON B. A. The properties and mode of action of the polymyxins. Bacteriol Rev. 1956 Mar;20(1):14–27. doi: 10.1128/br.20.1.14-27.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond M. H., Sykes R. B. The beta-lactamases of gram-negative bacteria and their possible physiological role. Adv Microb Physiol. 1973;9:31–88. doi: 10.1016/s0065-2911(08)60376-8. [DOI] [PubMed] [Google Scholar]
- Rosenthal K. S., Swanson P. E., Storm D. R. Disruption of Escherichia coli outer membranes by EM 49. A new membrane active peptide. Biochemistry. 1976 Dec 28;15(26):5783–5792. doi: 10.1021/bi00671a015. [DOI] [PubMed] [Google Scholar]
- Ross G. W., O'Callaghan C. H. Beta-lactamase assays. Methods Enzymol. 1975;43:69–85. doi: 10.1016/0076-6879(75)43081-6. [DOI] [PubMed] [Google Scholar]
- Shales S., Chopra I. Outer membrane composition in Escherichia coli and the poor activity of hydrophobic antibiotics against enteric bacteria. J Antimicrob Chemother. 1982 Apr;9(4):325–327. doi: 10.1093/jac/9.4.325. [DOI] [PubMed] [Google Scholar]
- Smith M. C., Chopra I. Energetics of tetracycline transport into Escherichia coli. Antimicrob Agents Chemother. 1984 Apr;25(4):446–449. doi: 10.1128/aac.25.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm D. R., Rosenthal K. S., Swanson P. E. Polymyxin and related peptide antibiotics. Annu Rev Biochem. 1977;46:723–763. doi: 10.1146/annurev.bi.46.070177.003451. [DOI] [PubMed] [Google Scholar]
- Vaara M., Vaara T. Outer membrane permeability barrier disruption by polymyxin in polymyxin-susceptible and -resistant Salmonella typhimurium. Antimicrob Agents Chemother. 1981 Apr;19(4):578–583. doi: 10.1128/aac.19.4.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaara M., Vaara T. Polycations as outer membrane-disorganizing agents. Antimicrob Agents Chemother. 1983 Jul;24(1):114–122. doi: 10.1128/aac.24.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaara M., Vaara T. Polycations sensitize enteric bacteria to antibiotics. Antimicrob Agents Chemother. 1983 Jul;24(1):107–113. doi: 10.1128/aac.24.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaara M., Vaara T. Sensitization of Gram-negative bacteria to antibiotics and complement by a nontoxic oligopeptide. Nature. 1983 Jun 9;303(5917):526–528. doi: 10.1038/303526a0. [DOI] [PubMed] [Google Scholar]
- Vaara M., Viljanen P. Binding of polymyxin B nonapeptide to gram-negative bacteria. Antimicrob Agents Chemother. 1985 Apr;27(4):548–554. doi: 10.1128/aac.27.4.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaara M., Viljanen P., Vaara T., Mäkelä P. H. An outer membrane-disorganizing peptide PMBN sensitizes E. coli strains to serum bactericidal action. J Immunol. 1984 May;132(5):2582–2589. [PubMed] [Google Scholar]
- Viljanen P., Vaara M. Susceptibility of gram-negative bacteria to polymyxin B nonapeptide. Antimicrob Agents Chemother. 1984 Jun;25(6):701–705. doi: 10.1128/aac.25.6.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamato I., Anraku Y., Hirosawa K. Cytoplasmic membrane vesicles of Escherichia coli. A simple method for preparing the cytoplasmic and outer membranes. J Biochem. 1975 Apr;77(4):705–718. doi: 10.1093/oxfordjournals.jbchem.a130774. [DOI] [PubMed] [Google Scholar]