Abstract
Reactive oxygen species (ROS) are ubiquitous signaling molecules in biological systems. Four members of the NADPH oxidase (Nox) enzyme family are important sources of ROS in the vasculature: Nox1, Nox2, Nox4 and Nox5. Signaling cascades triggered by stresses, hormones, vasoactive agents and cytokines control the expression and activity of these enzymes and of their regulatory subunits, among which p22phox, p47phox, Noxa1 and p67phox are present in blood vessels. Vascular Nox enzymes are also regulated by Rac, ClC-3, Poldip2 and PDI. Multiple Nox subtypes, simultaneously present in different subcellular compartments, produce specific amounts superoxide, some of which is rapidly converted to hydrogen peroxide. The identity and location of these ROS, and of the enzymes that degrade them, determine their downstream signaling pathways. Nox enzymes participate in a broad array of cellular functions including differentiation, fibrosis, growth, proliferation, apoptosis, cytoskeletal regulation, migration and contraction. They are involved in vascular pathologies such as hypertension, restenosis, inflammation, atherosclerosis and diabetes. As our understanding of the regulation of these oxidases progresses, so will our ability to alter their functions and associated pathologies.
Keywords: NADPH oxidase, Reactive Oxygen Species, Blood Vessels, Hypertension, Atherosclerosis
Introduction
Our knowledge of the signaling role of reactive oxygen species (ROS) in vascular physiology and pathophysiology has expanded tremendously in the last fifteen years. NADPH oxidases (Nox) have emerged as major sources of ROS in the vasculature, multiple Nox subtypes have been cloned and analyzed structurally and functionally, and the relationship of Nox enzymes to signaling pathways, cellular function and vascular disease has begun to be investigated.
The Nox family consists of seven catalytic homologues, four of which (Nox1, 2, 4 and 5) are found in the vasculature. These enzymes transfer electrons from NADPH to molecular oxygen producing superoxide (O2•−). Because O2•− does not readily cross membranes and is short-lived, its effect is mostly local. Depending on Nox subcellular location, O2•− is released either inside organelles or extracellularly, with corresponding internal signaling or paracrine effects. Superoxide dismutase rapidly converts O2•− to longer lasting and membrane-diffusible H2O2, thus modifying the signal and expanding its range of action. While some effects of Nox enzymes, such as inactivation of nitric oxide (NO•) in blood pressure regulation are mediated directly by O2•−, many are instead due to protein modification by H2O2, including growth signal transduction in vascular smooth muscle cells (VSMCs).
In this brief review, we will discuss our current understanding of vascular NADPH oxidases, and especially their roles in physiology and disease. We will first describe each oxidase separately, before presenting an overview of their interactions and connections with other systems.
Nox1
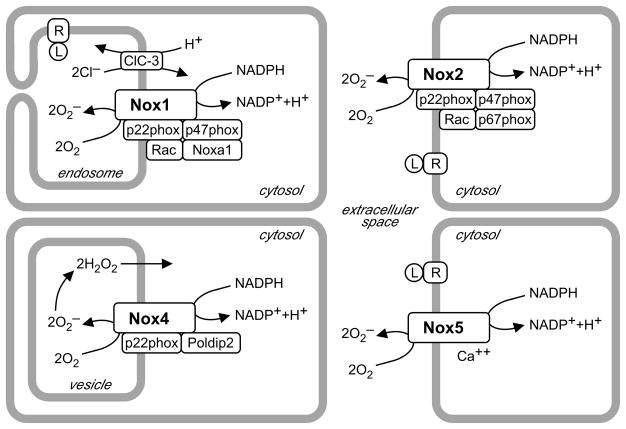
Nox1 is expressed in endothelium, smooth muscle and adventitial fibroblasts,1, 2 at the plasma membrane,3 caveolae4 and endosomes.5 Interestingly, in VSMC Nox1 is complexed with the novel activator Noxa1 and the phagocytic organizer p47phox (Figure 1).1, 6, 7 Unlike Noxo1, p47phox requires activation by phosphorylation, allowing for regulation of enzymatic activity. The identity of Nox1 regulatory subunits in other vascular cells is not known. Nox1 also closely associates with protein disulfide isomerase (PDI), a chaperone essential to its activity.8
Figure 1. Spatial and molecular organization of vascular Nox enzymes.
Nox1, 2 and 5 are represented here in different cellular compartments, but can be located either within cells or at the plasma membrane, thus releasing O2•− inside vesicles or extracellularly, following activation of receptor (R) by ligand (L). O2•− may affect cytosolic signaling after crossing membranes via anion channels, reversible protonation, or conversion to H2O2.9 In contrast, Nox4 is always intracellular, and constitutively produces a higher proportion of membrane-permeable H2O2 than other oxidases.116 All oxidases, except Nox5, form a membrane complex with p22phox. Cytosolic activators vary with oxidase subtype: Rac, p47phox and Noxa1 for Nox1 in VSMCs; Rac, p47phox and p67phox for Nox2; Poldip2 for Nox4; Ca++ for Nox5. Charge compensation mechanisms are unknown, except for VSMC endosomal ClC-3 that supports Nox1 activity. All vascular cells express multiple Nox subtypes simultaneously.
Other proteins essential to Nox1 activity do not necessarily associate with it. Because Nox enzymes carry electrons across membranes, they require charge compensation. Indeed, agonist-induced Nox1 activity in VSMC endosomes is blocked by deletion of the chloride/proton antiporter ClC-3 and rescued by transfection of intact channels.5 By exchanging protons for chloride anions, the antiporter prevents oxidase-induced cytosol acidification and accumulation of endosomal negative charges (Figure 1).9 Targeting anion channels could be used to inhibit oxidases, but their tissue and Nox subtype specificity remain to be investigated.
Nox1 is most highly expressed in intestinal epithelium, due to transcriptional upregulation of its promoter by cytokines involved in host defense.10–12 In VSMCs, Nox1 is induced by growth factors and vasoactive agents (Table 1), resulting from preferential activation of another promoter, located further upstream in the gene, in VSMCs stimulated to grow by injury or angiotensin II (AngII).13 The resulting longer transcript produces a protein with a slightly longer N-terminus, whose activity is identical to epithelial Nox1. This promoter is responsible for Nox1 upregulation by PDGF or PGF2α via a signaling cascade including PKCδ, EGFR transactivation, and either PI3K, ATF-1 and MEF2B, or ERK1/2 and JunB. Binding sites for transcription factors MEF2B and JunB have been identified.10, 14, 15
Table 1.
Examples of Nox1 signaling in vascular smooth muscle
| stimulus | upstream signals | Nox1 regulation | downstream signals | downstream effects | references |
|---|---|---|---|---|---|
| basal activity | caveolin phosphorylation AT1R at plasma membrane | 23 | |||
| AGE | upregulation | 26 | |||
| Aldosterone | upregulation | hypertrophy | 111 | ||
| AngII | Ca2+ | upregulation | P-Akt | hypertrophy | 1, 112 |
| PKC | activation | P-p38MAPK | |||
| Rac1 | SHP-2 | ||||
| Src | inactivation | ||||
| EGFR | |||||
| PI3K | |||||
| bFGF | PI3K | activation | JNK | migration | 29 |
| PKC | P-paxillin | ||||
| Rac1 | MMP2 MMP9 | ||||
| Cyclic stretch | upregulation | 113 | |||
| IL-1β | activation | NFκB | 5 | ||
| LDL | upregulation | 1, 25 | |||
| Oxidized LDL | |||||
| PDGF | PKCδ | upregulation | NFκB | hypertrophy | 1, 27, 114, 115 |
| PGF2α | EGFR | MCP-1 | migration | ||
| either PI3K | SSH1L | proliferation | |||
| ATF-1 | cofilin | ||||
| MEF2B | |||||
| or ERK | |||||
| JunB | |||||
| PMA | upregulation | 1 | |||
| Serum | upregulation | proliferation | 1, 27 | ||
| Statins | downregulation | 1 | |||
| T3 | upregulation | 28 | |||
| Thrombin | upregulation | proliferation | 1, 32 | ||
| TNFα | activation | NFκB | 5 | ||
Agonists also acutely activate Nox1 and its regulatory subunits by stimulating upstream signaling cascades, including phospholipases C and D, protein kinase C (PKC), Src and PI3K. These pathways are required for activation of p47phox and Rac.1 ROS produced by Nox1 then regulate downstream signaling pathways and essential cellular functions (Table 1) as discussed in greater detailed elsewhere.7
The role of Nox1 in cardiovascular disease has been studied mainly in hypertension. Vascular or kidney Nox1 is upregulated in experimental models, such as renin transgenic,1 2-kidney 1-clip,16 or Dahl salt-sensitive rats.17 AngII-induced hypertension is blunted by in vivo administration of p22phox siRNA18 or PKC inhibitor, which reduces Nox1 upregulation.1 Here p22phox and PKC likely activate Nox1, since Nox2 deletion has no effect (see below). Similarly, p47phox ablation prevents hypertension induced by BMP4, a Nox1-dependent endothelial cell (EC) agonist.19 More directly, Nox1 overexpression in SMC-targeted transgenics potentiates Ang-II induced hypertension and hypertrophy.20 Conversely, Nox1 deletion improves AngII-induced,21 but not ROS-independent norepinephrine-induced, hypertension.22 Unexpectedly, Nox1 deletion prevents AT1R cell surface expression.23 Whatever the mechanisms, these data implicate Nox1 in AngII-induced hypertension. In contrast, Nox1 deletion does not improve hypertension in renin transgenics,24 implying that other mechanisms control chronic hypertension.
Because growth factors stimulate ROS production in VSMC, the role of Nox1 in proliferative vascular disease was investigated. Nox1 is activated or upregulated in VSMCs treated with AngII, LDL, or AGEs1, 25, 26. Nox1 overexpression increases serum-induced proliferation and PDGF-induced migration in VSMCs.27 Conversely, Nox1 deletion inhibits VSMC proliferation induced by serum,1 thyroid hormone28 or PDGF,27 as well as PDGF or bFGF-induced migration,27, 29 which is rescued by Nox1 transfection.29 Moreover, Nox1 is activated or upregulated in vessels from diabetic animals.30, 31 Remarkably, not only is injury-induced intimal hyperplasia accompanied by Nox1 upregulation,1 but it is inhibited by Nox1 deletion.27 Thus, Nox1 can clearly mediate abnormal vascular growth and possibly atherosclerosis.
In this context, a possible link between Nox1 and vascular inflammation was also investigated. Thrombin increases VSMC Nox1 expression, IL-6 secretion, and NFκB transclocation. These latter effects may be mediated by Nox1, as they are inhibited by atorvastatin,32 which down-regulates Nox1.1 Furthermore, TNFα or IL-1β induce Nox1-dependent VSMC NFκB activation.5 Likewise, increased Nox1 expression and activity by hyperhomocysteinemia in rat coronary arteries appear to be mediated by TNFα.33 No less important, Nox1 in macrophages is required for foam cell formation, thereby contributing to vascular lesion formation.34 Therefore, Nox1 appears to mediate vascular inflammation via multiple mechanisms.
Although Nox1 is clearly implicated in vascular pathology, it also has beneficial physiological roles. For example, it is likely responsible for shear-induced outward vessel remodeling, since this response is blocked by deletion of p47phox, but not Nox2.35
Nox2
Nox2 is found in all vascular wall cells, except VSMCs from large arteries, and produces intra-or extracellular superoxide (Figure 1).1, 36, 37 Even in large arteries, endothelial cells and adventitial fibroblasts are important sources of Nox2-derived superoxide.2 Nox2 is activated by pathways similar to vascular Nox1 or phagocytic Nox2, such as p47phox phosphorylation by PKC and Src.38–40 Similar downstream signals are also affected by Nox1 and Nox2 as described at length in other reviews.7 For example Nox2 promotes EC proliferation by p38MAPK and Akt stimulation.37, 41 On a larger scale, Nox2 also affects vascular function and pathology.
Because O2•− rapidly neutralizes vasodilator NO•, Nox2 is expected to induce contraction. Indeed, Nox2 expression is inversely correlated with endothelium-dependent relaxation in isolated aorta.42, 43 Furthermore, Nox2 deletion inhibits PKC-mediated aortic contraction,40 restores acetylcholine-induced cerebral artery vasodilation44 and increases contraction in males.45 These results suggest that Nox2 may affect blood pressure.
A correlation between Nox2 expression and hypertension has been observed repeatedly. Aortic Nox2 is elevated in stroke-prone SHR, in rats exposed to aldosterone plus salt and AngII-infused mice.1, 46 Tempol improves both small intrapulmonary artery Nox2 expression and pulmonary hypertension in renin transgenic rats.47 As expected, hypertension is worsened by Nox2 overexpression in endothelial-targeted transgenics, after acute or chronic AngII treatment.48 In converse experiments, hypertension can be improved by Nox2 deletion, but results depend on the experimental model. Nox2 ablation in mice reduces systemic hypertension in 2-kidney 1-clip or DOCA salt animals49, 50 and prevents hypoxia-induced pulmonary hypertension.51 In contrast, Nox2 deletion does not prevent hypertension induced by AngII infusion, but decreases medial hypertrophy,1 possibly affecting blood pressure later on. Likewise, hypertension is unaffected in renin transgenics.52 These results suggest that treatments targeting Nox2 could improve some forms of hypertension.
Accumulating evidence suggests that Nox2 in resident ECs and adventitial fibroblasts as well as recruited macrophages promote inflammation and atherosclerosis.2, 53–55 Nox2 mRNA, O2•− production and monocyte binding are increased by oscillatory shear stress in ECs56 Furthermore, in animal models of surgical vascular injury or high cholesterol diet, Nox2 is significantly upregulated, at least during the chronic phase of the disease, even in the absence of inflammation.1, 57 Similarly, in human vessels Nox2 expression is correlated with lesion severity,1, 58 although part of the effect derives from macrophage infiltration in advanced lesions. Moreover, Nox2 deletion greatly reduces descending aortic lesion burden in hyperlipidemic mice.59 Overall these results favor a causal role of Nox2 in atherosclerosis.
Nox 4
Nox4 mRNA is present in all vascular wall cells and is significantly more abundant than other Nox enzymes.1, 60–63 The protein is located in perinuclear space or endoplasmic reticulum in ECs,64–67 nucleus in ECs and VSMCs,4, 68, 69 and focal adhesions and stress fibers in VSMCs.4, 60 Because the only regulatory subunit Nox4 shares with Nox1 and Nox2 is p22phox,70, 71 Nox4 was thought to be constitutively active,72 and responsible for basal ROS production,60, 73 but a recent report shows that Poldip2 enhances its activity (Figure 1).74
Nox4 is consistently upregulated and acutely activated by TGF-β in all cell types tested.7 In fact, TGF-β is responsible for Nox4 activation in response to other stimuli, such as hypoxia.75 Investigations of downstream signaling pathways suggest that p38MAPK is a target of Nox4.67, 76, 77 Nox4 also activates the Ras/ERK pathway, JNK and Akt.64, 76, 78 In addition to tyrosine kinase signaling pathways, Nox4 has been implicated in the activation of Rho in VSMCs, consistent with its effects on the cytoskeleton (see below).7, 74
Because there is no report to date of genetic models with altered Nox4 expression, current knowledge derives mostly from interventions in cell culture. Nox4 is linked to decreased vascular cell growth in many studies. For example, while serum withdrawal upregulates Nox4 mRNA in VSMCs and ECs,1, 61 growth-promoting agents such as AngII, PDGF, IL-1β, thrombin and phorbol esters downregulate Nox4 mRNA in VSMCs and adventitial fibroblasts,1, 62, 73 However, these results may need to be reevaluated in light of a recent study showing that Nox4 mRNA and protein expressions are not always correlated.79 Nevertheless, Nox4 is required to maintain the quiescent VSMC phenotype and TNF-α-induced apoptosis in ECs.60, 66, 80 In contrast, Nox4 appears to stimulate growth in other instances. Thus, proliferation induced by urotensin or hypoxia (via TGF-β) in pulmonary VSMCs requires Nox4.75, 76 Similarly, in ECs Nox4 promotes growth and inhibits apoptosis, and Nox4 knockdown impairs EGF- and HIV-tat-induced proliferation.64, 78, 81 Further studies are required to reconcile these conflicting results.
A similar conundrum holds for the role of Nox4 in migration. While Nox4 siRNA decreases PDGF-induced VSMCs migration and wounding-activated EC migration,74, 81 Nox4 overexpression also decreases migration in PDGF-stimulated VSMC and adventitial fibroblasts exposed to AngII.62, 74 It is likely that too little Nox4 prevents focal adhesion formation, while too much Nox4 prevents focal adhesion dissolution, both of which are required for cell motility.
In some cases Nox4 appears linked to pathways protecting vessels against disease. Indeed in ECs, Nox4 is upregulated by physiological shear stress and downregulated by pathological stress.56, 82 Similarly, Nox4 is increased during redifferentiation of smooth muscle after vascular injury.1 However, Nox4 expression is also correlated with deleterious responses. The proinflammatory mediators TNF-α and oxidized phospholipid Ox-PAPC increase Nox4 expression or activity, respectively.69, 83 Basal and urotensin-induced pro-fibrotic PAI-1 gene expression in pulmonary artery VSMCs is dependent upon Nox4, and Nox4 downregulation blocks IL-8, MCP-1, LDLR and ROS production in ECs stimulated with Ox-PAPC.76, 77, 83 Furthermore, Nox4 is elevated in cerebral aneurysms and in vessels from diabetic mice,30, 84, 85 (except31). Of interest, treatment with the PPARγ agonist rosiglitazone mitigates Nox4 upregulation.86
Overall, our knowledge of Nox4 in the vasculature is incomplete. Current evidence suggests that it is involved in numerous essential cellular pathways mediating differentiation, growth and migration. Its role is still controversial, suggesting that a central aspect of its function remains to be discovered.
Nox5
Nox5 differs from other Nox enzymes by its additional N-terminal regulatory domain. Nox5, which requires no additional subunit, is activated by calcium binding to N-terminal EF-hands (Figure 1). Calcium sensitivity is increased by calmodulin and phosphorylation by PKC.7 In transfected leukemia cells, Nox5 is activated by H2O2 in a positive feedback loop involving c-Abl.87 Five splice variants with different tissue distributions are known: Nox5α, β, γ, δ and Nox5S, a short form without calcium binding domain. Multiple isoforms are expressed in the vasculature.88 Within cells, Nox5 can be found in cytoskeletal fraction, endoplasmic reticulum or plasma membrane.89–91
Because Nox5 is not present in rodents, experimental models are limited to cultured cells and isolated tissue. In VSMCs, Nox5 participates in PDGF-induced proliferation via the JAK/STAT pathway.92 In ECs, Nox5 overexpression slightly increases proliferation and formation of capillary-like tubes, while Nox5 depletion reduces thrombin-stimulated growth and tube formation.89 In intact vessels, adenoviral-mediated Nox5 overexpression paradoxically increases eNOS activity, but as expected, reduces bioavailable NO• via inactivation by O2•−,93 leading to impaired endothelium-dependent relaxation and increased phenylephrine-induced contraction. One study shows that atherosclerosis increases Nox5 expression and calcium-sensitive oxidase activity in coronary arteries. Nox5 is detected in the endothelium of control arteries, in the neointima of diseased arteries, and in smooth muscle underlying complex lesions in arteries with advanced atherosclerosis.94
These studies are provocative, suggesting an important role for Nox5 in vascular function. Clearly, additional work is needed to further explore its physiological and pathological roles.
Interactions between oxidases
Because most cells simultaneously express multiple Nox enzymes, some of their functions may be redundant. For example, both Nox2 and Nox4 in ECs are required for the angiogenic response and participate in serum-induced proliferation.37, 41, 81, 95 Both enzymes are similarly regulated by shear stress56 and colocalize with endoplasmic reticulum markers,37 and knockdown of one subtype upregulates the other,96 suggesting that their functions are overlapping, although not entirely identical.41
Most often, however, Nox enzymes in the same cell mediate distinct functions. For example in VSMCs from large arteries, Nox1 is required for hypertrophy and proliferation,1 while Nox4 mediates differentiation.60, 80 These enzymes respond to different agonists,1, 60 participate in migration via different mechanisms27, 74 and have distinct localizations.4 Similarly in transfected HEK293 cells, Nox2 and Nox4 are expressed in different compartments, and each responds to different agonists and activates distinct signaling pathways.97 Therefore, subcellular localization seems to be an essential factor in determining Nox function.
With regard to vascular disease, multiple NADPH oxidases appear to contribute to the pathophysiological response. Both Nox1 and Nox2 have been implicated in hypertension,49–51, 98, 99 as they contribute directly to blood pressure elevation and to end-organ damage or remodeling.24, 98, 100 The expression of Nox1, Nox2 and Nox4 is altered after vascular injury, the former two mediating the proliferative response of VSMCs and ECs, while the latter most likely contributes to redifferentiation of VSMCs.1 In diabetic vascular disease, all three are upregulated,30, 85 but their specific contributions to diabetes-related endothelial dysfunction and inflammation remain to be elucidated.
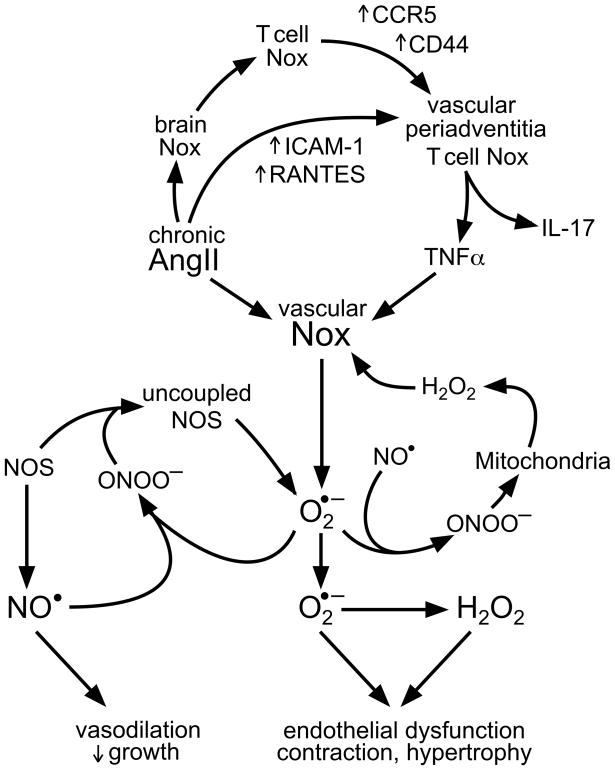
It is also important to consider that Noxes in multiple tissues contribute to the pathological response in a coordinated fashion. In hypertension, evidence exists for a role of brain, vascular, cardiac and kidney Nox enzymes.18, 101–103 Furthermore, a large part of the hypertensive response to AngII infusion or DOCA salt administration is dependent upon T lymphocytes.104 Harrison and Gongora105 have proposed that stimulation of Nox enzymes in circumventricular organs by AngII increases efferent sympathetic activity, leading to activation of T cells in lymph nodes, which home to vessels and kidneys. Importantly, T cells release cytokines that stimulate NADPH oxidases in these tissues, causing vasoconstriction and sodium retention (Figure 2). A similar coordinated response is likely to occur in the vessel wall in atherosclerosis, where infiltrated macrophages release cytokines in a Nox2-dependent manner, which then activate Nox1 and Nox2 in VSMCs, ECs and adventitial fibroblasts.2, 53–55
Figure 2. Nox enzymes in multiple tissues contribute to AngII-induced hypertension.
AngII infusion increases Nox activity in tissues involved in blood pressure regulation, including brain, kidney, heart and blood vessels.103 Brain Nox controls efferent sympathetic stimulation of lymphoid tissue, leading to activation of T lymphocytes and Nox-dependent upregulation of the CCR5 chemokine receptor and the CD44 hyaluronan receptor. Simultaneously, AngII upregulates the adhesion molecule ICAM-1 and the chemokine RANTES in vessels, leading to T cell migration to vascular periadventitia. AngII and Nox in T cells lead to secretion of TNFα, which upregulates vascular Nox.104, 105 In adventitial fibroblasts, VSMC and endothelial cells, Nox generates O2•−, which depletes NO• via conversion to peroxynitrate (ONOO−), leading to endothelial dysfunction. ROS production is further amplified by ONOO−-induced nitric oxide synthase (NOS) uncoupling and mitochondrial dysfunction.107 Whereas NO• induces vasodilation and growth arrest in healthy vessels, O2•− and H2O2 contribute to vessel contraction and hypertrophy, leading to sustained hypertension. Vascular inflammation produced by mediators such as IL-17 may also induce chronic vascular disease.
Once ROS production is initiated by Nox activation, other oxidase systems often amplify this initial response. For example, ECs exposed to pathological shear stress exhibit a Nox-dependent activation of xanthine oxidase.106 Similarly, AngII stimulation of these cells enhances mitochondrial ROS production in a Nox-dependent manner.107 In hypertension, Nox activation leads to the formation of peroxynitrite, which uncouples eNOS, leading to a dramatic enhancement of O2•− production.103 Moreover, ROS can also activate Nox enzymes and increase their own production in a positive feedback loop (Figure 2).47, 87, 103, 108–110 Such self-reinforcing loops may set new homeostatic points of ROS production and lock the organism in a sustained pathological state.
Conclusion
The evidence summarized in this review clearly shows that Nox enzymes are both essential to normal vascular function and participate in the development of vascular disease. They modulate intracellular signaling pathways to regulate cell survival, vessel contraction and relaxation. In pathological conditions, they contribute to VSMC proliferation and migration, angiogenesis, inflammation, hypertension and medial hypertrophy. Because of their varied and essential functions, it is not surprising that antioxidants designed to scavenge ROS produced from these enzymes provide ineffective protection against vascular disease. The work described here strongly argues for a change in therapeutic strategies to target only deleterious sources of ROS while leaving intact those that are required for normal vascular function.
That said, more research is necessary to fully understand the roles of different Nox enzymes in the vasculature. Additional studies should focus on identifying the specific molecular pathways that are targets of each Nox. The creation of tissue-specific, genetically modified animals will permit a better analysis of their roles in physiology and disease. Moreover, subtype-specific inhibitors are badly needed to allow the field to advance therapeutically once pathological targets have been identified. Studies such as these will allow us to truly test the oxidative hypothesis of vascular disease.
Acknowledgments
a) Sources of Funding
This work was supported by National Institutes of Health Grants HL38206, HL058863 and HL095070.
b) Acknowledgments
None
Footnotes
c) Disclosure
None
References
- 1.Lassègue B, Clempus RE. Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am J Physiol Regul Integr Comp Physiol. 2003;285:R277–297. doi: 10.1152/ajpregu.00758.2002. [DOI] [PubMed] [Google Scholar]
- 2.Csanyi G, Taylor WR, Pagano PJ. NOX and inflammation in the vascular adventitia. Free Radic Biol Med. 2009;47:1254–1266. doi: 10.1016/j.freeradbiomed.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang G, Zhang F, Muh R, Yi F, Chalupsky K, Cai H, Li PL. Autocrine/paracrine pattern of superoxide production through NAD(P)H oxidase in coronary arterial myocytes. Am J Physiol Heart Circ Physiol. 2007;292:H483–495. doi: 10.1152/ajpheart.00632.2006. [DOI] [PubMed] [Google Scholar]
- 4.Hilenski LL, Clempus RE, Quinn MT, Lambeth JD, Griendling KK. Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2004;24:677–683. doi: 10.1161/01.ATV.0000112024.13727.2c. [DOI] [PubMed] [Google Scholar]
- 5.Miller FJ, Jr, Filali M, Huss GJ, Stanic B, Chamseddine A, Barna TJ, Lamb FS. Cytokine activation of nuclear factor κ B in vascular smooth muscle cells requires signaling endosomes containing Nox1 and ClC-3. Circ Res. 2007;101:663–671. doi: 10.1161/CIRCRESAHA.107.151076. [DOI] [PubMed] [Google Scholar]
- 6.Ambasta RK, Schreiber JG, Janiszewski M, Busse R, Brandes RP. Noxa1 is a central component of the smooth muscle NADPH oxidase in mice. Free Radic Biol Med. 2006;41:193–201. doi: 10.1016/j.freeradbiomed.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 7.Brown DI, Griendling KK. Nox proteins in signal transduction. Free Radic Biol Med. 2009:1239–1253. doi: 10.1016/j.freeradbiomed.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janiszewski M, Lopes LR, Carmo AO, Pedro MA, Brandes RP, Santos CX, Laurindo FR. Regulation of NAD(P)H oxidase by associated protein disulfide isomerase in vascular smooth muscle cells. J Biol Chem. 2005;280:40813–40819. doi: 10.1074/jbc.M509255200. [DOI] [PubMed] [Google Scholar]
- 9.Lassègue B. How does the chloride/proton antiporter ClC-3 control NADPH oxidase? Circ Res. 2007;101:648–650. doi: 10.1161/CIRCRESAHA.107.161869. [DOI] [PubMed] [Google Scholar]
- 10.Lambeth JD, Kawahara T, Diebold B. Regulation of Nox and Duox enzymatic activity and expression. Free Radic Biol Med. 2007;43:319–331. doi: 10.1016/j.freeradbiomed.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adachi Y, Shibai Y, Mitsushita J, Shang WH, Hirose K, Kamata T. Oncogenic Ras upregulates NADPH oxidase 1 gene expression through MEK-ERK-dependent phosphorylation of GATA-6. Oncogene. 2008;27:4921–4932. doi: 10.1038/onc.2008.133. [DOI] [PubMed] [Google Scholar]
- 12.Valente AJ, Zhou Q, Lu Z, He W, Qiang M, Ma W, Li G, Wang L, Banfi B, Steger K, Krause KH, Clark RA, Li S. Regulation of NOX1 expression by GATA, HNF-1α, and Cdx transcription factors. Free Radic Biol Med. 2008;44:430–443. doi: 10.1016/j.freeradbiomed.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 13.Arakawa N, Katsuyama M, Matsuno K, Urao N, Tabuchi Y, Okigaki M, Matsubara H, Yabe-Nishimura C. Novel transcripts of Nox1 are regulated by alternative promoters and expressed under phenotypic modulation of vascular smooth muscle cells. Biochem J. 2006;398:303–310. doi: 10.1042/BJ20060300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cevik MO, Katsuyama M, Kanda S, Kaneko T, Iwata K, Ibi M, Matsuno K, Kakehi T, Cui W, Sasaki M, Yabe-Nishimura C. The AP-1 site is essential for the promoter activity of NOX1/NADPH oxidase, a vascular superoxide-producing enzyme: Possible involvement of the ERK1/2-JunB pathway. Biochem Biophys Res Commun. 2008;374:351–355. doi: 10.1016/j.bbrc.2008.07.027. [DOI] [PubMed] [Google Scholar]
- 15.Katsuyama M, Ozgur Cevik M, Arakawa N, Kakehi T, Nishinaka T, Iwata K, Ibi M, Matsuno K, Yabe-Nishimura C. Myocyte enhancer factor 2B is involved in the inducible expression of NOX1/NADPH oxidase, a vascular superoxide-producing enzyme. Febs J. 2007;274:5128–5136. doi: 10.1111/j.1742-4658.2007.06034.x. [DOI] [PubMed] [Google Scholar]
- 16.Wang P, Tang F, Li R, Zhang H, Chen S, Liu P, Huang H. Contribution of different Nox homologues to cardiac remodeling in two-kidney two-clip renovascular hypertensive rats: effect of valsartan. Pharmacol Res. 2007;55:408–417. doi: 10.1016/j.phrs.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 17.Nishiyama A, Yoshizumi M, Hitomi H, Kagami S, Kondo S, Miyatake A, Fukunaga M, Tamaki T, Kiyomoto H, Kohno M, Shokoji T, Kimura S, Abe Y. The SOD mimetic tempol ameliorates glomerular injury and reduces mitogen-activated protein kinase activity in Dahl salt-sensitive rats. J Am Soc Nephrol. 2004;15:306–315. doi: 10.1097/01.asn.0000108523.02100.e0. [DOI] [PubMed] [Google Scholar]
- 18.Modlinger P, Chabrashvili T, Gill PS, Mendonca M, Harrison DG, Griendling KK, Li M, Raggio J, Wellstein A, Chen Y, Welch WJ, Wilcox CS. RNA silencing in vivo reveals role of p22phox in rat angiotensin slow pressor response. Hypertension. 2006;47:238–244. doi: 10.1161/01.HYP.0000200023.02195.73. [DOI] [PubMed] [Google Scholar]
- 19.Miriyala S, Gongora Nieto MC, Mingone C, Smith D, Dikalov S, Harrison DG, Jo H. Bone morphogenic protein-4 induces hypertension in mice: role of noggin, vascular NADPH oxidases, and impaired vasorelaxation. Circulation. 2006;113:2818–2825. doi: 10.1161/CIRCULATIONAHA.106.611822. [DOI] [PubMed] [Google Scholar]
- 20.Dikalova A, Clempus R, Lassègue B, Cheng G, McCoy J, Dikalov S, San Martin A, Lyle A, Weber DS, Weiss D, Taylor WR, Schmidt HHW, Owens GK, Lambeth JD, Griendling KK. Nox1 overexpression potentiates angiotensin II-induced hypertension and vascular smooth muscle hypertrophy in transgenic mice. Circulation. 2005;112:2668–2676. doi: 10.1161/CIRCULATIONAHA.105.538934. [DOI] [PubMed] [Google Scholar]
- 21.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 22.Gavazzi G, Deffert C, Trocme C, Schappi M, Herrmann FR, Krause KH. NOX1 deficiency protects from aortic dissection in response to angiotensin II. Hypertension. 2007;50:189–196. doi: 10.1161/HYPERTENSIONAHA.107.089706. [DOI] [PubMed] [Google Scholar]
- 23.Basset O, Deffert C, Foti M, Bedard K, Jaquet V, Ogier-Denis E, Krause KH. Nadph Oxidase 1 Deficiency Alters Caveolin Phosphorylation and Angiotensin Ii Receptor Localization in Vascular Smooth Muscle. Antioxid Redox Signal. 2009:2371–2384. doi: 10.1089/ars.2009.2584. [DOI] [PubMed] [Google Scholar]
- 24.Yogi A, Mercure C, Touyz J, Callera GE, Montezano AC, Aranha AB, Tostes RC, Reudelhuber T, Touyz RM. Renal redox-sensitive signaling, but not blood pressure, is attenuated by Nox1 knockout in angiotensin II-dependent chronic hypertension. Hypertension. 2008;51:500–506. doi: 10.1161/HYPERTENSIONAHA.107.103192. [DOI] [PubMed] [Google Scholar]
- 25.Yin CC, Huang KT. H2O2 but not O2- elevated by oxidized LDL enhances human aortic smooth muscle cell proliferation. J Biomed Sci. 2007;14:245–254. doi: 10.1007/s11373-006-9132-4. [DOI] [PubMed] [Google Scholar]
- 26.San Martin A, Foncea R, Laurindo FR, Ebensperger R, Griendling KK, Leighton F. Nox1-based NADPH oxidase-derived superoxide is required for VSMC activation by advanced glycation end-products. Free Radic Biol Med. 2007;42:1671–1679. doi: 10.1016/j.freeradbiomed.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Lee MY, San Martin A, Mehta PK, Dikalova AE, Garrido AM, Datla SR, Lyons E, Krause KH, Banfi B, Lambeth JD, Lassegue B, Griendling KK. Mechanisms of vascular smooth muscle NADPH oxidase 1 (Nox1) contribution to injury-induced neointimal formation. Arterioscler Thromb Vasc Biol. 2009;29:480–487. doi: 10.1161/ATVBAHA.108.181925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X, Sun Z. Thyroid hormone induces artery smooth muscle cell proliferation: discovery of a new TRα1-Nox1 pathway. J Cell Mol Med. 2008 doi: 10.1111/j.1582-4934.2008.00489.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schroder K, Helmcke I, Palfi K, Krause KH, Busse R, Brandes RP. Nox1 mediates basic fibroblast growth factor-induced migration of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2007;27:1736–1743. doi: 10.1161/ATVBAHA.107.142117. [DOI] [PubMed] [Google Scholar]
- 30.San Martin A, Du P, Dikalova A, Lassegue B, Aleman M, Gongora MC, Brown K, Joseph G, Harrison DG, Taylor WR, Jo H, Griendling KK. Reactive oxygen species-selective regulation of aortic inflammatory gene expression in Type 2 diabetes. Am J Physiol Heart Circ Physiol. 2007;292:H2073–2082. doi: 10.1152/ajpheart.00943.2006. [DOI] [PubMed] [Google Scholar]
- 31.Wendt MC, Daiber A, Kleschyov AL, Mulsch A, Sydow K, Schulz E, Chen K, Keaney JF, Jr, Lassegue B, Walter U, Griendling KK, Munzel T. Differential effects of diabetes on the expression of the gp91phox homologues nox1 and nox4. Free Radic Biol Med. 2005;39:381–391. doi: 10.1016/j.freeradbiomed.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 32.Haloui M, Meilhac O, Jandrot-Perrus M, Michel JB. Atorvastatin limits the pro-inflammatory response of rat aortic smooth muscle cells to thrombin. Eur J Pharmacol. 2003;474:175–184. doi: 10.1016/s0014-2999(03)02043-0. [DOI] [PubMed] [Google Scholar]
- 33.Ungvari Z, Csiszar A, Edwards JG, Kaminski PM, Wolin MS, Kaley G, Koller A. Increased superoxide production in coronary arteries in hyperhomocysteinemia: role of tumor necrosis factor-α, NAD(P)H oxidase, and inducible nitric oxide synthase. Arterioscler Thromb Vasc Biol. 2003;23:418–424. doi: 10.1161/01.ATV.0000061735.85377.40. [DOI] [PubMed] [Google Scholar]
- 34.Lee JG, Lim EJ, Park DW, Lee SH, Kim JR, Baek SH. A combination of Lox-1 and Nox1 regulates TLR9-mediated foam cell formation. Cell Signal. 2008;20:2266–2275. doi: 10.1016/j.cellsig.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 35.Castier Y, Brandes RP, Leseche G, Tedgui A, Lehoux S. p47phox-dependent NADPH oxidase regulates flow-induced vascular remodeling. Circ Res. 2005;97:533–540. doi: 10.1161/01.RES.0000181759.63239.21. [DOI] [PubMed] [Google Scholar]
- 36.Chamseddine AH, Miller FJ., Jr gp91phox contributes to NADPH oxidase activity in aortic fibroblasts but not smooth muscle cells. Am J Physiol Heart Circ Physiol. 2003;285:H2284–2289. doi: 10.1152/ajpheart.00459.2003. [DOI] [PubMed] [Google Scholar]
- 37.Petry A, Djordjevic T, Weitnauer M, Kietzmann T, Hess J, Gorlach A. NOX2 and NOX4 mediate proliferative response in endothelial cells. Antioxid Redox Signal. 2006;8:1473–1484. doi: 10.1089/ars.2006.8.1473. [DOI] [PubMed] [Google Scholar]
- 38.Touyz RM, Yao G, Schiffrin EL. c-Src induces phosphorylation and translocation of p47phox: role in superoxide generation by angiotensin II in human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2003;23:981–987. doi: 10.1161/01.ATV.0000069236.27911.68. [DOI] [PubMed] [Google Scholar]
- 39.Chowdhury AK, Watkins T, Parinandi NL, Saatian B, Kleinberg ME, Usatyuk PV, Natarajan V. Src-mediated tyrosine phosphorylation of p47phox in hyperoxia-induced activation of NADPH oxidase and generation of reactive oxygen species in lung endothelial cells. J Biol Chem. 2005;280:20700–20711. doi: 10.1074/jbc.M411722200. [DOI] [PubMed] [Google Scholar]
- 40.Gupte SA, Kaminski PM, George S, Kouznestova L, Olson SC, Mathew R, Hintze TH, Wolin MS. Peroxide generation by p47phox-Src activation of Nox2 has a key role in protein kinase C-induced arterial smooth muscle contraction. Am J Physiol Heart Circ Physiol. 2009;296:H1048–1057. doi: 10.1152/ajpheart.00491.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peshavariya H, Dusting GJ, Jiang F, Halmos LR, Sobey CG, Drummond GR, Selemidis S. NADPH oxidase isoform selective regulation of endothelial cell proliferation and survival. Naunyn Schmiedebergs Arch Pharmacol. 2009;380:193–204. doi: 10.1007/s00210-009-0413-0. [DOI] [PubMed] [Google Scholar]
- 42.Takenouchi Y, Kobayashi T, Matsumoto T, Kamata K. Gender differences in age-related endothelial function in the murine aorta. Atherosclerosis. 2009;206:397–404. doi: 10.1016/j.atherosclerosis.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 43.Zemse SM, Hilgers RH, Webb RC. Interleukin-10 counteracts impaired endothelium-dependent relaxation induced by ANG II in murine aortic rings. Am J Physiol Heart Circ Physiol. 2007;292:H3103–3108. doi: 10.1152/ajpheart.00456.2006. [DOI] [PubMed] [Google Scholar]
- 44.Girouard H, Park L, Anrather J, Zhou P, Iadecola C. Angiotensin II attenuates endothelium-dependent responses in the cerebral microcirculation through nox-2-derived radicals. Arterioscler Thromb Vasc Biol. 2006;26:826–832. doi: 10.1161/01.ATV.0000205849.22807.6e. [DOI] [PubMed] [Google Scholar]
- 45.De Silva TM, Broughton BR, Drummond GR, Sobey CG, Miller AA. Gender influences cerebral vascular responses to angiotensin II through Nox2-derived reactive oxygen species. Stroke. 2009;40:1091–1097. doi: 10.1161/STROKEAHA.108.531707. [DOI] [PubMed] [Google Scholar]
- 46.Park YM, Lim BH, Touyz RM, Park JB. Expression of NAD(P)H oxidase subunits and their contribution to cardiovascular damage in aldosterone/salt-induced hypertensive rat. J Korean Med Sci. 2008;23:1039–1045. doi: 10.3346/jkms.2008.23.6.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.DeMarco VG, Habibi J, Whaley-Connell AT, Schneider RI, Heller RL, Bosanquet JP, Hayden MR, Delcour K, Cooper SA, Andresen BT, Sowers JR, Dellsperger KC. Oxidative stress contributes to pulmonary hypertension in the transgenic (mRen2)27 rat. Am J Physiol Heart Circ Physiol. 2008;294:H2659–2668. doi: 10.1152/ajpheart.00953.2007. [DOI] [PubMed] [Google Scholar]
- 48.Bendall JK, Rinze R, Adlam D, Tatham AL, de Bono J, Wilson N, Volpi E, Channon KM. Endothelial Nox2 overexpression potentiates vascular oxidative stress and hemodynamic response to angiotensin II: studies in endothelial-targeted Nox2 transgenic mice. Circ Res. 2007;100:1016–1025. doi: 10.1161/01.RES.0000263381.83835.7b. [DOI] [PubMed] [Google Scholar]
- 49.Jung O, Schreiber JG, Geiger H, Pedrazzini T, Busse R, Brandes RP. gp91phox-containing NADPH oxidase mediates endothelial dysfunction in renovascular hypertension. Circulation. 2004;109:1795–1801. doi: 10.1161/01.CIR.0000124223.00113.A4. [DOI] [PubMed] [Google Scholar]
- 50.Fujii A, Nakano D, Katsuragi M, Ohkita M, Takaoka M, Ohno Y, Matsumura Y. Role of gp91phox-containing NADPH oxidase in the deoxycorticosterone acetate-salt-induced hypertension. Eur J Pharmacol. 2006;552:131–134. doi: 10.1016/j.ejphar.2006.09.039. [DOI] [PubMed] [Google Scholar]
- 51.Liu JQ, Zelko IN, Erbynn EM, Sham JS, Folz RJ. Hypoxic pulmonary hypertension: role of superoxide and NADPH oxidase (gp91phox) Am J Physiol Lung Cell Mol Physiol. 2006;290:L2–10. doi: 10.1152/ajplung.00135.2005. [DOI] [PubMed] [Google Scholar]
- 52.Touyz RM, Mercure C, He Y, Javeshghani D, Yao G, Callera GE, Yogi A, Lochard N, Reudelhuber TL. Angiotensin II-dependent chronic hypertension and cardiac hypertrophy are unaffected by gp91phox-containing NADPH oxidase. Hypertension. 2005;45:530–537. doi: 10.1161/01.HYP.0000158845.49943.5e. [DOI] [PubMed] [Google Scholar]
- 53.Maiellaro K, Taylor WR. The role of the adventitia in vascular inflammation. Cardiovasc Res. 2007;75:640–648. doi: 10.1016/j.cardiores.2007.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Siow RC, Churchman AT. Adventitial growth factor signalling and vascular remodelling: potential of perivascular gene transfer from the outside-in. Cardiovasc Res. 2007;75:659–668. doi: 10.1016/j.cardiores.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 55.Haurani MJ, Pagano PJ. Adventitial fibroblast reactive oxygen species as autocrine and paracrine mediators of remodeling: bellwether for vascular disease? Cardiovasc Res. 2007;75:679–689. doi: 10.1016/j.cardiores.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 56.Hwang J, Ing MH, Salazar A, Lassegue B, Griendling K, Navab M, Sevanian A, Hsiai TK. Pulsatile versus oscillatory shear stress regulates NADPH oxidase subunit expression: implication for native LDL oxidation. Circ Res. 2003;93:1225–1232. doi: 10.1161/01.RES.0000104087.29395.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Collin B, Busseuil D, Zeller M, Perrin C, Barthez O, Duvillard L, Vergely C, Bardou M, Dumas M, Cottin Y, Rochette L. Increased superoxide anion production is associated with early atherosclerosis and cardiovascular dysfunctions in a rabbit model. Mol Cell Biochem. 2007;294:225–235. doi: 10.1007/s11010-006-9263-y. [DOI] [PubMed] [Google Scholar]
- 58.Guzik TJ, Sadowski J, Guzik B, Jopek A, Kapelak B, Przybylowski P, Wierzbicki K, Korbut R, Harrison DG, Channon KM. Coronary artery superoxide production and nox isoform expression in human coronary artery disease. Arterioscler Thromb Vasc Biol. 2006;26:333–339. doi: 10.1161/01.ATV.0000196651.64776.51. [DOI] [PubMed] [Google Scholar]
- 59.Judkins CP, Diep H, Broughton BRS, Mast AE, Hooker EU, Miller AA, Selemidis S, Dusting GJ, Sobey CG, Drummond GR. Direct evidence of a role for Nox2 in superoxide production, reduced nitric oxide bioavailability and early atherosclerotic plaque formation in ApoE−/− mice. Am J Physiol Heart Circ Physiol. 2009 doi: 10.1152/ajpheart.00799.2009. in press. [DOI] [PubMed] [Google Scholar]
- 60.Clempus RE, Sorescu D, Dikalova AE, Pounkova L, Jo P, Sorescu GP, Schmidt HH, Lassegue B, Griendling KK. Nox4 is required for maintenance of the differentiated vascular smooth muscle cell phenotype. Arterioscler Thromb Vasc Biol. 2007;27:42–48. doi: 10.1161/01.ATV.0000251500.94478.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ago T, Kitazono T, Ooboshi H, Iyama T, Han YH, Takada J, Wakisaka M, Ibayashi S, Utsumi H, Iida M. Nox4 as the major catalytic component of an endothelial NAD(P)H oxidase. Circulation. 2004;109:227–233. doi: 10.1161/01.CIR.0000105680.92873.70. [DOI] [PubMed] [Google Scholar]
- 62.Haurani MJ, Cifuentes ME, Shepard AD, Pagano PJ. Nox4 oxidase overexpression specifically decreases endogenous Nox4 mRNA and inhibits angiotensin II-induced adventitial myofibroblast migration. Hypertension. 2008;52:143–149. doi: 10.1161/HYPERTENSIONAHA.107.101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miller AA, Drummond GR, Schmidt HH, Sobey CG. NADPH oxidase activity and function are profoundly greater in cerebral versus systemic arteries. Circ Res. 2005;97:1055–1062. doi: 10.1161/01.RES.0000189301.10217.87. [DOI] [PubMed] [Google Scholar]
- 64.Chen K, Kirber MT, Xiao H, Yang Y, Keaney JF., Jr Regulation of ROS signal transduction by NADPH oxidase 4 localization. J Cell Biol. 2008;181:1129–1139. doi: 10.1083/jcb.200709049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Van Buul JD, Fernandez-Borja M, Anthony EC, Hordijk PL. Expression and localization of NOX2 and NOX4 in primary human endothelial cells. Antioxid Redox Signal. 2005;7:308–317. doi: 10.1089/ars.2005.7.308. [DOI] [PubMed] [Google Scholar]
- 66.Basuroy S, Bhattacharya S, Leffler CW, Parfenova H. Nox4 NADPH oxidase mediates oxidative stress and apoptosis caused by TNF-α in cerebral vascular endothelial cells. Am J Physiol Cell Physiol. 2009;296:C422–432. doi: 10.1152/ajpcell.00381.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goettsch C, Goettsch W, Muller G, Seebach J, Schnittler HJ, Morawietz H. Nox4 overexpression activates reactive oxygen species and p38 MAPK in human endothelial cells. Biochem Biophys Res Commun. 2009;380:355–360. doi: 10.1016/j.bbrc.2009.01.107. [DOI] [PubMed] [Google Scholar]
- 68.Kuroda J, Nakagawa K, Yamasaki T, Nakamura K, Takeya R, Kuribayashi F, Imajoh-Ohmi S, Igarashi K, Shibata Y, Sueishi K, Sumimoto H. The superoxide-producing NAD(P)H oxidase Nox4 in the nucleus of human vascular endothelial cells. Genes Cells. 2005;10:1139–1151. doi: 10.1111/j.1365-2443.2005.00907.x. [DOI] [PubMed] [Google Scholar]
- 69.Yoshida LS, Tsunawaki S. Expression of NADPH oxidases and enhanced H(2)O(2)-generating activity in human coronary artery endothelial cells upon induction with tumor necrosis factor-α. Int Immunopharmacol. 2008;8:1377–1385. doi: 10.1016/j.intimp.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 70.Ambasta RK, Kumar P, Griendling KK, Schmidt HH, Busse R, Brandes RP. Direct interaction of the novel Nox proteins with p22phox is required for the formation of a functionally active NADPH oxidase. J Biol Chem. 2004;279:45935–45941. doi: 10.1074/jbc.M406486200. [DOI] [PubMed] [Google Scholar]
- 71.Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal. 2006;18:69–82. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 72.Serrander L, Cartier L, Bedard K, Banfi B, Lardy B, Plastre O, Sienkiewicz A, Forro L, Schlegel W, Krause KH. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem J. 2007;406:105–114. doi: 10.1042/BJ20061903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ellmark SH, Dusting GJ, Fui MN, Guzzo-Pernell N, Drummond GR. The contribution of Nox4 to NADPH oxidase activity in mouse vascular smooth muscle. Cardiovasc Res. 2005;65:495–504. doi: 10.1016/j.cardiores.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 74.Lyle AN, Deshpande NN, Taniyama Y, Seidel-Rogol B, Pounkova L, Du P, Papaharalambus C, Lassegue B, Griendling KK. Poldip2, a novel regulator of nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circ Res. 2009;105:249–259. doi: 10.1161/CIRCRESAHA.109.193722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ismail S, Sturrock A, Wu P, Cahill B, Norman K, Huecksteadt T, Sanders K, Kennedy T, Hoidal J. NOX4 mediates hypoxia-induced proliferation of human pulmonary artery smooth muscle cells: the role of autocrine production of transforming growth factor-β1 and insulin-like growth factor binding protein-3. Am J Physiol Lung Cell Mol Physiol. 2009;296:L489–499. doi: 10.1152/ajplung.90488.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Djordjevic T, BelAiba RS, Bonello S, Pfeilschifter J, Hess J, Gorlach A. Human urotensin II is a novel activator of NADPH oxidase in human pulmonary artery smooth muscle cells. Arterioscler Thromb Vasc Biol. 2005;25:519–525. doi: 10.1161/01.ATV.0000154279.98244.eb. [DOI] [PubMed] [Google Scholar]
- 77.Jaulmes A, Sansilvestri-Morel P, Rolland-Valognes G, Bernhardt F, Gaertner R, Lockhart BP, Cordi A, Wierzbicki M, Rupin A, Verbeuren TJ. Nox4 mediates the expression of plasminogen activator inhibitor-1 via p38 MAPK pathway in cultured human endothelial cells. Thromb Res. 2009;124:439–446. doi: 10.1016/j.thromres.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 78.Wu RF, Ma Z, Myers DP, Terada LS. HIV-1 Tat activates dual Nox pathways leading to independent activation of ERK and JNK MAP kinases. J Biol Chem. 2007;282:37412–37419. doi: 10.1074/jbc.M704481200. [DOI] [PubMed] [Google Scholar]
- 79.Peshavariya H, Jiang F, Taylor CJ, Selemidis S, Chang CW, Dusting GJ. Translation-linked mRNA destabilization accompanying serum-induced Nox4 expression in human endothelial cells. Antioxid Redox Signal. 2009;11:2399–2408. doi: 10.1089/ars.2009.2579. [DOI] [PubMed] [Google Scholar]
- 80.Xiao Q, Luo Z, Pepe AE, Margariti A, Zeng L, Xu Q. Embryonic stem cell differentiation into smooth muscle cells is mediated by Nox4-produced H2O2. Am J Physiol Cell Physiol. 2009;296:C711–723. doi: 10.1152/ajpcell.00442.2008. [DOI] [PubMed] [Google Scholar]
- 81.Datla SR, Peshavariya H, Dusting GJ, Mahadev K, Goldstein BJ, Jiang F. Important role of Nox4 type NADPH oxidase in angiogenic responses in human microvascular endothelial cells in vitro. Arterioscler Thromb Vasc Biol. 2007;27:2319–2324. doi: 10.1161/ATVBAHA.107.149450. [DOI] [PubMed] [Google Scholar]
- 82.Goettsch C, Goettsch W, Arsov A, Hofbauer LC, Bornstein SR, Morawietz H. Long-Term Cyclic Strain Downregulates Endothelial Nox4. Antioxid Redox Signal. 2009 doi: 10.1089/ars.2009.2561. [DOI] [PubMed] [Google Scholar]
- 83.Lee S, Gharavi NM, Honda H, Chang I, Kim B, Jen N, Li R, Zimman A, Berliner JA. A role for NADPH oxidase 4 in the activation of vascular endothelial cells by oxidized phospholipids. Free Radic Biol Med. 2009;47:145–151. doi: 10.1016/j.freeradbiomed.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tada Y, Kitazato KT, Tamura T, Yagi K, Shimada K, Kinouchi T, Satomi J, Nagahiro S. Role of mineralocorticoid receptor on experimental cerebral aneurysms in rats. Hypertension. 2009;54:552–557. doi: 10.1161/HYPERTENSIONAHA.109.134130. [DOI] [PubMed] [Google Scholar]
- 85.Ding H, Hashem M, Triggle C. Increased oxidative stress in the streptozotocin-induced diabetic apoE-deficient mouse: changes in expression of NADPH oxidase subunits and eNOS. Eur J Pharmacol. 2007;561:121–128. doi: 10.1016/j.ejphar.2006.12.034. [DOI] [PubMed] [Google Scholar]
- 86.Hwang J, Kleinhenz DJ, Rupnow HL, Campbell AG, Thule PM, Sutliff RL, Hart CM. The PPARγ ligand, rosiglitazone, reduces vascular oxidative stress and NADPH oxidase expression in diabetic mice. Vascul Pharmacol. 2007;46:456–462. doi: 10.1016/j.vph.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 87.El Jamali A, Valente AJ, Lechleiter JD, Gamez MJ, Pearson DW, Nauseef WM, Clark RA. Novel redox-dependent regulation of NOX5 by the tyrosine kinase c-Abl. Free Radic Biol Med. 2008;44:868–881. doi: 10.1016/j.freeradbiomed.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Selemidis S, Sobey CG, Wingler K, Schmidt HH, Drummond GR. NADPH oxidases in the vasculature: molecular features, roles in disease and pharmacological inhibition. Pharmacol Ther. 2008;120:254–291. doi: 10.1016/j.pharmthera.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 89.BelAiba RS, Djordjevic T, Petry A, Diemer K, Bonello S, Banfi B, Hess J, Pogrebniak A, Bickel C, Gorlach A. NOX5 variants are functionally active in endothelial cells. Free Radic Biol Med. 2007;42:446–459. doi: 10.1016/j.freeradbiomed.2006.10.054. [DOI] [PubMed] [Google Scholar]
- 90.Jagnandan D, Church JE, Banfi B, Stuehr DJ, Marrero MB, Fulton DJ. Novel mechanism of activation of NADPH oxidase 5. calcium sensitization via phosphorylation. J Biol Chem. 2007;282:6494–6507. doi: 10.1074/jbc.M608966200. [DOI] [PubMed] [Google Scholar]
- 91.Serrander L, Jaquet V, Bedard K, Plastre O, Hartley O, Arnaudeau S, Demaurex N, Schlegel W, Krause KH. NOX5 is expressed at the plasma membrane and generates superoxide in response to protein kinase C activation. Biochimie. 2007;89:1159–1167. doi: 10.1016/j.biochi.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 92.Jay DB, Papaharalambus CA, Seidel-Rogol B, Dikalova AE, Lassegue B, Griendling KK. Nox5 mediates PDGF-induced proliferation in human aortic smooth muscle cells. Free Radic Biol Med. 2008;45:329–335. doi: 10.1016/j.freeradbiomed.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang Q, Malik P, Pandey D, Gupta S, Jagnandan D, Belin de Chantemele E, Banfi B, Marrero MB, Rudic RD, Stepp DW, Fulton DJ. Paradoxical activation of endothelial nitric oxide synthase by NADPH oxidase. Arterioscler Thromb Vasc Biol. 2008;28:1627–1633. doi: 10.1161/ATVBAHA.108.168278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Guzik TJ, Chen W, Gongora MC, Guzik B, Lob HE, Mangalat D, Hoch N, Dikalov S, Rudzinski P, Kapelak B, Sadowski J, Harrison DG. Calcium-dependent NOX5 nicotinamide adenine dinucleotide phosphate oxidase contributes to vascular oxidative stress in human coronary artery disease. J Am Coll Cardiol. 2008;52:1803–1809. doi: 10.1016/j.jacc.2008.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ushio-Fukai M, Tang Y, Fukai T, Dikalov SI, Ma Y, Fujimoto M, Quinn MT, Pagano PJ, Johnson C, Alexander RW. Novel role of gp91(phox)-containing NAD(P)H oxidase in vascular endothelial growth factor-induced signaling and angiogenesis. Circ Res. 2002;91:1160–1167. doi: 10.1161/01.res.0000046227.65158.f8. [DOI] [PubMed] [Google Scholar]
- 96.Pendyala S, Gorshkova IA, Usatyuk PV, He D, Pennathur A, Lambeth JD, Thannickal VJ, Natarajan V. Role of nox4 and nox2 in hyperoxia-induced reactive oxygen species generation and migration of human lung endothelial cells. Antioxid Redox Signal. 2009;11:747–764. doi: 10.1089/ars.2008.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Anilkumar N, Weber R, Zhang M, Brewer A, Shah AM. Nox4 and nox2 NADPH oxidases mediate distinct cellular redox signaling responses to agonist stimulation. Arterioscler Thromb Vasc Biol. 2008;28:1347–1354. doi: 10.1161/ATVBAHA.108.164277. [DOI] [PubMed] [Google Scholar]
- 98.Matsuno K, Yamada H, Iwata K, Jin D, Katsuyama M, Matsuki M, Takai S, Yamanishi K, Miyazaki M, Matsubara H, Yabe-Nishimura C. Nox1 is involved in angiotensin II-mediated hypertension: a study in Nox1-deficient mice. Circulation. 2005;112:2677–2685. doi: 10.1161/CIRCULATIONAHA.105.573709. [DOI] [PubMed] [Google Scholar]
- 99.Gavazzi G, Banfi B, Deffert C, Fiette L, Schappi M, Herrmann F, Krause KH. Decreased blood pressure in NOX1-deficient mice. FEBS Lett. 2006;580:497–504. doi: 10.1016/j.febslet.2005.12.049. [DOI] [PubMed] [Google Scholar]
- 100.Wang HD, Xu S, Johns DG, Du Y, Quinn MT, Cayatte AJ, Cohen RA. Role of NADPH oxidase in the vascular hypertrophic and oxidative stress response to angiotensin II in mice. Circ Res. 2001;88:947–953. doi: 10.1161/hh0901.089987. [DOI] [PubMed] [Google Scholar]
- 101.Zimmerman MC, Dunlay RP, Lazartigues E, Zhang Y, Sharma RV, Engelhardt JF, Davisson RL. Requirement for Rac1-dependent NADPH oxidase in the cardiovascular and dipsogenic actions of angiotensin II in the brain. Circ Res. 2004;95:532–539. doi: 10.1161/01.RES.0000139957.22530.b9. [DOI] [PubMed] [Google Scholar]
- 102.Rajagopalan S, Kurz S, Münzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation: contribution to alterations of vasomotor tone. J Clin Invest. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lassègue B, Griendling KK. Reactive oxygen species in hypertension; An update. Am J Hypertens. 2004;17:852–860. doi: 10.1016/j.amjhyper.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 104.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Harrison DG, Gongora MC. Oxidative stress and hypertension. Med Clin North Am. 2009;93:621–635. doi: 10.1016/j.mcna.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 106.McNally JS, Davis ME, Giddens DP, Saha A, Hwang J, Dikalov S, Jo H, Harrison DG. Role of xanthine oxidoreductase and NAD(P)H oxidase in endothelial superoxide production in response to oscillatory shear stress. Am J Physiol Heart Circ Physiol. 2003;285:H2290–2297. doi: 10.1152/ajpheart.00515.2003. [DOI] [PubMed] [Google Scholar]
- 107.Doughan AK, Harrison DG, Dikalov SI. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res. 2008;102:488–496. doi: 10.1161/CIRCRESAHA.107.162800. [DOI] [PubMed] [Google Scholar]
- 108.Yoshimoto T, Fukai N, Sato R, Sugiyama T, Ozawa N, Shichiri M, Hirata Y. Antioxidant effect of adrenomedullin on angiotensin II-induced reactive oxygen species generation in vascular smooth muscle cells. Endocrinology. 2004;145:3331–3337. doi: 10.1210/en.2003-1583. [DOI] [PubMed] [Google Scholar]
- 109.Li Q, Zhang Y, Marden JJ, Banfi B, Engelhardt JF. Endosomal NADPH oxidase regulates c-Src activation following hypoxia/reoxygenation injury. Biochem J. 2008;411:531–541. doi: 10.1042/BJ20071534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tian N, Moore RS, Phillips WE, Lin L, Braddy S, Pryor JS, Stockstill RL, Hughson MD, Manning RD., Jr NADPH oxidase contributes to renal damage and dysfunction in Dahl salt-sensitive hypertension. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1858–1865. doi: 10.1152/ajpregu.90650.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fan C, Kawai Y, Inaba S, Arakawa K, Katsuyama M, Kajinami K, Yasuda T, Yabe-Nishimura C, Konoshita T, Miyamori I. Synergy of aldosterone and high salt induces vascular smooth muscle hypertrophy through up-regulation of NOX1. J Steroid Biochem Mol Biol. 2008;111:29–36. doi: 10.1016/j.jsbmb.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 112.Tabet F, Schiffrin EL, Callera GE, He Y, Yao G, Ostman A, Kappert K, Tonks NK, Touyz RM. Redox-sensitive signaling by angiotensin II involves oxidative inactivation and blunted phosphorylation of protein tyrosine phosphatase SHP-2 in vascular smooth muscle cells from SHR. Circ Res. 2008;103:149–158. doi: 10.1161/CIRCRESAHA.108.178608. [DOI] [PubMed] [Google Scholar]
- 113.Grote K, Flach I, Luchtefeld M, Akin E, Holland SM, Drexler H, Schieffer B. Mechanical stretch enhances mRNA expression and proenzyme release of matrix metalloproteinase-2 (MMP-2) via NAD(P)H oxidase-derived reactive oxygen species. Circ Res. 2003;92:e80–86. doi: 10.1161/01.RES.0000077044.60138.7C. [DOI] [PubMed] [Google Scholar]
- 114.Fan C, Katsuyama M, Nishinaka T, Yabe-Nishimura C. Transactivation of the EGF receptor and a PI3 kinase-ATF-1 pathway is involved in the upregulation of NOX1, a catalytic subunit of NADPH oxidase. FEBS Lett. 2005;579:1301–1305. doi: 10.1016/j.febslet.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 115.Fan CY, Katsuyama M, Yabe-Nishimura C. PKCδ mediates up-regulation of NOX1, a catalytic subunit of NADPH oxidase, via transactivation of the EGF receptor: possible involvement of PKCδ in vascular hypertrophy. Biochem J. 2005;390:761–767. doi: 10.1042/BJ20050287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dikalov SI, Dikalova AE, Bikineyeva AT, Schmidt HH, Harrison DG, Griendling KK. Distinct roles of Nox1 and Nox4 in basal and angiotensin II-stimulated superoxide and hydrogen peroxide production. Free Radic Biol Med. 2008;45:1340–1351. doi: 10.1016/j.freeradbiomed.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]