Abstract
Bartonella quintana is a re-emerging pathogen and the causative agent of a variety of disease manifestations in humans including trench fever. Various typing methods have been developed for B. quintana, but these tend to be limited by poor resolution and, in the case of gel-based methods, a lack of portability. Multilocus sequence typing (MLST) has been used to study the molecular epidemiology of a large number of pathogens, including B. henselae, a close relative of B. quintana. We developed a MLST scheme for B. quintana based on the 7 MLST loci employed for B. henselae with two additional loci to cover underrepresented regions of the B. quintana chromosome. A total of 16 B. quintana isolates spanning over 60 years and three continents were characterized. Allelic variation was detected in five of the nine loci. Although only 8/4270 (0.002%) of the nucleotide sites examined were variable over all loci, these polymorphisms resolved the 16 isolates into seven sequence types (STs). We also demonstrate that MLST can be applied on uncultured isolates by direct PCR from cardiac valve tissue, and suggest this method presents a promising approach for epidemiological studies in this highly clonal organism. Phylogenetic and clustering analyses suggest that two of the seven STs form a distinct lineage within the population.
Introduction
Bartonella quintana is a fastidious, slow-growing, Gram-negative bacterium associated with a broad spectrum of disease manifestations, including trench fever, endocarditis, and bacillary angiomatosis [1], [2], [3]. Humans are the major reservoir for B. quintana and the human body louse is the principal vector [4], [5]. Recently, B. quintana was isolated by culture from the blood of a cynomolgus monkey [6] and B. quintana DNA was detected in specimens collected from cats and dogs and cat fleas, suggesting that other species may also be sporadically infected by this agent [7], [8], [9]. B. quintana is closely related to B. henselae, the agent of cat scratch disease, which can also cause bacillary angiomatosis, relapsing fever and endocarditis [3], [10]. Genome sequencing has revealed extensive genome reduction in B. quintana, suggesting that B. quintana is a genomic derivative of the more generalist species B. henselae [11].
Previous studies on genetic heterogeneity of B. quintana isolates have been carried out with different DNA fingerprinting methods including PCR–RFLP typing of the ribosomal intergenic spacer region, REP-PCR, ERIC-PCR, and pulsed-field gel electrophoresis (PFGE) [12], [13], [14]. These studies generally failed to reveal sufficient discrimination for epidemiological studies, suggesting that B. quintana is a highly conserved species. More recently, Foucault et al. described a Multispacer Typing (MST) method based on the sequence of highly polymorphic spacer regions of B. quintana [15]. This approach resolved only five MST profiles among 81 isolates and DNA extracts studied, which were mainly recovered from bacteremic homeless patients in France.
Multi-locus sequence typing (MLST) is based on the nucleotide sequences of housekeeping genes, and is a robust, standardizable and portable methodology that can be used in epidemiological and evolutionary studies [16], [17]. The aim of the present study was to develop a MLST scheme for B. quintana, based on the same genetic loci as those used previously for B. henselae [18], [19], [20], [21], [22]. We validated this scheme against a diverse sample of isolates representing three continents, over 60 years, and differing disease manifestations. Isolation of B. quintana by culture is often hampered by the fastidious nature of the organism, the requirement for prolonged incubation periods, and previous administration of antimicrobial chemotherapy. Therefore, the diagnosis is usually based on detection of bacterial DNA by PCR and/or serology. We sought to address the difficulties in culturing this organism by performing PCR directly from DNA collected from the cardiac valve tissue of a patient with culture-negative B. quintana endocarditis [23]. Analysis of the resulting data provides further evidence as to the clonality and phylogeny of the natural B. quintana population.
Results
Diversity among alleles and sequence types
All 16 isolates and the HROEH DNA extract were successfully sequenced at all nine MLST loci, with the sequenced alleles ranging in size between 416–510-bp at different loci (Table 1). Five of the nine loci were variable, atpF, ftsZ, groEL, nlpD, and rpoB, whereas bqtR, gap, gltA, and ribE were invariant. The average sequence divergence between all pairwise allelic comparisons was low, with the highest level being 0.4% observed in the atpF, groEL and rpoB locus, and no loci exhibited more than 3 alleles (Table 1). On average the data suggest that B. quintana is approximately 10% diverged from B. henselae at MLST genes (data not shown). The data resolved the 16 B. quintana isolates into 7 sequence types (STs), the most frequent being STs 1 and 2, represented by 5 and 6 isolates, respectively (Table 2). STs 3–7 were represented by single isolates. The polymorphisms observed within the ftsZ, groEL, and rpoB loci were consistent with previous studies [24], [25], [26]. The two copies of the sequenced Toulouse isolate included in this study (Freiburg and CIP 103739) displayed an identical allelic profile, which differed from the Toulouse strain/copy Uppsala deposited in Genbank (BX897700.1) by a single base change at both the rpoB and nlpD loci (corresponding to allele 2 at these loci in the current study, and allele 1 at these loci in the Uppsala copy).
Table 1. Characteristics of the nine loci evaluated for the B. quintana MLST scheme.
| Locus | Length of analysed sequence (bp) | No. of alleles | No. of variable sites | % sequence diversity [range (mean)] | Allelic polymorphism by reference to BX897700.1 (allele 1) |
| atpF | 495 | 2 | 2 | 0.4 | 2: C391858T, T391985C |
| bqtR | 510 | 1 | 0 | 0 | - |
| ftsZ | 416 | 2 | 1 | 0.2 | 2: C1036988G |
| gap | 504 | 1 | 0 | 0 | - |
| gltA | 450 | 1 | 0 | 0 | - |
| groEL | 466 | 3 | 2 | 0.2–0.4 (0.3) | 2: A1277454G, C1277746T 3: C1277746T |
| nlpD | 461 | 2 | 1 | 0.2 | 2: A585446G |
| ribE | 458 | 1 | 0 | 0 | - |
| rpoB | 510 | 3 | 2 | 0.2–0.4 (0.3) | 2: G847761A 3: G847761A, G847791A |
Table 2. Allelic profiles and sequence types (ST) obtained for the 16 B. quintana isolates and the HROEH DNA extract.
| Strains | atpF | bqtR | ftsZ | gap | gltA | groEL | nlpD | ribE | rpoB | ST |
| SH-Perm | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Oklahoma | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Jouhanneau | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| UR.BQ.MBA 263 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| UR.BQ.MNHP 295 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| JK-31 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 2 |
| BQ2D-70 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 2 |
| HROEH (DNA) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 2 |
| UR.BQ.TIE 326 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 2 |
| UR.BQ.MTF 357 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 2 |
| UR.BQ.MNHP 374 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 2 |
| Toulouse (copy Freiburg and CIP 103739) | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 2 | 3 |
| Munich | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 2 | 4 |
| Fuller | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 1 | 3 | 5 |
| UR.BQ.MTF 335 | 1 | 1 | 2 | 1 | 1 | 3 | 1 | 1 | 2 | 6 |
| Adelaide 1300/002 | 2 | 1 | 2 | 1 | 1 | 3 | 1 | 1 | 2 | 7 |
Stability of the allelic polymorphisms
In order to evaluate the stability of the polymorphisms, four B. quintana isolates from different countries that were assigned to different STs were subjected to serial in vitro passages. The subculture 20 isolates were subsequently subjected to MLST analysis. The allelic sequences and profiles obtained from the serially passages isolates were 100% consistent with the results obtained from the primary isolates (data not shown). In addition, the clonally related isolates JK-31 and BQ2-D70, which represent different copies of the same original isolate, and the two copies of the Toulouse isolate included in this study (Freiburg and CIP 103739), revealed an identical allelic profile, indicating a high stability of the allelic polymorphisms detected in this study.
Geographical and temporal distribution of B. quintana genotypes
There is little evidence for concordance between the MLST data and geographical source. Isolates from the same location exhibited different genotypes; for example, of the five isolates recovered from Marseille between 2002 and 2006, two corresponded to ST1, two to ST2, and one to ST6. Furthermore isolates from quite different locations exhibited the same genotypes; for example, ST1 corresponded to isolates from Russia, USA and France. The data also point to a remarkable temporal stability of this species. Most strikingly, the isolate SH-PERM (ST1), which was isolated in Russia during or shortly after the second world war, was identical over all nine loci (4270 bp) to an isolate from the USA sampled in 1990 and to three isolates from France sampled in the 1990ies, 2002 and 2003.
Clonal and phylogenetic analysis
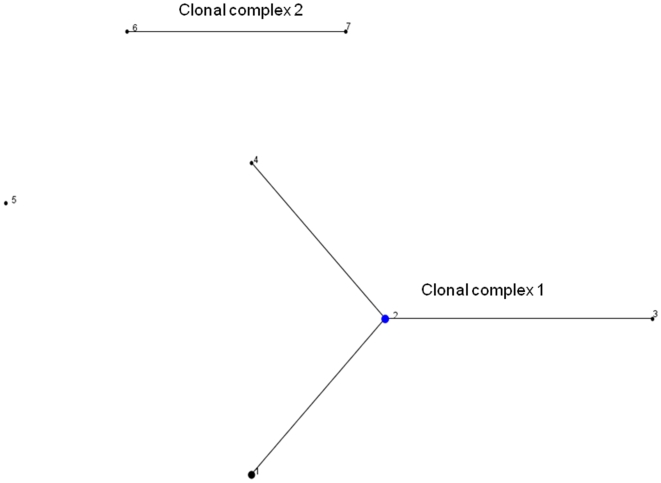
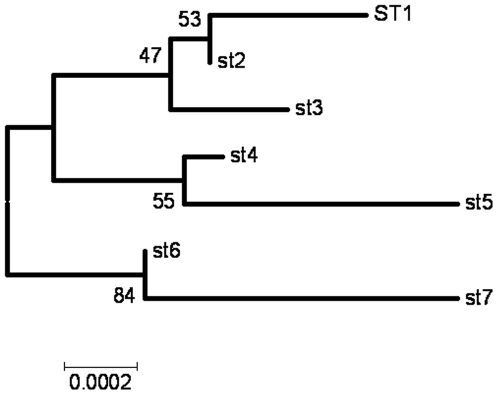
The relationships between the STs was first examined using eBURST, which uses allele profiles rather than sequences and does not attempt to reconstruct the relationships between the different clonal lineages. The majority of the isolates (13/16, 81.2%) corresponded to four STs, which formed a single clonal complex, clonal complex 1, with ST2 as primary founder, an assignment which is consistent with the fact this was the most commonly observed ST (Figure 1). STs 6 and 7 are single locus variants and ST5 is a singleton (differing at two or more genes from every other isolate). The clustering of STs 6 and 7 away from the other genotypes is also supported by neighbour-joining tree based on the concatenated sequences, with a bootstrap score of 84 (Figure 2).
Figure 1. Phylogenetic relationship between different B. quintana STs as determined by eBURST.
A clonal complex contains STs that have 8 out of 9 alleles in common. ST5 is assigned as a singleton since it differed in 3–7 alleles from all other STs. The size of the circles relates to the frequency of the corresponding ST, and illustrates that the assigned primary founder of the major clonal complex (ST2) is a common clone.
Figure 2. Neighbour-joining tree of the concatenated sequences of B. quintana STs as reconstructed by MEGA4.
1000 bootstrap replicates were used to examine the confidence in the tree. The clustering of STs 6 and 7 away from the other genotypes was supported by a bootstrap score of 84.
Discussion
Multi-locus sequence typing generates data which is highly discriminatory, reproducible, simple to perform, and portable, and here we describe a novel MLST scheme for B. quintana. The overall level of sequence divergence was very low, consistent with previous studies in this species. Four of the nine loci examined in this study were invariant, and future MLST studies on B. quintana might exclude these genes. Three of these invariant genes (batR, gltA, and ribC) have previously been found to be polymorphic in B. henselae [18], [20], [22], indicating that B. quintana is more homogenous than B. henselae. This observation supports the view that the former derived from the latter. Despite the low level of variation in B. quintana, the 16 diverse isolates were resolved into 7 STs, which represents a favourable degree of discrimination relative to other typing schemes in this species. For example, PCR–RFLP, REP-PCR, ERIC-PCR, and PFGE have failed to reveal any variation between B. quintana isolates [12], [13], and MST resolved 5 genotypes among 71 B. quintana isolates and 10 DNA extracts studied [15]. It is important to note that the majority of isolates (51/71) examined in the latter study were obtained from the blood of bacteremic homeless people in Marseille. The overrepresentation of isolates from a specific group of patients from the same geographic region might explain the relative low level of sequence polymorphism detected among the B. quintana isolates examined by MST. In contrast, our isolate collection is, although smaller in size, a global panel containing isolates associated with different disease manifestations that were collected at different time points in 10 distinct geographic regions on three continents. Hence, it is not possible to compare the discriminatory power of MLST with MST on the basis of the results of our study. Further studies using the same panel of isolates are necessary to allow a formal comparison of both methods.
The stability and reproducibility of the MLST results were confirmed by subjecting four diverse isolates to serial in vitro passages and repeating the MLST analysis with 100% consistency. MLST also gave identical results for the JK-31 and BQ2-D70 isolates, which represent different copies of the same original isolate prior and after in vivo passages. We also demonstrate that MLST is possible via direct PCR from clinical samples, without the need to culture the isolate. The fastidious growth requirements of this organism means this presents a considerable advantage, although caution is urged to guard against misleading results arising from mixed infection, which may be evident as mixed peaks on the sequence chromatograms.
Although the two copies of the Toulouse strain included in this study (copy Freiburg and CIP 103739) produced identical results, these differed from the Toulouse strain/copy Uppsala, which has been deposited in GenBank [11]. Whilst it remains formally possible that this variation reflects changes during in vitro passage, we consider this unlikely due to the stability of this species both in the laboratory and in the natural population. A re-examination of the sequence data obtained by the whole genome sequencing, and/or the identity of the Toulouse strain/copy Uppsala should help to resolve this discrepancy.
The MLST data provide no evidence of geographical structuring and indicate striking clonal stability. In particular, ST1 corresponds to strains isolated approximately 60 years apart (SH-PERM and UR.BQ.MNHP 295). This temporal stability may reflect, in part, long generation times, consistent with intimate host association, genome degradation and the fastidious growth requirements of this organism in the laboratory. The lack of geographical structure suggests that rates of global migration, most likely via the human host, have been sufficiently high to disseminate the rarely emerging variants. It is noteworthy that this species is particularly associated with the two world wars of the last century, events which precipitated intense large-scale human migration. Further studies on a larger collection of isolates would help to improve our understanding of the temporal and geographic distribution of B. quintana.
Because it is based on the sequences of multiple housekeeping loci which are predominantly under stabilising selection, MLST data can also be used to examine the evolutionary relationships between strains [17]. Although the number of informative sites and allele diversity was too low for comprehensive analysis, eBURST and phylogenetic analysis revealed a consistent division between STs 6 and 7 and the rest of the B. quintana population. These genotypes correspond to isolates from France and Australia respectively, which again implies a lack of concordance between genetic variation and geographical source.
In summary, the B. quintana MLST scheme provides favourable levels of discrimination, within the context of a highly clonal species, and can be applied directly to clinical specimens containing uncultured isolates. The data provide evidence for a high level of temporal stability, consistent with long generation-times, and a robust phylogenetic division within the population. We note little evidence of geographic structuring, which implies high rates of global migration via the human host, although further studies on a larger strain collection are required to improve our understanding of the natural population of this highly clonal organism.
Materials and Methods
B. quintana isolates and growth conditions
Sixteen B. quintana isolates from six countries were analysed in this study, 14 of which were epidemiologically unrelated. Table 3 summarises the clinical and epidemiological data of all isolates studied. The BQ2-D70 isolate was isolated from the blood of a Rhesus macaque at day 70 after its experimental infection with JK-31 [27], hence BQ2-D70 represents JK-31 after multiple in vivo passages. The B. quintana Toulouse isolate, which has been recently sequenced [11], was included twice: as copy Freiburg (obtained from the Bartonella collection of the Institute for Medical Microbiology at the University of Freiburg, Germany [28], and CIP 103739 (obtained from the Culture Collection of the Institute Pasteur, Paris). The SH-Perm isolate was probably isolated in the city of Perm, Russia, from a patient suffering from trench fever during or after the second World War [10], [15]. HROEH was a DNA extract from the cardiac valve of a patient with culture-negative endocarditis by B. quintana, who underwent cardiac surgery in Rostock, Germany [23]. Strains were stored at −20°C or −80°C until use. The isolates were grown on Columbia agar with 5% sheep blood (Becton Dickenson) at 37°C in 5% CO2 for 7–14 d, and passaged once on blood agar prior to isolation of bacterial DNA.
Table 3. Characteristics of the 16 B. quintana isolates studied.
| Isolate/DNA extract | Source of isolation, disease manifestation | Geographic origin | Isolation year | Reference |
| Fuller | Blood, trench fever | Yugoslavia | 1945 | [33] |
| SH-PERM | unknown, trench fever | Russia | World War II | [15] |
| Oklahoma | Blood, BA | Oklahoma city, USA | 1990 | [34] |
| JK-31 | Tissue, BA | California, USA | Unknown | [27] |
| BQ2-D70 | Blood, Rhesus macaque | California, USA | Unknown | [27] |
| Adelaide 1300/02 | Blood, endocarditis | Adelaide, Australia | 2002 | [35] |
| Toulouse -copy Freiburg -CIP 103739 | Blood, BA | Toulouse, France | 1992 | [36] |
| Jouhanneau | Blood, endocarditis | Paris, France | Unknown | this study |
| UR.BQ.MBA 263 | Blood, BA | Marseille, France | 2002 | this study |
| UR.BQ.MNHP 295 | Louse | Marseille, France | 2003 | this study |
| UR.BQ.TIE 326 | Cardiac valve, endocarditis | Toulouse, France | 2004 | this study |
| UR.BQ.MTF 335 | Blood, bacteraemia | Marseille, France | 2005 | this study |
| UR.BQ.MTF 357 | Blood, bacteraemia | Marseille, France | 2005 | this study |
| UR.BQ.MNHP 374 | Louse | Marseille, France | 2006 | this study |
| Munich | Blood, BA | Munich, Germany | 1994 | [37] |
| HROEH (DNA) | Cardiac valve, endocarditis | Rostock, Germany | 2007 | [23] |
HROEH was a DNA extract from the cardiac valve tissue of a patient with culture-negative endocarditis by B. quintana.
BA, bacillary angiomatosis.
Multi-locus sequence typing
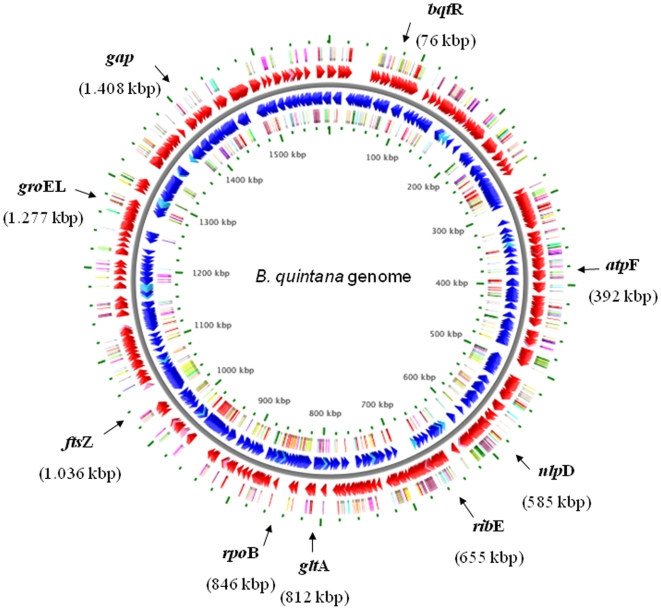
Nucleotide sequence data were collected from 9 genetic loci presented in Table 1. Seven of these loci, i.e. bqtR (homologue to batR), ftsZ, gltA, groEL, nlpD, ribE (homologue to ribC) and rpoB, are contained in the MLST scheme for B. henselae and were therefore selected for the MLST scheme for B. quintana [18], [22]. Two additional loci, atpF and gap, were selected because they are housekeeping genes that have been used in other MLST schemes [29], [30], and are located at underrepresented sites of the B. quintana chromosome. The distribution of alleles on the B. quintana chromosome is presented in Figure 3. Internal fragments of 531–622 length were amplified by using the primers presented in Table 4. PCR was performed in 50 µl volume as described previously [20]. Amplification was carried out for all loci by denaturation at 94°C for 5 min followed by 35 cycles (94°C for 1 min, 48°C for 30 s, and 72°C for 45 s) and a final extension step at 72°C for 6 min. The products were purified with a PCR purification kit from Quiagen Inc. (Hilden, Germany) and sequenced on both strands with the primers used for the initial amplification using the ABI PRISM BigDye Terminator cycle sequencing kit (Applied Biosystems) and a 3730XL DNA Analyzer (Applied Biosystems, Foster City, USA). The results were confirmed by repeats when necessary. The reliability of the sequence data was controlled by subjecting two randomly selected isolates in a blinded manner as “quality control strains” to the MLST analysis. The results of the quality control strains were 100% consistent with those obtained from the “original isolates”.
Figure 3. Localization of the 9 genetic loci used in the B. quintana MLST scheme on the chromosome of B. quintana.
The genomic map was adapted from http://wishart.biology.ualberta.ca/BacMap/graphs_cgview.html [32]. The position of the loci has been marked by arrows.
Table 4. Primers used for the amplification and sequencing of the nine genetic loci evaluated for the B. quintana MLST scheme.
| Locus | Putative gene product | Product size (bp) | Position on chromosome (bp) 1 | Forward primer (5′-3′) | Reverse primer (5′-3′) |
| atpF | ATP Synthase ß-chain | 606 | 391.779–392.384 | catcagagcatgcagatcgt | cgatgcacaatcatttctgg |
| bqtR | B. quintana transcriptional regulator | 622 | 76.463–77.084 | ttgcgacaaaacagcttcac | gatggagcatctgcactcaa |
| ftsZ | cell devision protein ftsZ | 531 | 1.036.753–1.037.283 | ccatagcaaaagcatcagca | aattgaagccacgcattacc |
| gap | glyceraldehyde 3-phosphate dehydrogenase | 612 | 1.408.521–1.409.132 | caaaaccaatcccacagctt | ttgcgtgctattgtggagag |
| gltA | citrat synthase | 558 | 811.826–812.383 | gcggttatcctatcgaccaa | ctgctgcgatacatgcaaat |
| groEL | Heat shock protein 60 chaperone | 569 | 1.277.324–1.277.892 | attggaggcattggagtgtc | cgcgtaaaccaaatcaaggt |
| nlpD | lipoprotein, outer membrane protein | 548 | 584.964–585.511 | gacgctttctcctgatggtc | tcaataaacgccctcgtacc |
| ribE | riboflavin synthase α-chain | 575 | 654.876–655.450 | gttcggaaatgaccgttgtt | gcttgtccccaagttgtcat |
| rpoB | RNA polymerase ß-subunit | 605 | 847.962–847.358 | tggtgatcgggtagagaagg | gacgcgcataaacattacga |
Corresponding to the complete genome sequence of B. quintana strain Toulouse/copy Uppsala, GenBank accession number BX897700.1.
In one set of experiments, the B. quintana isolates Adelaide, Jouhanneau, JK-30 and BQ2-D70 were serially passaged on Columbia blood agar for 20 times. DNA isolated from the 20th subculture was then subjected to MLST analysis again.
Analysis of MLST Data
The nucleotide sequences were analysed with the DNASTAR Lasergene software package 7 (DNASTAR, Madison, USA). Different sequences of a given locus were given allele number, and each unique combination of alleles, i.e. the allelic profile, was assigned a sequence type (ST). The sequence of the B. quintana Toulouse/copy Uppsala (GenBank accession number BX897700.1) was assigned as allele 1 for each locus, and the allelic profile of this strain was assigned ST1.
Phylogenetic analysis
The definition of clonal complexes and the examination of relationships between STs within clonal complexes were carried out by using eBURST (http://eburst.mlst.net). A neighbour-joining tree was reconstructed from the concatenated MLST alleles using the kimura-2-parameter distance measures as implemented in MEGA4 [31].
Nucleotide sequence accession number
The sequences of the alleles from the B. quintana MLST scheme presented here have been deposited in GenBank under the following accession numbers GU946557 to GU946572.
Acknowledgments
We thank Drs. Guillaume Arlet, Jane E. Koehler, Stefanie Kunz, Hans-Ulrich Schmidt and Mark W. Woolley for providing B. quintana isolates.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: These authors have no support or funding to report.
References
- 1.Brouqui P, Lascola B, Roux V, Raoult D. Chronic Bartonella quintana bacteremia in homeless patients. N Engl J Med. 1999;340:184–189. doi: 10.1056/NEJM199901213400303. [DOI] [PubMed] [Google Scholar]
- 2.Drancourt M, Mainardi JL, Brouqui P, Vandenesch F, Carta A, et al. Bartonella (Rochalimaea) quintana endocarditis in three homeless men. N Engl J Med. 1995;332:419–423. doi: 10.1056/NEJM199502163320702. [DOI] [PubMed] [Google Scholar]
- 3.Koehler JE, Quinn FD, Berger TG, LeBoit PE, Tappero JW. Isolation of Rochalimaea species from cutaneous and osseous lesions of bacillary angiomatosis. N Engl J Med. 1992;327:1625–1631. doi: 10.1056/NEJM199212033272303. [DOI] [PubMed] [Google Scholar]
- 4.Raoult D, Roux V. The body louse as a vector of reemerging human diseases. Clin Infect Dis. 1999;29:888–911. doi: 10.1086/520454. [DOI] [PubMed] [Google Scholar]
- 5.Maurin M, Raoult D. Bartonella (Rochalimaea) quintana infections. Clin Microbiol Rev. 1996;9:273–292. doi: 10.1128/cmr.9.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Rourke LG, Pitulle C, Hegarty BC, Kraycirik S, Killary KA, et al. Bartonella quintana in cynomolgus monkey (Macaca fascicularis). Emerg Infect Dis. 2005;11:1931–1934. doi: 10.3201/eid1112.030045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.La VD, Tran-Hung L, Aboudharam G, Raoult D, Drancourt M. Bartonella quintana in domestic cat. Emerg Infect Dis. 2005;11:1287–1289. doi: 10.3201/eid1108.050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breitschwerdt EB, Maggi RG, Sigmon B, Nicholson WL. Isolation of Bartonella quintana from a woman and a cat following putative bite transmission. J Clin Microbiol. 2007;45:270–272. doi: 10.1128/JCM.01451-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelly P, Rolain JM, Maggi R, Sontakke S, Keene B, et al. Bartonella quintana endocarditis in dogs. Emerg Infect Dis. 2006;12:1869–1872. doi: 10.3201/eid1212.060724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson B, Sims K, Regnery R, Robinson L, Schmidt MJ, et al. Detection of Rochalimaea henselae DNA in specimens from cat scratch disease patients by PCR. J Clin Microbiol. 1994;32:942–948. doi: 10.1128/jcm.32.4.942-948.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alsmark CM, Frank AC, Karlberg EO, Legault B-A, Ardell DH, et al. The louse-borne human pathogen Bartonella quintana is a genomic derivative of the zoonotic agent Bartonella henselae. PNAS. 2004;101:9716–9721. doi: 10.1073/pnas.0305659101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spach DH, Kanter AS, Dougherty MJ, Larson AM, Coyle MB, et al. Bartonella (Rochalimaea) quintana bacteremia in inner-city patients with chronic alcoholism. N Engl J Med. 1995;332:424–428. doi: 10.1056/NEJM199502163320703. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez-Barradas MC, Hamill RJ, Houston ED, Georghiou PR, Clarridge JE, et al. Genomic fingerprinting of Bartonella species by repetitive element PCR for distinguishing species and isolates. J Clin Microbiol. 1995;33:1089–1093. doi: 10.1128/jcm.33.5.1089-1093.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roux V, Raoult D. Inter- and intraspecies identification of Bartonella (Rochalimaea) species. J Clin Microbiol. 1995;33:1573–1579. doi: 10.1128/jcm.33.6.1573-1579.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foucault C, La Scola B, Lindroos H, Andersson SG, Raoult D. Multispacer typing technique for sequence-based typing of Bartonella quintana. J Clin Microbiol. 2005;43:41–48. doi: 10.1128/JCM.43.1.41-48.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feil EJ, Enright MC. Analyses of clonality and the evolution of bacterial pathogens. Curr Opin Microbiol. 2004;7:308–313. doi: 10.1016/j.mib.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Maiden MC. Multilocus sequence typing of bacteria. Annu Rev Microbiol. 2006;60:561–588. doi: 10.1146/annurev.micro.59.030804.121325. [DOI] [PubMed] [Google Scholar]
- 18.Iredell J, Blanckenberg D, Arvand M, Grauling S, Feil EJ, et al. Characterization of the natural population of Bartonella henselae by multilocus sequence typing. J Clin Microbiol. 2003;41:5071–5079. doi: 10.1128/JCM.41.11.5071-5079.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berghoff J, Viezens J, Guptill L, Fabbi M, Arvand M. Bartonella henselae exists as a mosaic of different genetic variants in the infected host. Microbiology. 2007;153:2045–2051. doi: 10.1099/mic.0.2007/006379-0. [DOI] [PubMed] [Google Scholar]
- 20.Arvand M, Viezens J. Evaluation of pulsed-field gel electrophoresis and multi-locus sequence typing for the analysis of clonal relatedness among Bartonella henselae isolates. Int J Med Microbiol. 2007;297:255–262. doi: 10.1016/j.ijmm.2007.02.001. Epub 2007 Mar 2030. [DOI] [PubMed] [Google Scholar]
- 21.Arvand M, Schubert H, Viezens J. Emergence of distinct genetic variants in the population of primary Bartonella henselae isolates. Microbes Infect. 2006;8:1315–1320. doi: 10.1016/j.micinf.2005.12.015. Epub 2006 Mar 1315. [DOI] [PubMed] [Google Scholar]
- 22.Arvand M, Feil EJ, Giladi M, Boulouis HJ, Viezens J. Multi-Locus Sequence Typing of Bartonella henselae Isolates from Three Continents Reveals Hypervirulent and Feline-Associated Clones. PLoS ONE. 2007;2:e1346. doi: 10.1371/journal.pone.0001346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yerebakan C, Westphal B, Aepinus C. Infective endocarditis due to Bartonella quintana: a challenging diagnostic entity. Acta Cardiol. 2008;63:519–521. doi: 10.2143/AC.63.4.2033053. [DOI] [PubMed] [Google Scholar]
- 24.Marston EL, Sumner JW, Regnery RL. Evaluation of intraspecies genetic variation within the 60 kDa heat-shock protein gene (groEL) of Bartonella species. Int J Syst Bacteriol. 1999;49 Pt 3:1015–1023. doi: 10.1099/00207713-49-3-1015. [DOI] [PubMed] [Google Scholar]
- 25.Birtles RJ, Raoult D. Comparison of partial citrate synthase gene (gltA) sequences for phylogenetic analysis of Bartonella species. Int J Syst Bacteriol. 1996;46:891–897. doi: 10.1099/00207713-46-4-891. [DOI] [PubMed] [Google Scholar]
- 26.Renesto P, Gouvernet J, Drancourt M, Roux V, Raoult D. Use of rpoB gene analysis for detection and identification of Bartonella species. J Clin Microbiol. 2001;39:430–437. doi: 10.1128/JCM.39.2.430-437.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang P, Chomel BB, Schau MK, Goo JS, Droz S, et al. A family of variably expressed outer-membrane proteins (Vomp) mediates adhesion and autoaggregation in Bartonella quintana. Proc Natl Acad Sci U S A. 2004;101:13630–13635. doi: 10.1073/pnas.0405284101. Epub 12004 Sep 13633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sander A, Penno S. Semiquantitative species-specific detection of Bartonella henselae and Bartonella quintana by PCR-enzyme immunoassay. J Clin Microbiol. 1999;37:3097–3101. doi: 10.1128/jcm.37.10.3097-3101.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meats E, Feil EJ, Stringer S, Cody AJ, Goldstein R, et al. Characterization of encapsulated and noncapsulated Haemophilus influenzae and determination of phylogenetic relationships by multilocus sequence typing. J Clin Microbiol. 2003;41:1623–1636. doi: 10.1128/JCM.41.4.1623-1636.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Homan WL, Tribe D, Poznanski S, Li M, Hogg G, et al. Multilocus sequence typing scheme for Enterococcus faecium. J Clin Microbiol. 2002;40:1963–1971. doi: 10.1128/JCM.40.6.1963-1971.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. Epub 2007 May 1597. [DOI] [PubMed] [Google Scholar]
- 32.Stothard P, Van Domselaar G, Shrivastava S, Guo A, O'Neill B, et al. BacMap: an interactive picture atlas of annotated bacterial genomes. Nucl Acids Res. 2005;33:D317–320. doi: 10.1093/nar/gki075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mooser H, Leemanns A, Chao SH, Gubler HU. Beobachtungen an Fünftagefieber. Schweizerische Zeitschrift für Pathologie und Bakteriologie. 1948;11:513–522. [PubMed] [Google Scholar]
- 34.Welch DF, Pickett DA, Slater LN, Steigerwalt AG, Brenner DJ. Rochalimaea henselae sp. nov., a cause of septicemia, bacillary angiomatosis, and parenchymal bacillary peliosis. J Clin Microbiol. 1992;30:275–280. doi: 10.1128/jcm.30.2.275-280.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woolley MW, Gordon DL, Wetherall BL. Analysis of the first Australian strains of Bartonella quintana reveals unique genotypes. J Clin Microbiol. 2007;45:2040–2043. doi: 10.1128/JCM.00175-07. Epub 2007 Apr 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maurin M, Roux V, Stein A, Ferrier F, Viraben R, et al. Isolation and characterization by immunofluorescence, sodium dodecyl sulfate-polyacrylamide gel electrophoresis, western blot, restriction fragment length polymorphism-PCR, 16S rRNA gene sequencing, and pulsed-field gel electrophoresis of Rochalimaea quintana from a patient with bacillary angiomatosis. J Clin Microbiol. 1994;32:1166–1171. doi: 10.1128/jcm.32.5.1166-1171.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmidt HU, Kaliebe T, Poppinger J, Buhler C, Sander A. Isolation of Bartonella quintana from an HIV-positive patient with bacillary angiomatosis. Eur J Clin Microbiol Infect Dis. 1996;15:736–741. doi: 10.1007/BF01691961. [DOI] [PubMed] [Google Scholar]