Abstract
The mechanism underlying blood pressure reduction in the high fruits and vegetables arm of the Dietary Approaches to Stop Hypertension (DASH) Study is unknown but may include potassium, magnesium and fiber. This study was designed to separate minerals and fiber from other components of DASH on blood pressure in abdominally obese metabolic syndrome subjects with prehypertension to Stage 1 hypertension (obese hypertensives). Fifteen obese hypertensives and 15 lean normotensives were studied on a standardized usual diet, randomized to DASH or usual diet supplemented with potassium, magnesium and fiber to match DASH, then crossed over to the complementary diet. All diets were three weeks long, isocaloric and matched for sodium and calcium. In obese hypertensives, blood pressure was lower after 3 weeks on DASH than usual diet (-7.6±1.4/-5.3±1.4 mmHg, p<0.001/0.02 and usual diet supplemented (-6.2±1.4/-3.7±1.4 p<0.005/0.06), whereas blood pressure was not significantly different on usual and supplemented diets. Blood pressure values were not different among the three diets in lean normotensives. Small artery elasticity was lower in obese hypertensives than lean normotensives on the usual and supplemented diets (p<0.02). This index of endothelial function improved in obese hypertensives (p<0.02) but not lean normotensives on DASH and was no longer different from values in lean normotensives (p>0.50). DASH is more effective than potassium, magnesium, and fiber supplements for lowering blood pressure in obese hypertensives, which suggest that high fruits and vegetables DASH lowers blood pressure and improves endothelial function in this group by nutritional factors in addition to potassium, magnesium and fiber.
Keywords: Blood pressure, Dietary Approaches to Stop Hypertension (DASH), obesity hypertension, metabolic syndrome, potassium, magnesium, fiber
INTRODUCTION
The majority of pre-hypertensive and hypertensive patients are overweight or obese.1,2 A substantial minority of pre-hypertensive and the majority of hypertensive patients have the metabolic syndrome.3 Weight loss ameliorates several facets of the metabolic syndrome including elevated blood pressure,4 yet sustained weight loss is an elusive long-term end-point for most obese people.5 Lifestyle interventions that reduce risk without weight loss could be useful in managing metabolic syndrome related risk and disease.
The Dietary Approaches to Stop Hypertension (DASH) Eating Plan, which is rich in fruits and vegetables, lowers blood pressure and improves the lipid profile without weight loss in hypertensive subjects.6 While DASH lowered blood pressure more with than without low fat dairy products, both diets reduced blood pressure in hypertensive patients. Both diets are high in potassium, magnesium, and fiber, which are associated with reduction of blood pressure.7-9
Obesity and the metabolic syndrome are linked to inflammation and oxidative stress, which contribute to endothelial dysfunction.10-12 Endothelial dysfunction is implicated in the pathophysiology of hypertension and cardiovascular disease.13 The fruits, vegetables and other whole foods in DASH contain numerous flavonoids and antioxidants, which may reduce oxidative stress, improve endothelial function and lower blood pressure.14 The high potassium, magnesium and fiber content of DASH may also improve endothelial and vascular function.15,16
Our study was designed to separate the effects of minerals and fiber from other components of DASH on blood pressure and markers of endothelial and vascular function in abdominally obese pre-hypertensive and hypertensive patients with the metabolic syndrome. Lean normotensive subjects without metabolic syndrome risk factors were studied as a healthy, time-control group. The comparators included a standardized typical Western diet, which is low in fruits, vegetables and other whole foods, the usual diet supplemented with potassium, magnesium, and fiber to match DASH, and DASH fruits and vegetables without emphasis on low fat dairy products given relatively poor adoption by free-living volunteers in our previous study.14 The diets were isocaloric and matched for sodium and calcium.
METHODS
Ethics
The protocol was reviewed and approved by the Office of Research Protection and Integrity at the Medical University of South Carolina (MUSC). Paid study volunteers were recruited from the staff and clinics at MUSC. Written informed consent was obtained from all volunteers prior to the screening evaluation.
Subjects
Volunteers were recruited and studied from 2003–2006. Inclusion criteria for lean normotensives were age 21–49 years, body mass index <25 kg/m2, waist circumference <40 inches for men and <35 inches for women, blood pressure consistently <130/85 mmHg on all 3 visits prior to the first study, fasting glucose <100 mg/dl, fasting triglycerides <125 mg/dl, HDL-cholesterol ≥40 mg/dl for men and ≥45mg/dl for women, total cholesterol <200 mg/dl and/or total cholesterol / HDL ≤3.5.
Inclusion criteria for abdominally obese patients included blood pressure 130–159/85–99 mmHg on the three screening visits, age 21–49 years, waist circumference >40 inches in men and >35 inches for women, and at least one other metabolic syndrome criterion (impaired fasting glucose [100–125 mg/dL], fasting triglycerides ≥150 mg/dl or HDL-cholesterol <50 for women and <40 for men).17
Exclusion criteria were diabetes mellitus (fasting glucose ≥126 mg/dl or treatment), clinically evident target organ, or history of stroke, transient ischemic attack, myocardial infarction, angina pectoris, or chronic heart failure.
Volunteers discontinued all non-essential prescription and non-prescription medications and supplements at least 3 weeks before beginning the study. Patients requiring medications to maintain BP <160/<100 mmHg were excluded. Medications, which volunteers identified as essential and that were determined to have minimal effects on blood pressure, were allowed. Examples include antihistamines for allergies, H2-receptor blockers and proton pump inhibitors for gastroesophageal reflux, and selective serotonin reuptake inhibitors for depression. Acetaminophen was allowed; non-steroidal anti-inflammatory medications were not.
Study diets
Subjects were studied first after 3 weeks on a standardized usual diet, with an average of one fruit and one vegetable (ULFV), ∼1700 mg potassium, 250 mg magnesium and 11 grams of fiber daily. They were randomized to ULFV supplemented with potassium, magnesium and fiber (ULFV-S) to match DASH, or DASH itself. Subjects completed the complementary phase of the randomized diet for three weeks. DASH without additional low-fat dairy was selected based on our prior study in free-living volunteers.14
The nutrient targets for the three diets were ∼50% carbohydrate, 35% fat, and 15% protein with 3000 mg sodium and 700 mg calcium daily. The calcium target was within the range attained in our previous study of the DASH combination diet in lean and obese volunteers.14 The ULFV diet was supplemented with potassium citrate (Upsher Smith Laboratories, Maple Grove, MN), or potassium chloride (Tower Laboratories Ltd., depending upon tolerability as both have similar blood pressure effects.5 Diets were also supplemented with magnesium oxide (Goldline Lab, Mason, OH) Centerbrook, CT) and (Metamucil®, Procter & Gamble, Cincinnati, OH) to match intake on DASH. The dietary potassium goal reflects levels achieved with fruits and vegetables DASH.7 The Minnesota Nutrition Data Systems (Nutrition Coordinating Center, Minneapolis, MN) was used to analyze food records and pictures and estimate nutrient intake.18
Study participants met individually with the GCRC dietician each week of the study. Sample menus of the ULFV and DASH were provided. Each subject’s isocaloric energy intake was estimated using the Harris Benedict equation.19 Volunteers kept a food diary and received a digital camera (PenCam VR, Aiptek, Irvine, to photograph all food and beverages consumed for 3 days before weekly visits with the dietician. Subjects collected weekly 24-hour urines for sodium and potassium. The dietician provided advice to further enhance dietary compliance and estimated nutrient consumption and dietary compliance from 3-day food records and urine data supplemented by photographs of food and beverages consumed.20 Calories were adjusted weekly to minimize weight changes.
Hemodynamic measurements
Blood pressure was measured with a random-zero sphygmomanometer, while participants were seated at three screening visits and weekly during the first two weeks of the three study diets. After three weeks on each diet, six supine (basal) BP readings were obtained with the random-zero device at 10-minute intervals.
Small and large artery elasticity were derived using the H.D.I./Pulse Wave CR-2000 (Eagan, MN).21 Central (aortic) blood pressure was derived with the Sphygmacor (SCOR—MX, West Ryde, NSW Australia).22 Aortic augmentation index was calculated.22
Metabolic assays
Plasma insulin was measured by radioimmunoassay.23 Homeostatic model assessment of insulin resistance (HOMAir) index was calculated.24 Triglycerides, total and HDL-cholesterol were measured;25 LDL-cholesterol was calculated.26
Study design and protocol
After 3 weeks on ULFV, subjects were randomized to DASH or the ULFV-S for the next 3 weeks. Volunteers crossed over to the complementary diet for 3 additional weeks. The dietary phases were not separated by a washout.
At the end of each 3-week dietary period, subjects were admitted to the outpatient GCRC at 8:00 AM after an overnight fast. Intravenous access was established. Supine hemodynamic data were obtained at 10 minute intervals for 60 minutes. Blood was then drawn for serum lipids, plasma glucose and plasma insulin.
Statistical considerations
Sample size estimates
The primary outcome variable was the last four of six supine laboratory blood pressures taken after three weeks on each diet. With 15 subjects, the study had a power of greater than 90% (β<0.10) to detect the pre-specified 5 mmHg (± 10 mmHg variance) difference in systolic BP between diets within group at p≤0.05.
Data analysis
Group comparisons for categorical variables, e.g., gender and ethnicity, were made using Chi-square tests. Data for continuous variables are presented as mean ± standard error. For baseline variables such as age, nutrient intake, and blood pressure, two-sample t-tests were used to compare obese and lean groups. One-sample t-tests were used to assess changes in nutritional variables between DASH and ULFV-S in obese hypertensives. Changes in blood pressure, nutritional and biochemical measurements across the three dietary phases within and between groups were made using linear mixed models for repeated measures. An order effect of diet order on blood pressure was examined by Grizzle’s method. Statistical analyses were performed with SAS Version 9.1. All statistical tests were two-sided, and p-values <0.05 were accepted as significant except Grizzle’s method where a p-value <0.10 indicates an order effect.
Results
Study volunteers
As shown, 102 volunteers, including 36 lean and 66 obese subjects, completed the baseline history, physical, and laboratory assessment (Figure 1). Sixty-five subjects did not qualify (screen failures), including 18 of 36 lean (50%) and 47 of 66 (71%) obese. Lean volunteers were generally excluded for having metabolic syndrome risk factors and obese subjects for having fewer than three. Of 102 volunteers screened, 37 qualified including 18 lean and 19 obese. Fifteen subjects in each group completed the three study phases and were included in the analysis.
Figure 1.
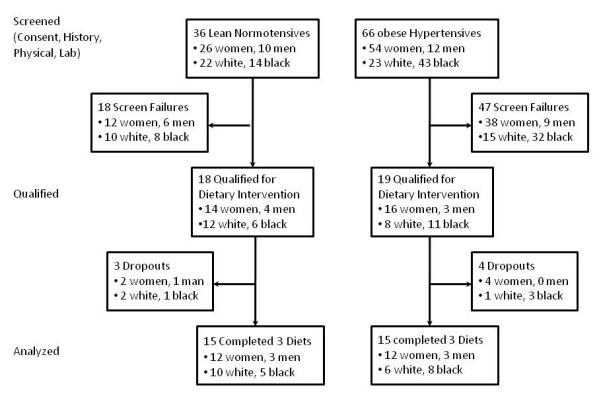
The study flow diagram shows the number of obese and lean volunteers that were screened, met inclusion and exclusion criteria, and completed the three dietary periods
Age, gender, and race were not different between the two groups (Table 1). Obese hypertensives had higher values than lean normotensives for blood pressure, body mass index, abdominal and hip circumferences, triglycerides, fasting glucose, and uric acid.
Table 1.
Selected baseline descriptive characteristics (mean ± SEM) of obese hypertensive and lean normotensive volunteers.
| Variables | Obese Hypertensives | Lean Normotensives | P-value |
|---|---|---|---|
| Age , years | 40.3 ± 1.7 | 36.7 ± 1.8 | 0.15 |
| Gender, F/M | 12 F/ 3 M | 12 F/ 3 M | 0.99 |
| Race, black/white | 8 B/7 W | 5 B / 10 W | 0.46 |
| Systolic BP, mm Hg | 136.3 ± 1.1 | 110.4 ± 0.8 | <0.001 |
| Diastolic BP, mm Hg | 88.6 ± 0.9 | 70.0 ± 0.7 | <0.001 |
| BMI, kg/m2 | 34.5 ± 1.3 | 22.8 ± 0.4 | <0.001 |
| Abdominal girth, in | 41.4 ± 1.1 | 31.7 ± 0.7 | <0.001 |
| Hip circumference, in | 46.9 ± 1.2 | 38.3 ± 0.4 | <0.001 |
| Total Chol, mg/dL | 188 ± 11 | 187 ± 7 | 0.95 |
| HDL-C, mg/dL | 47.0 ± 3.1 | 58.9 ± 4.0 | 0.03 |
| Triglycerides, mg/dL | 92 ± 10 | 57 ± 7 | 0.01 |
| LDL-C, mg/dL | 122 ± 9 | 117 ± 5 | 0.60 |
| Glucose, mg/dL | 96.1 ± 1.9 | 86.5 ± 3.4 | 0.02 |
| Sodium, mmol/L | 138.9 ± 0.5 | 138.5 ± 0.5 | 0.51 |
| Potassium, mmol/L | 4.2 ± 0.1 | 4.4 ± 0.1 | 0.38 |
| Creatinine, mg/dL | 0.83 ± 0.04 | 0.79 ± 0.03 | 0.54 |
| Calcium, mg/dL | 9.4 ± 0.1 | 9.4 ± 0.1 | 0.72 |
| Phosphorus, mg/dL | 3.6 ± 0.2 | 3.5 ± 0.1 | 0.64 |
| Uric acid, mg/dL | 5.1 ± 0.2 | 3.9 ± 0.3 | 0.002 |
Nutritional content of the diets
Fruits and vegetable on the usual and supplemented diet were lower than DASH (Table 2). Sodium intake was comparable across the three diets, while potassium, magnesium, and fiber were greater on ULFV-S and DASH than the baseline phase. Calcium intake was not different among the three dietary periods in lean normotensives or obese hypertensives, although mean values tended to be higher in obese hypertensives on DASH.
Table 2.
The nutrient goal and mean daily intake in all subjects combined for ULFV, ULFV-S, and DASH. Values are expressed as mean ± SEM.
| Nutrients (units) | All Subjects | |||||
|---|---|---|---|---|---|---|
| Goal ULFV |
Actual ULFV |
Goal ULFV-S |
Actual ULFV-S |
Goal DASH |
Actual DASH |
|
| Fruit, N/day | 1-2 | 0.9 ± 0.8## | 1-2 | 0.8 ± 0.8 | 5-6 | 4.5 ± 1.5** |
| Veg, N/day | 1-2 | 1.1 ± 0.6## | 1-2 | 1.1 ± 0.8 | 3-4 | 3.3 ± 1.5** |
| Kcals/day | 2000 | 1920 ± 43## | 2000 | 1991 ± 50 | 2000 | 2201 ± 44** |
| Protein, % kcal | 15 | 15.9 ± 0.3# | 15 | 14.9 ± 0.4 | 15 | 14.7 ± 0.3 |
| Carb, % kcals | 50 | 45.8 ± 0.8## | 50 | 48.5 ± 0.8+ | 50 | 51.2 ± 0.8* |
| Fat, % kcals | 35 | 37.7 ± 0.7## | 35 | 35.4 ± 0.7+ | 35 | 33.3 ± 0.7* |
| Alcohol, % kcals | 0.7 ± 0.2 | 1.3 ± 0.3 | 0.8 ± 0.2 | |||
| Chol, mg/d | 300 | 327 ± 17## | 300 | 297 ± 15 | 300 | 260 ± 14 |
| K+, mg/d | 1700 | 1861 ± 46## | >4100 | 3888 ± 80++ | >4100 | 3768 ± 80 |
| Fiber, mg/d | 9 | 11.9 ± 0.4## | 31.0 | 25.4 ± 0.5++ | 31.0 | 28.2 ± 0.8** |
| Na+, mg/d | 3000 | 3603 ± 103 | 3000 | 3736 ± 90 | 3000 | 3613 ± 101 |
| Mg++, mg/d | 175 | 194 ± 5## | 500 | 445 ± 16+++ | 500 | 401 ± 10** |
| Ca++, mg/d | 700 | 672 ± 29# | 700 | 688 ± 26 | 700 | 763 ± 27 |
Veg, = vegetables; N/day = servings per day; Kcal = 1000 calories or 1 Calorie, % kcal = % of total daily calories; Carb = carbohydrate.
indicate that ULFV-S and DASH are different at p<0.05
p<0.01, respectively.
indicate that ULFV-S and ULFV are different at p<0.05
p<0.01, respectively.
indicate that DASH and ULFV are different at p< 0.05
p< 0.01, respectively.
Nutrient differences between ULFV-S and DASH in obese hypertensives
Estimated intake of arginine, lycopene, Vitamins C and alpha E, and folate were higher on DASH than ULFV-S. Soluble fiber intake was higher during ULFV-S than DASH, whereas insoluble fiber was higher on DASH than ULFV-S. Trans-fatty acid intake was lower on DASH than ULFV-S. Values for fish oils (docosohexanoic, eicosopentanoic), linolenic acid, sodium, potassium, calcium, magnesium and selenium were not different between the two diets.
BP responses to dietary intervention
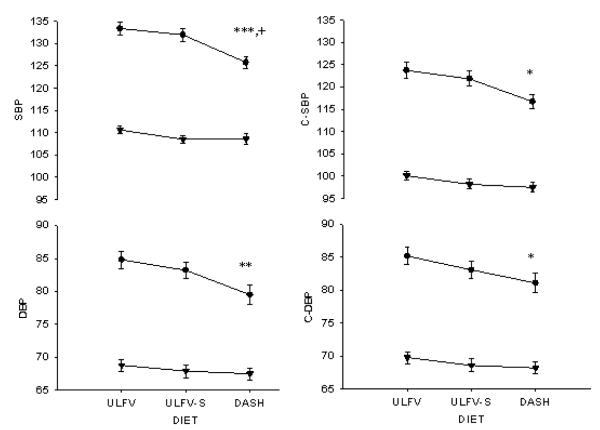
Peripheral (brachial) and estimated central (aortic) systolic and diastolic pressure at the end of 3 weeks on each diet are shown for obese hypertensives and lean normotensives in Figure 2. Systolic and diastolic pressures were lower in obese subjects after 3 weeks on DASH than either ULFV or ULFV-S. BPs were not different between ULFV and ULFV-S in the obese group. Central systolic and diastolic pressures were also lower in obese subjects on DASH than ULFV but were not significantly different from ULFV-S. Brachial and central systolic and diastolic blood pressures were not different among the three dietary periods in lean volunteers. Blood pressure differences between the dietary periods for both groups are shown in Figure 3.
Figure 2.
Peripheral (brachial cuff [left]) and central (right) systolic (top) and diastolic (bottom) blood pressures are provided for the obese hypertensive [ ●] and lean volunteers [▲] on the three dietary periods. Values reflect the means and standard error of multiple baseline, supine readings after three weeks on each diet. ULFV = usual diet low in fruits and vegetables; ULFV-S = usual diet supplemented with potassium, magnesium and fiber to match DASH; DASH = high fruits and vegetables DASH. * p<0.05, ** p<0.01, *** p<0.001 DASH vs ULFV; + p<0.05 DASH vs ULFV-S. P-values refer to comparisons within the obese group only as none of the blood pressure values among the three diets was significantly different in lean normotensives.
Figure 3.
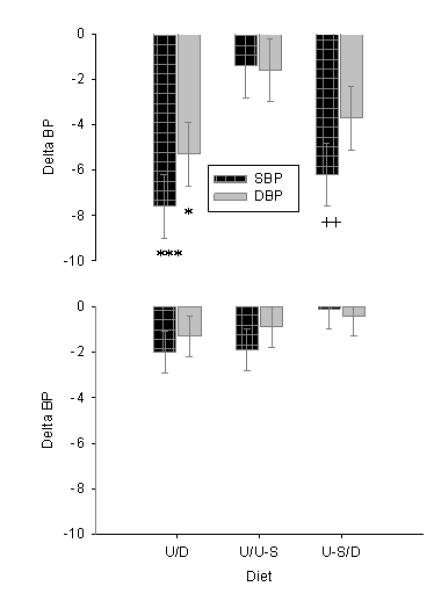
The differences in mean (± standard error) baseline supine blood pressures after three weeks are shown for systolic (dark bars) and diastolic BP (light bars) on ULFV (L) vs. DASH ([D] left), ULFV (U) vs. ULFV-S ([U-S] middle) and ULFV-S vs. DASH ([U-S/D] right) for both obese hypertensives (top) and lean normotensives (bottom). * p<0.05, *** p<0.001 DASH (D) vs ULFV (U); ++ p<0.01 DASH (D) vs ULFV-S (U-S).
Using Grizzle’s method, no carry over effect was found of diet sequence on systolic BP in lean normotensives (p=0.42), obese hypertensives (p=0.73) or all subjects (p=0.89). Corresponding p-values for diastolic pressure were 0.08 (lean), 0.87 and 0.83.
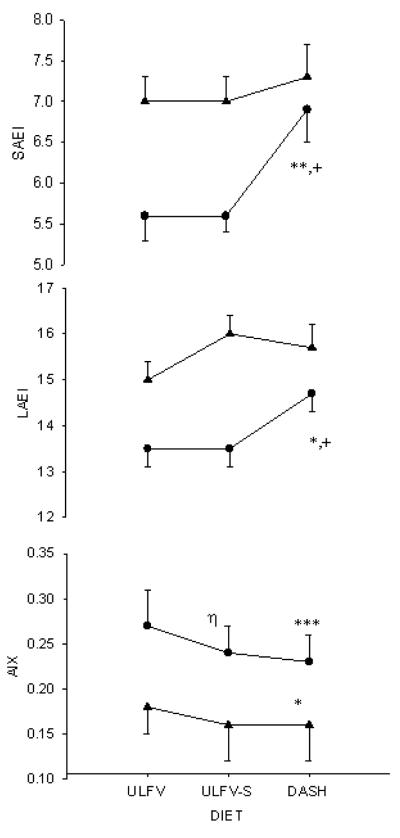
Vascular function
Nutritional effects on vascular stiffness were assessed with small and large artery elasticity and aortic augmentation index. Small and large artery elasticity values in obese hypertensives were higher on DASH than the other two diets, whereas values did not change significantly in lean normotensives. The aortic augmentation index was lower (improved) in both groups on DASH than ULFV-S. In obese hypertensives, this index was also lower on ULFV-S than the usual diet (Figure 4).
Figure 4.
Small (top panel) and large (middle panel) artery elasticity indices and aortic augmentation index (bottom panel) are shown for obese hypertensives [ ● ] and lean normotensives [▲]. * p<0.05, ** p<0.01, *** p<0.001 DASH vs ULFV; + p<0.05 DASH vs ULFV-S; η p<0.001 ULFV-S vs ULFV. P-values are for within group comparisons.
Metabolic variables
Weight, heart rate, metabolic and urine electrolyte values are shown for lean and obese volunteers during the three dietary periods (Table 4). The only significant changes occurred in urine potassium and magnesium, which were higher on DASH and ULFV-S than the usual diet (ULFV). No significant differences were observed for any of the variables in Table 4 between DASH and ULFV-S in either lean normotensives or obese hypertensives.
Table 4.
Selected metabolic and urine electrolyte data are shown for lean normotensive subjects (top) and obese hypertensives (bottom) on the three dietary periods.
| Lean Normotensive Volunteers | Obese Hypertensive Subjects | |||||
|---|---|---|---|---|---|---|
| ULFV | DASH | ULFV-S | ULFV | DASH | ULFV-S | |
| Weight, pounds | 141.4 ± 5.0 | 143.9 ± 5.1 | 142.3 ± 5.0 | 210.7 ± 8.1 | 210.1 ± 7.8 | 211 ± 8.2 |
| HR, beats/min | 64.5 ± 1.2 | 66.3 ± 1.4 | 64.8 ± 1.3 | 68.9 ± 0.9 | 68.2 ± 1.0 | 70.1 ± 1.2 |
| Insulin, μU/mL | 5.2 ± 0.7 | 6.0 ± 1.0 | 6.0 ± 0.9 | 12.4 ± 1.5 | 14.1 ± 1.6 | 18.2 ± 6.1 |
| Glucose, mg/dL | 82.4 ± 2 | 81.8 ± 1.9 | 84.3 ± 1.7 | 87.4 ± 2.2 | 88.5 ± 1.7 | 81.9 ± 5.3 |
| HOMAir | 1.0 ± 0.1 | 1.2 ± 0.2 | 1.3 ± 0.2 | 2.72 ± 0.3 | 3.03 ± 0.3 | 2.6 ± 0.4 |
| Cholesterol, mg/dL | 169 ± 7 | 162 ±12 | 158 ± 10 | 177 ± 10 | 167 ± 8 | 165 ± 8 |
| TG, mg/dL | 47 ± 5 | 49 ± 8 | 56 ± 6 | 91.3 ± 12.6 | 80.4 ± 9.7 | 85.2 ± 14.1 |
| LDL, mg/dL | 106 ± 5 | 110 ± 6 | 106± 6 | 116.7 ± 8.2 | 109.7 ± 6.8 | 108.2 ± 6.3 |
| HDL, mg/dL | 54 ± 4 | 53 ± 3 | 53 ± 4 | 42.2 ± 2.8 | 40.5 ± 2.63 | 40.6 ± 2.4 |
| VLDL, mg/dL | 9.4 ± 1.0 | 9.8 ± 1.7 | 10.8 ± 1.3 | 18.1 ± 2.5 | 16.7 ± 1.8 | 17.0 ± 2.8 |
| Urine Na+, mmol/d | 97 ± 8 | 93 ± 8 | 103 ± 8 | 108 ± 10 | 98 ± 7 | 102 ± 7 |
| Urine K+, mmol/d | 31 ± 2Ω,ΦΦ | 41 ± 3 | 47 ± 4 | 32 ± 3Ω, ΦΦ | 46 ± 5 | 51 ± 5 |
| Urine Mg++, mg/d | 56 ± 5Ω,Φ | 67 ± 8 | 69 ± 6 | 57 ± 5Φ | 63 ± 6 | 66 ± 6 |
HOMAir = homeostatic model assessment of insulin resistance
Chol = cholesterol; TG = triglycerides, LDL = low-density lipoprotein cholesterol; HDL — high-density lipoprotein cholesterol; VLDL = very low-density lipoprotein cholesterol; Na+ = sodium; K+ = potassium; Mg++ = magnesium.
p<0.05 vs DASH
p<0.05 vs ULFV-S
p<0.01 vs ULFV-S within the lean normotensive and obese hypertensive groups
Discussion
Abdominally obese subjects with the metabolic syndrome and (pre)hypertension had lower blood pressure on the DASH high fruits and vegetables eating plan than a usual diet low in fruits and vegetables ([ULFV] Figures 2, 3). Blood pressure was also lower on DASH than ULFV supplemented with potassium, magnesium and fiber (ULFV-S) to match DASH. In contrast, blood pressure was not lower in obese hypertensives on ULFV-S than ULFV (Table 3).
Table 3.
Nutrient intake (mean ± SEM) is shown for obese hypertensives on ULFV-S and DASH.
| Nutrient intake | ULFV-S | DASH | Mean Change DASH—ULFV-S |
P-Value |
|---|---|---|---|---|
| Na+, mg/d | 3854±185 | 3563±231 | -291 ± 183 | 0.13 |
| K+, mg/d | 3791 ± 227 | 3873 ± 241 | 83 ± 193 | 0.67 |
| Ca++, mg/d | 666 ± 56 | 807 ± 46 | 141 ± 72 | 0.07 |
| Mg++, mg/d | 455 ± 35 | 410 ± 27 | -45 ± 29 | 0.14 |
| Lycopene, mcg/d | 3125 ± 791 | 6546 ± 1213 | 3422 ±1313 | 0.02 |
| Selenium, mg/d | 285 ± 159 | 126 ± 10 | -159 ± 159 | 0.34 |
| Vitamin C, mg/d | 44 ± 7 | 216 ± 18 | 172 ± 20 | <.0001 |
| Vitamin E, mg/d | 8.3 ± 0.6 | 14.8 ± 1.5 | 6.5 ± 1.5 | <0.001 |
| Alpha Vit E, mg/d | 6.1 ± 0.5 | 12.5 ± 1.5 | 6.4 ± 1.5 | <0.001 |
| Beta Vit E, mg/d | 0.75 ± 0.4 | 0.40 ± 0.04 | -0.29 ± 0.40 | 0.52 |
| Folate, μg/d | 488 ± 30 | 666 ± 40 | 178 ± 35 | <0.001 |
| Arginine, g/d | 4.5 ± 0.2 | 5.1 ± 0.2 | 0.5 ± 0.2 | 0.03 |
| Arachidonic Acid | 0.10 ± 0.02 | 0.14 ± 0.01 | 0.04 ± 0.02 | 0.12 |
| Linoleic Acid, g/d | 14.1 ± 1.1 | 16.1 ± 1.3 | 2.0 ± 1.5 | 0.18 |
| Linolenic, g/d | 1.5 ± 0.1 | 1.7 ± 0.1 | 0.2 ± 0.2 | 0.22 |
| DHA, g/d | 0.17 ± 0.05 | 0.18 ± 0.08 | 0.01 ± 0.07 | 0.67 |
| EPA, g/d | 0.06 ± 0.01 | 0.06 ± 0.02 | 0.007 ± 0.02 | 0.73 |
| Total trans fat, g/d | 6.3 ± 0.6 | 4.2 ± 0.4 | 2.1 ± 0.5 | 0.001 |
| Insoluble Fiber, g/d | 11.1 ± 0.7 | 19.2 ± 1.2 | 8.5 ± 1.1 | <.0001 |
| Soluble Fiber, g/d | 14.3 ± 0.8 | 8.5 ± 0.8 | -5.8 ± 0.5 | <.0001 |
| Caffeine, mg/d | 78 ± 13 | 67 ± 12 | 11 ± 17 | 0.50 |
Previous work raised the possibility that the high content of potassium, magnesium, and fiber in the DASH Eating Plan explains the beneficial blood pressure effects.7-9 Other studies suggest these minerals and fiber may not fully account for the blood pressure effects of DASH.14-16 This study was designed to separate the effects of minerals and fiber from other components of DASH which may include antioxidants and flavonoids,27,28 on blood pressure and markers of endothelial and vascular function. The findings suggest DASH lowers blood pressure beyond the content of potassium, magnesium and fiber in abdominally obese (pre)hypertensives.
Sodium can have important blood pressure effects.29 While controversial,30 calcium may have modest BP effects.31 Sodium intake was similar across the dietary periods, although somewhat lower than a typical Western diet. The moderate sodium restriction may have limited the effects of the supplements on blood pressure, especially potassium, which has natriuretic properties.7 Mean calcium intake was ∼140 mg/d higher on DASH than ULFV-S (p=0.07). Based on previous studies on the blood pressure effects of calcium, this modest difference is unlikely to explain the lower blood pressure on DASH.15,16,31
One meta-analysis found that supplemental fiber lowered blood pressure ∼1.1–1.3 mmHg and reported soluble and mixed fiber lowers blood pressure, whereas insoluble fiber does not.9 Soluble fiber intake was higher during ULFV-S than DASH. Thus, it is unlikely the greater blood pressure reduction with DASH than ULFV-S is explained by fiber composition.
Among obese hypertensives, DASH improved small artery elasticity (Figure 4). Small artery elasticity serves as an index of endothelial function.32,33 The link between small artery elasticity and endothelial function is consistent with evidence that raising fatty acids systemically14,33 that inhibit endothelial cell nitric oxide synthase and induce reactive oxygen species in vitro,34,35 impaired small artery elasticity in vivo. Our findings suggest nutritional components of DASH other than potassium, magnesium, sodium, calcium and fiber improved endothelial function in obese hypertensives.
Stiffness of the vascular system predicts adverse outcomes independently of blood pressure.36,37 DASH improved small and large distensibility and reduced the aortic augmentation index, a general measure of arterial system stiffness in obese hypertensives. DASH improved the augmentation index in lean subjects, despite a non-significant blood pressure effect. These findings are consistent with evidence that DASH-like eating patterns improve cardiovascular outcomes38 and raise the possibility that beneficial effects on arterial function may contribute. Supplements improved the aortic augmentation index in obese hypertensives, which aligns with evidence that dietary potassium, magnesium and fiber are linked to better vascular outcomes.39-41
Extensive efforts were made to estimate intake of nutrients, which could explain the observed effects on blood pressure and markers of endothelial and vascular function in the obese subjects. Arginine, lycopene, Vitamins C and E, and folate intake were all higher on DASH than ULFV-S (Table 3) and may have contributed to beneficial effects of DASH in obese volunteers with DASH. In contrast, intake of, selenium, caffeine, and omega-3 fatty acids (linolenic, eicosapentanoic and docosahexanoic), which may affect BP, were not different.26,42
In obese subjects, blood pressure and indices of vascular function were more favorable on DASH than the control diet supplemented with potassium, magnesium and fiber to match DASH. It may be useful to examine nutritional differences between the two diets which could explain beneficial effects of DASH. Intake of arginine, the precursor for nitric oxide, was higher in obese hypertensives on DASH than ULFV-S. Arginine improves endothelium-dependent vasodilation and small artery elasticity.43,44 and lowers blood pressure26,45 Lycopene intake was also greater in obese subjects on DASH than ULFV-S, and may have lowered blood pressure.26,46
Vitamins C intake was higher on DASH than ULFV-S. Vitamin C lowers blood pressure26,47 and improves endothelial function, especially when a deficiency exists.26 Vitamin C may lower blood pressure by enhancing nitric oxide synthase activity.48 Vitamin C and E supplements in rats markedly attenuated glutathione deficiency-induced hypertension.49 Vitamin C and E reduced arterial stiffness and improved endothelial function in hypertensives.50 Vitamins C and E may lower blood pressure by scavenging reactive oxygen species, down-regulating NADPH oxidase, and increasing nitric oxide synthase.50,51 Yet, Vitamin C and grape-seed polyphenols together46 or alpha-tocopherol alone can raise blood pressure.52
Estimated folate consumption was greater on DASH than ULFV-S. Studies suggest folate lowers blood pressure,53,54 reduces hypertension incidence,55 and improves endothelial function.56,57 Several studies cited above indicate specific components of DASH, other than potassium, magnesium and fiber, lower blood pressure and improve endothelial function. DASH could also reduce consumption of factors such as refined sugar, which may raise blood pressure.
Limitations
Volunteers prepared their own meals, which may have compromised adherence in contrast to DASH, in which volunteers received food and beverages from a metabolic kitchen. The weekly three-day food records, photographs and timed urine collections suggested good adherence. Moreover, our data in free-living volunteers may be more applicable to patients in community-based clinical settings than metabolic feeding studies.
The dietary interventions were three weeks long and not separated by washout periods. The DASH report and our experience6,14 indicated that most of the blood pressure effect occurred within 2–3 weeks. An order effect was not observed in our previous or current study.14 The dietary interventions were unblinded. Thus, patient and investigator expectations may have influenced results. To minimize investigator bias, a random zero sphygmomanometer was used to measure blood pressure, the primary outcome variable. The limited effect of the supplemented diet on blood pressure may be partially due to the comparatively low sodium intake of ∼3000 mg daily. In our previous work, despite numerous prompts from the study dietician, free-living volunteers continued to consume significantly less than one low-fat dairy product daily. Thus, this study examined only the high fruits and vegetables DASH, and we cannot extrapolate our results to the combination diet.
We studied lean normotensives without and obese pre-hypertensives and hypertensives with the metabolic syndrome and cannot extrapolate beyond the groups studied. However, we believe the study findings are important, since obese, metabolic syndrome subjects comprise a large proportion of all patients with pre-hypertension and hypertension. Compared to the control diet, blood pressure effects of high fruits and vegetables DASH in our study are comparable to the original report.6 Specifically, blood pressure changes in the current study and DASH fruits and vegetables were -2.0/-1.3 (lean normtensives [current]) vs. -0.8/-0.3 (non-hypertensives [DASH]) and -7.6/-5.3 (obese hypertensives [current]) vs. -7.2/-2.8 (hypertensives [DASH]). We cannot exclude the possibility that the potassium, magnesium and fiber contents of food in the high fruits and vegetables eating plan lower blood pressure by interacting with other nutrients in the high fruits and vegetables eating plan.14
Our study shows that the DASH fruits and vegetables eating plan lowers blood pressure in obese, pre-hypertensive and hypertensive patients with the metabolic syndrome. Moreover, DASH improved their endothelial function and reduced vascular stiffness. The beneficial effects were less evident in obese subjects when a usual diet was supplemented with potassium, magnesium and fiber to match DASH. The data suggest DASH lowers blood pressure and improves endothelial function in obese, metabolic syndrome subjects with elevated blood pressure beyond potassium, magnesium and fiber.
Table 5.
Summary.
| What is known about topic: |
| • The high fruits and vegetables DASH Eating Plan lowers blood pressure and improves markers endothelial function in obese subjects with the metabolic syndrome and elevated blood pressures, but it is not clear whether this effect reflects the favorable mineral and fiber composition or other components of whole foods. |
| What this study adds: |
| • The high fruits and vegetables Eating Plan lowers blood pressure and improves markers of endothelial function more in abdominally obese subjects with elevated blood pressures than an isocaloric diet with comparable sodium and calcium content that is supplemented with potassium, magnesium and fiber to match DASH. Thus, components of whole foods appear to mediate the beneficial effects of DASH on blood pressure and vascular function beyond or in addition to their mineral and fiber content. |
Acknowledgements
The authors thank Kelley Martin, RD, MS, General Clinical Research Nutritionist, for her extraordinary efforts in assisting volunteers to comply with the study diets. We also thank the entire General Clinical Research Center staff for their dedication to the integrity of the research protocol as well as Kim Edwards for administrative support.
This research was supported by grants from the National Institutes of Health (HL58794, HL04290), MD00267 from the National Center for Minority Health and Disparities, and the General Clinical Research Center (RR-01070) from the Division of Research Resources.
References
- 1.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999 – 2000. JAMA. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y, Wang Q-J. The prevalence of prehypertension and hypertension among US adults according to the new Joint National Committee Guidelines: New challenges of the old problem. Arch Intern Med. 2004;164:2126–2134. doi: 10.1001/archinte.164.19.2126. [DOI] [PubMed] [Google Scholar]
- 3.Egan BM. Should metabolic syndrome patients with pre-hypertension receive antihypertensive therapy? In: Bakris GL, editor. Therapeutic Strategies in Hypertension. Clin Publ; Oxford, UK: 2006. pp. 9–25. ch 2. [Google Scholar]
- 4.Case CC, Jones PH, Nelson K, Smith E O’Brian, Ballantyne CM. Impact of weight loss on the metabolic syndrome. Diab Obes Metab. 2002;4:407–414. doi: 10.1046/j.1463-1326.2002.00236.x. [DOI] [PubMed] [Google Scholar]
- 5.Mark AL. Weight reduction for treatment of obesity-associated hypertension: Nuances and challenges. Curr Hypertens Rep. 2007;9:368–372. doi: 10.1007/s11906-007-0068-5. [DOI] [PubMed] [Google Scholar]
- 6.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, Lin PH, Karanja N. A clinical trial of the effects of dietary patterns on blood pressure: DASH Collaborative Research Group. N Engl J Med. 1997;336:1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 7.He FJ, Markandu ND, Coltart R, Barron J, MacGregor GA. Effect of short-term supplementation of potassium chloride and potassium citrate on blood pressure in hypertensives. Hypertension. 2005;45:571–574. doi: 10.1161/01.HYP.0000158264.36590.19. [DOI] [PubMed] [Google Scholar]
- 8.Jee SH, Miller ER, Guallar E, Singh VK, Appel LJ, Klag MJ. The effect of magnesium supplementation on blood pressure: A meta-analysis of randomized clinical trials. Am J Hypertension. 2002;15:691–696. doi: 10.1016/s0895-7061(02)02964-3. [DOI] [PubMed] [Google Scholar]
- 9.Streppel MT, Arends LR, van’t Veer P, Grobbee DE, Gelenijnse JM. Dietary fiber and blood pressure: A meta-analysis of randomized placebo-controlled trials. Arch Intern Med. 2005;9:95–97. doi: 10.1001/archinte.165.2.150. [DOI] [PubMed] [Google Scholar]
- 10.Pou KM, Massaro JM, Hoffman U, Vasan RS, Maurovich-Horvat P, Larson MG, Keaney JF, Meigs JB, Lipinska I, Kathiresen S, Marabito JM, O’Donnell CJ, Benjaminm EJ, Fox CS. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: The Framingham Heart Study. Circulation. 2007;116:1234–1241. doi: 10.1161/CIRCULATIONAHA.107.710509. [DOI] [PubMed] [Google Scholar]
- 11.Van Guilder GP, Hoetzer ?GL, Greiner JJ, Stauffer BL, Desouza CA. Influence of metabolic syndrome on biomarkers of oxidative stress and inflammation in obese adults. Obesity. 2006;14:2127–2131. doi: 10.1038/oby.2006.248. [DOI] [PubMed] [Google Scholar]
- 12.Vincent HK, Innes KE, Vincent KR. Oxidative stress and potential interventions to reduce oxidative stress in overweight and obesity. Diab Obes Metab. 2007;9:813–839. doi: 10.1111/j.1463-1326.2007.00692.x. [DOI] [PubMed] [Google Scholar]
- 13.Meyer MR, Gokce N. Endothelial dysfunction in obesity: Etiological role in atherosclerosis. Curr Opin Endrocinol Diab Obes. 2007;14:365–369. doi: 10.1097/MED.0b013e3282be90a8. [DOI] [PubMed] [Google Scholar]
- 14.Lopes HF, Martin KL, Nashar K, Morrow JD, Goodfriend TL, Egan BM. DASH diet lowers blood pressure and lipid-induced oxidative stress in obesity. Hypertension. 2003;41:422–30. doi: 10.1161/01.HYP.0000053450.19998.11. [DOI] [PubMed] [Google Scholar]
- 15.Whelton PK, Kumanyika SK, Cook NR, Cutler JA, Borhani NO, Hennekens CH, Kuller LH, Langford H, Jones DW, Satterfield S, Lasser NL, Cohen JD. Efficacy of nonpharmacologic interventions in adults with high-normal blood pressure: Results from phase 1 of the Trials of Hypertension Prevention. Trials of Hypertension Prevention Collaborative Group. Am J Clin Nutr. 1997;65(Suppl 2):652S–660S. doi: 10.1093/ajcn/65.2.652S. [DOI] [PubMed] [Google Scholar]
- 16.Beyer FR, Dickinson HO, Nicolson DJ, Ford GA, Mason J. Combined calcium, magnesium and potassium supplementation for the management of primary hypertension in adults. Coch Data Sys Rev. 2006;3 doi: 10.1002/14651858.CD004805.pub2. CDC004805. [DOI] [PubMed] [Google Scholar]
- 17.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Spertus JA, Costa F. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 18.Schakel S, Buzzard I, Gebhardt, S. Procedures for estimating nutrient values for food composition databases. J Food Comp Anal. 1997;10:102–114. [Google Scholar]
- 19.Harris J, Benedict F. A biometric study of human basal metabolism. Proc Natl Acad Sci USA. 1918;4:370–3. doi: 10.1073/pnas.4.12.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simmons SF, Reuben D. Nutritional intake monitoring for nursing home residents: A comparison of staff documentation, direct observation, and photography methods. J Am Ger Soc. 2000;48:209–213. doi: 10.1111/j.1532-5415.2000.tb03914.x. [DOI] [PubMed] [Google Scholar]
- 21.McVeigh GE, Bratteli CW, Morgan DJ, Alinder CM, Glasser SP, Finkelstein SM, Cohn JN. Age-related abnormalities in arterial compliance identified by pressure pulse contour analysis: Aging and arterial compliance. Hypertension. 1999;33:1392–1398. doi: 10.1161/01.hyp.33.6.1392. [DOI] [PubMed] [Google Scholar]
- 22.Lemogoum D, Flores G, Van den Abeele W, Ciarka A, Leeman M, Dagaute JP, van de Borne P, Van Bortel L. Validity of pulse pressure and augmentation index as surrogate measures of arterial stiffness during beta-adrenergic stimulation. J Hypertens. 2004;22:511–517. doi: 10.1097/00004872-200403000-00013. [DOI] [PubMed] [Google Scholar]
- 23.Besch W, Woltanski KP, Keilacker H, Diaz-Alonso JM, Schulz B, Amendt P, Kohnert KD, Ziegler M. Measurement of insulin in human sera using a new RIA kit, 1: insulin determination in the absence of insulin antibodies— conventional assay and micro modification. Exp Clin Endocrinol. 1987;90:264–270. doi: 10.1055/s-0029-1210700. [DOI] [PubMed] [Google Scholar]
- 24.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and ß-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 25.De Bruin TW, Brouwer CB, Gimpel JA, Erkelens DW. Postprandial decrease in HDL cholesterol and HDL apo A-I in normal subjects in relation to triglyceride metabolism. Am J Physiol. 1991;260:E492–E498. doi: 10.1152/ajpendo.1991.260.3.E492. [DOI] [PubMed] [Google Scholar]
- 26.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 27.Houston MC. Nutraceuticals, vitamins, antioxidants, and minerals in the prevention and treatment of hypertension. Prog Cardiov Dis. 2005;47:396–449. doi: 10.1016/j.pcad.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 28.Moline J, Bukharovich IF, Wolff MS, Phillips R. Dietary flavonoids and hypertension: Is there a link? Med Hypoth. 2000;55:306–309. doi: 10.1054/mehy.2000.1057. [DOI] [PubMed] [Google Scholar]
- 29.Elliott P, Stamler J, Nichol R, Dyer AR, Stamler R, Kesteloot H, Marmot M. Intersalt revisited: Further analyses of 24-hour sodium excretion and blood pressure within and across populations. Intersalt Cooperative Research Group. Brit Med J. 1996;312:1249–1253. doi: 10.1136/bmj.312.7041.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dickinson HO, Nicolson DJ, Cook JV, Campbell F, Beyer FR, Ford GA, Mason J. Calcium supplementation for the management of primary hypertension in adults. Coch Data Sys Rev. 2006;2 doi: 10.1002/14651858.CD004639.pub2. CD004639. [DOI] [PubMed] [Google Scholar]
- 31.Bucher HC, Cook RJ, Guyatt GH, Lang JD, Cook DJ, Hatala R, Hunt DL. Effects of dietary calcium supplementation on blood pressure: a meta-analysis of randomized controlled trials. JAMA. 1996;275:1016–1022. doi: 10.1001/jama.1996.03530370054031. [DOI] [PubMed] [Google Scholar]
- 32.McVeigh GE, Bratteli CW, Morgan DJ, Alinder CM, Glasser SP, Finkelstein SM, Cohn JN. Age-related abnormalities in arterial compliance identified by pressure pulse contour analysis: Aging and arterial compliance. Hypertension. 1999;33:1392–1398. doi: 10.1161/01.hyp.33.6.1392. [DOI] [PubMed] [Google Scholar]
- 33.Stojiljkovic MP, Zhang D, Lopes HF, Lee CG, Goodfriend TL, Egan BM. Hemodynamic effects of lipids in humans. Am J Physiol. 2001;R280:1674–1679. doi: 10.1152/ajpregu.2001.280.6.R1674. [DOI] [PubMed] [Google Scholar]
- 34.Davda RK, Stepniakowski KT, Lu G, Ullian ME, Goodfriend TL, Egan BM. Oleic acid inhibits endothelial cell nitric oxide synthase by a PKC-independent mechanism. Hypertension. 1995;26:764–770. doi: 10.1161/01.hyp.26.5.764. [DOI] [PubMed] [Google Scholar]
- 35.Lu G, Greene EL, Nagai T, Egan BM. Reactive oxygen species are critical in the oleic acid-mediated mitogenic signaling pathway in vascular smooth muscle cells. Hypertension. 1998;32:1003–1010. doi: 10.1161/01.hyp.32.6.1003. [DOI] [PubMed] [Google Scholar]
- 36.Cernes R, Zimiichman R, Shargorodsky M. Arterial elasticity in cardiovascular disease: Focus on hypertension, metabolic syndrome and diabetes. Adv Cardiol. 2008;45:65–81. doi: 10.1159/000115188. [DOI] [PubMed] [Google Scholar]
- 37.Hansen TW, Staessen JA, Torp-Pedersen C, Rasmussen S, Thijs L, Ibsen H, Jeppesen J. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006;113:664–670. doi: 10.1161/CIRCULATIONAHA.105.579342. [DOI] [PubMed] [Google Scholar]
- 38.Feng TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Int Med. 2008;168:713–720. doi: 10.1001/archinte.168.7.713. [DOI] [PubMed] [Google Scholar]
- 39.Khaw KT, Barrett-Connor E. Dietary potassium and stroke-associated mortality. A 12-year prospective population study. N Engl J Med. 1987;316:235–240. doi: 10.1056/NEJM198701293160502. [DOI] [PubMed] [Google Scholar]
- 40.Khaw KT, Barrett-Connor E. Dietary fiber and reduced ischemic heart disease mortality rates in men and women: A 12-year prospective study. Am J Epidemiol. 1987;126:1093–1102. doi: 10.1093/oxfordjournals.aje.a114748. [DOI] [PubMed] [Google Scholar]
- 41.Garside PS, Gleuck CJ. The important role of modifiable dietary and behavioral characteristics in the causation and prevention of coronary heart disease hospitalization and mortality: The prospective NHANES I follow-up study. J Am Coll Nutr. 1995;14:71–79. doi: 10.1080/07315724.1995.10718476. [DOI] [PubMed] [Google Scholar]
- 42.Djoussé L, Arnett DK, Pankow JS, Hopkins PN, Province MA, Ellison RC. Dietary linolenic acid is associated with a lower prevalence of hypertension in the NHLBI Family Heart Study. Hypertension. 2005;45:368–373. doi: 10.1161/01.HYP.0000154679.41568.e6. [DOI] [PubMed] [Google Scholar]
- 43.Preli RB, Klein KP, Herrington DM. Vascular effects of dietary L-arginine supplementation. Atherosclerosis. 2002;162:1–15. doi: 10.1016/s0021-9150(01)00717-1. [DOI] [PubMed] [Google Scholar]
- 44.Lekakis JP, Papathanassiou S, Papaioannou TG, Papamichael CM, Zakopoulos N, Kotsis V, Dagre AG, Stamatelopoulos K, Protogerou A, Stamatelopoulos SF. Oral L-arginine improves endothelial dysfunction in patients with essential hypertension. Int J Cardiol. 2002;86:317–323. doi: 10.1016/s0167-5273(02)00413-8. [DOI] [PubMed] [Google Scholar]
- 45.Castejon AM, Hoffmann IS, Jimenez E, Cubeddu RJ, Baldonedo RM, Cubeddu LX. Comparative effects of L-arginine infusion in obese hypertensive and lean normotensive: Blood pressure, heart rate and insulin levels. J Hum Hypertens. 2002;16(Suppl 1):S33–S36. doi: 10.1038/sj.jhh.1001359. [DOI] [PubMed] [Google Scholar]
- 46.Engelhard YN, Gazer B, Paran E. Natural antioxidants from tomato extract reduce blood pressure in patients with grade-1 hypertension: A double-blind, placebo-controlled pilot study. Am Heart J. 2006;151:100. doi: 10.1016/j.ahj.2005.05.008. (abstract) [DOI] [PubMed] [Google Scholar]
- 47.Ward NC, Hodgson JM, Croft KD, Burke V, Beilin LJ, Puddey IB. The combination of vitamin C and grape-seed polyphenols increases blood pressure: A randomized, double-blind, placebo-controlled trial. J Hypertens. 2005;23:427–434. doi: 10.1097/00004872-200502000-00026. [DOI] [PubMed] [Google Scholar]
- 48.D’Uscio LV, Milstein S, Richardson D, Smith L, Katusic ZS. Long-term vitamin C treatment increases vascular tetrahydrobiopterin levels and nitric oxide synthase activity. Circ Res. 2003;92:88–95. doi: 10.1161/01.res.0000049166.33035.62. [DOI] [PubMed] [Google Scholar]
- 49.Nosratola D, Vaziri D, Wang XQ, Oveisi F, Rad B. Induction of oxidative stress by glutathione depletion causes severe hypertension in normal rats. Hypertension. 2000;36:142–146. doi: 10.1161/01.hyp.36.1.142. [DOI] [PubMed] [Google Scholar]
- 50.Plantinga Y, Ghiadoni L, Magagna A, Giannarelli C, Franzoni F, Taddei S, Salvetti A. Supplementation with Vitamins C and E improves arterial stiffness and endothelial function in essential hypertensive patients. Am J Hypertens. 2007;20:392–397. doi: 10.1016/j.amjhyper.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 51.Heller R, Werner-Felmayer G, Werner ER. Alpha-tocopherol and endothelial nitric oxide synthesis. Ann NY Acad Sci. 2004;1031:74–85. doi: 10.1196/annals.1331.007. [DOI] [PubMed] [Google Scholar]
- 52.Ward NC, Wu JHY, Clarke MW, Puddey IB, Burke V, Croft KD, Hodgson JM. The effect of vitamin E on blood pressure in individuals with type 2 diabetes: A randomized,double-blind, placebo-controlled trial. J Hypertens. 2007;25:227–234. doi: 10.1097/01.hjh.0000254373.96111.43. [DOI] [PubMed] [Google Scholar]
- 53.van Etten RW, de Koning EJ, Verhaar MC, Gaillard CA, Rabelink TJ. Impaired NO-dependent vasodilation in patients with type II (non—insulin-dependent) diabetes mellitus is restored by acute administration of folate. Diabetologia. 2002;45:1004–1010. doi: 10.1007/s00125-002-0862-1. [DOI] [PubMed] [Google Scholar]
- 54.van Dijk RA, Rauwerda JA, Steyn M, Twisk JW, Stehouwer CD. Long-term homocysteine-lowering treatment with folic acid plus pyridoxine is associated with decreased blood pressure but not with improved brachial artery endothelium-dependent vasodilation or carotid artery stiffness: a 2-year, randomized, placebo-controlled trial. Arterioscler Thromb Vasc Biol. 2001;21:2072–2079. doi: 10.1161/hq1201.100223. [DOI] [PubMed] [Google Scholar]
- 55.Forman JP, Rimm EB, Stampfer MJ, Curhan GC. Folate intake and the risk of incident hypertension among US women. JAMA. 2005;293:320–329. doi: 10.1001/jama.293.3.320. [DOI] [PubMed] [Google Scholar]
- 56.Doshi SN, McDowell IF, Moat SJ, Lang D, Newcombe RG, Kredan MB, Lewis MJ, Goodfellow J. Folate improves endothelial function in coronary artery disease: an effect mediated by reduction of intracellular superoxide? Arterioscler Thromb Vasc Biol. 2001;21:1196–1202. doi: 10.1161/hq0701.092000. [DOI] [PubMed] [Google Scholar]
- 57.Verhaar MC, Wever RM, Kastelein JJ, van Dam T, Koomans HA, Rabelink TJ. 5-methyltetrahydrofolate, the active form of folic acid, restores endothelial function in familial hypercholesterolemia. Circulation. 1998;97:237–241. doi: 10.1161/01.cir.97.3.237. [DOI] [PubMed] [Google Scholar]