Abstract
Neurons of the cerebellar nuclei generate the non-vestibular output of the cerebellum. Like other neurons, they integrate excitatory and inhibitory synaptic inputs and filter them through their intrinsic properties to produce patterns of action potential output. The synaptic and intrinsic features of cerebellar nuclear cells are unusual in several respects, however: these neurons receive an overwhelming amount of basal and driven inhibition from Purkinje neurons, but are also spontaneously active, producing action potentials even without excitation. Moreover, not only is spiking by nuclear cells sensitive to the amount of inhibition, but the strength of inhibition is also sensitive to the amount of spiking, through multiple forms of long-term plasticity. Here, we review the properties of synaptic excitation and inhibition, their short-term plasticity, and their influence on action potential firing of cerebellar nuclear neurons, as well as the interactions among excitation, inhibition, and spiking that produce long-term changes in synaptic strength. The data provide evidence that electrical and synaptic signaling in the cerebellar circuit is both plastic and resilient: the strength of IPSPs and EPSPs readily changes as the activity of cerebellar nuclear cells is modified. Notably, however, many of the identified forms of plasticity have an apparently homeostatic effect, responding to perturbations of input by restoring cerebellar output toward pre-perturbation values. Such forms of self-regulation appear consistent with the role of cerebellar output in coordinating movements. In contrast, other forms of plasticity in nuclear cells, including a long-term potentiation of excitatory postsynaptic currents (EPSCs) and excitation-driven increases in intrinsic excitability, are non-homeostatic, and instead appear suited to bring the circuit to a new set point. Interestingly, the combinations of inhibitory and excitatory stimuli that potentiate EPSCs resemble patterns of activity predicted to occur during eyelid conditioning, suggesting that this form long-term potentiation, perhaps amplified by intrinsic plasticity, may represent a cellular mechanism that is engaged during cerebellar learning.
Keywords: Purkinje, Deep cerebellar nuclei, Rebound, Delay eyeblink, Long-term potentiation (LTP), Long-term depression (LTD)
Introduction
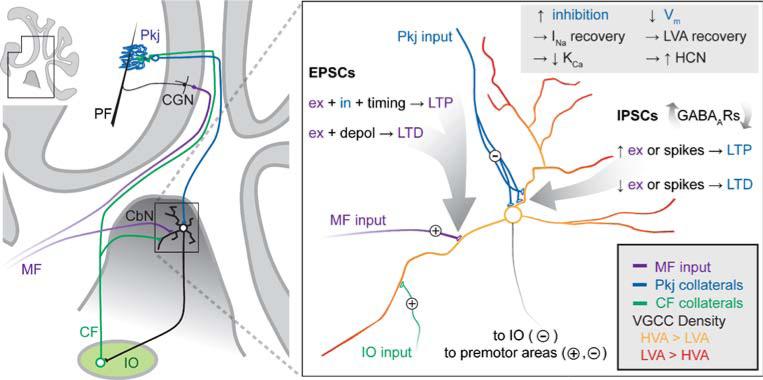
The output of the cerebellum, with the exception of vestibular information, is carried exclusively by neurons of the cerebellar nuclei. A subset of neurons, primarily with large diameter somata (>15 μm), project to premotor areas, thereby directly influencing motor control, while other, smaller nuclear neurons project to the inferior olive, providing a negative feedback that likely corrects errors of movement (Fig. 1; [1–5]). In the cerebellar nuclei, therefore, converging excitatory and inhibitory synaptic inputs must be integrated and transformed by the intrinsic properties of nuclear neurons to yield the final output of the cerebellum. An understanding of the cellular mechanisms of this encoding of information by the cerebellum ideally includes identifying what the properties of synaptic inputs to the nuclei are, how the excitability of nuclear neurons is affected by these stimuli, and which patterns of activity modify synaptic inputs and spike outputs to yield motor coordination and learning. The synaptic inputs to the nuclei, and the sites and signs of plasticity discussed in this review are illustrated in Fig. 1.
Fig. 1.
Schematic of sites of plasticity in the cerebellar nuclei. Left Schematic of a parasagittal section of the cerebellum (inset) with expanded region indicating that cerebellar nuclear neurons (CbN) project either to premotor areas (not shown) or to the inferior olive (IO). The IO forms the climbing fiber (CF) that targets Purkinje neurons (Pkj), with collaterals excite the CbN cells. Mossy fibers (MF) excite CbN cells as well as cerebellar granule neurons (CGN), which in turn form the parallel fibers (PF) that excite Pkj cells. Right Expansion of the cerebellar nuclear cell and its inputs, along with the sites and bases of plasticity. Note that small, usually inhibitory, cells project to the IO and large, usually excitatory cells project to premotor areas. Vm membrane voltage, INa voltage-gated Na current, LVA low-voltage activated (T-type) Ca current, KCa Ca-activated K current, HCN hyperpolarization-gated cyclic nucleotide-gated channels, VGCC voltage-gated Ca channels, HVA high-voltage activated Ca channels, ex excitatory input, in inhibitory input, depol depolarization. Figure by J. S. Bant
Spontaneous Firing and Synaptic Inputs
Neurons of the cerebellar nuclei belong to the class of neurons that fire action potentials spontaneously, even without synaptic input. Recordings from in vitro preparations from rodents indicate that the intrinsic firing rates of these cells generally lie between 10 and 50 Hz [6–12]. Thus, like any other spontaneously active neuron, the basal activity of cerebellar nuclear cells lies in the middle of their dynamic range, from which it can be reduced by inhibition, or increased by excitation. What distinguishes cerebellar nuclear cells from most other neurons, however, is the amount of inhibition that they receive. Although cerebellar nuclear neurons are diverse in their morphologies, transmitter contents, and projections, they all appear to be targets of Purkinje cells of the cerebellar cortex, which are GABAergic [1, 13–17]. Both large (primarily excitatory) and small (primarily inhibitory) nuclear cells receive input from dozens of Purkinje cells, which form dense, inhibitory synaptic contacts on nuclear cell somata and proximal dendrites (Fig. 1; [1, 18]). Purkinje cells, too, are spontaneously active, firing ~50 spikes per second in vitro with synaptic transmission blocked or removed [19–21], as well as in vivo when the animal is not actively engaged in cerebellar behaviors [22, 23]. Since somatic spikes in Purkinje cells propagate reliably for several hundred microns along myelinated axons [24, 25], most are likely to invade the synaptic terminal where they can trigger release of GABA. Thus, in the basal state, each nuclear cell is subject to a barrage of more than a thousand inhibitory postsynaptic potentials (IPSPs) per second.
In addition to this large amount of inhibitory input, nuclear cells receive excitatory contacts from both mossy fibers and inferior olivary fibers (Fig. 1). The anatomy of these synaptic connections, however, leads to the prediction that elevated excitatory input to nuclear cells will frequently coincide with elevated inhibitory input. Mossy fibers have a low basal activity, but during cerebellar behaviors, can fire at rates exceeding 100 Hz [26]. These inputs directly excite nuclear cells, but also indirectly excite Purkinje cells, via granule cells. Thus, mossy fiber activity is likely to increase both excitation and inhibition of nuclear neurons nearly simultaneously. An additional source of excitation comes from inferior olivary fibers, which split into the massive climbing fiber contact onto Purkinje cells, which elicits complex spikes and the subsequent pauses, and an anatomically less impressive excitatory contact onto nuclear cells [1].
It is striking, therefore, that although inhibition frequently dominates excitation, especially in the basal state, activity of cerebellar nuclear neurons persists in vivo [22, 27, 28], indicating that the tendency of nuclear neurons to fire can resist even substantial inhibition. This maintenance of firing is in part supported by the intrinsic leak currents of cerebellar nuclear cells: in contrast to many neurons whose resting membrane potential is set largely by K channels, cerebellar nuclear cells primarily express voltage-independent non-selective cationic leak channels that reverse near –30 mV [10], whose properties resemble NALCN channels [29]. As a consequence, cells continually seek a potential that is suprathreshold, eliciting spikes whenever spontaneous synaptic inhibition transiently drops (e.g. reference [11], discussed below).
Firing rates of cerebellar nuclear cells are modulated during specific motor behaviors, however, consistent with their participation in regulating movements [22, 27, 30]; at certain times during a behavior, excitation dominates, whereas at other times, inhibition becomes more effective. Importantly, motor learning can modify patterns of nuclear cell firing [31], likely owing to plasticity of both synaptic inputs and intrinsic conductances. Such observations raise the question of how excitatory postsynaptic currents (EPSCs) and inhibitory postsynaptic currents (IPSCs) change in the short term and long term, interact in nuclear cells, and regulate the rate and timing of cerebellar nuclear action potentials.
Short-Term Synaptic Excitation
Remarkably few studies have focused directly on the properties of excitatory synaptic transmission in the cerebellar nuclei. Most of the existing research on synaptic transmission has concentrated on the larger nuclear cells, which include, but are not limited to, glutamatergic neurons that project to premotor areas [13, 17]. During prolonged stimulus trains at ≥100 Hz, excitatory postsynaptic potentials (EPSPs) in these cells consistently raise firing above spontaneous rates, suggesting that excitation remains effective for several hundred milliseconds [32]. Voltage-clamp studies illustrate that the short-term plasticity of underlying EPSCs is variable, with some cells depressing and others facilitating during 100-Hz trains of stimulation; the average response across cells shows nearly no net change in EPSC amplitude [32]. A distinctive feature of these synapses is that N-methyl-D-aspartic acid (NMDA) receptors contribute a substantial component of the total synaptic current even at negative voltages [33, 34], owing to the expression of NR2D NMDA receptor subunits, which generate channels that are only weakly blocked by Mg++ [35–37]. The contribution of NMDA receptors is particularly evident during trains of evoked EPSCs, which permit the NMDA-receptor-mediated component to summate; after 20 stimuli at 100 Hz, its amplitude exceeds 60% of the AMPA-receptor mediated component, even at –65 mV in 1 mM Mg++ [32]. Consequently, NMDA receptors in the cerebellar nuclei permit substantial Ca influx even when cells are not spiking [38], a feature that may be significant to long-term plasticity (discussed below).
Short-Term Synaptic Inhibition
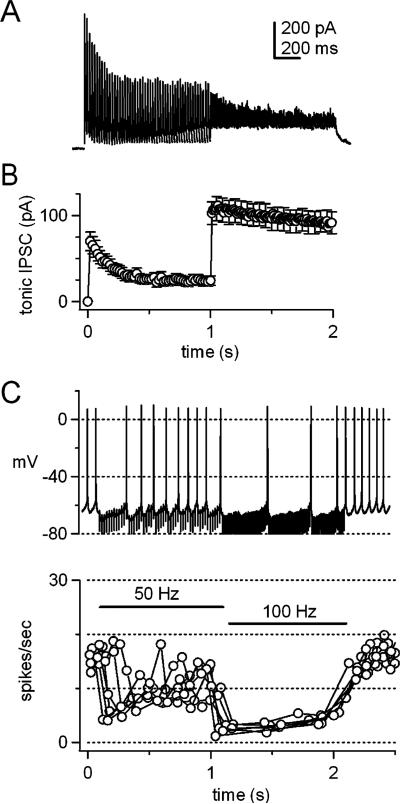
Purkinje-mediated IPSCs in large cerebellar nuclear cells are plastic but remain robust during trains of stimuli. Their plasticity can be conveniently measured in cerebellar slices, in which most Purkinje axons are severed from their spontaneously firing somata. Consequently, IPSCs can be elicited without a background of ongoing inhibition or a history of either facilitation or depression. Under these conditions, stimulating Purkinje axons elicits synaptic currents that are apparently mediated exclusively by GABAA receptors. When IPSCs are evoked at 50 Hz, they fall to about half their initial amplitude over the course of a second ([39]; Fig. 2a), suggesting that spontaneous activity of Purkinje cells tonically depresses IPSCs by about 50% in the intact cerebellum. Each synaptic current decays rapidly, with a time constant of about 5 ms near physiological temperatures, so that little tonic (residual) current remains between IPSCs evoked at 50 Hz (Fig. 2b); lapses of the inhibitory conductance between release events from afferent Purkinje neurons may provide a temporal window in which spikes are generated [11]. Consistent with this idea, action potentials in these cerebellar nuclear cells often persist during 50-Hz IPSPs elicited by coherent activation of about five to ten afferents (Fig. 2c). Increasing the stimulation rate above 50 Hz, as is expected to occur during cerebellar behaviors, mildly increases the amount of depression of IPSCs. Nevertheless, inhibition becomes more effective, for the simple reason that the synaptic current does not decay completely between stimuli, thus shunting action potential firing (Fig. 2a–d,[39]); a similar shunt can probably be achieved by asynchronous inhibitory inputs [11]. Mechanistically, the resistance of IPSCs to strong synaptic depression at high stimulus rates results from an effective spillover-mediated transmission, owing to release of GABA from boutons with multiple release sites but without GABA transporters [40, 41]. These features allow a low vesicular release probability, which minimizes depletion, to evoke a postsynaptic response with high probability. Functionally, it permits the efficacy of inhibition to vary with the firing rate of afferent Purkinje cells, in a manner that is relatively independent of short-term depression [8].
Fig. 2.
Short-term synaptic depression and the efficacy of inhibition. a IPSCs in a cerebellar nuclear neuron in a cerebellar brain slice from a ~2-week-old mouse. Currents were evoked at –40 mV by stimulating Purkinje axons for 1-s at 50 Hz followed by a 1-s at 100 Hz; 31°C, ECl=–70 mV. Peak IPSC amplitudes depress by about 50% during the 50-Hz train and depression is slightly increased during the 100-Hz train. b The tonic IPSC, defined as the inhibitory synaptic current remaining just before the subsequent stimulation, during the 50-Hz and 100-Hz trains. Mean of 25 cells. c Spontaneous action potentials recorded in a cerebellar nuclear neuron with no holding current subjected to IPSPs evoked at 50 Hz (1 s) followed by 100 Hz (1 s). The firing rate is effectively decreased before the synaptic current has depressed (at the beginning of the 50-Hz train) or when the tonic IPSC is increased by rapid stimulation (during the 100-Hz train). d Instantaneous firing rate (spikes/s) for the cell in c, over six trials of identical stimuli. Data from [39], reproduced with permission
Disinhibition and Voltage-Gated Na Currents
Although inhibition most obviously acts to reduce firing by nuclear cells, a long-standing question is how nuclear cells respond to the offset of IPSPs [7, 42]. As mentioned above, because of the intrinsic tendency of nuclear cells to spike, whenever inhibition subsides, firing invariably resumes [8, 10, 11]. Elevated inhibition that interrupts firing for a few hundred milliseconds, however, can change the availability of channels, e.g., permitting the recovery of voltage-gated Na or Ca channels that have inactivated during spiking, deactivating KCa or other channels that require tonic Ca influx to open, or activating HCN channels. If these changes favor the availability and/or activation of depolarizing currents—as they seem likely to do—then firing will not only resume but may also be accelerated whenever a prolonged inhibition is abated.
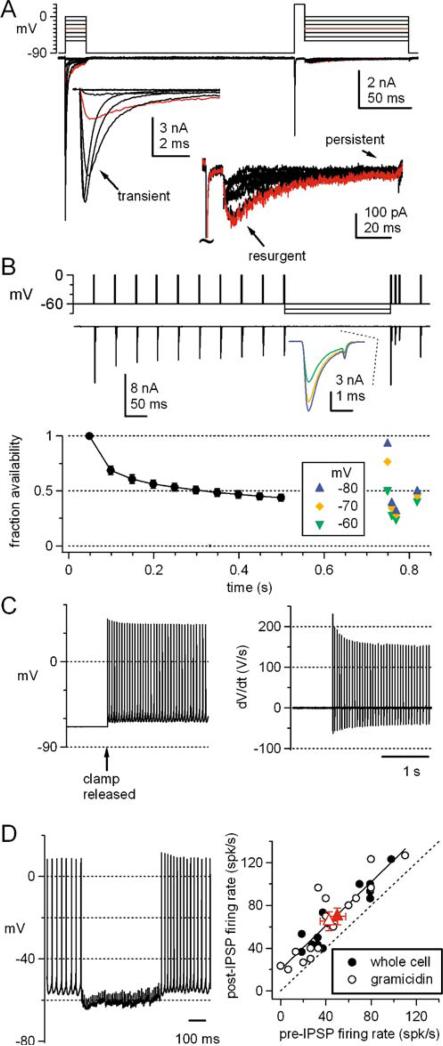
One current that is highly sensitive to the mean membrane potential in cerebellar nuclear cells is voltage-gated Na current. Voltage-clamped Na currents of both large and small nuclear neurons have a transient, persistent, and resurgent component (Fig. 3a); the transient component has a relatively depolarized half-inactivation voltage of –52 mV and a marked tendency for slow inactivation [10, 43, 44]. During spiking, the rapidly deactivating voltage-gated K currents prevent deep after-hyperpolarizations, keeping most interspike intervals above –65 mV and mean membrane potentials near –50 mV [6, 8–12, 16, 39]. As a result, spontaneously firing nuclear cells operate with a remarkably low availability of Na channels [10, 44]. Even interruptions of firing on the order of a few hundred milliseconds, however, can permit recovery of Na channels from fast and slow inactivation. This effect is evident when tetrodotoxin-sensitive Na currents are evoked by 20-Hz trains of brief, spike-like depolarizations, applied from –60 mV. The amplitude of the transient Na current decreases by more than 50% over the first ten stimuli. A gap of 250 ms at –70 mV, however, can restore the current to 80% of its initial amplitude. Such recovery is likely to facilitate firing immediately upon disinhibition ([44], Fig. 3b). Moreover, the availability of Na channels is likely to be increased for a few hundred milliseconds after firing resumes. Evidence for this idea comes from holding cells at –68 mVand then releasing the voltage clamp to allow spiking to occur. Plotting the time derivative of the resulting action potentials illustrates that the maximal rate of rise of each spike drops with successive spikes, reflecting the decreasing Na channel availability over the course of about half a second (Fig. 3c). Indeed, after trains of IPSPs (at 100 Hz for 500 ms), the rate of spontaneous action potential firing in large neurons in slices is increased by about 20 Hz for at least 300 ms, regardless of the initial rate of firing (Fig. 3d, [45]). Thus, Na channels form one class of channels that is sensitive to periods of inhibition, and which is likely to influence firing patterns after inhibition is relieved.
Fig. 3.
Na currents, inactivation, and firing. a Transient, persistent, and resurgent tetrodotoxin-sensitive Na current evoked in a cerebellar nuclear cell acutely isolated from a ~2-week-old mouse. Currents were evoked by the voltage protocol shown. Responses to depolarizing and repolarizing steps are expanded in the insets. Red trace indicates steps to –30 mV. b Top panel Tetrodotoxin-sensitive Na currents evoked by step depolarizations from −60 to 0 mV applied at 20 Hz (10 stimuli), and interrupted by a 250-ms step to –60, –70, or –80 mV. After this interval, three depolarizations are applied at 100 Hz to simulate a burst of action potentials. The first current evoked after the 250-ms pause is expanded in the inset. Bottom panel Mean currents evoked by this protocol in five cells. During the 20-Hz train, Na channels inactivate by about 50%, and the availability of Na channels after the pause depends on the voltage during the interval. Data from [44], reproduced with permission. c Spontaneous action potentials with no holding current (left) and their derivatives (right) in an isolated cerebellar nuclear cell after a period of voltage clamp at –68 mV. Note the decrease in dV/dt over time, reflecting the decreasing availability of Na channels. Data from [10], reproduced with permission. d Left Action potentials and IPSPs evoked at 100 Hz in a cerebellar nuclear neuron in a slice (33°C). No holding current. Right Firing rate after the IPSPs plotted against the rate before the IPSPs, for 13 cells recording in the whole-cell configuration and 16 cells recorded in the perforated-patch configuration with gramicidin. Red symbols indicate the means of the two data sets. Solid line is a linear fit to the data, with a fitted slope of 1.0 and intercept of 20 Hz (R2=0.82), indicating that after the period of inhibition, the firing rate increases by 20 Hz. Data from [45], reproduced with permission
Disinhibition and T-Type Ca Currents
After experimentally applied hyperpolarizations to strongly negative potentials, both small and large cerebellar nuclear cells in slice preparations often generate bursts of spikes that are superimposed on an underlying depolarization [7, 9, 12, 46]. The term “rebound” has come to be applied to the depolarization, the spike burst, and occasionally the period of prolonged firing following hyperpolarization. The rebound response elicited after hyperpolarizations beyond about –90 mV is likely to result in part from activation of recovered T-type Ca channels, which are present at high densities on nuclear cell dendrites [38, 45, 47, 48], and whose density correlates with the number of spikes in the post-hyperpolarization burst [46, 49].
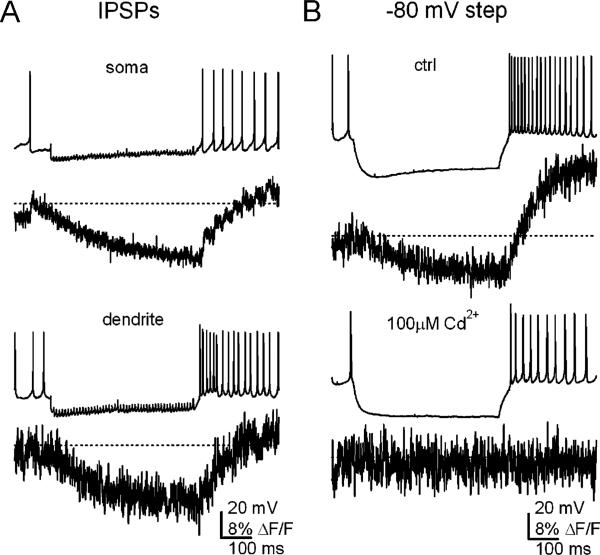
Whether rebound bursts with a similar ionic mechanism are activated by GABAA-mediated synaptic inhibition, however, is still debated (e.g. [7, 42, 45, 50, 51]). The recruitment of T-type or any other channels necessarily depends on the duration and extent of hyperpolarization by inhibition. Activation of GABAA receptors can hyperpolarize cells no further than ECl, which has repeatedly been measured to be near –75 mV in these neurons, in different species (guinea pig, rat, and mouse) and at different ages (from P5 to young adult) [9, 42, 45, 50, 52]. The voltage-dependence of T-type channels (measured in large neurons), however, makes it unlikely that they recover substantially even at ECl. Moreover, imaging studies reveal no evidence of large, brief T-type Ca transients even in dendrites after trains of IPSPs (Fig. 4a). Instead, when spontaneous firing is interrupted by synaptic inhibition, the Ca signal drops, and when firing resumes, it is restored to baseline levels. Thus, it is difficult to conceive of a scenario in which T-type currents are recruited by GABAA-mediated inhibition alone. Only when cells are hyperpolarized by current injections beyond ECl does the Ca signal overshoot the basal level [45]. These results are consistent with previous work demonstrating that post-hyperpolarization Ca signals are roughly proportional to firing rate in these cells, indicating that most of the Ca influx is generated by high-voltage-activated (HVA) Ca currents activated during spiking [53].
Fig. 4.
Ca signals during IPSPs and inhibition-like hyperpolarizations. a Spontaneous action potentials (no holding current) and IPSPs evoked at 100 Hz for 500 ms and somatic and dendritic Ca signals recorded with Fluo-4 (Kd=345 nM). ΔF/F values are calculated relative to the Ca signal when cells were held silent at –80 mV. Dotted lines indicate mean ΔF/F during spontaneous firing. Note that transients on the time scale of individual action potentials are evident. During IPSPs, Ca signals drop, indicating that firing neurons sustain a Ca load. After inhibition, Ca levels increase gradually at a rate proportional to the rate of firing, but do not rapidly exceed the value associated with spontaneous firing. b Experiments as in a, but with a current injection to –80 mV, a value about 5 mV negative to ECl, and about 10 mV hyperpolarized to the value reached during synaptic inhibition. With this hyperpolarization, the post-hyperpolarization dendritic Ca signal is large (top). The recording is repeated in the same cell (bottom) but with 100 μM Cd++ in the solution, which nearly fully blocks HVA channels but T-type channels by only 50%. The Ca changes are abolished, suggesting that they are mediated by HVA channels. Data from [45], reproduced with permission
In some preparations, the post-inhibitory propensity to burst has been reported to increase with the strength of stimulation of Purkinje afferents [51]. Since the depth of hyperpolarization does not change significantly with increased synaptic inhibition, this observation raises the interesting possibility that stronger stimulation facilitates post-inhibitory bursts by engaging additional mechanisms, such as modulation of T-type currents or activation of other ionic conductances. In fact, the elevations of firing rate observed after strong inhibition persists for several hundred milliseconds, far outlasting the duration of T-type current, which decays within 50 ms [32, 45, 54], possibly indicative of multiple underlying processes.
Nevertheless, the presence of T-type currents in nuclear cells suggests that they are indeed activated by specific stimuli, and it is of interest to identify what these stimuli may be. Substantial recovery of T-type currents may well require sustained activation of K currents, which can hyperpolarize cells beyond ECl. Which K currents may be involved is yet unknown; inward rectifiers that are stimulated by GABAB receptors seem a likely candidate, but GABAB receptors are not readily activated even with prolonged Purkinje cell stimulation, at least in slice studies [8, 40, 55]. Another possibility is that ECl itself becomes more negative under certain conditions. During development, ECl does appear to shift negatively as a result of changes in the relative density of chloride transporters NKCC1 and KCC2. In cerebellar slices from very young (P2-P3) rats, ECl of nuclear neurons is relatively depolarized (–55 mV) and shifts about 10 mV negative in response to experimentally induced synaptic activity that drives expression of the potassium chloride co-transporter KCC2 [56]. The shift appears to be complete by P5, however, making it seem unlikely that further developmental shifts bring ECl significantly below the value of –75 mV. A relevant point has recently been raised by research on neocortical neurons, however, namely, that young (nursing) mammals often rely on ketone bodies rather than glucose for metabolism. Application of ketone bodies to neocortical slices from P4-P8 rats, before pyramidal cells express KCC2, shifts ECl from about –55 mV to about –80 mV, apparently by actions on the chloride/bicarbonate transporter [57]. If chloride/bicarbonate transporters influence ECl in the cerebellar nuclei even after expression of KCC2, it is possible that GABAA receptor activation in vivo may hyperpolarize cells more strongly than in vitro, in which ACSF is usually supplemented only by glucose. Notably, however, relief of 500-ms hyperpolarizations even to –80 mV fails to engage significant T-type current in cerebellar nuclear cell dendrites (Fig. 4b,[45]).
The Long-Term Influence of Excitation on Inhibition
Because NMDA receptors in the nuclei are only weakly blocked by Mg++ as described above, an NMDA-receptor-dependent Ca influx is not restricted to periods of postsynaptic depolarization. These receptors therefore likely do not carry out their common role as coincidence detectors that drive plasticity when presynaptic and post-synaptic spiking temporally overlap. Nevertheless, NMDA receptors in the nuclei do indeed participate in plasticity. For instance, in nuclear cells studied without selection based on size, a variety of protocols that elicit moderate Ca influx, including low-frequency stimulation of NMDA receptors, induce a long-term depression (LTD) of inhibitory synaptic currents; depolarizations that activate L-type Ca channels can substitute for the NMDA receptor activation. This form of LTD does not require activation of GABAA receptors, and consequently, it is not synapse-specific but cell-wide [52, 58]. Moreover, LTD appears to be postsynaptically expressed, since it reduces the sensitivity to GABAA agonists. LTD does not, however, change the chloride reversal potential or the kinetics of IPSCs [58].
Conversely, protocols that generate a large Ca influx, such as 100-Hz stimulation of NMDA receptors, induce a cell-wide long-term potentiation (LTP) of IPSCs that depends on postsynaptic exocytosis, implicating GABAA receptor insertion [59]. Like LTD, however, the LTP of IPSCs in large and small cells does not have an absolute requirement for NMDA receptor activation. With fast glutamatergic transmission blocked, LTP of IPSPs can be evoked by 10-pulse, 100-Hz trains of IPSPs evoked twice per second, a protocol that produces relatively large postsynaptic Ca influx via post-inhibitory action potential firing: more action potentials generate a larger intracellular Ca signal and a more consistent potentiation [53]. Interestingly, this same protocol induces LTD if the number of post-inhibitory action potentials, as well as the corresponding Ca signal, is reduced by tonic hyperpolarization [53]. As mentioned above, more recent imaging studies in large cells confirm that the amplitude of Ca signals in nuclear neurons correlates with the rate of action potential firing [45], and that these spike-related signals result from flux through HVA Ca channels [45, 54, 60]. Regarding the role of specific Ca channels in inducing plasticity, the LTD-induction protocol likely evokes more T-type than HVA Ca channel current, because tonic hyperpolarization is expected to permit T-type channel recovery, and the low number of post-inhibitory action potentials are expected to minimize HVA Ca channel activation. In contrast, the LTP-induction protocol favors activation of HVA Ca channels over T-type channels, because cells are relatively more depolarized both during and after inhibition, and higher post-inhibitory firing rates are attained. L-type channels in particular, unlike other HVA channels, do not contribute strongly to dendritic Ca influx, but are present at high densities in the somata, making them well placed to interact with GABAA receptors [45].
Together, these data on LTD and LTP provide insight into the regulation of inhibition in the cerebellar nuclei. The olivocerebellar circuit forms a negative feedback loop [5]: a drop in spiking by small nuclear cells, e.g. because of a reduction in excitatory drive from mossy fibers, decreases the inhibitory drive onto inferior olivary neurons, which increases climbing fiber activity, thereby evoking complex spikes in Purkinje cells. Because complex spikes induce LTD of parallel fiber synapses onto Purkinje cells, they have the effect of reducing excitation and slowing the driven rate of Purkinje cells [61]. The resulting decrease in inhibition from Purkinje cells will have the tendency to restore firing in the nuclei. The negative feedback is incomplete, however, since reductions in the driven firing of Purkinje cells by definition do not influence spontaneous firing of Purkinje cells. Thus, the LTD of Purkinje IPSCs is well suited to carry out the missing component of the negative feedback, by making nuclear cells less responsive even to basal inhibition. Conversely, high-frequency excitation that drives rapid firing in the nuclei may exert a homeostatic strengthening of inhibition via the negative feedback circuit as well as by LTP of individual inhibitory synapses. Thus, multiple negative feedback systems are engaged that apparently interact to stabilize nuclear cell output to a new set point of incoming excitation.
The Long-Term Influence of Inhibition on Excitation
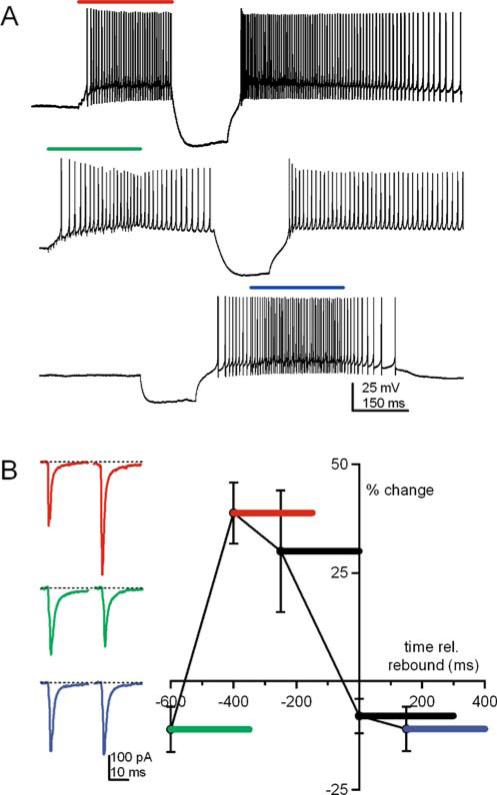
Based on systems and behavioral studies, cerebellar models have long predicted that motor learning requires Purkinje-mediated inhibition to regulate the strength of synaptic excitation and the consequent spike output of Purkinje target neurons [62, 63]. Cellular support for this hypothesis has come from cerebellar slice studies of large nuclear neurons, which demonstrate that specific combinations of excitation and inhibition can induce long-term changes in excitatory synaptic strength in cerebellar nuclear cells. Specifically, when high-frequency trains of synaptic excitation precede a period of inhibition and disinhibition, EPSCs undergo a synapse-specific LTP (Fig. 5,[32, 38]). What makes this pattern of plasticity of interest is that the induction protocols resemble the patterns of stimulation that are expected to occur during associative learning in the cerebellum ([63]; reviewed in [64]). Shifting the relative timing of the EPSPs and inhibition changes the polarity of plasticity: if the train of excitation is too early relative to disinhibition, EPSCs tend to depress weakly. Likewise, if excitation is superimposed on the post-hyperpolarization action potentials, depression is favored (Fig. 5,[38]). A similar LTD of EPSCs can be induced by simultaneous synaptic excitation and depolarization, in a manner that depends on metabotropic glutamate receptor activation [65]. Both these forms of LTD (which may be convergent in their mechanisms) are suggestive of another negative feedback-like form of plasticity in the nuclei, since coincident synaptic excitation and postsynaptic spiking tends to diminish excitatory synaptic strength.
Fig. 5.
Long-term potentiation of EPSCs. a Responses of cerebellar nuclear neurons to three induction protocols, consisting of a 250 ms train of EPSPs at 133 Hz, applied during the colored bar and a 150-ms hyperpolarization. b Left EPSCs evoked at –65 mV before and after 30 applications of the induction protocol. Right Efficacy of the various induction protocols in inducing long-term potentiation of EPSCs, plotted as the percent change in EPSC amplitude, against the onset of the excitatory synaptic stimulation relative to the time of disinhibition. Bars indicate the duration of the EPSPs. Data from [38], reproduced with permission
The dependence of the LTP of EPSCs on a fixed temporal pattern excitation and inhibition raises the question of the mechanisms underlying the plasticity. To induce LTP effectively, the period of excitation must activate NMDA receptors, and the disinhibition—which can be generated either by current injection or stimulation of Purkinje afferents—must activate voltage-gated Ca channels. Although voltage-clamp experiments demonstrate that activation of T-type Ca currents are sufficient to generate the disinhibition-dependent Ca signal [32], they do not appear to be necessary, since potentiation can be induced by real synaptic inhibition that activates high-voltage-activated Ca channels but not T-type currents [38, 45].
The requirement for at least two sources of Ca to induce LTP suggests that multiple signaling cascades are engaged by the induction protocol. In fact, this form of LTP is consistently prevented by blockade of the Ca-dependent phosphatase calcineurin, which unmasks an LTD driven by the same stimulus protocol. The plasticity is also prevented, however, by blockade of the Ca-dependent kinase CaMKII [38]. Thus, both dephosphorylation and phosphorylation appear necessary for potentiation of EPSCs. A possible scenario involves activation of at least one of these enzymes, e.g. calcineurin, by NMDA receptor activation and another enzyme, e.g. CaMKII, by disinhibition, although it is equally possible that an additional signaling mechanism is activated by the drop in Ca resulting from the suppression of spiking during inhibition [45]. Such a situation exists in medial vestibular neurons, which are also targets of Purkinje cells, and in which CaMKII activity is down-regulated by synaptic inhibition [66, 67]. Thus, synaptic excitation may serve to prime individual synapses for potentiation, which is subsequently triggered if and only if the priming event is followed relatively rapidly by inhibition that interrupts firing [38].
The most appealing interpretation of this form of potentiation is that it may form part of the cellular mechanism of cerebellar learning, such as delay eyelid conditioning ([68], reviewed in [69]). Whole-animal studies demonstrate that during training, Purkinje neurons change their responses to mossy fiber input that carries the information associated with the conditioned stimulus [70]: initially they fire throughout the conditioned stimulus, but after repeated exposure to paired conditioned and unconditioned stimuli, they fire during the early phase but less during the late phase of the conditioned stimulus [71]. At the level of the cerebellar nuclei, such a situation predicts that the excitation of cerebellar nuclei (via the mossy fibers that are active during the conditioned stimulus) overlaps with a sequence of inhibition and disinhibition, which could potentiate EPSCs. In this way, plasticity in the cortex would drive plasticity in the nuclei, as predicted by the Medina–Mauk model [63]. Disinhibition of cerebellar nuclear cells that project to premotor areas, combined with the increased sensitivity to excitatory input from mossy fibers, might be sufficient to drive conditioned responses, i.e., to evoke the reflex-like eyelid closure in response to the conditioned stimulus alone.
Such an increased responsiveness to mossy fiber input may be further augmented by an unusual form of intrinsic plasticity that is present in the cerebellar nuclei. Bursts of high-frequency EPSPs that evoke Ca influx through NMDA receptors induce neurons to become more excitable in response to fixed current injections [72, 73]. Along with the LTP of EPSCs, this form of plasticity is one of the few examples of a positive feedback in the cerebellar nuclei, as stimuli that (intermittently) facilitate firing actually increase excitability. Strikingly, however, IPSP bursts that permit post-inhibitory spiking and an associated intracellular Ca rise can also induce this intrinsic activity [73], suggesting that the response is not necessarily sensitive to the polarity of synaptic input, as long as firing is increased. Whatever the means by which excitability is modulated, it is possible that intrinsic plasticity may amplify synaptic plasticity, making the response of nuclear cells to mossy fiber input even more robust.
Pathophysiological Plasticity of Synaptic Inhibition
Studies of mutant mice with disrupted Purkinje cell input provide interesting parallels with the plasticity of inhibitory inputs onto cerebellar nuclear cells. For instance, in heterozygous Lurcher mice (Lc/+), in which the GluRδ2 gene is mutated, Purkinje cells degenerate between P8 and P26, rendering animals ataxic [74–76]. Multiple changes occur at the level of the cerebellar nuclei, however, that are suggestive of compensation for reduced inhibitory input. For example, inhibitory boutons enlarge, and even the number of cerebellar nuclear cells that co-label for GABA and glycine increases [77]. Consistent with these anatomical changes, during the period of Purkinje cell degeneration, the amplitude of spontaneous and miniature IPSCs increases [78]. In addition, aggregates of gephyrin, which clusters GABAA receptors at synapses, increase in size [79]. Together, these data are consistent with the idea that strong regulatory mechanisms operate to keep inhibition onto cerebellar nuclear cells within some operationally acceptable range: low levels of inhibition, both physiological and pathophysiological, trigger multiple compensatory processes that raise the sensitivity to inhibitory input.
Complementary data come from studies of dystonic rats [80]. This work not only illustrates pathophysiological plasticity but also provides information that may address the question of whether bursting contributes to signals involved in motor control. In these animals, basal firing rates of Purkinje cells are increased [81]. Climbing fiber input also decreases, which is expected to correlate with a loss of long-term depression of excitatory parallel fiber inputs, and therefore a further increase in Purkinje neuronal activity [81]; these changes are likely to increase inhibition of cerebellar nuclear cells. As in Lurcher mice, however, examination of GABAA receptors reveals compensatory changes: autoradiographic studies of dystonic rats indicate that the density of GABA receptors in the nuclei is reduced [82], suggestive of a down-regulation of sensitivity to GABA that may offset increased Purkinje-mediated inhibition. Intriguingly, however, measurements of the changes in firing by cerebellar nuclear neurons in these animals reveals neither a net increase nor a decrease in firing; instead the primary modification is that spike output of nuclear cells consists of more burst-like clusters of action potentials [83]. Most strikingly, the symptoms of dystonia are relieved by ablation of the cerebellum, suggesting that this aberrant cerebellar output generates the pathological co-contraction of opposing muscle groups [80]. Consistent with the idea that excessively abrupt changes of nuclear cell spike rate disrupt motor coordination, studies of mice with mutations of P-type Ca channels have shown that irregularities in firing by Purkinje cells correlate with ataxia. Moreover, the motor disorders in these mice can be largely reversed by application of SK agonists that regularize Purkinje cell firing [84]. Together, these studies raise the interesting point that smooth modulation of spike output of cerebellar nuclear neurons may be regulated by Purkinje cells and may constitute an important component of normal motor control.
Acknowledgments
We would like to acknowledge all the members of the laboratory whose research is reviewed in this article: Fatemeh Afshari, Teresa Aman, Amy Gustafson, Zayd Khaliq, Dan Padgett, Jason Pugh, and Petra Telgkamp. We are especially grateful to Jason Bant and Teresa Aman for their contributions to Figures. Supported by NIH NS39395 (IMR).
Contributor Information
Nan Zheng, Northwestern University Interdepartmental Neuroscience Program, Evanston, IL 60208, USA.
Indira M. Raman, Department of Neurobiology and Physiology, Northwestern University, 2205 Tech Drive, Evanston, IL 60208, USA
References
- 1.Chan-Palay V. Organization, Cytology, and Transmitters. Springer; New York: 1977. Cerebellar dentate nucleus. [Google Scholar]
- 2.Teune TM, van der Burg J, Ruigrok TJ. Cerebellar projections to the red nucleus and inferior olive originate from separate populations of neurons in the rat: a non-fluorescent double labeling study. Brain Res. 1995;673(2):313–319. doi: 10.1016/0006-8993(94)01431-g. [DOI] [PubMed] [Google Scholar]
- 3.Teune TM, van der Burg J, van der Moer J, Voogd J, Ruigrok TJ. Topography of cerebellar nuclear projections to the brain stem in the rat. Prog Brain Res. 2000;124:141–172. doi: 10.1016/S0079-6123(00)24014-4. [DOI] [PubMed] [Google Scholar]
- 4.Fredette BJ, Mugnaini E. The GABAergic cerebello-olivary projection in the rat. Anat Embryol (Berl) 1991;184(3):225–243. doi: 10.1007/BF01673258. [DOI] [PubMed] [Google Scholar]
- 5.Medina JF, Nores WL, Mauk MD. Inhibition of climbing fibres is a signal for the extinction of conditioned eyelid responses. Nature. 2002;416(6878):330–333. doi: 10.1038/416330a. [DOI] [PubMed] [Google Scholar]
- 6.Jahnsen H. Electrophysiological characteristics of neurones in the guinea-pig deep cerebellar nuclei in vitro. J Physiol. 1986;372:129–147. doi: 10.1113/jphysiol.1986.sp016001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Llinás R, Mühlethaler M. Electrophysiology of guinea-pig cerebellar nuclear cells in the in vitro brain stem-cerebellar preparation. J Physiol. 1988;404:241–258. doi: 10.1113/jphysiol.1988.sp017288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mouginot D, Gähwiler BH. Characterization of synaptic connections between cortex and deep nuclei of the rat cerebellum in vitro. Neuroscience. 1995;64(3):699–712. doi: 10.1016/0306-4522(94)00456-f. [DOI] [PubMed] [Google Scholar]
- 9.Aizenman CD, Linden DJ. Regulation of the rebound depolarization and spontaneous firing patterns of deep nuclear neurons in slices of rat cerebellum. J Neurophysiol. 1999;82(4):1697–1709. doi: 10.1152/jn.1999.82.4.1697. [DOI] [PubMed] [Google Scholar]
- 10.Raman IM, Gustafson AE, Padgett DE. Ionic currents and spontaneous firing in neurons isolated from the cerebellar nuclei. J Neurosci. 2000;20(24):9004–9016. doi: 10.1523/JNEUROSCI.20-24-09004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gauck V, Jaeger D. The control of rate and timing of spikes in the deep cerebellar nuclei by inhibition. J Neurosci. 2000;20(8):3006–3016. doi: 10.1523/JNEUROSCI.20-08-03006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Czubayko U, Sultan F, Thier P, Schwarz C. Two types of neurons in the rat cerebellar nuclei as distinguished by membrane potentials and intracellular fillings. J Neurophysiol. 2001;85(5):2017–2029. doi: 10.1152/jn.2001.85.5.2017. [DOI] [PubMed] [Google Scholar]
- 13.Monaghan PL, Beitz AJ, Larson AA, Altschuler RA, Madl JE, Mullett MA. Immunocytochemical localization of glutamate-, glutaminase- and aspartate aminotransferase-like immunoreactivity in the rat deep cerebellar nuclei. Brain Res. 1986;363(2):364–370. doi: 10.1016/0006-8993(86)91024-3. [DOI] [PubMed] [Google Scholar]
- 14.Chen S, Hillman DE. Colocalization of neurotransmitters in the deep cerebellar nuclei. J Neurocytol. 1993;22(2):81–91. doi: 10.1007/BF01181572. [DOI] [PubMed] [Google Scholar]
- 15.Teune TM, van der Burg J, de Zeeuw CI, Voogd J, Ruigrok TJ. Single Purkinje cell can innervate multiple classes of projection neurons in the cerebellar nuclei of the rat: a light microscopic and ultrastructural triple-tracer study in the rat. J Comp Neurol. 1998;392(2):164–178. doi: 10.1002/(sici)1096-9861(19980309)392:2<164::aid-cne2>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 16.Uusisaari M, Obata K, Knöpfel T. Morphological and electrophysiological properties of GABAergic and non-GABAergic cells in the deep cerebellar nuclei. J Neurophysiol. 2007;97(1):901–911. doi: 10.1152/jn.00974.2006. [DOI] [PubMed] [Google Scholar]
- 17.Bagnall MW, Zingg B, Sakatos A, Moghadam S, Zeilhofer HU, du Lac S. Glycinergic projection neurons of the cerebellum. J Neurosci. 2009;29(32):10104–10110. doi: 10.1523/JNEUROSCI.2087-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palkovits M, Mezey E, Hamori J, Szentagothai J. Quantitative histological analysis of the cerebellar nuclei in the cat. I. Numerical data on cells and on synapses. Exp Brain Res. 1977;28(1–2):189–209. doi: 10.1007/BF00237096. [DOI] [PubMed] [Google Scholar]
- 19.Häusser M, Clark BA. Tonic synaptic inhibition modulates neuronal output pattern and spatiotemporal synaptic integration. Neuron. 1997;19(3):665–678. doi: 10.1016/s0896-6273(00)80379-7. [DOI] [PubMed] [Google Scholar]
- 20.Raman IM, Bean BP. Resurgent sodium current and action potential formation in dissociated cerebellar Purkinje neurons. J Neurosci. 1997;17(12):4517–4526. doi: 10.1523/JNEUROSCI.17-12-04517.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nam SC, Hockberger PE. Analysis of spontaneous electrical activity in cerebellar Purkinje cells acutely isolated from postnatal rats. J Neurobiol. 1997;33(1):18–32. doi: 10.1002/(sici)1097-4695(199707)33:1<18::aid-neu3>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 22.Thach WT. Discharge of Purkinje and cerebellar nuclear neurons during rapidly alternating arm movements in the monkey. J Neurophysiol. 1968;31(5):785–797. doi: 10.1152/jn.1968.31.5.785. [DOI] [PubMed] [Google Scholar]
- 23.Latham A, Paul DH. Spontaneous activity of cerebellar Purkinje cells and their responses to impulses in climbing fibres. J Physiol. 1971;213(1):135–156. doi: 10.1113/jphysiol.1971.sp009373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khaliq ZM, Raman IM. Axonal propagation of simple and complex spikes in cerebellar Purkinje neurons. J Neurosci. 2005;25(2):454–463. doi: 10.1523/JNEUROSCI.3045-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monsivais P, Clark BA, Roth A, Häusser M. Determinants of action potential propagation in cerebellar Purkinje cell axons. J Neurosci. 2005;25(2):464–472. doi: 10.1523/JNEUROSCI.3871-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Kan PL, Gibson AR, Houk JC. Movement-related inputs to intermediate cerebellum of the monkey. J Neurophysiol. 1993;69(1):74–94. doi: 10.1152/jn.1993.69.1.74. [DOI] [PubMed] [Google Scholar]
- 27.Armstrong DM, Edgley SA. Discharges of nucleus interpositus neurones during locomotion in the cat. J Physiol. 1984;351:411–432. doi: 10.1113/jphysiol.1984.sp015253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McDevitt CJ, Ebner TJ, Bloedel JR. Relationships between simultaneously recorded Purkinje cells and nuclear neurons. Brain Res. 1987;425(1):1–13. doi: 10.1016/0006-8993(87)90477-x. [DOI] [PubMed] [Google Scholar]
- 29.Lu B, Su Y, Das S, Liu J, Xia J, Ren D. The neuronal channel NALCN contributes resting sodium permeability and is required for normal respiratory rhythm. Cell. 2007;129(2):371–383. doi: 10.1016/j.cell.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 30.Cody FW, Moore RB, Richardson HC. Patterns of activity evoked in cerebellar interpositus nuclear neurones by natural somatosensory stimuli in awake cats. J Physiol. 1981;317:1–20. doi: 10.1113/jphysiol.1981.sp013810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sánchez-Campusano R, Gruart A, Delgado-García JM. The cerebellar interpositus nucleus and the dynamic control of learned motor responses. J Neurosci. 2007;27(25):6620–6632. doi: 10.1523/JNEUROSCI.0488-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pugh JR, Raman IM. Potentiation of mossy fiber EPSCs in the cerebellar nuclei by NMDA receptor activation followed by postinhibitory rebound current. Neuron. 2006;51(1):113–123. doi: 10.1016/j.neuron.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 33.Audinat E, Gähwiler BH, Knöpfel T. Excitatory synaptic potentials in neurons of the deep nuclei in olivo-cerebellar slice cultures. Neuroscience. 1992;49(4):903–911. doi: 10.1016/0306-4522(92)90366-a. [DOI] [PubMed] [Google Scholar]
- 34.Anchisi D, Scelfo B, Tempia F. Postsynaptic currents in deep cerebellar nuclei. J Neurophysiol. 2001;85(1):323–331. doi: 10.1152/jn.2001.85.1.323. [DOI] [PubMed] [Google Scholar]
- 35.Akazawa C, Shigemoto R, Bessho Y, Nakanishi S, Mizuno N. Differential expression of five N-methyl-D-aspartate receptor subunit mRNAs in the cerebellum of developing and adult rats. J Comp Neurol. 1994;347(1):150–160. doi: 10.1002/cne.903470112. [DOI] [PubMed] [Google Scholar]
- 36.Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12(3):529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 37.Momiyama A, Feldmeyer D, Cull-Candy SG. Identification of a native low-conductance NMDA channel with reduced sensitivity to Mg2+ in rat central neurones. J Physiol. 1996;494(Pt 2):479–492. doi: 10.1113/jphysiol.1996.sp021507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pugh JR, Raman IM. Mechanisms of potentiation of mossy fiber EPSCs in the cerebellar nuclei by coincident synaptic excitation and inhibition. J Neurosci. 2008;28(42):10549–10560. doi: 10.1523/JNEUROSCI.2061-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Telgkamp P, Raman IM. Depression of inhibitory synaptic transmission between Purkinje cells and neurons of the cerebellar nuclei. J Neurosci. 2002;22(19):8447–8457. doi: 10.1523/JNEUROSCI.22-19-08447.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Telgkamp P, Padgett DE, Ledoux V, Woolley CS, Raman IM. Maintenance of high-frequency inhibitory transmission at Purkinje to cerebellar nuclear synapses by spillover from boutons with multiple release sites. Neuron. 2004;41:113–126. doi: 10.1016/s0896-6273(03)00802-x. [DOI] [PubMed] [Google Scholar]
- 41.Pugh JR, Raman IM. GABAA receptor kinetics in the cerebellar nuclei: evidence for detection of transmitter from distant release sites. Biophys J. 2005;88(3):1740–1754. doi: 10.1529/biophysj.104.055814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jahnsen H. Extracellular activation and membrane conductances of neurones in the guinea-pig deep cerebellar nuclei in vitro. J Physiol. 1986;372:149–168. doi: 10.1113/jphysiol.1986.sp016002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Afshari FS, Ptak K, Khaliq ZM, Grieco TM, Slater NT, McCrimmon DR, Raman IM. Resurgent Na currents in four classes of neurons of the cerebellum. J Neurophysiol. 2004;92(5):2831–2843. doi: 10.1152/jn.00261.2004. [DOI] [PubMed] [Google Scholar]
- 44.Aman TK, Raman IM. Subunit dependence of Na channel slow inactivation and open channel block in cerebellar neurons. Biophys J. 2007;92(6):1938–1951. doi: 10.1529/biophysj.106.093500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zheng N, Raman IM. Ca currents activated by spontaneous firing and synaptic disinhibition in neurons of the cerebellar nuclei. J Neurosci. 2009;29(31):9826–9838. doi: 10.1523/JNEUROSCI.2069-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Molineux ML, McRory JE, McKay BE, Hamid J, Mehaffey WH, Rehak R, Snutch TP, Zamponi GW, Turner RW. Specific T-type calcium channel isoforms are associated with distinct burst phenotypes in deep cerebellar nuclear neurons. Proc Natl Acad Sci USA. 2006;103(14):5555–5560. doi: 10.1073/pnas.0601261103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muri R, Knöpfel T. Activity induced elevations of intracellular calcium concentration in neurons of the deep cerebellar nuclei. J Neurophysiol. 1994;71(1):420–428. doi: 10.1152/jn.1994.71.1.420. [DOI] [PubMed] [Google Scholar]
- 48.Gauck V, Thomann M, Jaeger D, Borst A. Spatial distribution of low- and high-voltage-activated calcium currents in neurons of the deep cerebellar nuclei. J Neurosci. 2001;21(15):RC158. doi: 10.1523/JNEUROSCI.21-15-j0004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Molineux ML, Mehaffey WH, Tadayonnejad R, Anderson D, Tennent AF, Turner RW. Ionic factors governing rebound burst phenotype in rat deep cerebellar neurons. J Neurophysiol. 2008;100(5):2684–2701. doi: 10.1152/jn.90427.2008. [DOI] [PubMed] [Google Scholar]
- 50.Alviña K, Walter JT, Kohn A, Ellis-Davies G, Khodakhah K. Questioning the role of rebound firing in the cerebellum. Nat Neurosci. 2008;11(11):1256–1258. doi: 10.1038/nn.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tadayonnejad R, Mehaffey WH, Anderson D, Turner RW. Reliability of triggering postinhibitory rebound bursts in deep cerebellar neurons. Channels (Austin) 2009;3(3):149–155. doi: 10.4161/chan.3.3.8872. [DOI] [PubMed] [Google Scholar]
- 52.Morishita W, Sastry BR. Long-term depression of IPSPs in rat deep cerebellar nuclei. Neuroreport. 1993;4(6):719–722. doi: 10.1097/00001756-199306000-00030. [DOI] [PubMed] [Google Scholar]
- 53.Aizenman CD, Manis PB, Linden DJ. Polarity of long-term synaptic gain change is related to postsynaptic spike firing at a cerebellar inhibitory synapse. Neuron. 1998;21(4):827–835. doi: 10.1016/s0896-6273(00)80598-x. [DOI] [PubMed] [Google Scholar]
- 54.Alviña K, Ellis-Davies G, Khodakhah K. T-type calcium channels mediate rebound firing in intact deep cerebellar neurons. Neuroscience. 2009;158(2):635–641. doi: 10.1016/j.neuroscience.2008.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morishita W, Sastry BR. Pharmacological characterization of pre- and postsynaptic GABAB receptors in the deep nuclei of rat cerebellar slices. Neuroscience. 1995;68(4):1127–1137. doi: 10.1016/0306-4522(95)00206-x. [DOI] [PubMed] [Google Scholar]
- 56.Ouardouz M, Sastry BR. Activity-mediated shift in reversal potential of GABA-ergic synaptic currents in immature neurons. Brain Res Dev Brain Res. 2005;160(1):78–84. doi: 10.1016/j.devbrainres.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 57.Rheims S, Holmgren CD, Chazal G, Mulder J, Harkany T, Zilberter T, Zilberter Y. GABA action in immature neocortical neurons directly depends on the availability of ketone bodies. J Neurochem. 2009;110:1330–1338. doi: 10.1111/j.1471-4159.2009.06230.x. [DOI] [PubMed] [Google Scholar]
- 58.Morishita W. Sastry BR Postsynaptic mechanisms underlying long-term depression of GABAergic transmission in neurons of the deep cerebellar nuclei. J Neurophysiol. 1996;76(1):59–68. doi: 10.1152/jn.1996.76.1.59. [DOI] [PubMed] [Google Scholar]
- 59.Ouardouz M, Sastry BR. Mechanisms underlying LTP of inhibitory synaptic transmission in the deep cerebellar nuclei. J Neurophysiol. 2000;84(3):1414–1421. doi: 10.1152/jn.2000.84.3.1414. [DOI] [PubMed] [Google Scholar]
- 60.Alviña K, Khodakhah K. Selective regulation of spontaneous activity of neurons of the deep cerebellar nuclei by N-type calcium channels in juvenile rats. J Physiol. 2008;586(10):2523–2538. doi: 10.1113/jphysiol.2007.148197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Linden DJ, Connor JA. Cellular mechanisms of long-term depression in the cerebellum. Curr Opin Neurobiol. 1993;3(3):401–406. doi: 10.1016/0959-4388(93)90133-j. [DOI] [PubMed] [Google Scholar]
- 62.Miles FA. Lisberger SG Plasticity in the vestibulo-ocular reflex: a new hypothesis. Annu Rev Neurosci. 1981;4:273–299. doi: 10.1146/annurev.ne.04.030181.001421. [DOI] [PubMed] [Google Scholar]
- 63.Medina JF, Mauk MD. Simulations of cerebellar motor learning: computational analysis of plasticity at the mossy fiber to deep nucleus synapse. J Neurosci. 1999;19(16):7140–7151. doi: 10.1523/JNEUROSCI.19-16-07140.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pugh JR, Raman IM. Nothing can be coincidence: synaptic inhibition and plasticity in the cerebellar nuclei. Trends Neurosci. 2009;32(3):170–177. doi: 10.1016/j.tins.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang W, Linden DJ. Long-term depression at the mossy fiber-deep cerebellar nucleus synapse. J Neurosci. 2006;26(26):6935–6944. doi: 10.1523/JNEUROSCI.0784-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nelson AB, Krispel CM, Sekirnjak C, Du Lac S. Long-lasting increases in intrinsic excitability triggered by inhibition. Neuron. 2003;40(3):609–620. doi: 10.1016/s0896-6273(03)00641-x. [DOI] [PubMed] [Google Scholar]
- 67.Nelson AB, Gittis AH, du Lac S. Decreases in CaMKII activity trigger persistent potentiation of intrinsic excitability in spontaneously firing vestibular nucleus neurons. Neuron. 2005;46(4):623–631. doi: 10.1016/j.neuron.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 68.McCormick DA, Thompson RF. Cerebellum: essential involvement in the classically conditioned eyelid response. Science. 1984;223(4633):296–299. doi: 10.1126/science.6701513. [DOI] [PubMed] [Google Scholar]
- 69.Medina JF, Nores WL, Ohyama T, Mauk MD. Mechanisms of cerebellar learning suggested by eyelid conditioning. Curr Opin Neurobiol. 2000;10(6):717–724. doi: 10.1016/s0959-4388(00)00154-9. [DOI] [PubMed] [Google Scholar]
- 70.Hesslow G, Svensson P, Ivarsson M. Learned movements elicited by direct stimulation of cerebellar mossy fiber afferents. Neuron. 1999;24(1):179–185. doi: 10.1016/s0896-6273(00)80831-4. [DOI] [PubMed] [Google Scholar]
- 71.Jirenhed DA, Bengtsson F, Hesslow G. Acquisition, extinction, and reacquisition of a cerebellar cortical memory trace. J Neurosci. 2007;27(10):2493–2502. doi: 10.1523/JNEUROSCI.4202-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Aizenman CD, Linden DJ. Rapid, synaptically driven increases in the intrinsic excitability of cerebellar deep nuclear neurons. Nat Neurosci. 2000;3(2):109–111. doi: 10.1038/72049. [DOI] [PubMed] [Google Scholar]
- 73.Zhang W, Shin JH, Linden DJ. Persistent changes in the intrinsic excitability of rat deep cerebellar nuclear neurones induced by EPSP or IPSP bursts. J Physiol. 2004;561(Pt 3):703–719. doi: 10.1113/jphysiol.2004.071696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Caddy KW, Biscoe TJ. Structural and quantitative studies on the normal C3H and Lurcher mutant mouse. Philos Trans R Soc Lond B Biol Sci. 1979;287(1020):167–201. doi: 10.1098/rstb.1979.0055. [DOI] [PubMed] [Google Scholar]
- 75.Wetts R, Herrup K. Interaction of granule. Purkinje and inferior olivary neurons in lurcher chimaeric mice. I. Qualitative studies. J Embryol Exp Morphol. 1982;68:87–98. [PubMed] [Google Scholar]
- 76.Zuo J, De Jager PL, Takahashi KA, Jiang W, Linden DJ, Heintz N. Neurodegeneration in Lurcher mice caused by mutation in δ2 glutamate receptor gene. Nature. 1997;388(6644):769–773. doi: 10.1038/42009. [DOI] [PubMed] [Google Scholar]
- 77.Sultan F, König T, Möck M, Thier P. Quantitative organization of neurotransmitters in the deep cerebellar nuclei of the Lurcher mutant. J Comp Neurol. 2002;452(4):311–323. doi: 10.1002/cne.10365. [DOI] [PubMed] [Google Scholar]
- 78.Linnemann C, Sultan F, Pedroarena CM, Schwarz C, Thier P. Lurcher mice exhibit potentiation of GABA(A)-receptor-mediated conductance in cerebellar nuclei neurons in close temporal relationship to Purkinje cell death. J Neurophysiol. 2004;91(2):1102–1107. doi: 10.1152/jn.00163.2003. [DOI] [PubMed] [Google Scholar]
- 79.Garin N, Hornung JP, Escher G. Distribution of postsynaptic GABA(A) receptor aggregates in the deep cerebellar nuclei of normal and mutant mice. J Comp Neurol. 2002;447(3):210–217. doi: 10.1002/cne.10226. [DOI] [PubMed] [Google Scholar]
- 80.LeDoux MS, Lorden JF, Ervin JM. Cerebellectomy eliminates the motor syndrome of the genetically dystonic rat. Exp Neurol. 1993;120(2):302–310. doi: 10.1006/exnr.1993.1064. [DOI] [PubMed] [Google Scholar]
- 81.LeDoux MS, Lorden JF. Abnormal spontaneous and harmaline-stimulated Purkinje cell activity in the awake genetically dystonic rat. Exp Brain Res. 2002;145(4):457–467. doi: 10.1007/s00221-002-1127-4. [DOI] [PubMed] [Google Scholar]
- 82.Beales M, Lorden JF, Walz E, Oltmans GA. Quantitative autoradiography reveals selective changes in cerebellar GABA receptors of the rat mutant dystonic. J Neurosci. 1990;10(6):1874–1885. doi: 10.1523/JNEUROSCI.10-06-01874.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.LeDoux MS, Hurst DC, Lorden JF. Single-unit activity of cerebellar nuclear cells in the awake genetically dystonic rat. Neuroscience. 1998;86(2):533–545. doi: 10.1016/s0306-4522(98)00007-4. [DOI] [PubMed] [Google Scholar]
- 84.Walter JT, Alviña K, Womack MD, Chevez C, Khodakhah K. Decreases in the precision of Purkinje cell pacemaking cause cerebellar dysfunction and ataxia. Nat Neurosci. 2006;9(3):389–397. doi: 10.1038/nn1648. [DOI] [PubMed] [Google Scholar]