Abstract
Netrin-1 was recently proposed to control tumorigenesis by inhibiting apoptosis induced by the dependence receptors DCC (Deleted in colorectal cancer) and UNC5H. While a loss of these dependence receptors’ expression has been described as a selective advantage for tumor growth and progression in numerous cancers, recent observations have shown that some tumors may utilize an alternative strategy to block dependence receptor-induced programmed cell death: the autocrine expression of netrin-1. This alternative strategy has been observed in a large fraction of aggressive breast cancers, neuroblastoma, pancreatic adenocarcinoma, and lung cancer. This observation is of potential interest regarding future targeted therapy, since in such cases interfering with the ability of netrin-1 to inhibit DCC or UNC5H-induced cell death is associated with apoptosis of netrin-1-expressing tumor cells in vitro, and with inhibition of tumor growth or metastasis in different mouse tumor models. The understanding of the mechanism by which netrin-1 inhibits cell death is therefore of interest. Here, we show that netrin-1 triggers the multimerization of both DCC and UNC5H2 receptors, and that multimerization of the intracellular domain of DCC and UNC5H2 is the critical step to inhibit the pro-apoptotic effects of both of these receptors. Taking advantage of this property, we utilized a recombinant specific domain of DCC that (i) interacts with netrin-1 and (ii) inhibits netrin-1- induced multimerization, to trigger apoptosis in netrin-dependent tumor cells.
Keywords: Animals; Apoptosis; Cell Line; Chickens; Disease Models, Animal; Humans; Neoplasms; metabolism; Nerve Growth Factors; pharmacology; Protein Multimerization; drug effects; Receptors, Cell Surface; metabolism; Recombinant Proteins; pharmacology; Tumor Suppressor Proteins; metabolism; pharmacology
Introduction
Dependence receptors form a group of receptors that share the ability to induce cell death when expressed in settings in which their trophic ligands are unavailable (1). The survival of cells that express such receptors is hypothesized to be dependent on the suppression of the receptor-mediated cell death by the receptors’ ligands. This functional receptor family includes the common neurotrophin receptor p75NTR (2), the GDNF receptor RET (3), the Sonic Hedgehog receptor Patched (4), some integrins (5), the RGM receptor neogenin (6), ALK (7), the NT-3 receptor TrkC (8), the EphrinB3 receptor EphA4 (9) and the netrin-1 receptors, DCC (10) and UNC5H (11).
Netrin-1 was initially discovered as a laminin-related molecule, produced by the floor plate, that attracts commissural axons as they migrate from the dorsal to the ventral spinal cord during the development of the nervous system (12, 13). It was predicted from genetic screens that the C. elegans netrin-1 -UNC6- interacted with UNC40 and with UNC5 (14). Four orthologues of UNC5 were identified in mammals: UNC5H1, H2, H3, and H4; and UNC40 was found to be the orthologue of the vertebrate DCC (Deleted in Colorectal Cancer) (15). The DCC gene was initially discovered by Vogelstein and colleagues as a gene located in the minimal region of chromosome 18q, which is deleted in most colorectal cancers. The fact that DCC expression is lost in the majority of colorectal cancers, but also in many other tumors, prompted it to be considered as a tumor suppressor gene (16). However, although an initial series of reports supported the fact that DCC acted as a tumor suppressor (for a review see (17)), doubts have arisen, mainly because of the rarity of point mutations in the DCC coding sequence and because of the lack of tumor predisposition in DCC hemizygous mice (18). The observation that DCC is a dependence receptor and, as such, is able to trigger cell death in the absence of netrin-1 strengthened the initial hypothesis. Indeed, from this point of view, DCC would act as a tumor suppressor, limiting tumor development by inducing apoptosis of tumor cells that would otherwise proliferate in environmental settings of netrin-1 absence (19). Interestingly, the UNC5H netrin-1 receptors were also recently proposed to be tumor suppressors: UNC5H genes were shown to be down-regulated in many cancers, especially in colorectal cancer (20), and inactivation of UNC5H3 in mice is associated with intestinal tumor progression (21). Moreover, ectopic expression of netrin-1 in the mouse intestine is associated with decreased apoptosis in the intestinal epithelium, as well as tumoral predisposition (22). Thus, UNC5H (and probably DCC, as well) actually represent a novel class of tumor suppressors—conditional tumor suppressors—which suppress tumor formation in a milieu-dependent fashion: in the absence of trophic support from netrin-1, they induce cell death and tumor suppression; however, in the presence of high concentrations of netrin-1, they support tumor development (19, 22).
The tumor suppressive effects of UNC5H and DCC, coupled with the observation that their expression is decreased or lost in numerous cancers, raises the possibility that these may be therapeutic targets. Theoretically, tumor development and metastasis can be enhanced either by loss of the pro-apoptotic effect of these receptors or by gain of the ligand’s anti-apoptotic effect, and both strategies have been observed: in some cancer types–e.g., colorectal cancer—netrin-1 receptor expression is lost or markedly decreased; in contrast, in a large fraction of other tumor types, such as metastatic breast cancer (23), lung cancer (24), neuroblastoma (25), and pancreatic adenocarcinoma (26), we and others have observed that netrin-1 is up-regulated. This up-regulation of netrin-1 has been shown to confer a selective growth advantage similar to that conferred by the loss of netrin-1 receptors (23, 24). Furthermore, this autocrine production of netrin-1 is a mechanism that lends itself more readily to therapeutic attack than does the receptor loss: indeed, we have found that its inhibition, either by inhibiting netrin-1 production via RNA interference or by extracellular titration of netrin-1, is associated with cell death in vitro and tumor growth/metastasis inhibition in vivo (23, 24). The search for compounds that may interfere with netrin-1’s ability to block DCC/UNC5H-induced cell death is consequently of great potential for the development of alternative anti-cancer strategies.
The finding that the cognate ligands for DCC, UNC5H, and other dependence receptors blocks their cell death induction raises the question of how this is achieved mechanistically. DCC has been shown to homomultimerize in the presence of netrin-1, an important feature for the positive signaling of the receptor during axon guidance (27). Because classic death receptors like TNFr or Fas promote apoptosis in the presence of ligand by homomultimerization, and because dependence receptors have been suggested to mirror death receptors functionally (28), we analyzed whether netrin-1- induced DCC and/or UNC5H multimerization is a critical step for DCC and/or UNC5H-mediated pro-apoptotic activity. We show here that DCC and/or UNC5H multimerize in response to netrin-1, and that this event is sufficient to inhibit apoptosis. We also demonstrate that a domain of DCC called DCC-5Fbn that triggers cell death in vitro and tumor inhibition in animal models (23, 24)) does so by interfering with netrin-1-induced multimerization.
Results and Discussion
To analyze whether DCC is primarily monomeric in the absence of netrin-1, we transiently co-expressed an HA-tagged full-length DCC together with Myc-tagged full-length DCC in HEK293T cells. Immunoprecipitation was then performed using an anti- Myc antibody, and as shown in Fig. 1A, despite good expression of both HA and Myc tagged-DCC, HA-DCC was only modestly included in the Myc-DCC pull-down in the absence of ligand, suggesting that DCC was mainly present as a monomer when expressed in HEK293T in the absence of netrin-1. However, under the same experimental conditions, when netrin-1 was added to the culture medium (Fig. 1A) or when a netrin-1 expression construct was co-expressed with the DCC-expressing constructs (not shown and Fig. 3C), HA-DCC was clearly included in the Myc-DCC pull-down, demonstrating that netrin-1 triggers dimerization or multimerization of DCC. This result is in agreement with data from Tessier-Lavigne and colleagues, who first reported netrin-1-induced multimerization (27), even though in our culture and immunoprecipitation conditions, DCC displayed a modest level of multimerization in the absence of netrin-1. This constitutive, low multimerization level could either be attributed to the low affinity of DCC receptors for themselves in the absence of ligand or to the system used, which is based on forced expression of high levels of transmembrane receptors.
Figure 1. Netrin-1 mediates DCC and UNC5H2 multimerization.
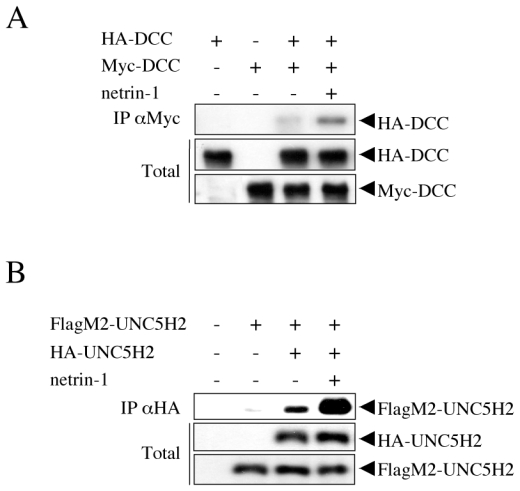
(A) DCC multimerization in the presence of netrin-1 in HEK293T cells. Lysates of HEK293T cells transiently transfected with HA-DCC and/or Myc-DCC expressing constructs together plus recombinant netrin-1 (300 ng/ml) were subjected to Myc pull-down (IP αMyc). DCC-HA presence was revealed with an anti-HA antibody. As control DCC surface expression in the presence or absence of netrin-1 was analyzed by FACs analysis and showed no significant change (not shown). (B) UNC5H2 dimerization in the presence of netrin-1 in HEK293T cells. Cell transfection and cell lysate preparation were done as in (A) but with HA-UNC5H2 and/or FlagM2-UNC5H2 expressing constructs. Cell lysates were subjected to HA pull-down (IP αHA). FlagM2-UNC5H2 presence was revealed with an anti-HA antibody. Total: Western blot on lysate before pull-down.
Figure 3. The recombinant soluble fifth fibronectin domain of DCC (DCC-5Fbn) inhibits netrin-1-induced DCC-multimerization.
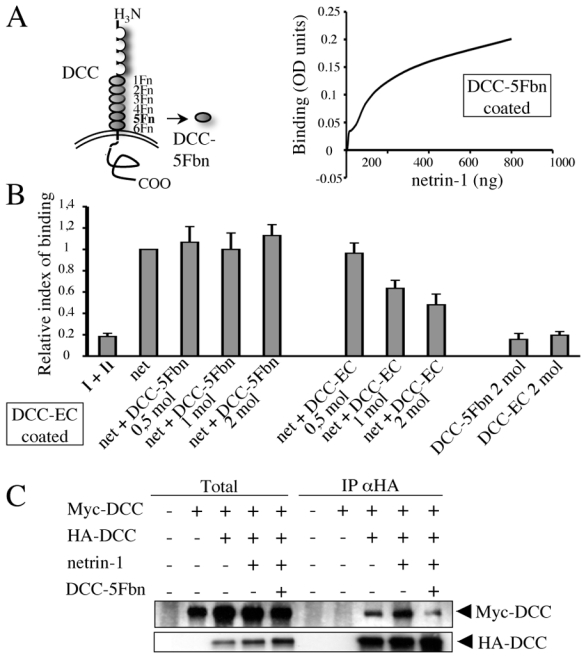
(A) Scheme showing DCC-5Fbn as one of the six fibronectin domain (Fn) of DCC. Affinity curve of netrin-1 on DCC-5Fbn measured by ELISA test shows that DCC-5Fbn is able to bind netrin-1. DCC-5Fbn (100 ng) or IL3-R (600 ng) was coated and increasing doses of netrin-1 were added (0 to 800 ng). The IL3 values were substracted from the DCC-5Fbn values. The approximate Kd of DCC-5Fbn/netrin-1 was estimated at 5 nM. (B) Competition assay. As in (A) but the complete extracellular domain of DCC (DCC-EC, 150 ng) was coated instead of DCC-5Fbn and netrin-1 (net) was added (50 ng) in the presence of increasing concentrations of either DCC-5Fbn or the complete DCC-EC. Note that DCC-5Fbn failed to compete with DCC/netrin-1 interaction. I+II (primary and secondary antibody), DCC-5Fbn 2 mol, DCC-EC 2 mol: controls performed in the absence of netrin-1. (C) Netrin-1-induced DCC multimerization is inhibited by DCC-5Fbn. Lysates of HEK293T cells transiently transfected with HA-DCC and/or Myc- DCC and/or netrin-1 (1/3 of total DNA) expressing constructs with or without DCC-5Fbn (900 ng/ml) were subjected to HA pull-down (IP αHA). Myc-DCC presence was revealed with anti-Myc antibody. Anti-HA immunoblot was also performed to detect HA-DCC precipitate. Total: Western blot on lysate before pull-down.
We then investigated whether the other netrin-1 receptors, UNC5H, share a similar behavior. HEK293T cells were transiently transfected with an HA-tagged full-length UNC5H2 together with Flag-tagged full-length UNC5H2 in the presence or absence of netrin-1. Immunoprecipitation was then performed using an anti-HA antibody. As shown in Fig. 1B, the presence of netrin-1 triggers an efficient immunoprecipitation of Flag-UNC5H2 with HA-UNC5H2. Thus, while in the absence of netrin-1, DCC and UNC5H2 are mainly monomeric, both DCC and UNC5H2 show an increased propensity to multimerize in the presence of netrin-1.
To determine whether netrin-1-induced multimerization is the crucial step for inhibiting DCC/UNC5H2 pro-apoptotic cell death, we developed a chimeric system in which protein dimerization can be induced by a chemical agent. This system was successfully used to show both the role of caspase-8 dimerization in caspase-8 activation (29) and the importance of p75ntr-multimerization in blocking p75ntr pro-apoptotic activity (28). This system is derived from the ability of the Fk1012 compound to cross-dimerize the FkBP motif. DCC and UNC5H2 intracellular domains were fused in their N-termini to derived Fv2e FkBP motifs, and dimerization was induced using the AP20187 chemical compound (Fig. 2A). We first analyzed whether the developed system recapitulates netrin-1-induced multimerization of the UNC5H2 intracellular domain. HEK293T cells were co-transfected with an HA-tagged Fv2e-UNC5H2-IC together with Myc-tagged Fv2e-UNC5H2-IC and co-immunoprecipitations were performed using an anti-Myc antibody. As shown in Fig. 2B, without addition of AP20187, HA-Fv2e-UNC5H2-IC was barely detectable in the Myc-Fv2e-UNC5H2-IC pull-down, supporting the notion that Fv2e-UNC5H2-IC is expressed in HEK293T cells mainly as a monomer. As expected, the addition of AP20187 led to the efficient pull-down of HA-Fv2e-UNC5H2-IC with Myc-Fv2e-UNC5H2-IC. Similar results were obtained with Fv2e-DCC-IC (not shown). Thus, this dimerization system recapitulated the dimerization of the intracellular domain of the netrin-1 receptors DCC and UNC5H2.
Figure 2. Forced DCC dimerization blocks DCC pro-apoptotic activity.
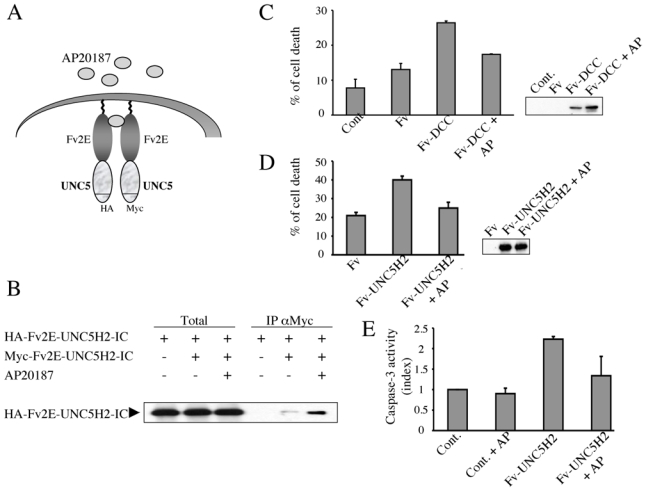
(A) Schematic representation of Fv2e-UNC5H2 fusion constructions showing the two constructs (one tagged HA, the other one tagged Myc) used to validate the artificial dimerization system. (B) Lysates of HEK293T cells transiently transfected with Fv2E-UNC5H2 tagged HA or Myc with or without the dimerization drug (AP20187, 10 nM) were subjected to Myc pull-down (IP αMyc). Total: Western blot on lysate before pull-down. HA-Fv2E-UNC5H2 presence was revealed with anti-HA antibody. (C) DCC- induced cell death is inhibited by dimerization induced by AP20187, as measured by trypan blue exclusion. HEK293T cells were transfected with mock plasmid (Cont.), Fv2E (Fv), Fv2E-DCC-IC (Fv-DCC) with or without 10 nM AP20187 (AP). In all conditions, cells were also transfected with the surface marker pKk. Transfected cells expressing the marker were magnetically labeled with MACSelect Microbeads and separated using a MACS Separator and Separation Columns. Trypan blue exclusion was assayed on these purified cells. A western blot was done using anti HA antibody. (D) UNC5H2- induced cell death is inhibited by dimerization induced by AP20187, as measured by trypan blue exclusion as in (C). Cells were transfected with pMACSKk and Fv2E (Fv), Fv2E-UNC5H2-IC (Fv-UNC5H2) with or without AP20187 (AP). A western blot was done using anti HA antibody. (E) UNC5H2-induced caspase activation is inhibited by dimerization induced by AP20187, as measured by relative caspase-3 activity. HEK293T cells were transfected with mock vector pCMV (Cont.), Fv2E (Fv), Fv2E-UNC5H2- IC (Fv-UNC5H2) with or without 10 nM AP20187 (AP). Index of relative caspase activity is presented as the ratio between the caspase activity of the sample and that measured in HEK293T cells transfected with pCMV. Standard deviations are indicated (n=3).
Because this chemically-inducible DCC/UNC5H2 dimerization system appears to work adequately to mimic netrin-1-induced DCC/UNC5H2 multimerization, we then assessed whether the dimerization of DCC/UNC5H2 was sufficient to inhibit DCC/UNC5H2 pro-apoptotic activity. HEK293T cells were forced to express Fv2e-DCC-IC in the presence or absence of AP20187, and cell death was assessed by trypan blue staining, as previously described, to measure DCC-induced cell death (10) (30). As shown in Fig. 2C, expression of Fv2e-DCC-IC was associated with increased cell death compared to expression of the Fv2e motives without the DCC-IC fusion. Interestingly, when AP20187 was added, cell death induced by Fv2e-DCC-IC was reduced (Fig. 2C). Similarly, while Fv2e-UNC5H2-IC triggered cell death (Fig. 2D) and caspase activation (Fig. 2E) when expressed in HEK293T in the absence of AP20187, the addition of the dimerizing drug was sufficient to reduce significantly Fv2e-UNC5H2-IC-induced cell death (Fig. 2D) and caspase activation (Fig. 2E). Thus, while monomeric DCC-IC and UNC5H2-IC were pro-apoptotic, the multimeric forms of DCC-IC or UNC5H2-IC did not display pro-apoptotic activity. Therefore, the ability of netrin-1 to inhibit DCC/UNC5H2 pro-apoptotic activity is intrinsically linked to the ability of netrin-1 to multimerize DCC or UNC5H2, as this multimerization process is sufficient to block DCC and UNC5H2 pro-apoptotic activity.
Although the mechanisms underlying netrin-1-induced receptor multimerization are yet to be described, the observation that netrin-1-induced DCC/UNC5H2 multimerization is sufficient to inhibit DCC/UNC5H2-induced cell death may represent an interesting tool to turn on DCC or UNC5H pro-apoptotic activity in vivo, in tumors in which netrin-1 is expressed in an autocrine manner. Of relevance to this goal, we have demonstrated that netrin-1 overexpression in mouse gastrointestinal tract is associated with intestinal tumor development because of apoptosis inhibition (22), and we and others have recently observed that netrin-1 is overexpressed in a large fraction of metastastic breast cancers (23), lung cancer (24), aggressive neuroblastoma (25), and pancreatic cancers (26). Therefore, the inhibition of DCC/UNC5H-induced dimerization might potentially represent a method to trigger tumor cell apoptosis.
In order to evaluate this possibility, we employed the fifth fibronectin domain of DCC, which has been shown to be a domain of interaction with netrin-1 (Fig. 3A and (31)), even though conflicting data have also been reported (32). We thus first assessed whether a recombinant soluble fifth fibronectin domain of DCC (DCC-5Fbn) could bind to recombinant netrin-1. ELISA assay demonstrated that DCC-5Fbn specifically binds to netrin-1, as opposed to the extracellular domain of an unrelated receptor, IL3-R (Fig. 3A). The approximate Kd for DCC-5Fbn/netrin-1 was estimated to be approximately 5 nM, in keeping with the order of magnitude of the described DCC/netrin-1 Kd. We next investigated whether this domain was sufficient to displace DCC/netrin-1 interaction. As shown in Figure 3B, using an ELISA assay in which the extracellular domain of DCC was coated and netrin-1/DCC interaction was detected by netrin-1 immunoreactivity, we observed that, while as a positive control the complete extracellular domain of DCC (DCC-EC) was sufficient to displace DCC/netrin-1 interaction, DCC-5Fbn failed to interfere. Thus, DCC-5Fbn interacts with netrin-1 but is not sufficient to inhibit DCC/netrin-1 interaction. We next investigated whether DCC-5Fbn could influence DCC multimerization. We performed co-immunoprecipitation in HEK293T transiently transfected with HA-tagged full-length DCC together with Myc-tagged full-length DCC in the presence or absence of netrin-1. As shown in Fig. 1A, the presence of netrin-1 triggered the immunoprecipitation of Myc-DCC with HA-DCC, demonstrating netrin-1- induced DCC multimerization (Fig. 3C). However, when the cells incubated with netrin-1 were also simultaneously treated with DCC-5Fbn, the HA-DCC/Myc-DCC interaction returned to netrin-1 untreated levels. Thus, DCC-5Fbn interacts with netrin-1 and inhibits netrin-1-induced DCC-multimerization.
We therefore tested whether DCC-5Fbn triggers netrin-1 receptor-induced cell death. To this end, HEK293T cells were transfected to express DCC in the presence or absence of netrin-1, with or without DCC-5Fbn, and cell death was determined by trypan blue exclusion assay (Fig. 4A) or caspase assay (not shown). As shown in Fig. 4A, while DCC triggered cell death in the absence of netrin-1, a pro-apoptotic activity that was blocked by the presence of netrin-1; the presence of DCC-5Fbn was sufficient to block the inhibitory activity of netrin-1, thus leading to DCC-induced cell death. A similar effect was observed with UNC5H2 when cell death was measured by TUNEL staining (Fig. 4B). Thus DCC-5Fbn interfered with netrin-1-mediated receptor multimerization, and triggered cell death.
Figure 4. DCC-5Fbn triggers cell death via disruption of netrin-1-mediated inhibition of DCC/UNC5H2 pro-apoptotic activity.
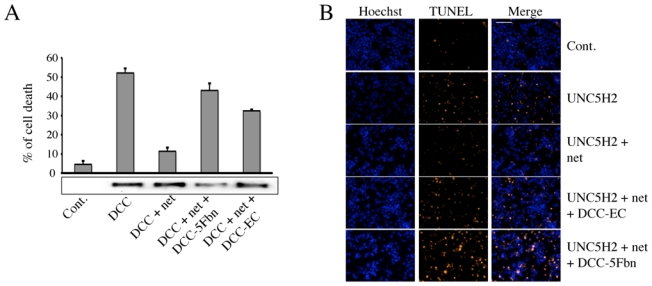
(A) HEK293T cells were transiently transfected with a mock (Cont.) or a full-length DCC construct (DCC) and incubated with or without netrin-1 (net) (150 ng/ml) and/or DCC- 5Fbn (800 ng/ml) or the complete extracellular domain of DCC (DCC-EC) (200 ng/ml). Cell death was assessed by trypan blue staining. Inset: DCC immunoblot showing DCC expression level in the different tested conditions. (B) HEK293T cells were transiently transfected with a mock (Cont.) or a full length UNC5H2 construct (UNC5H2) and incubated with or without netrin-1 (net) (500 ng/ml) and/or DCC-5Fbn (1000 ng/ml) or the complete extracellular domain of DCC (DCC-EC) (200 ng/ml). Cell death was measured by fluorescent TUNEL staining. Hoechst staining shows cell nuclei. For each condition, at least 5 randomly chosen fields were analyzed and photographed under epifluorescence microscopy. A representative field is presented from 3 independent experiments. Superimposed photographs (Merge) are shown. Scale bar represents 100 μM.
DCC-5Fbn not only triggered apoptosis in HEK293T cells ectopically expressing DCC/UNC5H2, but also was active in a more pathophysiologically relevant setting: previously we reported than DCC-5Fbn not only induces apoptosis of endogenously netrin-1 expressing metastatic breast cancer lines and Non small Cell Lung cancer cell lines or neuroblastoma cell lines in vitro, but also inhibits tumor growth and metastasis in different animal models (23–25). Similarly, the colorectal cancer cell line HCT116 expresses netrin-1 receptors and a high level of netrin-1 at the RNA level (Fig. 5A), which is associated with secretion of netrin-1 protein, as shown by netrin-1 immunohistochemistry performed on non-permeabilized cells (Fig. 5B). When these HCT116 cells were treated with DCC-5Fbn, apoptotic cell death was induced, as measured by trypan blue exclusion and caspase-3 activity assays (Fig. 5C); furthermore, this death effect was specifically related to an inhibition of netrin-1 activity, since the addition of recombinant netrin-1 in excess blocked DCC-5Fbn-mediated killing. To provide further support for the in vivo efficacy of DCC-5Fbn to limit tumor progression (23–25), HCT116 cells were grafted onto the chorioallantoic membrane (CAM) of 10-day-old chick embryos, a model that has been shown to recapitulate both tumour growth at a primary site— i.e., within the CAM—as well as tumour invasion and dissemination at a secondary site—metastasis to the lung – (25). HCT116 cells were loaded onto 10-day-old CAM and embryos were treated on day 10 and day 13 with PBS or DCC-5Fbn, then 17-day-old chicks were analyzed for metastases in the lung. As shown in Fig. 5D, DCC-5Fbn dramatically reduced lung metastasis formation.
Figure 5. DCC-5Fbn triggers HCT116 cell death in vitro and inhibits HCT116 cell metastasis in a chicken model.
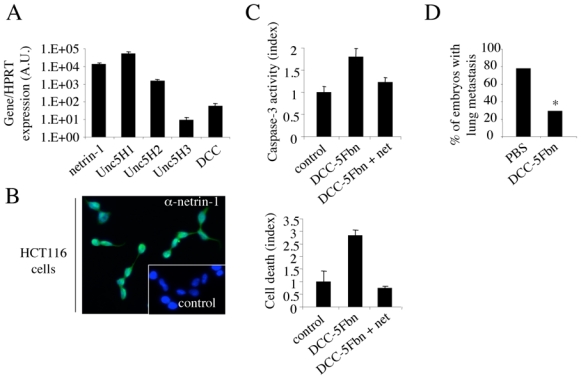
(A) Quantification of netrin-1 and netrin-1 receptors. Ratio of netrin-1 and netrin-1 receptors expression to HPRT housekeeping gene is presented. (B) Representative netrin-1 immunohistochemistry on HCT116 tumor cell line. Inset: Control without primary antibody is presented. (C) Quantitative analysis of cell death in HCT116 cell line treated with DCC-5Fbn, with or without addition of netrin-1 in excess to reverse the effect of DCC-5Fbn. Cell death was quantified by trypan blue exclusion assay (below panel) while apoptosis was monitored by measuring relative caspase-3 activity (upper panel). (D) HCT116 cells were grafted in chick chorioallantoic membrane at day 10 and DCC- 5Fbn or PBS was injected on day 10 and day 13. Tumours and lungs were harvested on day 17. Percentage of embryos with lungs invaded by human HCT116 cells after two injections (day 10 and day 13) of either DCC-5Fbn or PBS. * indicates a p<0,05 calculated using a χ-squared test.
Taken together, these results demonstrate that the multimerization of the dependence receptors DCC and UNC5H is a sufficient mechanism to block their pro-apoptotic activity. Interestingly, this inhibitory mechanism appears to mirror what is observed with death receptors. Indeed, it is known that TNFr or Fas requires trimerization to induce apoptosis (33). This intrinsic difference may therefore suggest a therapeutic strategy that utilizes dependence receptors. Indeed, the search for therapeutic molecules in the past has mainly led to hits that act on the inhibition of cellular processes –e.g., kinase inhibitors, IAP inhibitors- rather than activators. As a consequence, inhibition of netrin-1 receptors’ multimerization via the use of recombinant DCC-5Fbn or via any compound screened to interfere with receptor multimerization appears to be a tempting strategy for the treatment of cancers in which netrin-1 autocrine expression has been acquired.
Materials and Methods
Cell cultures, transfection procedures, reagents and immunoblots
Transient transfections of Human Embryonic Kidney 293T cells (HEK293T) were performed as previously described (4) according to a modified calcium phosphate procedure or using Lipofectamine according to the manufacturer’s instructions (Invitrogen). Immunoblots were performed as described previously (10) using anti-Myc (Sigma, 1/1000), anti-FlagM2 (Sigma, 1/10000), anti-HA (Sigma, 1/5000), anti-DCC Ab20 (Tebu, 1/1000). The artificial dimerizing agent AP20187 was from Ariad Pharmaceuticals. The complete extracellular domain of DCC (DCC-EC) was purchased from R&D system. Netrin-1 (with a FlagM2 tag) was from Apotech. For cell death analysis, caspase activity measurement and immunoprecipitation, AP20187 was used at a final concentration of 10 nM, netrin-1 was used at a final concentration of 300 ng/ml.
Site directed mutagenesis and plasmid construction
pCMV and pGNET1 were described previously (10). pKk was described in (34). HA-DCC was obtained by introducing a HA tag in the template pCMV-DCC (described in (10)) by QuikChange site-directed mutagenesis system (Stratagene) using the following primers: DCC-HA F: 5′ CACAGGCTCAGCCTTTTATCCATATGATGTACCGGATTATGCATAACATGTATTTCT GAATG 3′, DCC-HA R: 5′ CATTCAGAAATACATGTTATGCATAATCCGGTACATCATATGGATAAAAGGCTGAGCCTGTG 3′. Myc-DCC was also obtained by introducing a Myc tag in the template pCMV-DCC by QuikChange using the following primers: DCC-Myc F: 5′ CACAGGCTCAGCCTTTGAGCAGAAGTTGATAAGTGAGGAAGATCTGTAACATGTATTTCTGAATG 3′. DCC-Myc R: 5′ CATTCAGAAATACATGTTACAGATCTTCCTCACTTCTCAACTTCTGCTCAAAGGCTGAGCCTGTG 3′. HA-Fv2E encoding expression vector (in pC4M) from the Argent Regulated Homodimerization kit is from Ariad Pharmaceuticals. From this plasmid, the HA-Fv2E-DCC-IC plasmid was constructed. A PCR fragment of the intracellular domain of DCC (1122–1447) was obtained with the primers: F 5′ TATGTCGACCGACGCTCTTCAGCCCAGCAGAGA 3′ and R 5′ TATGAATTCTTAGTCGAGTGCGTAGTCTGGTACGTCGTACGGATAAAAGGCTGAGCCTGTGATGGCATTAAG 3′. The reverse primer fused the HA tag to C-terminal end of DCC. The PCR fragment was subcloned in HA-Fv2E by SalI and EcoRI restriction digestion. The Myc-Fv2E-DCC-IC was obtained using the QuikChange site-directed mutagenesis system (Stratagen) with pC4M-Fv2E-DCC-IC-HA as template and the following primers: primer F: 5′ CTTAATGCCATCACAGGCTCAGCCTTTGAACAGAAACTCATCTCTGAAGAGGATCTGTAAGAATTCATAAAGGGCAAT 3′ and primer R: 5′ ATTGCCCTTTATGAATTCTTACAGATCCTCTTCAGAGATGAGTTTCTGTTCAAAGGCTGAGCCTGTGATGGCATTAAG 3′. HA-UNC5H2 (in pcDNA3.1) has already been described (11); the constructs encoding FlagM2-UNC5H2 was generated by cloning in p3xFlag-CMV™-7.1 (Sigma) the NotI-EcoRI PCR fragment derived from HA-UNC5H2 as template and the following primers: primer F 5′-GCGCGGCCGCAGGGCCCGGAGCGGG-3′ and primer R 5′-CGGAATTCTCAGCAATCGCCATCAGTGGTC-3′. HA-Fv2E-UNC5H2-IC- and Myc- Fv2E-UNC5H2-IC in pC4M were generated by PCR amplification of the UNC5H2 intracellular domain using the following primers: UNC5H2-HA F 5′-CGGTCGACGTGTACCGGAGAAACTGC-3′ and UNC5H2-HA R 5′-GCGAATTCTCATGCATAATCCGGCACATCATACGGATAGCAATCGCCATCAGTGGT C-3′, and UNC5H2-Myc F 5′-CGGTCGACGTGTACCGGAGAAACTGC-3′ and UNC5H2- Myc R 5′-GCGAATTCTCACAGATCCTCTTCTGAGATGAGTTTTTGTTCGCAATCGCCATCAGTGGTC-3′ respectively. The PCR fragments were cloned in HA-Fv2E by SalI and EcoRI restriction digestion. The cDNA encoding the HA-Fv2E-UNC5H2-IC and Myc-Fv2E-UNC5H2- IC fusion proteins were then subcloned in pcDNA3.1-TOPO by PCR using the following primers: Fv2E F 5′-CCACCATGGGGAGTAGCA-3′ and UNC5H2-HA R 5′-TCATGCATAATCCGGCACATCATACGGATAGCAATCGCCATCAGTGGTC-3′, and Fv2E F 5′-CCACCATGGGGAGTAGCA-3′ and UNC5H2-Myc R 5′-TCACAGATCCTCTTCTGAGATGAGTTTTTGTTCGCAATCGCCATCAGTGGTC-3′ respectively and HA-Fv2E-UNC5H2-IC and Myc-Fv2E-UNC5H2-IC in pC4M as respective templates. pLIM01-DCC-5Fbn-His allowing bacterial expression of the fifth fibronectin type III domain of DCC was done by M. Noirclerc-Savoye and B. Gallet from the laboratory RoBioMol at the Institut de Biologie Structurale, Grenoble. This plasmid was created by using the Ligation Independent Cloning strategy (Novagen). A PCR fragment of DCC-5Fbn was produced using plasmid pQE-Flag-DCC-5Fbn as template and the primers NAME SEQUENCE and NAME SEQUENCE. The PCR product was inserted into pLIM01 by spontaneous hybridization. The pQE-Flag-DCC-5Fbn construct was done by Apotech by inserting human DCC-5Fbn fragment in PstI and BamHI sites in plasmid pQE-Flag.
DCC-5Fbn production
DCC-5Fbn production was performed using a standard procedure. Briefly, BL21 cells were forced to express DCC-5Fbn in response to IPTG and the BL21 lysate was subjected to affinity chromatography using His purification on Ni-NTA columns (Quiagen).
Immunoprecipitation
Coimmunoprecipitations were carried out on HEK293T cells transfected with various tagged constructs as described previously (30). Briefly, HEK293T cells were lysed in 50 mM HEPES pH 7.6, 150 mM NaCl, 5 mM EDTA and 0.1% NP-40 in the presence of protease inhibitor and sonicated 2 pulses of 5 secondes, and further incubated with anti- HA (Sigma), anti-Myc antibody (Sigma), anti-FlagM2 (Sigma) and protein-A Sepharose (Sigma). Washes were done in 50 mM HEPES pH 7.6, 150 mM NaCl, 5 mM EDTA.
Binding assay and ELISA competition assay
DCC-5Fbn (100 ng) or IL3-R (R&D systems, 600 ng) was coated on maxisorp plate (Nunc) and increasing doses of netrin-1 (Apotech) were added (0 to 800 ng) for binding assay. DCC-EC (R&D systems,150 ng) was coated on maxisorp plate for ELISA competition assay. Netrin-1 (Apotech, 50 ng) and competitor DCC-EC or DCC-5Fbn were then added simultaneously in increasing concentration. After washes, for both binding assay or ELISA competition assay, residual netrin-1 still fixed was revealed with an anti-netrin-1 antibody (R&D systems) and OPD revelation (Sigma).
Cell death analysis and caspase activity measurement
Cell death was analysed 48h after transfection using trypan blue staining procedures as described previously (10). To select transfected cells, cells were co-transfected with the surface marker pKk and the plasmid encoding genes of interest. Transfected cells expressing the marker were magnetically labeled with MACSelect Microbeads and separated using a MACS Separator and Separation Columns (Miltenyi Biotec). Trypan blue exclusion was assayed on these purified cells. Caspase-3 activity was measured by using the Caspase-3 assay from BioVision. Caspase activity is presented as the ratio between the caspase activity of the sample and that measured in HEK293T cells transfected with pCMV. For cell death analysis and caspase activity measurement, AP20187 or/and netrin-1 or/and DCC-5Fbn were added in cell culture medium 20 hours and 1 hour before collecting cells. TUNEL staining was done as previously described (30) (10)but with fluorescent staining using streptavidine-conjugated Cy3 antibody (Jackson ImmunoResearch, 1/1000) and Hoechst (Sigma, 1/1000).
Metastasis quantification in chicken model
2. 107 HCT116 colorectal tumoral cells were seeded on 10-day-old (day 10) chick chorioallantoic membrane (CAM). HCT116 cells were suspended in a 100μL of either DCC-5Fbn solution (0,05μg/μL) or PBS. A second injection of DCC-5Fbn or PBS was performed on the tumor at day 13 (100μL de DCC-5Fbn 0,05μg/μL or 100μL of PBS). To assess metastasis, lungs were harvested from the tumour-bearing embryos and genomic DNA was extracted with NucleoSpin Tissue kit (Macherey Nagel). Metastasis was quantified by polymerase chain reaction (PCR)-based detection of the human Alu sequence using the primers 5′-ACGCCTGTAATCCCAGCACTT-3′ (sense) and 5′-TCGCCCAGGCTGGAGTGCA-3′ (antisense) with chick glyceraldehyde-3-phosphate dehydrogenase-specific primers (sense, 5′-GAGGAAAGGTCGCCTGGTGGATCG-3′; antisense, 5′-GGTGAGGACAAGCAGTGAGGAACG-3′) as controls. For both couple of primers, metastasis was assessed by polymerase activation at 95°C for 2 min followed by 30 cycles at 95°C for 30 s, 63°C for 30 s and 72°C for 30 s. Genomic DNA extracted from lungs of healthy chick embryos were used to determine the threshold between HCT116 cells invaded and non invaded lungs.
Acknowledgments
We wish to thank C. Forcet, C. Bonod-Bidaud, C. Maisse and A. Paradisi for preliminary works and Jonathan Blachier for excellent technical help. We thank M. Noirclerc-Savoye and B. Gallet (RoBioMol at the Institut de Biologie Structurale, Grenoble) for materials. This work was supported by the Ligue Contre le Cancer, the Agence Nationale de la Recherche (P.M), the Institut National du Cancer (P.M), the Rhône-Alpes Region (P.M), the Centre National de la Recherche Scientifique (P.M) and the NIH (P.M and D.E.B). F.M was supported by a Rhône-Alpes Region fellowship.
References
- 1.Mehlen P, Bredesen DE. The dependence receptor hypothesis. Apoptosis. 2004;9:37–49. doi: 10.1023/B:APPT.0000012120.66221.b2. [DOI] [PubMed] [Google Scholar]
- 2.Rabizadeh S, Oh J, Zhong LT, Yang J, Bitler CM, Butcher LL, et al. Induction of apoptosis by the low-affinity NGF receptor. Science. 1993 Jul 16;261( 5119):345–348. doi: 10.1126/science.8332899. [DOI] [PubMed] [Google Scholar]
- 3.Bordeaux MC, Forcet C, Granger L, Corset V, Bidaud C, Billaud M, et al. The RET proto-oncogene induces apoptosis: a novel mechanism for Hirschsprung disease. Embo J. 2000 Aug 1;19( 15):4056–4063. doi: 10.1093/emboj/19.15.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thibert C, Teillet MA, Lapointe F, Mazelin L, Le Douarin NM, Mehlen P. Inhibition of neuroepithelial patched-induced apoptosis by sonic hedgehog. Science. 2003 Aug 8;301( 5634):843–846. doi: 10.1126/science.1085405. [DOI] [PubMed] [Google Scholar]
- 5.Stupack DG, Puente XS, Boutsaboualoy S, Storgard CM, Cheresh DA. Apoptosis of adherent cells by recruitment of caspase-8 to unligated integrins. J Cell Biol. 2001 Oct 29;155( 3):459–470. doi: 10.1083/jcb.200106070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsunaga E, Tauszig-Delamasure S, Monnier PP, Mueller BK, Strittmatter SM, Mehlen P, et al. RGM and its receptor neogenin regulate neuronal survival. Nat Cell Biol. 2004 Aug;6( 8):749–755. doi: 10.1038/ncb1157. [DOI] [PubMed] [Google Scholar]
- 7.Mourali J, Benard A, Lourenco FC, Monnet C, Greenland C, Moog-Lutz C, et al. Anaplastic lymphoma kinase is a dependence receptor whose proapoptotic functions are activated by caspase cleavage. Mol Cell Biol. 2006 Aug;26( 16):6209–6222. doi: 10.1128/MCB.01515-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tauszig-Delamasure S, Yu LY, Cabrera JR, Bouzas-Rodriguez J, Mermet- Bouvier C, Guix C, et al. The TrkC receptor induces apoptosis when the dependence receptor notion meets the neurotrophin paradigm. Proc Natl Acad Sci U S A. 2007 Aug 14;104( 33):13361–13366. doi: 10.1073/pnas.0701243104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furne C, Ricard J, Cabrera JR, Pays L, Bethea JR, Mehlen P, et al. EphrinB3 is an Anti-apoptotic Ligand that Inhibits the Dependence Receptor Functions of EphA4 Receptors during adult neurogenesis. BBA Biol Mol Cell. 2008 doi: 10.1016/j.bbamcr.2008.09.009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehlen P, Rabizadeh S, Snipas SJ, Assa-Munt N, Salvesen GS, Bredesen DE. The DCC gene product induces apoptosis by a mechanism requiring receptor proteolysis. Nature. 1998 Oct 22;395( 6704):801–804. doi: 10.1038/27441. [DOI] [PubMed] [Google Scholar]
- 11.Llambi F, Causeret F, Bloch-Gallego E, Mehlen P. Netrin-1 acts as a survival factor via its receptors UNC5H and DCC. Embo J. 2001 Jun 1;20( 11):2715–2722. doi: 10.1093/emboj/20.11.2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serafini T, Kennedy TE, Galko MJ, Mirzayan C, Jessell TM, Tessier-Lavigne M. The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans UNC-6. Cell. 1994 Aug 12;78( 3):409–424. doi: 10.1016/0092-8674(94)90420-0. [DOI] [PubMed] [Google Scholar]
- 13.Serafini T, Colamarino SA, Leonardo ED, Wang H, Beddington R, Skarnes WC, et al. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell. 1996 Dec 13;87( 6):1001–1014. doi: 10.1016/s0092-8674(00)81795-x. [DOI] [PubMed] [Google Scholar]
- 14.Hedgecock EM, Culotti JG, Hall DH. The unc-5, unc-6, and unc-40 genes guide circumferential migrations of pioneer axons and mesodermal cells on the epidermis in C. elegans. Neuron. 1990 Jan;4( 1):61–85. doi: 10.1016/0896-6273(90)90444-k. [DOI] [PubMed] [Google Scholar]
- 15.Chan SS, Zheng H, Su MW, Wilk R, Killeen MT, Hedgecock EM, et al. UNC-40, a C. elegans homolog of DCC (Deleted in Colorectal Cancer), is required in motile cells responding to UNC-6 netrin cues. Cell. 1996 Oct 18;87( 2):187–195. doi: 10.1016/s0092-8674(00)81337-9. [DOI] [PubMed] [Google Scholar]
- 16.Fearon ER, Cho KR, Nigro JM, Kern SE, Simons JW, Ruppert JM, et al. Identification of a chromosome 18q gene that is altered in colorectal cancers. Science. 1990 Jan 5;247( 4938):49–56. doi: 10.1126/science.2294591. [DOI] [PubMed] [Google Scholar]
- 17.Mehlen P, Fearon ER. Role of the dependence receptor DCC in colorectal cancer pathogenesis. J Clin Oncol. 2004 Aug 15;22( 16):3420–3428. doi: 10.1200/JCO.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 18.Fazeli A, Dickinson SL, Hermiston ML, Tighe RV, Steen RG, Small CG, et al. Phenotype of mice lacking functional Deleted in colorectal cancer (Dcc) gene. Nature. 1997 Apr 24;386( 6627):796–804. doi: 10.1038/386796a0. [DOI] [PubMed] [Google Scholar]
- 19.Grady WM. Making the case for DCC and UNC5C as tumor-suppressor genes in the colon. Gastroenterology. 2007 Dec;133( 6):2045–2049. doi: 10.1053/j.gastro.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 20.Thiebault K, Mazelin L, Pays L, Llambi F, Joly MO, Saurin JC, et al. The netrin-1 receptors UNC5H are putative tumor suppressors controlling cell death commitment. Proc Natl Acad Sci U S A. 2003;100( 7):4173–4178. doi: 10.1073/pnas.0738063100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bernet A, Mazelin L, Coissieux MM, Gadot N, Ackerman SL, Scoazec JY, et al. Inactivation of the UNC5C Netrin-1 receptor is associated with tumor progression in colorectal malignancies. Gastroenterology. 2007 Dec;133( 6):1840–1848. doi: 10.1053/j.gastro.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mazelin L, Bernet A, Bonod-Bidaud C, Pays L, Arnaud S, Gespach C, et al. Netrin-1 controls colorectal tumorigenesis by regulating apoptosis. Nature. 2004 Sep 2;431( 7004):80–84. doi: 10.1038/nature02788. [DOI] [PubMed] [Google Scholar]
- 23.Fitamant J, Guenebeaud C, Coissieux MM, Guix C, Treilleux I, Scoazec JY, et al. Netrin-1 expression confers a selective advantage for tumor cell survival in metastatic breast cancer. Proc Natl Acad Sci U S A. 2008 Mar 25;105( 12):4850–4855. doi: 10.1073/pnas.0709810105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Delloye C, Brambilla E, Coissieux M, Guenebeaud C, Pedeux R, Brambilla C, et al. Interference with netrin-1 triggers tumor cell death in Non Small Cell Lung Cancer. J Natl Cancer Ins. 2009 doi: 10.1093/jnci/djn491. In press. [DOI] [PubMed] [Google Scholar]
- 25.Delloye C, Fitamant J, Douc-Rasy S, Cappellen D, Racquin MA, Stupack D, et al. Netrin-1 acts as a survival factor for aggressive neuroblatoma. doi: 10.1084/jem.20082299. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Link BC, Reichelt U, Schreiber M, Kaifi JT, Wachowiak R, Bogoevski D, et al. Prognostic Implications of Netrin-1 Expression and Its Receptors in Patients with Adenocarcinoma of the Pancreas. Ann Surg Oncol. 2007 Jun 5;14( 9):2591–2599. doi: 10.1245/s10434-007-9469-6. [DOI] [PubMed] [Google Scholar]
- 27.Stein E, Zou Y, Poo M, Tessier-Lavigne M. Binding of DCC by netrin-1 to mediate axon guidance independent of adenosine A2B receptor activation. Science. 2001 Mar 9;291( 5510):1976–1982. doi: 10.1126/science.1059391. [DOI] [PubMed] [Google Scholar]
- 28.Wang JJ, Rabizadeh S, Tasinato A, Sperandio S, Ye X, Green M, et al. Dimerization-dependent block of the proapoptotic effect of p75(NTR) J Neurosci Res. 2000 Jun 1;60( 5):587–593. doi: 10.1002/(SICI)1097-4547(20000601)60:5<587::AID-JNR3>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 29.Yang X, Chang HY, Baltimore D. Autoproteolytic activation of pro-caspases by oligomerization. Mol Cell. 1998 Jan;1( 2):319–325. doi: 10.1016/s1097-2765(00)80032-5. [DOI] [PubMed] [Google Scholar]
- 30.Forcet C, Ye X, Granger L, Corset V, Shin H, Bredesen DE, et al. The dependence receptor DCC (deleted in colorectal cancer) defines an alternative mechanism for caspase activation. Proc Natl Acad Sci U S A. 2001 Mar 13;98( 6):3416–3421. doi: 10.1073/pnas.051378298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geisbrecht BV, Dowd KA, Barfield RW, Longo PA, Leahy DJ. Netrin binds discrete subdomains of DCC and UNC5 and mediates interactions between DCC and heparin. J Biol Chem. 2003 Aug 29;278( 35):32561–32568. doi: 10.1074/jbc.M302943200. [DOI] [PubMed] [Google Scholar]
- 32.Kruger RP, Lee J, Li W, Guan KL. Mapping netrin receptor binding reveals domains of Unc5 regulating its tyrosine phosphorylation. J Neurosci. 2004 Dec 1;24( 48):10826–10834. doi: 10.1523/JNEUROSCI.3715-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muppidi JR, Tschopp J, Siegel RM. Life and death decisions: secondary complexes and lipid rafts in TNF receptor family signal transduction. Immunity. 2004 Oct;21( 4):461–465. doi: 10.1016/j.immuni.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 34.Llambi F, Lourenco FC, Gozuacik D, Guix C, Pays L, Del Rio G, et al. The dependence receptor UNC5H2 mediates apoptosis through DAP-kinase. Embo J. 2005 Mar 23;24( 6):1192–1201. doi: 10.1038/sj.emboj.7600584. [DOI] [PMC free article] [PubMed] [Google Scholar]


