Abstract
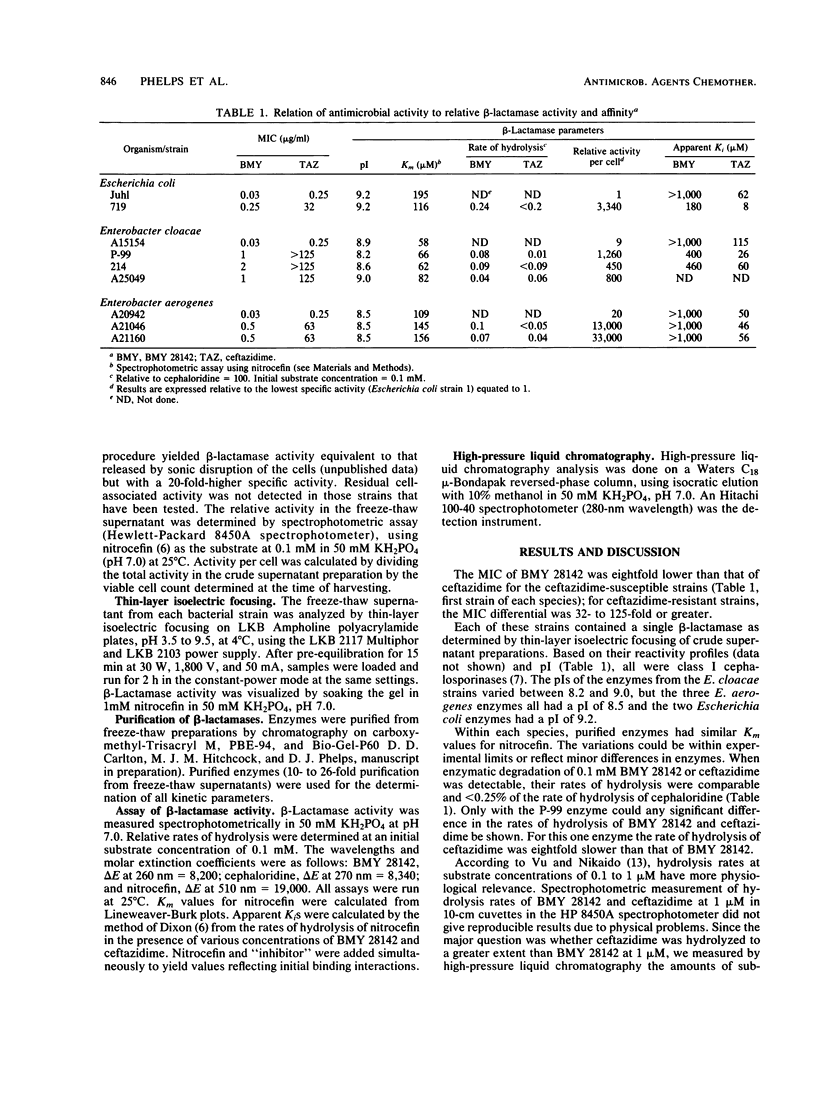
Strains of Escherichia coli, Enterobacter aerogenes, and Enterobacter cloacae that were resistant to ceftazidime (MIC greater than 16 micrograms/ml) but susceptible to BMY 28142 (MIC less than 4 micrograms/ml) were found to contain higher levels of beta-lactamase activity (50- to 3,340-fold) than control strains of the corresponding species. Ceftazidime was at least as resistant as BMY 28142 to hydrolysis by these enzymes. However, the apparent Ki of BMY 28142 for each enzyme was larger (8- to greater than 20-fold) than that of ceftazidime; i.e., the affinity of these enzymes for BMY 28142 appeared to be lower than that for ceftazidime. Thus, BMY 28142 was affected less than ceftazidime by a mechanism of resistance that depends, at least in part, on the relative affinities of cephalosporins for the beta-lactamases of these species. These results indicate that the affinity between a beta-lactamase and a cephalosporin may be a distinguishing factor in the evaluation of beta-lactamase-resistant cephalosporins and suggest that affinity can play a major role in susceptibility to highly beta-lactamase-resistant cephalosporins.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cullmann W., Opferkuch W., Stieglitz M., Dick W. Influence of spontaneous and inducible beta-lactamase production on the antimicrobial activity of recently developed beta-lactam compounds. Chemotherapy. 1984;30(3):175–181. doi: 10.1159/000238265. [DOI] [PubMed] [Google Scholar]
- Cullmann W., Opferkuch W., Stieglitz M. Relation between beta-lactamase production and antimicrobial activity: comparison of the new compound HR 810 with cefotaxime. Eur J Clin Microbiol. 1983 Aug;2(4):350–352. doi: 10.1007/BF02019466. [DOI] [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutmann L., Williamson R. A model system to demonstrate that beta-lactamase-associated antibiotic trapping could be a potential means of resistance. J Infect Dis. 1983 Aug;148(2):316–321. doi: 10.1093/infdis/148.2.316. [DOI] [PubMed] [Google Scholar]
- Kessler R. E., Bies M., Buck R. E., Chisholm D. R., Pursiano T. A., Tsai Y. H., Misiek M., Price K. E., Leitner F. Comparison of a new cephalosporin, BMY 28142, with other broad-spectrum beta-lactam antibiotics. Antimicrob Agents Chemother. 1985 Feb;27(2):207–216. doi: 10.1128/aac.27.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond M. H., Sykes R. B. The beta-lactamases of gram-negative bacteria and their possible physiological role. Adv Microb Physiol. 1973;9:31–88. doi: 10.1016/s0065-2911(08)60376-8. [DOI] [PubMed] [Google Scholar]
- Sanders C. C. Inducible beta-lactamases and non-hydrolytic resistance mechanisms. J Antimicrob Chemother. 1984 Jan;13(1):1–3. doi: 10.1093/jac/13.1.1. [DOI] [PubMed] [Google Scholar]
- Seeberg A. H., Tolxdorff-Neutzling R. M., Wiedemann B. Chromosomal beta-lactamases of Enterobacter cloacae are responsible for resistance to third-generation cephalosporins. Antimicrob Agents Chemother. 1983 Jun;23(6):918–925. doi: 10.1128/aac.23.6.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi I., Sawai T., Ando T., Yamagishi S. Cefoxitin resistance by a chromosomal cephalosporinase in Escherichia coli. J Antibiot (Tokyo) 1980 Sep;33(9):1037–1042. doi: 10.7164/antibiotics.33.1037. [DOI] [PubMed] [Google Scholar]
- Tolxdorff-Neutzling R. M., Wiedemann B. HR 810, a cephalosporin with low affinity for Enterobacter cloacae beta-lactamase. Eur J Clin Microbiol. 1983 Aug;2(4):352–354. doi: 10.1007/BF02019467. [DOI] [PubMed] [Google Scholar]
- Vu H., Nikaido H. Role of beta-lactam hydrolysis in the mechanism of resistance of a beta-lactamase-constitutive Enterobacter cloacae strain to expanded-spectrum beta-lactams. Antimicrob Agents Chemother. 1985 Mar;27(3):393–398. doi: 10.1128/aac.27.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Yokota T. Beta-lactamase-directed barrier for penicillins of Escherichia coli carrying R plasmids. Antimicrob Agents Chemother. 1977 Jun;11(6):936–940. doi: 10.1128/aac.11.6.936. [DOI] [PMC free article] [PubMed] [Google Scholar]



