Abstract
Acute cerebral cortical trauma often leads to paroxysmal activities which terminate in a few hours, but several months later, patients can develop epilepsy. The process occurring between the initial acute triggered seizures and the onset of spontaneous unprovoked seizures is termed epileptogenesis. Here we summarize recent morphological, electro-physiological and computational studies demonstrating that partial cortical isolation increases the number and duration of silent states in the cortical network, boosting neuronal connectivity and network excitability. These changes develop progressively, and after several weeks their synergetic action leads to epilepsy.
Keywords: Sleep, wake, epilepsy, epileptogenesis, synaptic
Introduction
As indicated by the World Health Organization, trauma together with brain infection are the primary sources of acquired epilepsy at any age, and may account for a higher incidence of epilepsy in developing countries. It is well known that acute cerebral cortical trauma leads to paroxysmal activities; within the first 24 hours, up to 80% of patients with penetrating wounds display clinical seizures that usually stop after a 48 hour period (Dinner 1993). Vietnam and Croatia post-war epidemiological studies report that 10–15 years after the trauma of war, about 50% of patients with penetrating cranial wounds develop epilepsy (Marcikic and others 1998; Salazar and others 1985). Despite complex medical therapy, including new antiepileptic drugs, trauma induced epilepsy is still poorly controlled. Early administration of anticonvulsant medication decreases the frequency of early posttraumatic seizures, but not that of chronic epilepsy (Chang and Lowenstein 2003). Thus, understanding the mechanisms of trauma-induced epileptogenesis may help the development of new preventive approaches.
Partially isolated neocortex is a well-established model of trauma induced epileptogenesis (Hoffman and others 1994; Li and others 2005; Nita and others 2006; Nita and others 2007; Prince and Tseng 1993; Topolnik and others 2003). This model closely reproduces the major features of human posttraumatic epilepsy but within a faster time frame. Undercutting cortical fibers in the white matter induces acute seizures that start at ~3 hours after trauma and last for ~5 hours (Topolnik and others 2003).
In chronic conditions, electrographic seizures were recorded as early as day 4 after deafferentation. The incidence of seizures decreased by the 4th week and then recovered, reaching a steady level by weeks 5 and 6 (Nita and others 2007). In the early stages of the undercut paroxysmal activity was primarily focal, being recorded in regions neighbouring the undercut cortex, and secondary propagated to other cortical areas; involving most of the neocortex in chronic stages of the undercut (Nita and others 2007). The involvement of larger cortical regions during paroxysmal discharges was accompanied by an increased synchrony between different recording electrodes and increased speed of paroxysmal activity propagation (Marchenko and Pasikova 2008; Nita and others 2006; Nita and others 2007). At approximately 6 weeks after trauma, half of animals with undercut had behavioural seizures consisting of sudden freezing of activity (absence-like seizures) or paroxysmal muscular jerks (clonic-like seizures), resembling the epileptogenetic pattern of trauma-induced seizures in humans.
Trauma-induced epileptogenesis
Epileptogenesis is a hidden continuous process occurring between the initial cortical insult and the explicit onset of late epilepsy. The trademark of epilepsy is the expression of unprovoked seizures, characterized by paroxysmal neuronal activities and high synchronization. In the present review article we focus on the different processes underlying epileptogenesis following cortical trauma and chronic deafferentation. Two major factors can be involved in neocortical trauma-induced epileptogenesis: a) reorganization of the cortical network secondary to deafferentation, and b) modulation of intrinsic neuronal excitability1. After undercut, most of the neurons that do not branch extensively within neocortex die triggering reactive gliosis. As a consequence, the intracortical connectivity pattern and synaptic efficiency are remodelled, leading to an abnormal propagation of electrical activity within the neocortex and to the generation of paroxysmal discharges.
Neocortical seizures and states of vigilance
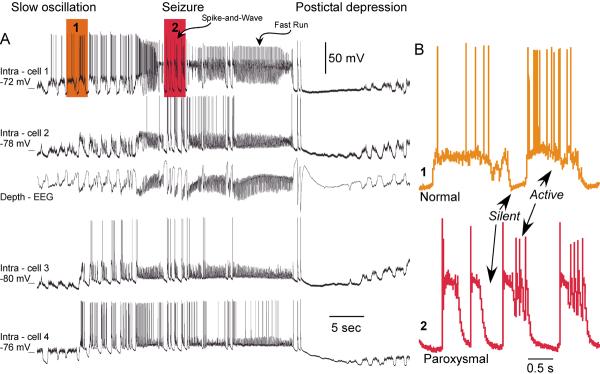
Studies on experimental animals point to an intracortical origin of some specific types of seizures occurring during slow-wave sleep, characterized by spike-wave or polyspike-wave complexes at 1.5–3 Hz, intermingled with episodes of fast-runs EEG patterns at 7–16 Hz (reviewed in (Timofeev and Steriade 2004). These seizures develop from the cortical slow oscillation without discontinuation. Similar patterns of activity can be found over large cortical territories (Fig. 1). Multiple evidence suggests that the silent periods occurring during slow-wave sleep are the key factors promoting an increased cortical excitability and the prompting of seizure onset.
Figure 1. Cortical electrographic seizure.
A. Simultaneous depth-EEG and quadruple intracellular recordings. The electrodes were equally distributed from anterior to posterior parts of suprasylvian gyrus. The intra-cell 1 was in the anterior part of area 5 and the intra-cell 4 was in the posterior part of area 21. Shadowed areas are expanded in B. B. Fragment of intracellular activity during slow oscillation. (1) During slow oscillation neurons oscillate between silent (hyperpolarized) and active (depolarized) states. (2) During paroxysmal spike-wave complexes neurons also reveal alterations of depolarized and hyperpolarized states (modified from (Boucetta and others 2008).
Structural changes associated with cortical trauma
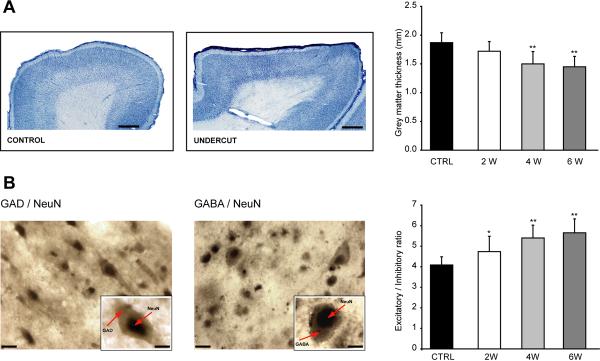
Early studies in both humans and animals demonstrated that isolated cortical islands, or cortical slabs, develop abnormal hyperexcitability (Echlin and others 1952) accompanied by general neuronal loss and gliosis (Szentagothai 1965). A study conducted on layer 5 neurons from chronically isolated neocortical slabs identified intense axonal sprouting, increase in synaptic button density and reduction in somatic cellular area (Salin and others 1995). These data suggest that an increase in neuronal connectivity can take place in traumatized cortex. In a recent study, we showed that following deafferentation, the thickness of cortical grey matter decreases (Fig. 2A) and that the normal layer architecture is altered (Avramescu and others 2008). This was associated with a decrease in the neuronal density for both excitatory and inhibitory neurons; however, the reduction in the number of inhibitory neurons was larger compared to excitatory ones (Fig. 2B).
Figure 2. Cortical undercut results in a reduction of cortical thickness and change in the ratio excitatory/inhibitory neurons towards reduced inhibition.
A. Left and middle panels, sagital section of the suprasylvian gyrus of cat in control conditions and 6 weeks after undercut. Scale bars represent 1 mm. Right panel, reduction of neocortical thickness at different time delays from isolation. B. Double staining GAD & NeuN (left panel) and GABA & NeuN (middle panel). Insets depict the double labelling of inhibitory neurons. Note the nucleus labelled in grey-black (DAB - Ni, Cr enhancement) and the cytoplasm in brown (DAB). Scale bars represent 20 μm (10 μm in the insets). Right panel depicts the relative increase in the excitatory-inhibitory ratio in the late stages of the undercut. CTRL - control, 2W, 4W, 6W – 2, 4 and 6 weeks, respectively. (modified from (Avramescu and others 2008).
Intracellular activities during states of vigilance in the undercut cortex
During slow-wave sleep cortical neurons oscillate between active (depolarizing) and silent (hyperpolarizing) states (Timofeev and others 2001). The presence of long-lasting periods of disfacilitation (silence) during slow-wave sleep is the distinct feature of this state of vigilance (Volgushev and others 2006). During other states of vigilance (wake or REM sleep), most cortical neurons are relatively depolarized and fire action potentials.
In order to generate and maintain spontaneous active states, a minimal number of neurons is needed. In small slabs of cat neocortex (~6×10 mm), active states are generated only occasionally; however, when the slab size is increased to ~20×20 mm, the active states are generated at frequencies similar to those observed in the intact cortex (Timofeev and others 2000).
Recently we showed a reduction in the total number of neurons by ~20% 6 weeks after cortical undercut in the superficial cortical layers, while in the deep layers that are strongly involved in the generation of active states, the total number of neurons decreased by ~50% from its initial value (Avramescu and others 2008). Because the total number of neurons decreases we expected to observe an increased frequency or duration of silent periods in chronically undercut cortex.
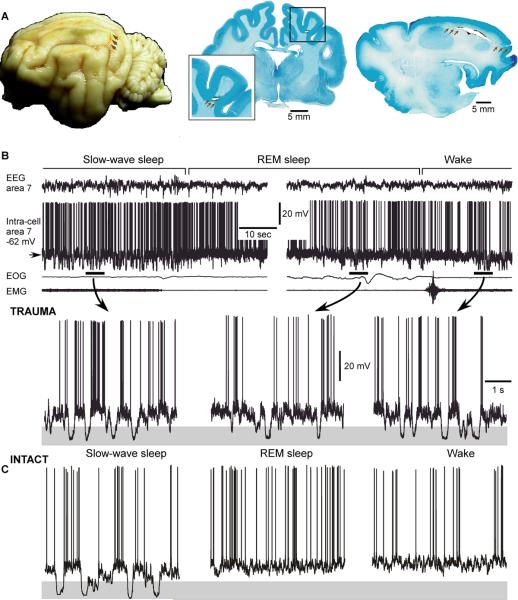
Neurons in both intact and injured cortex display a bi-stable behavior, alternating between active and silent states during slow-wave sleep. Differently from intact cortex preparations (Timofeev and others 2001) in the undercut cortex, the silent epochs occur also during REM sleep and waking state (Fig. 3). Our follow up study conducted on anesthetized cats showed that the mean duration of silent states progressively increased with time from initial trauma to 6 weeks following undercut (Avramescu and Timofeev 2008).
Figure 3. Long-lasting silent periods occur in all states of vigilance in the undercut cortex.
A. Cat brain depicting the localization of the undercut (arrows), global view – left, frontal section – middle and sagital section – right. B. Intracellular and field potential recordings during different states of vigilance. Epochs indicated by horizontal bars are expanded below. Note the presence of large amplitude hyperpolarizing potentials, indicated by shadowed area, during REM sleep and waking state. C. Intracellular neuronal recording in the intact cortex (B, modified from (Nita and others 2007); C, modified from (Timofeev and others 2001).
Plastic changes of synaptic connectivity in the undercut cortex
The increased network excitability (Echlin and others 1952; Prince and Tseng 1993) and axonal sprouting (Salin and others 1995) in the undercut cortex led to the hypothesis that major synaptic reorganization in the deafferented cortex may trigger a shift in the balance between excitation and inhibition towards excitation.
Experiments performed on cortical slices using photostimulation-induced glutamate uncaging demonstrated an increased density of hot spots and incidence of multipeaked flash-evoked EPSCs (Jin and others 2006). Moreover, the frequency of spontaneous and miniature EPSCs was increased compared to control, in the undercut cortex (Li and Prince 2002). The same group, using relatively large electrical stimulation, demonstrated a higher incidence of short-term synaptic depression in the undercut cortex compared to control (Li and others 2005), which is indicative of higher release probability (Zucker and Regehr 2002). However, because these results were obtained using electrical stimulation and not stimulation of individual presynaptic neurons, complex network-base interactions could affect the extent of reported short-term synaptic depression in these experiments. In addition, these experiments were done using higher than physiological concentrations of extracellular Ca2+, the ion that influences the release probability.
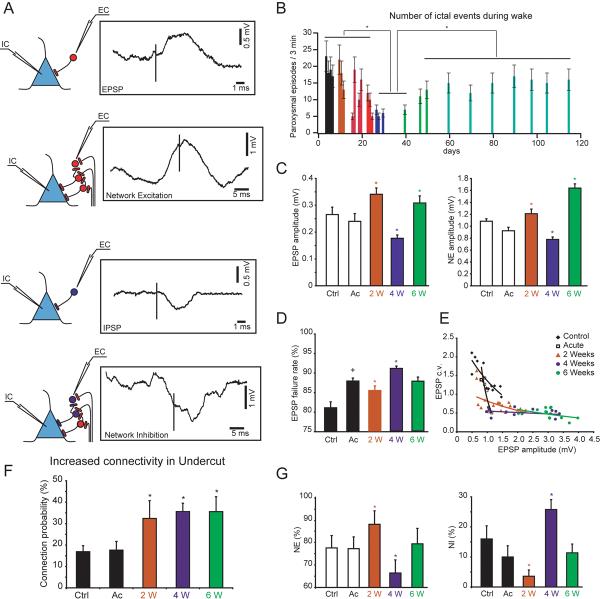
In order to avoid technical artifacts associated with in vitro preparation and electrical stimulation, we performed in vivo experiments in which pairs of directly or indirectly connected neurons were recorded simultaneously (Avramescu and Timofeev 2008). In these experiments, a presynaptic neuron was recorded extracellularly and a postsynaptic neuron was recorded intracellularly. To create preparation with chronically deafferented cortex, cortical fibers have been undercut in the white matter. Four different types of interactions were observed: pure EPSP, pure IPSP, network excitation (NE) and network inhibition (NI) (Fig. 4A). The last two (NE and NI) represented effect of activation of many synaptic connections simultaneously. We found that the overall connectivity was increased in chronically deafferented cortex (Fig. 4F). In agreement with our previous study, the failure rates and the coefficient of variation (c.v.) of EPSP amplitude at excitatory connections in vivo was very high (Crochet and others 2005). In the undercut cortex the failure rate was further increased compared to the intact cortex, with the highest levels of failure rates observed at 4 weeks after undercut (Fig. 4D). As expected, the coefficient of variation was high for low-amplitude single axon EPSPs and low for high-amplitude single axon EPSPs (Fig. 4E). The amplitude of direct EPSPs and NE was increased at 2 and 6 weeks after the undercut; however, it was decreased 4 weeks after the undercut (Fig. 4C). The NE evolution followed the same temporal profile and showed the largest decrease 4 weeks after undercut (Fig. 4G). Surprisingly, the NI underwent opposite changes, and was highest at 4 weeks from the undercut. These synaptic changes were well correlated with a decrease in the incidence of paroxysmal discharges recorded ~30 days (4 weeks) following undercut in non-anesthetized cats and its higher occurrence before and after that time (Fig. 4B), thus suggesting the leading role of these synaptic modifications in the epileptogenesis triggered by cortical undercut.
Figure 4. Properties of connectivity patterns in the undercut cortex and their relation to paroxysmal discharges.
Patterns of connectivity in recorded pairs of neurons. The postsynaptic neuron was recorded intracellularly (IC) and the presynaptic neuron was recorded extracelularly (EC). EPSP and IPSP - direct connection, network excitation (NE) and network inhibition (NI) - indirect connections. B. Variation in time of the number of ictal events. C. Variation in time of the EPSP and NE amplitudes. D. Variation in time of the EPSP's failure rates. E. Coefficient of variation versus EPSP amplitude in control and in injured cortex. F. Variation of direct connection probability at different time delays after cortical injury. G. Incidence of NE and NI in control and after cortical deafferentation. Ctrl - control; Ac - acute; W - weeks. (A, C–G modified from (Avramescu and Timofeev 2008); B, modified from (Nita and others 2007).
Our more recent experiments using recordings from connected neurons in vivo, demonstrate that short-term synaptic depression between pairs of connected cortical neurons in the undercut cortex in vivo is minor and its effects do not exceed 10 ms interspike intervals (Timofeev, Avramescu, Chauvette, unpublished). This suggests low release probability that may reduce overall cortical excitability. Therefore, despite general increase in connection probability, the dynamics of synaptic changes in the traumatized cortex are not linear, and apparently excitatory and inhibitory synapses are controlled differently.
Homeostatic plasticity and chronic epileptogenesis
Evidences from in vitro studies suggest that chronic blockade of activity may modify synaptic strengths and intrinsic neuronal excitability. After a few days of pharmacological blockade of activity in cortical cell cultures, the amplitudes of mEPSCs and EPSCs in pyramidal cells increase (Turrigiano and others 1998). Conversely, prolonged enhanced activity levels induced by blockade of synaptic inhibition reduce the size of mEPSCs (Turrigiano and others 1998). Synaptic scaling occurs in part postsynaptically by changes in the number of open channels (Turrigiano and others 1998), although all synaptic components may increase (Murthy and others 2001). These observations suggest the existence of a fundamental mechanism, termed “homeostatic plasticity” (Turrigiano 1999), that controls the levels of neuronal activity (Murthy and others 2001; Turrigiano and others 1998). Some of these processes may also occur in vivo (Desai and others 2002; Echegoyen and others 2007).
Our recordings from undercut cortex during epileptogenesis showed a dramatic increase in the overall amount of silent epochs, based on the occurrence of silent states during wake and REM sleep (Nita and others 2007) and in the increased duration of the silent periods during slow oscillation (Avramescu and Timofeev 2008). The increase in the duration of silent states throughout the process of epileptogenesis and corresponding increase of spontaneous firing rates (Avramescu and Timofeev 2008) suggest up-regulation of neuronal excitability induced by deafferentation.
We hypothesize that a mechanism comparable to homeostatic plasticity could underlie the pathological trauma-induced increase in excitability and its failure, at the upper end, will lead to overt epilepsy. We suggest that homeostatic synaptic plasticity is able to compensate the decrease in synaptic input and to re-establish the overall activity within certain limits, but the dramatically decreased activity in the undercut cortex leads to an uncontrollable cortical hyperexcitability and seizure generation.
Epileptogenesis in silico
Potential role of homeostatic plasticity (HSP) in trauma induced epileptogenesis was explored in network models of cortical population with activity dependent control of excitatory and inhibitory conductances (Houweling and others 2005; Frohlich and others 2008). The plasticity rule involved the up-regulation of synaptic coupling between pyramidal neurons and the down-regulation of connections from inhibitory interneurons to pyramidal cells as a function of the average firing rate of pyramidal cells. The “intact” network was set to display asynchronous spontaneous activity with average firing rates about 5 and 10 Hz for PY and IN cells respectively. The model of deafferentation consisted in removing the extrinsic excitatory inputs from all pyramidal (PY) and inhibitory (IN) neurons (Houweling and others 2005). This reduced spontaneous firing rates and activated HSP mechanisms, which then modified synaptic weights to bring average firing rates to the original (before deafferentation) level.
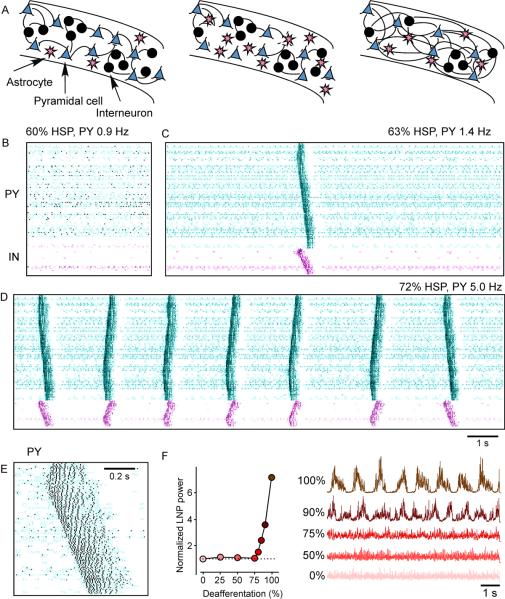
Homeostatic synaptic plasticity had initially little effect on network activity (Houweling and others 2005). Up to 60% HSP (60% upregulation of the excitatory synapses between pyramidal cells and 30% downregulation of inhibitory synapses), patterns of spontaneous network activity were similar to those in the acutely deafferented network (Fig. 5B). After 63% HSP, the spontaneous activity of the network changed in a qualitative manner (Fig. 5C). Occasionally, locally generated active states propagated through the network. They were approximately 200–400 ms in duration and involved multiple spikes in PY and IN cells (see Fig. 5E). Bursts were generated by network interactions because none of the cells possessed intrinsic bursting mechanisms (all PY cells were of the regular-spiking type). As the average number of spikes per PY cell measured over long periods of time (1.4 Hz) was still below the homeostasis target frequency of 5 Hz, further HSP increased the occurrence of network bursts (Fig. 5D, 65% HSP). Eventually, a steady state was reached where bursts repeated at frequencies of about 0.5 Hz and the average PY cell firing rate (5.0 Hz) equaled the homeostasis target firing rate (Fig. 5D, 72% HSP). Transitions to active states (bursts) in this model depend on a small fraction of cortical neurons which remain spontaneously active after deafferentation.
Figure 5. Structural changes in neocortex accompanying trauma-induced epileptogenesis and computer model of propagating burst discharges in deafferented cortex after homeostatic synaptic plasticity.
A. Cortical reorganization from control conditions to chronic stages. B–E. Raster plots of the network activity: neuron index (y-axis) vs. time (x-axis). Each dot represents the spike from a single model neuron. The pyramidal (PY) cells are in the top part of the diagram (cyan) and the inhibitory (IN) cells are shown in the bottom part (violet). These spike rasterplots show network activity after (B) 60% HSP, (C) 63% HSP, (D) 72% HSP. After 72% HSP, a steady state was reached for which PY cells fired on average 5.0 Hz. (E) An expanded spontaneous burst at 72% HSP. (F) Synaptic scaling in intact cortex modulated the average firing rate of PY cells while maintaining a low-amplitude irregular averaged local field potential (LFP) oscillations. Homeostatic synaptic plasticity restored a low-amplitude irregular LFP after partial (<80%) deafferentation. The LFP traces (right panel) are scaled by the reverse of the square root of their means for comparison purposes. (B–F, modified from (Houweling and others 2005).
Because cortical trauma may not necessarily lead to complete (100%) deafferentation, the degree of deafferentation (fraction of the excitatory afferents removed from the network) was systematically varied and the effects of homeostatic synaptic plasticity were studied (Houweling and others 2005). Surprisingly, only nearly complete deafferentation resulted in slow oscillatory network activity (Fig. 5F). When the model was deprived of only a fraction (<80%) of extrinsic inputs (which can correspond to less severe deafferentation in vivo), homeostatic synaptic plasticity restored a low-amplitude irregular LFP similar to that of intact cortex. Similar threshold value for partial cortical deafferentation required to trigger pathological periodic discharges was found in (Frohlich and others 2008). These results indicate that only after severe deafferentation is homeostatic synaptic plasticity unable to restore an asynchronous state and periodic paroxysmal activities take place.
Conclusion
We propose the following sequence of events that mediate epileptogenesis and trigger trauma-induced epilepsy (Fig. 5A). Acute penetrating wounds damage multiple cellular elements within cerebral cortex, which result in early seizures and activation of astrocytes. Reactive glial cells remove damaged neurons and promote axonal sprouting and the formation of new synapses among remaining neurons. With a decreased number of neurons, the generation of cortical active states is impaired, and cortical network spends more time in silent states, which triggers homeostatic processes trying to restore the excitability in the traumatized cortex.
We believe that at least one of the mechanisms of the increased intracortical excitability is based on an increased neuronal connectivity, which is accompanied by an increase in EPSP's amplitude. One counterbalancing process that may reduce high excitability is the increase in the failure rate of synaptic transmission, which is likely an efficient method of control during early stages of epileptogenesis. However, approaching 6 weeks from deafferentation, the failure rates of synaptic transmission are no longer different from the initial condition and at this period of time, previously focal electrographic seizures become generalized and animals start to experience behavioral seizures.
If homeostatic plasticity is involved in the development of epileptogenesis induced by trauma, it can be possible to design experiments to prevent homeostatic changes. Thus, electrical stimulation applied during particular phases of paroxysmal oscillations might be effective in the preventing of homeostatic plasticity. The major problem with this approach, however, is that low intensity electrical stimulation of the same electrode may elicit kindling. To avoid this problem, low intensity stimulation with a random pattern across the array of electrodes could be possibly applied either continuously or periodically. Alternatively, local pharmacological agents could be used to either prevent hyperpolarization (reduction of potassium currents) or to increase depolarizing influences, which can increase the level of spontaneous activity otherwise reduced because of deafferentation. One possible way to reduce the level of hyperpolarization is by dialysis of neuromodulators, which blocks potassium currents. Unlike the use of direct blockers of inhibition (bicuculline, picrotoxin, etc), these agents should not lead to the development of paroxysmal activities, but would increase locally the levels of spontaneous activities, preventing homeostatic plasticity from developing.
ACKNOWLEDGEMENTS
IT is supported by Canadian Institutes of Health Research, Natural Science and Engineering Research Council of Canada, Fonds de la recherche en santé du Québec and Nation Institute of Health Research. MB is supported by Nation Institute of Health Research. SA was partially supported by Savoy Foundation.
Footnotes
Although important, the role of changes in intrinsic excitability to epileptogenesis will not be explored in the current update.
References
- Avramescu S, Nita D, Timofeev I. Neocortical post-traumatic epileptogenesis is associated with the loss of GABAergic neurons. J Neurotrauma. 2008 doi: 10.1089/neu.2008.0739. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avramescu S, Timofeev I. Synaptic strength modulation following cortical trauma: a role in epileptogenesis. J Neurosci. 2008;28:6760–6772. doi: 10.1523/JNEUROSCI.0643-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucetta S, Chauvette S, Bazhenov M, Timofeev I. Focal generation of paroxysmal fast runs during electrographic seizures. Epilepsia. 2008;49:1925–1940. doi: 10.1111/j.1528-1167.2008.01707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang BS, Lowenstein DH. Practice parameter: antiepileptic drug prophylaxis in severe traumatic brain injury: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2003;60:10–16. doi: 10.1212/01.wnl.0000031432.05543.14. [DOI] [PubMed] [Google Scholar]
- Crochet S, Chauvette S, Boucetta S, Timofeev I. Modulation of synaptic transmission in neocortex by network activities. Eur J Neurosci. 2005;21:1030–1044. doi: 10.1111/j.1460-9568.2005.03932.x. [DOI] [PubMed] [Google Scholar]
- Desai NS, Cudmore RH, Nelson SB, Turrigiano GG. Critical periods for experience-dependent synaptic scaling in visual cortex. Nat Neurosci. 2002;5:783–789. doi: 10.1038/nn878. [DOI] [PubMed] [Google Scholar]
- Dinner D. Posttraumatic epilepsy. In: E. W, editor. The Treatment of Epilepsy: Principles. Lea & Fibinger; Philadelphia: 1993. pp. 654–658. [Google Scholar]
- Echegoyen J, Neu A, Graber KD, Soltesz I. Homeostatic plasticity studied using in vivo hippocampal activity-blockade: synaptic scaling, intrinsic plasticity and age-dependence. PLoS ONE. 2007;2:e700. doi: 10.1371/journal.pone.0000700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echlin FA, Arnet V, Zoll J. Paroxysmal high voltage discharges from isolated and partially isolated human and animal cerebral cortex. Electroencephalography and Clinical Neurophsysiology. 1952;4:147–164. doi: 10.1016/0013-4694(52)90004-7. [DOI] [PubMed] [Google Scholar]
- Fröhlich F, Bazhenov M, Sejnowski TJ. Pathological effect of homeostatic synaptic scaling on network dynamics in diseases of the cortex. J Neuroscience. 2008;28(7):1709–20. doi: 10.1523/JNEUROSCI.4263-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman SN, Salin PA, Prince DA. Chronic neocortical epileptogenesis in vitro. J Neurophysiol. 1994;71:1762–1773. doi: 10.1152/jn.1994.71.5.1762. [DOI] [PubMed] [Google Scholar]
- Houweling AR, Bazhenov M, Timofeev I, Steriade M, Sejnowski TJ. Homeostatic synaptic plasticity can explain post-traumatic epileptogenesis in chronically isolated neocortex. Cereb Cortex. 2005;15:834–845. doi: 10.1093/cercor/bhh184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Prince DA, Huguenard JR. Enhanced excitatory synaptic connectivity in layer V pyramidal neurons of chronically injured epileptogenic neocortex in rats. J Neurosci. 2006;26:4891–4900. doi: 10.1523/JNEUROSCI.4361-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Bandrowski AE, Prince DA. Cortical injury affects short-term plasticity of evoked excitatory synaptic currents. J Neurophysiol. 2005;93:146–156. doi: 10.1152/jn.00665.2004. [DOI] [PubMed] [Google Scholar]
- Li H, Prince DA. Synaptic activity in chronically injured, epileptogenic sensory-motor neocortex. J Neurophysiol. 2002;88:2–12. doi: 10.1152/jn.00507.2001. [DOI] [PubMed] [Google Scholar]
- Marchenko VG, Pasikova NV. Synchronization of electrical field potentials in neocortex of rat after isolation of a cortex island in the contralateral brain hemisphere. Zhurnal vysshei nervnoi deiatelnosti imeni I P Pavlova. 2008;58:88–97. in Russian. [PubMed] [Google Scholar]
- Marcikic M, Melada A, Kovacevic R. Management of war penetrating craniocerebral injuries during the war in Croatia. Injury. 1998;29:613–618. doi: 10.1016/s0020-1383(98)00146-6. [DOI] [PubMed] [Google Scholar]
- Murthy VN, Schikorski T, Stevens CF, Zhu Y. Inactivity produces increases in neurotransmitter release and synapse size. Neuron. 2001;32:673–682. doi: 10.1016/s0896-6273(01)00500-1. [DOI] [PubMed] [Google Scholar]
- Nita D, Cissé Y, Timofeev I, Steriade M. Increased propensity to seizures after chronic cortical deafferentation in vivo. J Neurophysiol. 2006;95:902–913. doi: 10.1152/jn.00742.2005. [DOI] [PubMed] [Google Scholar]
- Nita DA, Cisse Y, Timofeev I, Steriade M. Waking-sleep modulation of paroxysmal activities induced by partial cortical deafferentation. Cereb Cortex. 2007;17:272–283. doi: 10.1093/cercor/bhj145. [DOI] [PubMed] [Google Scholar]
- Prince DA, Tseng GF. Epileptogenesis in chronically injured cortex: in vitro studies. JNeurophysiol. 1993;69:1276–1291. doi: 10.1152/jn.1993.69.4.1276. [DOI] [PubMed] [Google Scholar]
- Salazar A, Jabbari B, Vance S, Grafman J, Amin D, Dillon J. Epilepsy after penetrating head injury. I. Clinical correlates: a report of the Vietnam Head Injury Study. Neurology. 1985;35:1406–1414. doi: 10.1212/wnl.35.10.1406. [DOI] [PubMed] [Google Scholar]
- Salin P, Tseng G-F, Hoffman S, Parada I, Prince DA. Axonal sprouting in layer V pyramidal neurons of chronically injured cerebral cortex. J Neurosci. 1995;15:8234–8245. doi: 10.1523/JNEUROSCI.15-12-08234.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szentagothai J. The use of degeneration methods in the investigation of short neuronal connections. In: Singer M, Schadé P, editors. Degeneration patterns in the nervous system, progress in brain research. Elsevier; Amsterdam: 1965. pp. 1–32. [PubMed] [Google Scholar]
- Timofeev I, Grenier F, Bazhenov M, Sejnowski TJ, Steriade M. Origin of slow cortical oscillations in deafferented cortical slabs. Cereb Cortex. 2000;10:1185–1199. doi: 10.1093/cercor/10.12.1185. [DOI] [PubMed] [Google Scholar]
- Timofeev I, Grenier F, Steriade M. Disfacilitation and active inhibition in the neocortex during the natural sleep-wake cycle: An intracellular study. Proc Natl Acad Sci U S A. 2001;98:1924–1929. doi: 10.1073/pnas.041430398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timofeev I, Steriade M. Neocortical seizures: initiation, development and cessation. Neuroscience. 2004;123:299–336. doi: 10.1016/j.neuroscience.2003.08.051. [DOI] [PubMed] [Google Scholar]
- Topolnik L, Steriade M, Timofeev I. Partial cortical deafferentation promotes development of paroxysmal activity. Cereb Cortex. 2003;13:883–893. doi: 10.1093/cercor/13.8.883. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG. Homeostatic plasticity in neuronal networks: the more things change, the more they stay the same. Trends Neurosci. 1999;22:221–227. doi: 10.1016/s0166-2236(98)01341-1. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391:892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- Volgushev M, Chauvette S, Mukovski M, Timofeev I. Precise long-range synchronization of activity and silence in neocortical neurons during slow-wave sleep. J Neurosci. 2006;26:5665–5672. doi: 10.1523/JNEUROSCI.0279-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]