Abstract
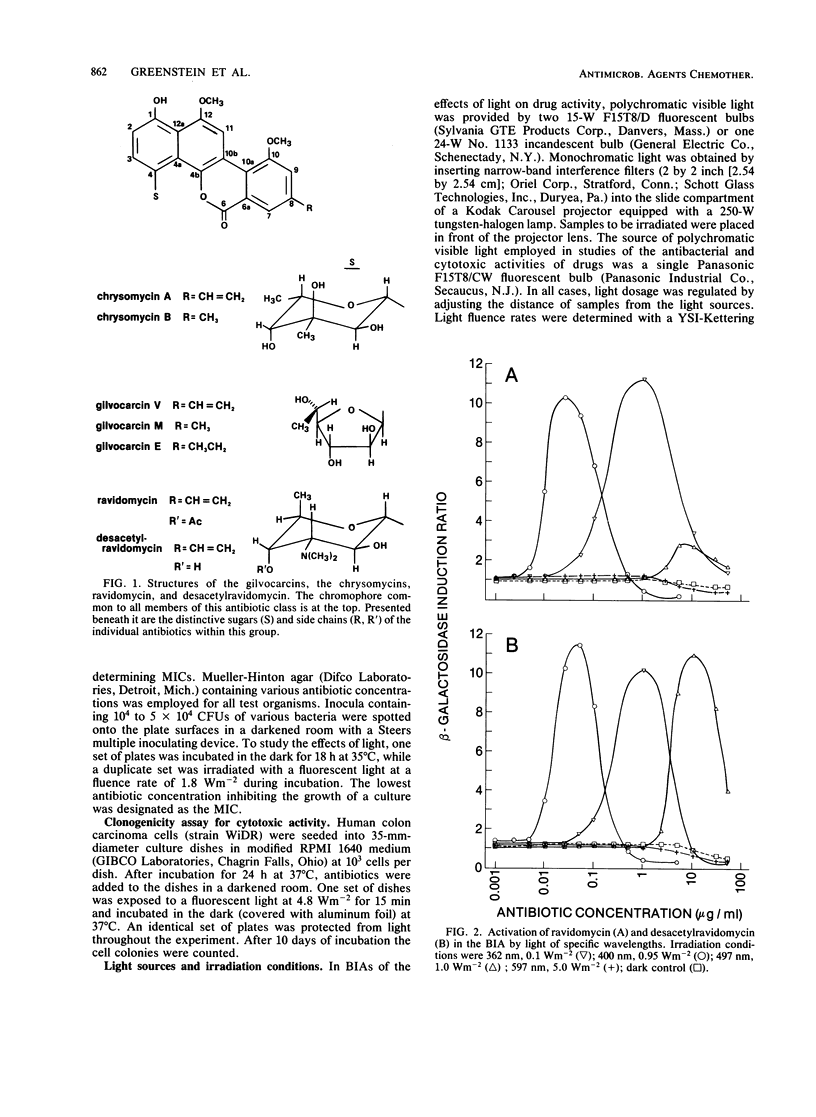
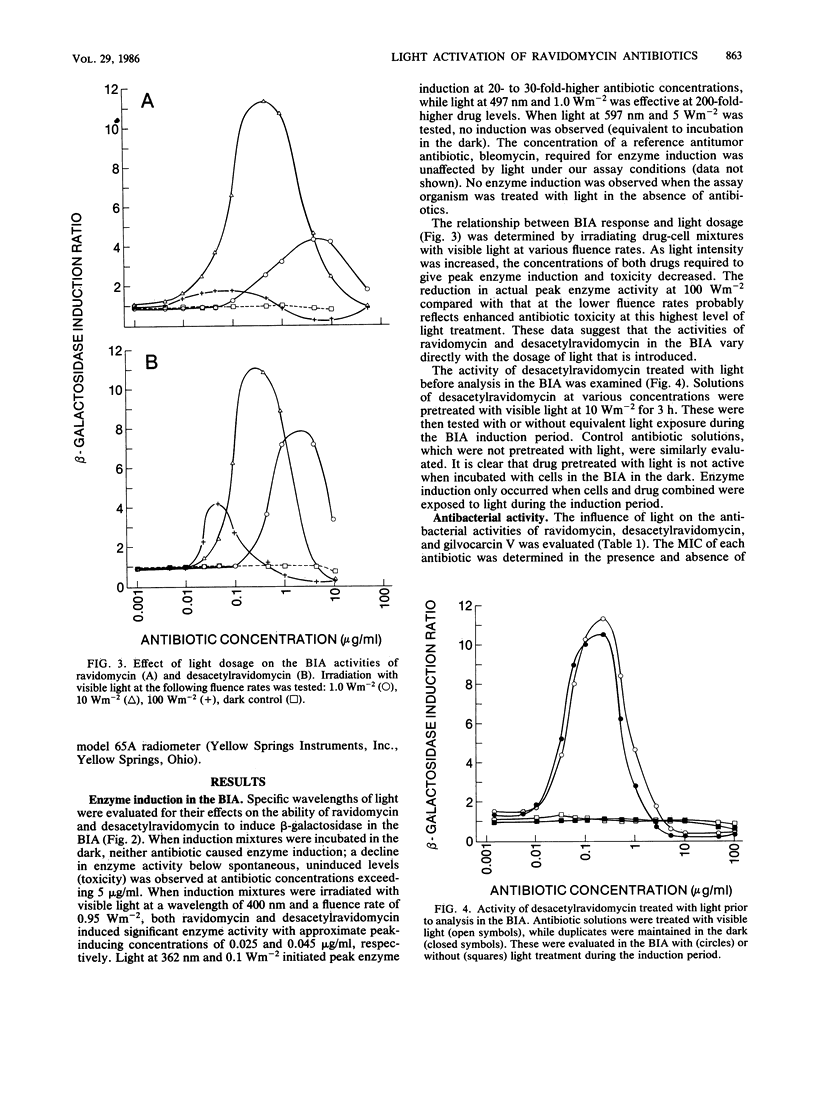
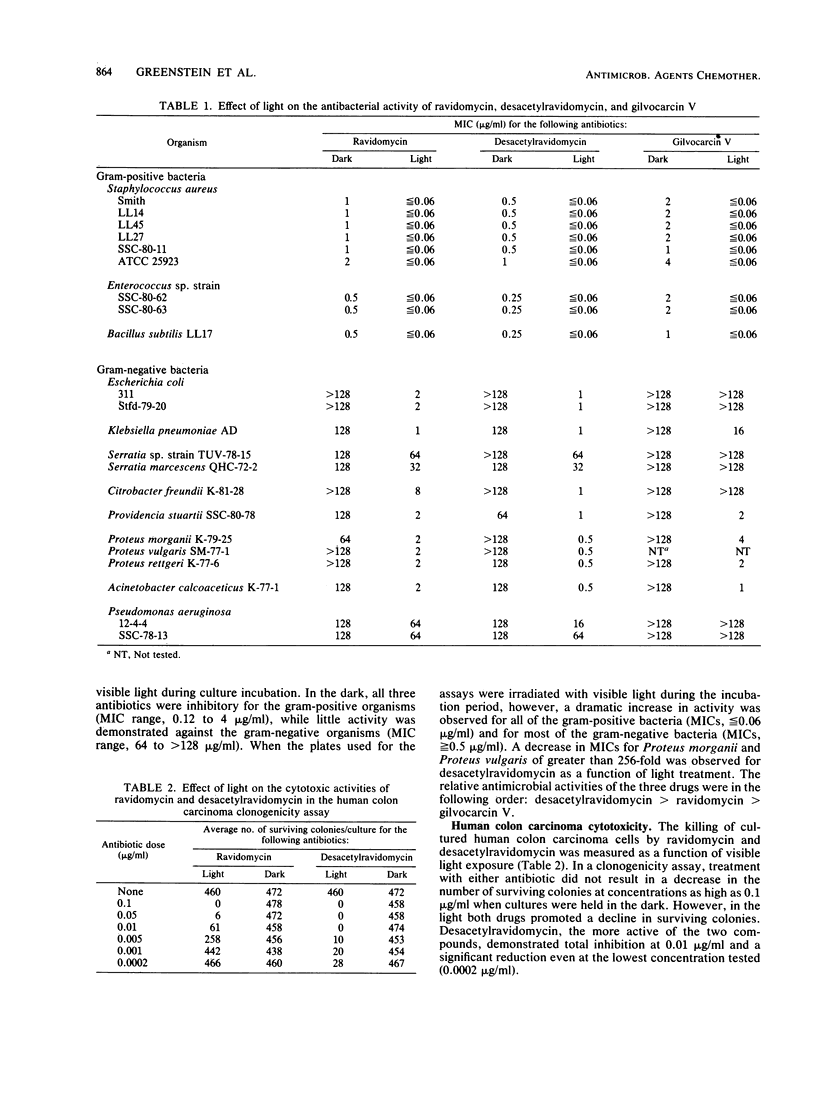
The antitumor antibiotics ravidomycin and desacetylravidomycin were studied by the biochemical lambda prophage induction assay. In this assay, induction of the enzyme beta-galactosidase is measured as a specific indication of the ability of an agent to directly or indirectly damage DNA. Induction was observed only when these two antibiotics were irradiated with light in the presence of the indicator organism. Drug treated with light followed by incubation with the indicator organism in the dark did not cause induction. Light in both the near UV and visible wave length ranges activated these antibiotics; near UV and visible blue wavelengths were most effective, while 597-nm light was totally ineffective. The amount of induction caused by these drugs varied directly with the dosage of light provided. Bacterial growth inhibition, as well as cytotoxicity for a human colon carcinoma cell line, was also dramatically enhanced by light. These data suggest that ravidomycin and desacetylravidomycin are potent photosensitizing, DNA-damaging agents.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balitz D. M., O'Herron F. A., Bush J., Vyas D. M., Nettleton D. E., Grulich R. E., Bradner W. T., Doyle T. W., Arnold E., Clardy J. Antitumor agents from Streptomyces anandii: gilvocarcins V, M and E. J Antibiot (Tokyo) 1981 Dec;34(12):1544–1555. doi: 10.7164/antibiotics.34.1544. [DOI] [PubMed] [Google Scholar]
- Chang C. H., Meares C. F. Light-induced nicking of deoxyribonucleic acid by cobalt(III) bleomycins. Biochemistry. 1982 Dec 7;21(25):6332–6334. doi: 10.1021/bi00268a001. [DOI] [PubMed] [Google Scholar]
- Dougherty T. J. Hematoporphyrin as a photosensitizer of tumors. Photochem Photobiol. 1983 Sep;38(3):377–379. doi: 10.1111/j.1751-1097.1983.tb02687.x. [DOI] [PubMed] [Google Scholar]
- Dougherty T. J. Photoradiation therapy. Urology. 1984 Mar;23(3 Suppl):61–64. doi: 10.1016/s0090-4295(84)80119-3. [DOI] [PubMed] [Google Scholar]
- Douglas K. T., Thakrar N., Minter S. J., Davies R. W., Scazzochio C. Differences in the cleavage of DNA by bleomycin under light (300-350 nm) and dark conditions. Cancer Lett. 1982 Sep;16(3):339–345. doi: 10.1016/0304-3835(82)90016-7. [DOI] [PubMed] [Google Scholar]
- Elespuru R. K., Gonda S. K. Activation of antitumor agent gilvocarcins by visible light. Science. 1984 Jan 6;223(4631):69–71. doi: 10.1126/science.6229029. [DOI] [PubMed] [Google Scholar]
- Elespuru R. K., Yarmolinsky M. B. A colorimetric assay of lysogenic induction designed for screening potential carcinogenic and carcinostatic agents. Environ Mutagen. 1979;1(1):65–78. doi: 10.1002/em.2860010113. [DOI] [PubMed] [Google Scholar]
- Gilchrest B. A. Methoxsalen photochemotherapy for mycosis fungoides. Cancer Treat Rep. 1979 Apr;63(4):663–667. [PubMed] [Google Scholar]
- Hsiang Y. H., Hertzberg R., Hecht S., Liu L. F. Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I. J Biol Chem. 1985 Nov 25;260(27):14873–14878. [PubMed] [Google Scholar]
- Lown J. W., Chen H. H. Studies on the effects of the antitumor agent camptothecin and derivatives on deoxyribonucleic acid. Mechanism of the scission of deoxyribonucleic acid by photoactivated camptothecin. Biochem Pharmacol. 1980 Mar 15;29(6):905–915. doi: 10.1016/0006-2952(80)90221-x. [DOI] [PubMed] [Google Scholar]
- Morimoto M., Okubo S., Tomita F., Marumo H. Gilvocarcins, new antitumor antibiotics. 3. Antitumor activity. J Antibiot (Tokyo) 1981 Jun;34(6):701–707. doi: 10.7164/antibiotics.34.701. [DOI] [PubMed] [Google Scholar]
- Nakano H., Matsuda Y., Ito K., Ohkubo S., Morimoto M., Tomita F. Gilvocarcins, new antitumor antibiotics. 1. Taxonomy, fermentation, isolation and biological activities. J Antibiot (Tokyo) 1981 Mar;34(3):266–270. doi: 10.7164/antibiotics.34.266. [DOI] [PubMed] [Google Scholar]
- Parrish J. A. Therapeutic in vivo photochemistry: photochemical toxicity studies in humans. J Natl Cancer Inst. 1982 Jul;69(1):273–278. [PubMed] [Google Scholar]
- Pfyffer G. E., Towers G. H. Photochemical interaction of dictamnine, a furoquinoline alkaloid, with fungal DNA. Can J Microbiol. 1982 May;28(5):468–473. doi: 10.1139/m82-071. [DOI] [PubMed] [Google Scholar]
- Rakhit S., Eng C., Baker H., Singh K. Chemical modification of ravidomycin and evaluation of biological activities of its derivatives. J Antibiot (Tokyo) 1983 Nov;36(11):1490–1494. doi: 10.7164/antibiotics.36.1490. [DOI] [PubMed] [Google Scholar]
- STRELITZ F., FLON H., ASHESHOV I. N. Chrysomycin: a new antibiotic substance for bacterial viruses. J Bacteriol. 1955 Mar;69(3):280–283. doi: 10.1128/jb.69.3.280-283.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K. Studies on the mechanism of action of ravidomycin (AY-25,545). J Antibiot (Tokyo) 1984 Jan;37(1):71–73. doi: 10.7164/antibiotics.37.71. [DOI] [PubMed] [Google Scholar]
- Song P. S., Tapley K. J., Jr Photochemistry and photobiology of psoralens. Photochem Photobiol. 1979 Jun;29(6):1177–1197. doi: 10.1111/j.1751-1097.1979.tb07838.x. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Yoshida M., Tomita F., Shirahata K. Gilvocarcins, new antitumor antibiotics. 2. Structural elucidation. J Antibiot (Tokyo) 1981 Mar;34(3):271–275. doi: 10.7164/antibiotics.34.271. [DOI] [PubMed] [Google Scholar]
- Tomita F., Takahashi K., Tamaoki T. Gilvocarcins, new antitumor antibiotics. 4. Mode of action. J Antibiot (Tokyo) 1982 Aug;35(8):1038–1041. doi: 10.7164/antibiotics.35.1038. [DOI] [PubMed] [Google Scholar]
- Wei T. T., Byrne K. M., Warnick-Pickle D., Greenstein M. Studies on the mechanism of actin of gilvocarcin V and chrysomycin A. J Antibiot (Tokyo) 1982 Apr;35(4):545–548. doi: 10.7164/antibiotics.35.545. [DOI] [PubMed] [Google Scholar]
- Wei T. T., Chan J. A., Roller P. P., Weiss U., Stroshane R. M., White R. J., Byrne K. M. Detection of gilvocarcin antitumor complex by a biochemical induction assay (BIA). J Antibiot (Tokyo) 1982 Apr;35(4):529–532. doi: 10.7164/antibiotics.35.529. [DOI] [PubMed] [Google Scholar]
- Weiss U., Yoshihira K., Highet R. J., White R. J., Wei T. T. The chemistry of the antibiotics chrysomycin A and B. Antitumor activity of chrysomycin A. J Antibiot (Tokyo) 1982 Sep;35(9):1194–1201. doi: 10.7164/antibiotics.35.1194. [DOI] [PubMed] [Google Scholar]
- Wilson B. C., Jeeves W. P., Lowe D. M., Adam G. Light propagation in animal tissues in the wavelength range 375-825 nanometers. Prog Clin Biol Res. 1984;170:115–132. [PubMed] [Google Scholar]


