Abstract
Objective
Determine effect of body mass index (BMI) on response to bacterial vaginosis (BV) treatment.
Study Design
Secondary analysis of two multicenter trials of therapy for BV and Trichomonas vaginalis. Gravida screened for BV between 8-22 weeks and randomized between 16-23 weeks to metronidazole or placebo. Of 1,497 gravida with asymptomatic BV and preconceptional BMI, 738 were randomized to metronidazole; BMI divided into categories: <25, 25-29.9, and ≥ 30. Rates of BV persistence at follow-up compared using Mantel-Haenszel Chi square. Multiple logistic regression used to evaluate effect of BMI on BV persistence at follow-up, adjusting for potential confounders.
Results
No association identified between BMI and BV rate at follow-up (p=0.21). BMI was associated with maternal age, smoking, marital status, and black race. Compared to women with BMI of <25, adjusted OR of BV at follow-up were: BMI 25-29.9: OR=0.66, 95% CI 0.43-1.02; BMI ≥ 30: OR=0.83, 95% CI 0.54-1.26.
Conclusion
Persistence of BV after treatment was not related to BMI.
Keywords: body mass index, bacterial vaginosis, pregnancy, treatment
Introduction
Excessive weight has become one of the major health problems in the United States. The most commonly used method to define obesity is the body mass index (BMI), defined as weight in kilograms divided by height in meters, squared (kg/m2). Data from the National Health and Nutrition Examination Survey (NHANES) 1999 which included males and non-pregnant females indicated that 61% of adults in the United States are either overweight (BMI=25 to 29) or obese (BMI of 30 or more).1
Obese individuals have a higher percentage of fat and lower percentage of lean tissue and water that can affect drug distribution in the tissues.2 Increased cardiac output and total blood volume are demonstrated in the obese. Blood flow per gram of fat in nonpregnant obese patients is less than in the non obese.2 However, the blood flow per gram of fat in pregnancy is unknown. Obese women may have an increased volume of distribution compared to their lean counterparts, and these changes may be particularly important with regard to drug therapy during pregnancy.
Bacterial vaginosis (BV) is common and affects approximately 800,000 pregnant women per year in the United States with persistence of BV after therapy reported in 22.2% of women.3 Koumans and co-investigators4 report that women delivered prematurely are more likely to have an upper genital tract infection, and organisms associated with identified infections are typically those found in women with BV. Bacterial vaginosis has been reported to be associated with an increased risk of preterm delivery and delivery of low birth-weight infants, especially in high-risk women.5-10 Our objective was to determine the effect of body mass index (BMI) on the response to treatment among pregnant women with a confirmed diagnosis of bacterial vaginosis.
Methods
This is a secondary analysis of data originally collected as part of two multicenter clinical trials of therapy for BV and Trichomonas vaginalis.3 All patients with asymptomatic BV at randomization who had follow-up results and data on preconceptional BMI were included (N=1497). Women with symptomatic vaginitis or those with current or planned antibiotic therapy (Chlamydia trachomatis, syphilis, gonorrhea) were excluded prior to randomization. Women were also ineligible for randomization if antibiotics were administered in the past 2 weeks or antibiotic therapy was planned for any time prior to the onset of labor.3 BMI was calculated using the reported height and pre-pregnant weight (weight in kilograms/height in meters2). Pregnant women were screened for BV between 8 and 22 weeks gestation and were randomized between 16 and 23 weeks to metronidazole (2 gm orally, repeated in 48 hours) or identical appearing placebo. Follow-up visits were scheduled between 24.0 and 29.6 weeks gestation and were performed at least two weeks after randomization. BV was diagnosed using Nugent's criteria.11 Reliability of diagnosing bacterial vaginosis is improved by a standardized method of Gram stain interpretation11 and a vaginal pH >4.4 as described in the primary publication.3
BMI was first evaluated as a continuous variable then categorized narrowly to assess a dose-response relationship. We used previously defined categories for BMI. The categories were then collapsed into three categories to increase sample sizes within strata: < 25 (normal/underweight, n=712), 25-29.9 (overweight, n=377), and ≥ 30 (obese, n=408). The relationship between these BMI categories and other variables including T. vaginalis at randomization, smoking, alcohol, number of lifetime sexual partners, number of recent sexual encounters, ethnicity, marital status, source of medical care payment, parity, and age was assessed in univariate analyses. Categorical variables were compared using the Chi-square test and continuous variables were compared using the Kruskal-Wallis test. Rates of BV persistence at follow up were compared using Mantel-Haenszel Chi-square test for trend. Variables found to be significant in univariate analysis were included in a multivariate logistic regression to evaluate the effect of BMI on persistence of BV at follow-up controlling for these potential confounders
Results
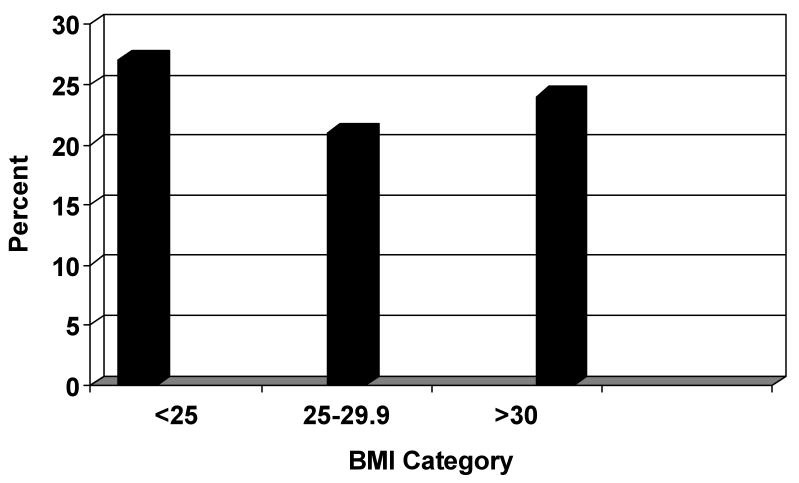
Of the 2,570 patients randomized in the trials, there were 1733 women with asymptomatic BV at randomization. A total of 1,497 had both preconceptional BMI data and follow up results available. Seven hundred thirty eight were randomized to treatment and 759 received placebo. BMI ranged from 15-71 (median 25.5 kg/m2). Of the 738 women in the treatment arm, 339 (46%) women had a BMI<25, 200 (27%) women had a BMI of 25-29.9, and 199 (27%) had a BMI ≥30. Among the 738 women receiving therapy, there was no association between BMI and rate of BV at follow-up: <25: 27%; 25-29.9: 21%; ≥30: 24%, p=0.21 (Figure 1). BMI was associated with maternal age, smoking, marital status and black race (Table 1); therefore logistic regression was used to adjust for the effect of these variables. Compared to women with a BMI of <25, the adjusted odds ratios of BV at follow-up for the heavier categories were: BMI 25-29.9: OR=0.66, 95% CI 0.43, 1.02, and BMI≥30: OR=0.83, 95% CI 0.54, 1.26 (Table 2). Furthermore, odds of BV at follow-up were not significantly different when BMI was included in the multivariate model as either a continuous variable or as a quadratic variable. No significance differences were identified in the population among this cohort and women who were excluded.
Figure 1. Persistence of BV at follow up by BMI category.
Table 1. Summary of baseline variables by BMI category.
| < 25 (n=339) | 25-29.9 (n=200) | ≥ 30 (n=199) | p-value | |
|---|---|---|---|---|
| TV + at randomization | 42 (12%) | 16 (8%) | 23 (12%) | 0.27 |
| Black race | 240 (71%) | 145 (73%) | 167 (84%) | 0.002 |
| Married | 96 (28%) | 76 (38%) | 55 (28%) | 0.03 |
| Maternal age | 22.5 ± 5.4 | 24.1 ±5.7 | 24.2 ± 5.4 | <0.0001 |
| Source of medical care payment | 0.30 | |||
| Government | 288 (85%) | 165 (83%) | 179 (90%) | |
| Private insurance | 19 (6%) | 12 (6%) | 7 (4%) | |
| None/self-paid | 32 (9%) | 23 (12%) | 13 (7%) | |
| Smoked during pregnancy | 74 (22%) | 26 (13%) | 30 (15%) | 0.02 |
| Alcohol during pregnancy | 29 (9%) | 15 (8%) | 13 (7%) | 0.69 |
| Lifetime sexual partners | 0.21 | |||
| 1-2 | 85 (25%) | 47 (24%) | 40 (20%) | |
| 3-5 | 155 (46%) | 78 (39%) | 85 (43%) | |
| 6 or more | 99 (29%) | 74 (37%) | 73 (37%) | |
| Number of times/week patient had intercourse | 0.85 | |||
| Up to 1 | 188 (55%) | 117 (59%) | 109 (55%) | |
| 2-4 | 127 (37%) | 72 (36%) | 79 (40%) | |
| 5 or more | 24 (7%) | 11 (6%) | 11 (6%) | |
| Parity | 0.13 | |||
| 0 | 163 (48%) | 76 (38%) | 85 (43%) | |
| 1 | 98 (29%) | 61 (31%) | 56 (28%) | |
| 2 or more | 78 (23%) | 63 (32%) | 58 (29%) | |
Table 2. Adjusted Odds Ratios* for BV positive at Follow-up by BMI category.
| OR | 95% Confidence Interval | |
|---|---|---|
| Obese (BMI ≥ 30) | 0.83 | 0.54 – 1.26 |
| Overweight (BMI 25-29.9) | 0.66 | 0.43 – 1.02 |
| Normal/underweight (BMI < 25) | reference |
Adjusted for maternal age, smoking, marital status and black race.
Discussion
Although the literature is replete with articles addressing antibiotic therapy during pregnancy, there is a paucity of information regarding the pharmacokinetics of various antibiotics during gestation, especially in obese pregnant women. There does, however, appear to be a decrease in the serum levels of most antibiotics during pregnancy.12,13 Pregnant women have an increased blood volume and increase in body fat. Serum albumin levels are decreased which affects the distribution of drugs into maternal tissues. Additional physiologic alterations in pregnancy that may affect drug distribution and bioavailability are slowed gut motility and the transplacental transfer of drugs.
Metronidazole is an antiprotozoal and antibacterial agent with the major route of elimination the urine (60-80%), the main site of metabolism the liver, and volume of distribution that of total body water.14 A linear relationship exists between metronidazole doses of 200 to 2000 mg and plasma concentration, and average effective concentrations of the drug are 8mcg/mL for most susceptible protozoa and bacteria.14 Bioavailability of metronidazole in the non-pregnant individual approaches 100% with an elimination half-life of about 8 hours.14 Dosage recommendations for children with amebiasis are weight-based. For anaerobic infections in adults, intravenous metronidazole is administered on a weight basis.
With the increasing prevalence of overweight and obese adults in the U.S., more overweight and obese women are now encountered in obstetric practices across the country. Moreover, the effects of obesity on drug distribution during pregnancy are largely unknown. Our hypothesis is that women with an increased BMI would have more treatment failures due to a larger volume of distribution, decreased plasma levels of metronidazole, and an increased renal clearance. BMI in this study was evaluated as a continuous variable prior to being categorized narrowly due to sample size. Originally we looked at five categories of BMI using a standard definition. Since the N were small in the two extreme groups (underweight and morbidly obese), we combined them with the next severe group. We found that among the 738 women receiving treatment, there was no association between BMI and rate of BV at follow-up. When logistic regression analysis was utilized to adjust for the effect of the potential confounders (maternal age, smoking, marital status and black race), the adjusted odds ratio of BV at follow-up for the heavier women was not increased as compared to their lean counterparts.
Of note, since the time of the original study, updated Sexually Transmitted Diseases Treatment Guidelines published by the Centers for Disease Control and Prevention (2006)15 caution that metronidazole 2-g single-dose therapy has the lowest efficacy for BV and is no longer a recommended treatment regimen. In conclusion, persistence of BV after treatment in pregnant women is not related to BMI after controlling for potential confounding factors, and failure of therapy was not more common among obese women. Further studies are needed to see if our data can be extrapolated to a less socioeconomically-deprived population.
Acknowledgments
Supported by grants from the National Institute of Child Health and Human Development (HD21410, HD21414, HD27860, HD27861, HD27869, HD27883, HD27889, HD27905, HD27915, HD27917, HD34116, HD34122, HD34136, HD34208, HD34210, HD19897 and HD36801) and the National Institute of Allergy and Infectious Diseases (AI 38514).
In addition to the authors, other members of the National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network are as follows:
University of Alabama at Birmingham — R.L. Copper, A. Northen, and W.W. Andrews
University of Chicago — P. Jones and M.D. Lindheimer
University of Cincinnati — N. Elder and T.A. Siddiqi
The George Washington University Biostatistics Center — E.Thom, S. Leindecker, and M.L. Fischer
University of Pittsburgh — R. Phillip Heine, Steven N. Caritis, T. Kamon, and J Roberts.
University of Miami — S. Beydoun, C. Alfonso, and F. Doyle
National Institute of Child Health and Human Development — D. McNellis, C. Catz and S.J. Yaffe
Ohio State University — Wayne Trout, F. Johnson, and M.B. Landon
University of Oklahoma — G. Thurnau and A. Meier
Medical University of South Carolina — B.A. Collins, F. LeBoeuf, and R.B. Newman
University of Tennessee — B.M. Mercer and R. Ramsey
University of Texas at San Antonio — M. Berkus and S. Nicholson
University of Texas Southwestern Medical Center — M.L. Sherman. And S. Bloom,
Thomas Jefferson University — M. DiVito and J. Tolosa
University of Utah — D. Dudley and L. Reynolds
Wake Forest University — P. Meis, E. Mueller-Heubach, and M. Swain
Wayne State University — S.F. Bottoms (deceased) and G.S. Norman.
Consultants: Sharon L. Hillier, Ph.D., University of Pittsburgh
Robert P. Nugent, Ph.D., M.P.H., National Institute of Child Health and Human Development
Contributor Information
Joan M. Mastrobattista, Department of Obstetrics, Gynecology and Reproductive Sciences, The University of Texas Medical School at Houston, 6431 Fannin Street, Suite 3.274, Houston, Texas 77030, Joan.M.Mastrobattista@uth.tmc.edu, (713) 500-6412, Fax: (713) 500-7860.
Mark A. Klebanoff, National Institute of Child Health and Human Development, Division of Epidemiology, Statistics & Prevention Research (DESPR), 6100 Executive Boulevard, Room 7B05F, MSC 7510, Bethesda, MD 20892-7510, klebanom@mail.nih.gov, (301) 496-5267, Fax: (301) 496-3790.
J. Christopher Carey, Denver Health and Professor, University of Colorado School of Medicine, 777 Bannock St. MC 0660, Denver, CO, 80204, J.Chris.Carey@dhha.org, (303) 602-9715, Fax: (303) 602-9734.
John C. Hauth, Department of Obstetrics and Gynecology, University of Alabama, Birmingham, 618 South 20th Street, Birmingham, AL 35233-7333, jchauth@uab.edu, (205) 934-9414, Fax: (205) 975-5723.
Cora A. MacPherson, Department of Epidemiology and Biostatistics, The George Washington University Biostatistics Center, 6110 Executive Boulevard, Suite 750, Rockville, MD, 20852, cora.mac@verizon.net, (301) 881-9260, Fax: (301) 816-0385.
J.M Ernest, Department of Obstetrics and Gynecology, Wake Forest University, Medical Center Boulevard, Winston-Salem, NC 27157, jernest@wfubmc.edu, (336) 716-1025, Fax: (336) 716-6937.
Margaret Cotroneo, Magee Women's Hospital, Department of Obstetrics, Gynecology and Reproductive Sciences, 300 Halket Street, Pittsburgh, PA 15213, mcotroneo@mail.magee.edu, (412) 641-4055, Fax: (412) 641-5518.
Kenneth J. Leveno, Department of Obstetrics and Gynecology, The University of Texas Southwestern Medical Center at Dallas, 5323 Harry Hines Boulevard, Dallas, TX 75390-9032, Kenneth.leveno@utsouthwestern.edu, (214) 648-2316, Fax: (214) 648-4763.
Ronald Wapner, Division of Maternal Fetal Medicine, Department of Obstetrics and Gynecology, Columbia Medical University, 16 East 60th Street, Room 480, New York, NY 10021, rw2191@columbia.edu, (212) 305-6293, Fax: (212) 305-4380.
Michael Varner, University of Utah Health Sciences Center Department of Obstetrics and Gynecology, 30 North 1900 East, Suite 2B200, Salt Lake City, UT 84132, michael.varner@hsc.utah.edu, (801) 585-5156, Fax: (801) 585-2594.
Jay D. Iams, Ohio State University, Department of Obstetrics and Gynecology, 563 Means Hall, 1654 Upham Drive, Columbus, OH 43210-1228, Jay.Iams@osumc.edu, (614) 293-8736, Fax: (614) 293-8993.
Atef Moawad, Department of Obstetrics and Gynecology, University of Chicago Medical Center, 5841 S. Maryland MC 2050, Chicago, IL 60637, amoawad@babies.bsd.uchicago.edu, (773) 702-5200, Fax: (773) 702-5160.
Baha M. Sibai, University of Cincinnati, Department of Obstetrics and Gynecology, 231 Albert Sabin Way, Cincinnati, OH 45267-0526, baha.sibai@uc.edu, (513) 558-8448, Fax: (513) 558-6138.
Menachem Miodovnik, Department of Obstetrics and Gynecology, Washington Hospital Center, 110 Irving Street, NW, Room 5B-63, Washington, DC 20010, Menachem.miodovnik@medstar.net, (202) 877-9663, Fax: (202) 877-5435.
Mitchell Dombrowski, Department of Obstetrics and Gynecology, St. John Hospital, 22151 Moross, Detroit, MI, 48236, Mitchell.Dombrowski@stjohn.org, (313) 343-7798, Fax: (313) 343-4932.
Mary J. O'Sullivan, University of Miami, Department of Obstetrics and Gynecology, Division of Research, R 136, Miami, FL, 33123, MOSullivan@med.miami.edu, (305) 243-7364.
J. Peter VanDorsten, Medical University of South Carolina, Department of Obstetrics and Gynecology, 96 Jonathan Lucas Street, Suite 634, Charleston, SC 29425, vandorsp@musc.edu, (843) 792-1668, Fax: 843-792-0533.
Oded Langer, Department of Obstetrics and Gynecology, St. Luke's-Roosevelt Hospital Center, 1000 10th Avenue, Suite 10C-01, New York, NY 10019, olanger@chpnet.org, (212) 523-5750, Fax: (212) 523-8066.
References
- 1.CDC National Center for Chronic Disease Prevention and Health Promotion: Nutrition and Physical Activity. Defining overweight and obesity. Available at: http://www.cdc.gov/nccdphp/dnps/obesity/defining.htm.
- 2.Cheymol G. Drug pharmacokinetics in the obese. Fundam Clin Pharmacol. 1988;2(3):239–256. doi: 10.1111/j.1472-8206.1988.tb00635.x. [DOI] [PubMed] [Google Scholar]
- 3.Carey JC, Klebanoff MA, Hauth JC, et al. Metronidazole to prevent preterm delivery in pregnant women with asymptomatic bacterial vaginosis. N Engl J Med. 2000;342:534–540. doi: 10.1056/NEJM200002243420802. [DOI] [PubMed] [Google Scholar]
- 4.Koumans EL, Markowitz LE, Hogan V, CDC BV Working Group Indications for therapy and treatment recommendations for bacterial vaginosis in nonpregnant and pregnant women: A synthesis of data. Clin Infect Dis. 2002;35(Suppl 2):S152–172. doi: 10.1086/342103. [DOI] [PubMed] [Google Scholar]
- 5.Hauth JC, Goldenberg RL, Andrews WW, DuBard MB, Copper RL. Reduced incidence of preterm delivery with metronidazole and erythromycin in women with bacterial vaginosis. N Engl J Med. 1995;333:1732–1736. doi: 10.1056/NEJM199512283332603. [DOI] [PubMed] [Google Scholar]
- 6.Hillier SL, Nugent RP, Eschenbach DA, et al. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. N Engl J Med. 1995;333:1737–1742. doi: 10.1056/NEJM199512283332604. [DOI] [PubMed] [Google Scholar]
- 7.Leitich H, Bodner-Adler B, Brunbauer M, Kaider A, Egarter C, Husslei P. Bacterial vaginosis as a risk factor for preterm delivery: A meta-analysis. Am J Obstet Gynecol. 2003;189:139–147. doi: 10.1067/mob.2003.339. [DOI] [PubMed] [Google Scholar]
- 8.Carey JC, Klebanoff MA, National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network What have we learned about vaginal infections and preterm birth? Semin Perinatol. 2003;27:212–216. doi: 10.1016/s0146-0005(03)00020-x. [DOI] [PubMed] [Google Scholar]
- 9.Kekki M, Kurki T, Kotomaki T, Sintonen H, Paavonen J. Cost-effectiveness of screening and treatment for bacterial vaginosis in early pregnancy among women at low risk for preterm birth. Acta Obstet Gynecol Scand. 2004;83:27–36. doi: 10.1111/j.1600-0412.2004.00262.x. [DOI] [PubMed] [Google Scholar]
- 10.Tebes CC, Lynch C, Sinnott J. The effect of treating bacterial vaginosis on preterm labor. Infect Dis Obstet Gynecol. 2003;11:123–129. doi: 10.1080/10647440300025509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of Gram stain interpretation. J Clin Microbiol. 1991;29:297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chow AW, Jewesson PJ. Pharmacokinetics and safety of antimicrobial agents during pregnancy. Rev Infect Dis. 1985;7:287–313. doi: 10.1093/clinids/7.3.287. [DOI] [PubMed] [Google Scholar]
- 13.Niebyl JR. Antibiotics and other anti-infective agents in pregnancy and lactation. Am J Perinatol. 2003;20:405–414. doi: 10.1055/s-2003-45391. [DOI] [PubMed] [Google Scholar]
- 14.Tracy JW, Webster LT., Jr . Drugs used in the chemotherapy of protozoal infections. In: Hardman JG, Limbird LE, Gilman AG, editors. Goodman & Gilman's The Pharmacologic Basis of Therapeutics. 10th. New York: McGraw-Hill Medical Publishing Division; 2001. pp. 1097–1120. [Google Scholar]
- 15.Centers for Disease Control and Prevention. Workowski KA, Berman SM. Sexaully transmitted diseases treatment guidelines 2006. MMWR Recomm Rep. 2006;55(No RR11):1–94. [PubMed] [Google Scholar]