Abstract
Objective
An absence of cysteine-rich protein 2 (CRP2) enhances vascular smooth muscle cell (VSMC) migration and increases neointima formation following arterial injury; therefore CRP2 plays an important role in the response to vascular injury. The goal of the present study was to elucidate the molecular mechanisms that preserve CRP2 expression in the adult vasculature and thus might serve to inhibit the response to injury.
Methods and Results
We generated a series of transgenic mice harboring potential Csrp2 regulatory regions with a lacZ reporter. We determined that the 12-kb first intron was necessary for transgene activity in adult but not developing vasculature. Within the intron we identified a 6.3-kb region that contains two CArG boxes. SRF preferentially bound to CArG2 box in gel mobility shift and chromatin immunoprecipitation assays; additionally, SRF coactivator myocardin factors activated CRP2 expression via the CArG2 box. Mutational analysis revealed that CArG2 box was important in directing lacZ expression in VSMCs of adult vessels.
Conclusions
Although CRP2 expression during development is independent of CArG box regulatory sites, CRP2 expression in adult VSMCs requires CArG2 element within the first intron. Our results suggest that distinct mechanisms regulate CRP2 expression in VSMCs that are controlled by separate embryonic and adult regulatory modules.
Keywords: CRP2, VSMC, adult element, CArG box, intron
Cysteine-rich protein (CRP) 2 is a member of the LIM-only CRP family with two tandem LIM domains.1, 2 On the basis of the prominent cytoskeletal association, a cytoarchitectural role has been proposed for CRP proteins2 and was demonstrated in mice lacking CRP3/MLP; those mice developed dilated cardiomyopathy with dramatic disruption of cardiomyocyte cytoarchitecture.3 In addition to a potential cytoarchitectural function, CRP2 also has nuclear functions by strongly enhancing serum response factor (SRF)-mediated smooth muscle (SM) gene expression.4 CRP2 is strongly expressed in rat carotids5 and mouse femoral arteries.6 Importantly CRP2 levels decrease after balloon or wire artery injury,5, 6 suggesting an important role for CRP2 in vascular remodeling. Indeed, using Csrp2 (mouse CRP2 gene symbol)-deficient mice, we demonstrated that an absence of CRP2 enhances VSMC migration and increases neointima formation following arterial injury.6
In light of the critical role of CRP2 in the response to vascular injury, we sought to elucidate the molecular mechanisms that preserve CRP2 expression in adult blood vessels. Maintaining or upregulating CRP2 expression in adult vessels might blunt VSMC migration and potentially protect against intimal thickening and restenosis. To this end, we recently determined that TGFβ induces CRP2 expression via a cAMP response promoter element.7 To identify elements that confer VSMC expression of CRP2 in vivo, we analyzed the Csrp2 promoter using promoter-lacZ reporter gene in transgenic mice. We previously showed that the 5’-flanking region between bp -573 and -550 that does not contain a CArG box (CC(A/T)6GG) is required for lacZ transgene expression in VSMCs of the developing vasculature.8 However, this embryonic module was not able to direct transgene expression in VSMCs of blood vessels in adult transgenic mice.
Given that the large intron 1 of SM marker genes SM-MHC9 and SM α-actin10 are needed for in vivo SMC expression during development and in adult, we hypothesized that the 12-kb first intron of the Csrp2 gene might harbor required elements for adult VSMC expression. The aim of this study was to identify regulatory elements that confer CRP2 expression in VSMCs of adult vasculature. Importantly, we show that Csrp2 intron 1 contains two CArG boxes and these regulatory sequences are necessary for directing gene expression in adult vasculature.
Methods
Detailed methods are described in the expanded Methods section in the data supplement.
Luciferase Reporter and Expression Plasmid Constructs
Luciferase reporter -825Int1-Csrp2-luc, pGL2P-6.3, and CArG mutant constructs were generated. Expression plasmids for myocardin factors were cloned into pFLAG-CMV vector.
Transgenic mice
Transgenic mice harboring bp -825 to +11752 of the Csrp2 gene and CArG box mutations were generated and analyzed.
Cell Culture and Transient Transfection Assays
Luciferase reporters, expression plasmids, and pCMVβ were cotransfected into VSMCs. Luciferase and β-galactosidase activities were measured after 2 days.
Electrophoretic Mobility Shift Assays (EMSA)
EMSAs were performed using VSMC nuclear extracts and oligonucleotides containing CArG1, CArG2 elements, or with CArG mutations.
Chromatin Immunoprecipitation (ChIP) Assays
ChIP assays were performed using VSMCs with Millipores's EZ-ChIP kit.
Results
Csrp2 Intron 1 Is Required for LacZ Transgene Expression in VSMCs of Adult Blood Vessels
Since CRP2 is highly expressed in adult vasculature5, 6 and functions in part to slow VSMC migration in response to injury,6 we sought to define the mechanisms that control CRP2 expression in adults. To this end, we examined lacZ expression in adult transgenic mice harboring the longest 5’ promoter (-4855Csrp2-lacZ)11 (Figure 1A). LacZ staining of major arteries from 8-week-old -4855Csrp2 mice revealed very little blue staining in vessels and in medial SMCs (Figure 1B, 1E, and 1H). Additional transgenic mice harboring 5’ deletions8 (-3513, -2663, and -795) exhibited little lacZ expression in adult vessels (data not shown). Because the large intron 1 of SM marker genes SM-MHC9 and SM α-actin10 are required for in vivo SMC expression during development and in adult, we hypothesized that the 12-kb first intron of the Csrp2 gene might harbor required elements for adult VSMC expression. To test this hypothesis, we generated -825Int1 transgenic mice harboring a -825 bp promoter that is sufficient for embryonic expression and the first intron upstream of lacZ reporter gene (Figure 1A). Compared with promoter only mice (Figure 1B, and reference [8]), two independent founder lines of 8-week-old -825Int1 mice consistently showed intense reporter activity in great arteries and in the medial SMCs but not endothelial cells (ECs) of vessels (Figure 1C, 1F, and 1I), consistent with endogenous CRP2 expression.5, 6 Arterial lacZ expressions persisted in 24-week-old mice (Figure 1D, 1G, and 1J), indicating intron 1 is important for CRP2 expression in adult vessels.
Figure 1.
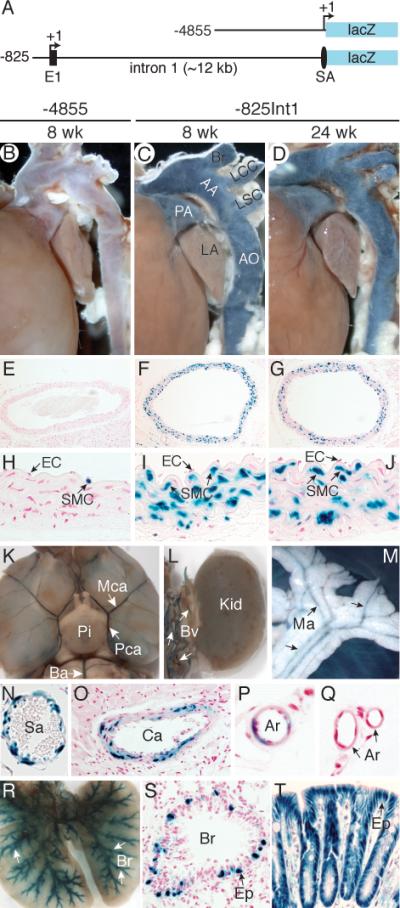
Csrp2 intron 1 is required for lacZ transgene expression in adult blood vessels. A, Diagram of transgenic constructs. SA, splice acceptor. B-D, Whole mount β-galactosidase staining (blue) of major arteries in adult transgenic mice from -4855 (B, 8 weeks), and -825Int1 at 8 weeks (C) and 24 weeks old (D). Br, brachiocephalic artery; LCC, left common carotid artery; LSC, left subclavian artery; AA, aortic arch; AO, aorta; PA, pulmonary artery; LA, left atrium. E-G, Sections of stained aortas. H-J, LacZ expression in SMC but not EC of the arteries. K-T, Staining of adult organs from -825Int1 transgenic mice. K, Adult brain: Ba, basilar artery; Mca, middle cerebral artery; Pca, posterior communicating artery; Pi, pituitary. L, Kidney and surrounding blood vessels (Bv). M, Mesenteric arteries (Ma). Blue staining in small arteries (N) and in some but not all coronary artery (O) and arterioles (P-Q). R-T, LacZ activity in bronchial epithelial cells (Ep) in the lung bronchioles (Br) and intestinal epithelial cells in the colon (T). Independent founder lines of -4855 (2 lines) and -825Int1 (2 lines) showed comparable expression patterns.
β-Galactosidase activity was also detected in other vascular beds of adult -825Int1 mice, including arteries in the brain, abdominal vessels, and mesenteric arteries (Figure 1K-1M). Histological analysis revealed blue staining in small arteries (Figure 1N), in some but not all coronary arteries (Figure 1O) and arterioles (Figure 1Q). In contrast to persistent blue staining in various size arteries in young and adult -825Int1 mice, lacZ expression in different size vessels of -4855 mice was diminished at 3-week-old and was only weakly detected in adults (≥6-8 weeks) (Figure 1B and data not shown). Staining of several other Csrp2-expressing tissues12, 13 of adult -825Int1 mice revealed strong lacZ activity in bronchial epithelial cells in the lung bronchioles and intestinal epithelial cells in the colon (Figure 1R-1T). LacZ activity was not detected in the kidney (Figure 1L), indicating that the promoter-intron fragment recapitulated the endogenous expression of Csrp2 in most tissues.
Two CArG Elements Are Present in Intron 1 of the Csrp2 Gene
To identify potential regulatory sites, we cloned and sequenced the mouse Csrp2 intron 1. We identified two consensus CArG elements, CArG1 at +5548 (CCTTATTTGG) and CArG2 at +10672 (CCTTTTAAGG) (Figure 2A-2B). Because of the central role of SRF/CArG-box interactions in mediating SMC-specific transcription,14, 15 we performed EMSAs to determine whether SRF can bind to these two CArG boxes. Incubation of mouse VSMC nuclear extracts with CArG1 probe resulted in a specific DNA-protein complex, which was competed by excess unlabeled identical probe, but not by a mutated CArG1 probe (Figure 2C). SRF but not unrelated Sp1 antibodies completely supershifted the complex (Figure 2C), indicating SRF bound to CArG1 element. A more prominent specific DNA-protein complex was observed when CArG2 probe was used, which was completely supershifted by SRF but not Sp1 antibodies (Figure 2C), demonstrating SRF bound to CArG2 box. ChIP assays revealed a low level SRF binding to CArG1 whereas SRF binding to CArG2 was substantially higher (Figure 2D, left panel). We then performed real-time quantitative PCR to quantify binding levels. Compared with 0.06±0.02% binding to CArG1, 0.31±0.04% of SRF bound to CArG2 (Figure 2D, right panel).
Figure 2.
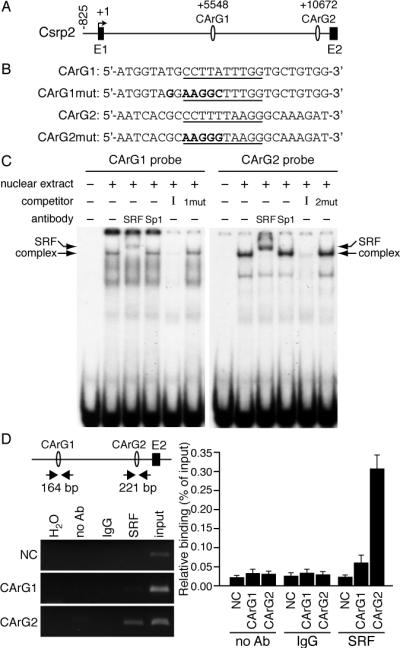
Two CArG elements are present in the Csrp2 intron 1. A, Positions of CArG1 and CArG2 in Csrp2 intron 1. B, Oligonucleotide sequences used in the gel mobility shift assays with core sequences of CArG underlined and mutated sequences in boldface type. C, Addition of VSMC nuclear extracts resulted in a retarded DNA-protein complex (complex), which was abolished by addition of identical unlabeled oligonucleotides (I) but not by mutant oligonucleotides (CArG1mut or CArG2mut). SRF but not Sp1 antibody supershifted the complex (SRF). D, ChIP assays of the Csrp2 intronic CArG boxes. Left panel, The positions of PCR primers to amplify a 164-bp CArG1 and a 221-bp CArG2 fragments are indicated. Chromatin was immunoprecipitated with no antibody, normal rabbit IgG, or SRF antibody. As an additional negative control (NC), a 270-bp fragment within the Csrp1 gene was amplified. PCR products were separated on 2% agarose gel. Right panel, Real-time quantitative PCR was performed and binding activity is expressed as percentage relative to input DNA. Values are mean±SE of 4 experiments.
To investigate the function of the CArG-containing region, we cloned the 6.3-kb fragment (bp +5479 to +11752) that contains both CArG boxes upstream of an SV40 promoter to generate pGL2P-6.3 luciferase construct and cotransfected luciferase reporters with a dominant-negative SRF (DN-SRF). In contrast to SRF-independent activity of -795 promoter,8 DN-SRF repressed 77% of pGL2P-6.3 activity (Figure 3A), indicating the 6.3-kb intronic fragment contained elements responsible for SRF-mediated activity. To determine the role of CArG boxes, we generated mutant constructs with CArG mutations within the context of pGL2P-6.3. Transfection assays revealed that mutation at CArG1 slightly decreased luciferase activity while CArG2 mutation decreased activity to 65±3% of wild-type and double mutation further reduced activity to 55±4% (Figure 3B). These data suggested that both CArG elements, particularly CArG2, contributed to the transcriptional activity of the 6.3-kb fragment. To further test the role of intron and CArG elements in concert with the native promoter, we generated a luciferase construct -825Int1-Csrp2-luc, corresponding to the -825Int1-Csrp2-lacZ transgenic construct, and constructs with CArG mutations. Even in the presence of the 5’-promoter,8 mutation of one or both CArG boxes dampened luciferase activity (Figure 3C), demonstrating the importance of intronic CArG boxes in mediating transcriptional activity of Csrp2.
Figure 3.
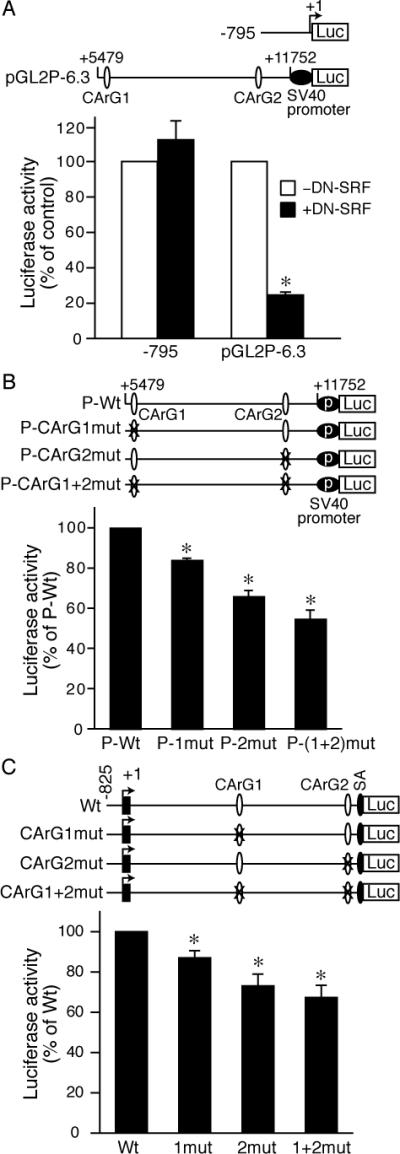
The first intron of the Csrp2 gene contains SRF/CArG-dependent activity. A, VSMCs were transiently transfected with -795Csrp2-luc or pGL2P-6.3-luc and DN-SRF or empty vector. Luciferase activity of each construct without DN-SRF was set at 100%. B, Luciferase plasmids pGL2P-6.3-luc (P-Wt) or with CArG box mutations were transfected into VSMCs. Luciferase activity of P-Wt was set at 100%. C, Luciferase reporters -825Int1-luc (Wt) or with CArG box mutations were transiently transfected into VSMCs. Luciferase activity of Wt was set at 100%. Values are mean±SE of 3-4 experiments in A-C. *P<0.05.
SRF Coactivators Upregulate Csrp2 Transcription via Intron 1 Sequences in VSMCs
As SRF mediates the activity of Csrp2 intron 1 and that SRF coactivators, which include myocardin, myocardin-related transcription factor A (MRTF-A/MKL1) and B (MRTF-B/MKL2), play important roles in regulating transcription of SRF target genes,16-19 we hypothesized that SRF coactivators may participate in the regulation of CRP2 expression. C-terminal deletion of the transactivation domain of MRTF-A (DN-A630 and DN-A723) function as dominant-negative mutants18 and are effective in repressing SRF coactivator-induced transactivation of the SM22α promoter.20 Thus, to test our hypothesis, transfection experiments were performed by cotransfecting luciferase constructs with DN-MRTF-A into VSMCs. Consistent with previous reports,20, 21 DN-MRTF-A decreased SM22α promoter activity (Figure 4A). Interestingly, the repression of -825Int1 luciferase activity by DN-MRTF-A was similar to that of SM22α promoter (Figure 4A), indicating a critical role of SRF coactivators in the regulation of CRP2 expression.
Figure 4.
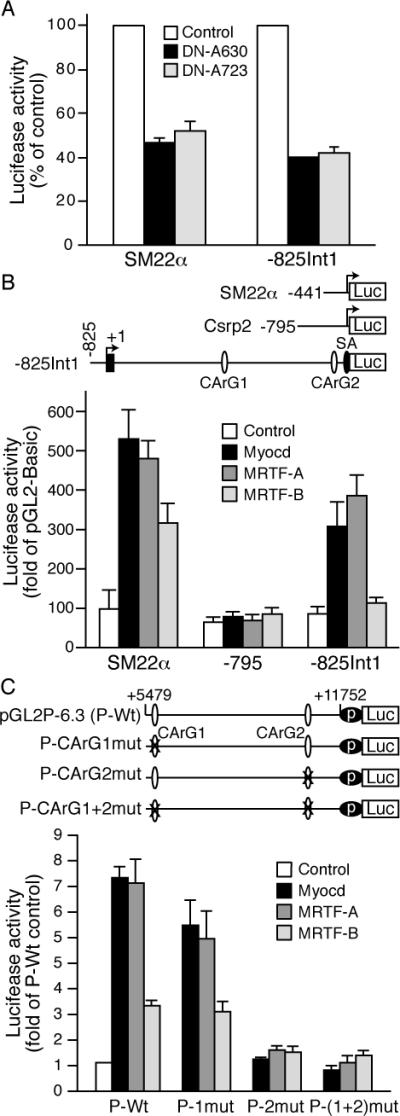
SRF coactivators contribute to Csrp2 expression via intronic sequences in VSMCs. A, VSMCs were cotransfected with luciferase reporter -441SM22α-luc or -825Int1-Csrp2-luc and dominant-negative MRTF-A expression plasmids DN-MRTF-A630 or DN-MRTF-A723. Empty expression vector was used as a control and set at 100%. B, VSMCs were cotransfected with pGL2-Basic, SM22α, -795Csrp2-luc, or -825Int1-Csrp2-luc and myocardin (Myocd), MRTF-A, MRTF-B or empty expression vector pFLAG-CMV (Control). Luciferase activity of pGL2-Basic with pFLAG-CMV was set at 1. C, VSMCs were cotransfected with Csrp2 reporters pGL2P-6.3-luc (P-Wt), or with CArG box mutations and expression plasmids as in B. Luciferase activity of P-Wt with pFLAG-CMV was set at 1. Values are mean±SE of 4 experiments in A-C.
We next sought to determine whether myocardin factors mediated Csrp2 induction via intron by transfection experiments. As reported previously17, 22 myocardin and MRTF-A activated SM22α promoter, from 98-fold to 500-fold of pGL2-Basic while MRTF-B activated to 316-fold (Figure 4B). Interestingly, myocardin factors had very little activation on -795 promoter, which was 60-fold of pGL2-Basic (Figure 4B), as its transcriptional activity is independent of SRF/CArG box.8 Importantly, myocardin and MRTF-A increased -825Int1 activity from 86-fold of pGL2-Basic to 308- and 390-fold, respectively, and MRTF-B slightly activated -825Int1 activity (Figure 4B). These data demonstrated that myocardin and MRTF-A are critical in upregulating Csrp2 transcription through intronic sequences. To further identify the elements, we cotransfected Csrp2 reporter pGL2P-6.3 and CArG mutant constructs with expression plasmids into VSMCs. Both myocardin and MRTF-A activated pGL2P-6.3 activity 7-fold while MRTF-B activated 3-fold (Figure 4C), demonstrating myocardin and MRTF-A and to a lesser degree MRTF-B upregulated Csrp2 transcription through intronic sequences. These activations were maintained with CArG1 mutation while mutation of CArG2 alone or both CArGs abolished transactivation (Figure 4C), suggesting CArG2 played a critical role in mediating the induction of Csrp2 by myocardin factors.
Intronic CArG Elements Are Important for LacZ Transgene Expression in VSMCs of Adult Vessels
To determine in vivo function of the two CArG boxes, we generated CArG1+2mut transgenic mice with both CArGs mutated (Figure 5A). LacZ staining revealed intense staining of major arteries in E18.5 -825Int1 transgenic embryos (Figure 5B). Mutation of CArGs did not affect lacZ activity in E18.5 blood vessels (Figure 5C), consistent with the notion that CArG is not required for CRP2 expression in the developing vessels.8, 11 Staining of CArG1+2mut neonates revealed strong lacZ expression in day 1 neonatal vessels (data not shown). Blue staining was maintained at 1 to 2 weeks (Figure 5D-5E). Interestingly, β-galactosidase activity was substantially reduced in 3-week-old CArG1+2mut mice (Figure 5F) while -825Int1 mice retained high lacZ activity (Figure 5G), suggesting that intronic CArGs potentially become important around weaning age. Strong lacZ expression was observed in the aorta of 12-week-old -825Int1 transgenic mice whereas only faint blue staining was detected in the aorta from 12-week-old CArG1+2mut mice (Figure 5H-5I). Compared with many positive blue nuclei in SM layers of -825Int1 mouse aorta, few blue SMCs were detected in CArG1+2mut aorta (Figure 5J-5K). To quantitate lacZ expression, we measured aortic β-galactosidase activity, which was barely detectable in non-transgenic mice (0.2±0.1 A405/mg protein/h). Robust activity was detected in -825Int1 transgenic mouse aorta while mutation of both CArG boxes substantially reduced β-galactosidase activity (30.0±0.9 vs 3.2±0.3 A405/mg protein/h) (Figure 5L), correlating with the intensity of whole mount staining results. Together, these data suggest that Csrp2 intronic CArGs are required for directing lacZ transgene expression in adult VSMCs but they are dispensable in embryonic vasculature.
Figure 5.
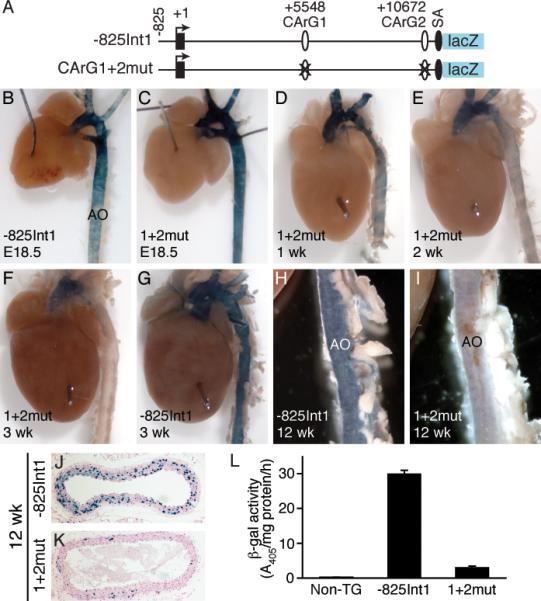
Csrp2 intronic CArGs are important for transgene expression in adult vessels. A, Transgenic constructs of -825Int1 and CArG double mutant (CArG1+2mut). B-C, β-Galactosidase staining of arteries from E18.5 transgenic embryos. AO, aorta. D-F, Staining of aortas from CArG1+2mut mice at 1-week (D), 2-week (E), and 3-week (F). G, Staining from 3-week-old -825Int1 mice. H-K, LacZ staining of aortas from 12-week-old -825Int1 (H, J) and CArG1+2mut (I, K) mice. L, Quantitative β-galactosidase activity from aortas of non-transgenic (Non-TG), -825Int1, and CArG1+2mut transgenic mice. Values are mean±SE of three experiments. Data are representative for 2 independent lines of -825Int1 and one line of CArG1+2mut mice.
CArG2 Is Important for Csrp2 Expression in Adult Blood Vessels
In light of the importance of intronic CArGs for VSMC expression in adult vessels (Figure 5), we sought to determine whether a single CArG or both CArGs are required. To this end, we generated transgenic mice harboring CArG1 or CArG2 mutation (Figure 6A) and then assessed lacZ transgene expression. Mutation of CArG1 retained lacZ activity in the arteries of 12-week-old mice (Figure 6B) whereas 2 independent lines of CArG2 mutation showed substantially diminished lacZ staining in the blood vessels (Figure 6C). Histological analysis revealed many blue SMCs from CArG1 mutant mice (Figure 6D) while few blue cells were detected in medial layer of CArG2 mutant mice (Figure 6E). Together, these data suggest that while CArG1 is dispensable, CArG2 is important for CRP2 expression in VSMCs of adult vasculature.
Figure 6.
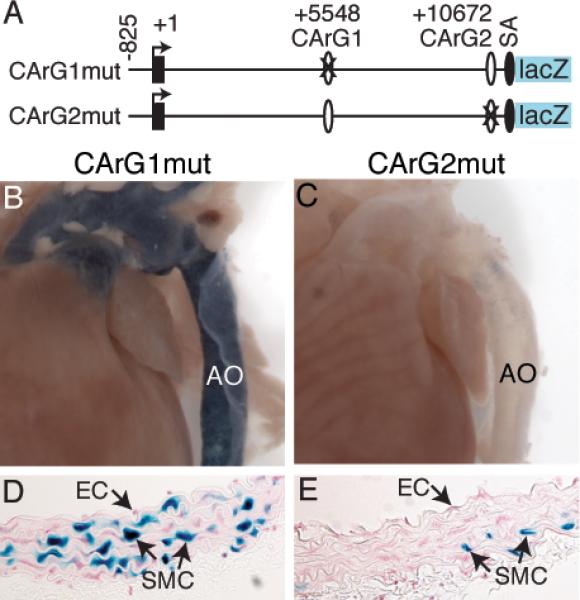
CArG2 of Csrp2 intron 1 is essential for lacZ transgene expression in adult blood vessels. A, Transgenic constructs with CArG1 and CArG2 mutations. B-C, LacZ staining of aortas (AO) from 12-week-old CArG1mut (B) and CArG2mut (C) transgenic mice. D-E, LacZ expression in SMC but not EC of the arteries. Expression patterns are representative for one line of CArG1mut and two lines CArG2mut mice.
Discussion
Using Csrp2-deficient mice we have determined a critical role of CRP2 in the response to vascular injury.6 The goal of the present study was to elucidate the molecular mechanisms that preserve CRP2 expression in the adult vasculature and thus might serve to inhibit the response to injury.
We have shown that the 5’-flanking Csrp2 promoter is sufficient to direct reporter gene expression in the developing vasculature and that the promoter activity is not dependent on CArG box or SRF.8 Of interest, this SRF-CArG independent regulation was only found in a few SMC-selective genes including aortic carboxypeptidase-like protein (ACLP)23 and focal adhesion kinase related non-kinase (FRNK).24 Intriguingly, sequences of a 13-bp fragment (GAAACCCGAAGCC) within the Csrp2 embryonic module were also found in the proximal promoter of ACLP, but not FRNK (GenBank accession # NC_000081). Whether these sequences control ACLP expression in embryos remains to be determined. However, unlike ACLP and FRNK, we found that lacZ expression was substantially reduced in VSMCs of adult vessels in all Csrp2-promoter transgenic mice we previously generated8 (Figure 1 and data not shown), suggesting that in addition to an embryonic control module a separate adult regulatory module(s) control CRP2 expression in VSMCs of adult vessels. Indeed, transgenic studies suggest CArGs in the first intron of Csrp2 are the potential adult VSMC regulatory elements for CRP2 expression (Figure 5). However, it is unknown whether the CArG elements are functioning or not at E18.5 because the proximal promoter of Csrp2 is sufficient to direct reporter gene expression in embryonic vessels.8 The regulation of Csrp2 expression in VSMCs by separate embryonic and adult regulatory modules represents a previously unrecognized distinct mechanism for VSMC gene expression. Interestingly, this mode of regulation is similar to the EC-specific gene TIE2.25 The 5’-flanking region of the TIE2 promoter is capable of directing transgene expression to vascular ECs of transgenic mouse embryos whereas a first intron fragment is required for transgene expression in adulthood.25
The cis-acting element CArG box mediates expression of many SMC marker genes. These SMC-selective CArG boxes are present in 5’ promoters as for SM22α and telokin,26, 27 in the first intron as for CRP1,28 or in both 5’ promoters and first introns as for SM α-actin and SM-MHC.9, 10, 29 These CArG boxes are needed for SMC expression both during embryonic development and in adults. In this regard, the transcriptional regulation of Csrp2 differs from these SMC genes.
Many SM-specific genes contain at least two or more CArG boxes in their regulatory regions and function in concert to control SMC-selective expression.10, 30, 31 Interestingly, there are two CArG boxes in the first intron of Csrp2 and both CArGs contributed to transcriptional activity in vitro (Figure 3). The findings that SRF/CArG regulated Csrp2 transcription are consistent with a previous report that Csrp2 is an SRF target gene.32 Mutational analysis in transgenic mice (2 lines of CArG2mut and 1 line of CArG1mut) indicated that CArG2 is important for CRP2 expression in adult vessels (Figure 6). Supporting a major functional role for CArG2 instead of CArG1, CArG2 seemed to form a more prominent complex with SRF in EMSAs and SRF preferentially bound to CArG2 in the intact chromatin in ChIP assays (Figure 2C-2D). In line with a critical role of CArG2 in regulating Csrp2 transcription in adult mice, transfection experiments demonstrated that SRF cofactor myocardin and MRTF-A significantly transactivated Csrp2 expression through the CArG2 element (Figure 4). Further supporting the preferential utilization of one CArG box in Csrp2 gene regulation, a recent study demonstrated that there is a strong bias for myocardin-dependent transactivation through CArG2 of the CArG box-rich human ACTG2 promoter.33 Of interest, there are two degenerate CArGs that exhibit reduced SRF binding affinity in the SM α-actin promoter10 and the degeneracy of the 5′ CArGs is critical for injury-induced SM α-actin downregulation.34 Although CArG1 in intron 1 of the Csrp2 gene is not required for CRP2 expression under basal conditions, whether CArG1 plays a role in CRP2 expression in response to vascular injury remains to be determined.
Intriguingly, while there appears to be a bias for CArG elements to reside within 4 kb of the transcription start site,15 the CArG2 of Csrp2 is located 10 kb away from the transcription start site at +10672. Whether these differences are involved in the unique functional regulation of Csrp2 gene transcription requires further investigation. Importantly, although CArG1 does not appear to be conserved across several species examined, CArG2 (CCTTTTAAGG) is highly conserved in CSRP2 gene of human at +13949, cow at +12861, and rhesus monkey (with a smaller gene size of 8.1 kb) at +1238. A CArG2-like box with one base divergence (CCTTTTATGG) is located at +3815 of the 7.7 kb chicken CSRP2 gene whereas a CArG-like box with one base divergent (CCTTTTATGC) from chicken CArG is located at +8789 of the rat Csrp2 gene. Interestingly, we found TCF (ternary complex factor) site (GGAA) in the flanking sequences of CArG2 in CSRP2 gene in the species examined. TCFs have been shown to modulate SMC gene expression via binding to TCF site adjacent to CArG box.35 Whether TCF site and CArG2 coordinately regulate Csrp2 transcription requires further investigation.
Csrp1 and Csrp2 are CRP family members with highly similar gene structure11, 36 and are both expressed in VSMCs.5, 13 However, their mechanisms of expression appear to differ. An intronic CArG box is essential for CRP1 expression in both embryonic and adult vessels28 while CArG2 box is required for CRP2 expression in adult vasculature (Figure 5 and 6). Furthermore, although Csrp1 is an SRF target gene, an expression profiling study identified Csrp1 to be an MRTF-A-independent gene.37 In contrast, MRTF-A upregulated CRP2 expression (Figure 4).
In this study, although only 1-2 independent founder lines of each transgenic construct were analyzed a total of 6 novel transgenic founder mouse lines were studied (supplemental Table 1 and supplemental Figure 1). Moreover, the transgenic results were consistent with the transfection and chromatin immuoprecipitation results. In conclusion, although CRP2 expression during development is independent of CArG box its expression in adult VSMCs requires CArG2 within intron 1 and might be mediated by myocardin factors. Our results suggest that separate embryonic and adult regulatory modules might control CRP2 expression, which represents a unique mechanism in controlling VSMC gene expression.
Supplementary Material
Sources of Funding
This work was supported in part by National Health Research Institutes (Taiwan) Grant CS-098-PP-06 (to S.-F.Y.), National Science Council (Taiwan) Grants 96-2321-B-400-004-MY2 and 98-2320-B-400-004-MY3 (to S.-F.Y.), and National Institutes of Health Grant HL-078869 (to M.D.L.).
Footnotes
Disclosures
None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weiskirchen R, Pino JD, Macalma T, Bister K, Beckerle MC. The cysteine-rich protein family of highly related LIM domain proteins. J Biol Chem. 1995;270:28946–28954. doi: 10.1074/jbc.270.48.28946. [DOI] [PubMed] [Google Scholar]
- 2.Louis HA, Pino JD, Schmeichel KL, Pomies P, Beckerle MC. Comparison of three members of the cysteine-rich protein family reveals functional conservation and divergent patterns of gene expression. J Biol Chem. 1997;272:27484–27491. doi: 10.1074/jbc.272.43.27484. [DOI] [PubMed] [Google Scholar]
- 3.Arber S, Hunter JJ, Ross J, Jr., Hongo M, Sansig G, Borg J, Perriard JC, Chien KR, Caroni P. MLP-deficient mice exhibit a disruption of cardiac cytoarchitectural organization, dilated cardiomyopathy, and heart failure. Cell. 1997;88:393–403. doi: 10.1016/s0092-8674(00)81878-4. [DOI] [PubMed] [Google Scholar]
- 4.Chang DF, Belaguli NS, Iyer D, Roberts WB, Wu SP, Dong XR, Marx JG, Moore MS, Beckerle MC, Majesky MW, Schwartz RJ. Cysteine-rich LIM-only proteins CRP1 and CRP2 are potent smooth muscle differentiation cofactors. Dev Cell. 2003;4:107–118. doi: 10.1016/s1534-5807(02)00396-9. [DOI] [PubMed] [Google Scholar]
- 5.Jain MK, Fujita KP, Hsieh C-M, Endege WO, Sibinga NE, Yet S-F, Kashiki S, Lee W-S, Perrella MA, Haber E, Lee M-E. Molecular cloning and characterization of SmLIM, a developmentally regulated LIM protein preferentially expressed in aortic smooth muscle cells. J Biol Chem. 1996;271:10194–10199. doi: 10.1074/jbc.271.17.10194. [DOI] [PubMed] [Google Scholar]
- 6.Wei J, Gorman TE, Liu X, Ith B, Tseng A, Chen Z, Simon DI, Layne MD, Yet S-F. Increased neointima formation in cysteine-rich protein 2-deficient mice in response to vascular injury. Circ Res. 2005;97:1323–1331. doi: 10.1161/01.RES.0000194331.76925.5c. [DOI] [PubMed] [Google Scholar]
- 7.Lin D-W, Chang I-C, Tseng A, Wu ML, Chen C-H, Patenaude CA, Layne MD, Yet S-F. Transforming growth factor β up-regulates cysteine-rich protein 2 in vascular smooth muscle cells via activating transcription factor 2. J Biol Chem. 2008;283:15003–15014. doi: 10.1074/jbc.M801621200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang Y-F, Wei J, Liu X, Chen Y-H, Layne MD, Yet S-F. Identification of a CArG-independent region of the cysteine-rich protein 2 promoter that directs expression in the developing vasculature. Am J Physiol Heart Circ Physiol. 2003;285:H1675–1683. doi: 10.1152/ajpheart.00165.2003. [DOI] [PubMed] [Google Scholar]
- 9.Madsen CS, Regan CP, Hungerford JE, White SL, Manabe I, Owens GK. Smooth muscle-specific expression of the smooth muscle myosin heavy chain gene in transgenic mice requires 5'-flanking and first intronic DNA sequence. Circ Res. 1998;82:908–917. doi: 10.1161/01.res.82.8.908. [DOI] [PubMed] [Google Scholar]
- 10.Mack CP, Owens GK. Regulation of smooth muscle α-actin expression in vivo is dependent on CArG elements within the 5' and first intron promoter regions. Circ Res. 1999;84:852–861. doi: 10.1161/01.res.84.7.852. [DOI] [PubMed] [Google Scholar]
- 11.Yet S-F, Folta SC, Jain MK, Hsieh C-M, Maemura K, Layne MD, Zhang D, Marria PB, Yoshizumi M, Chin MT, Perrella MA, Lee M-E. Molecular cloning, characterization, and promoter analysis of the mouse Crp2/SmLim gene: Preferential expression of its promoter in the vascular smooth muscle cells of transgenic mice. J Biol Chem. 1998;273:10530–10537. doi: 10.1074/jbc.273.17.10530. [DOI] [PubMed] [Google Scholar]
- 12.Okano I, Yamamoto T, Kaji A, Kimura T, Mizuno K, Nakamura T. Cloning of CRP2, a novel member of the cysteine-rich protein family with two repeats of an unusual LIM/double zinc-finger motif. FEBS Letters. 1993;333:51–55. doi: 10.1016/0014-5793(93)80373-3. [DOI] [PubMed] [Google Scholar]
- 13.Henderson JR, Brown D, Richardson JA, Olson EN, Beckerle MC. Expression of the gene encoding the LIM protein CRP2: a developmental profile. J Histochem Cytochem. 2002;50:107–111. doi: 10.1177/002215540205000112. [DOI] [PubMed] [Google Scholar]
- 14.Yoshida T, Owens GK. Molecular determinants of vascular smooth muscle cell diversity. Circ Res. 2005;96:280–291. doi: 10.1161/01.RES.0000155951.62152.2e. [DOI] [PubMed] [Google Scholar]
- 15.Sun Q, Chen G, Streb JW, Long X, Yang Y, Stoeckert CJ, Jr., Miano JM. Defining the mammalian CArGome. Genome Res. 2006;16:197–207. doi: 10.1101/gr.4108706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang D, Chang PS, Wang Z, Sutherland L, Richardson JA, Small E, Krieg PA, Olson EN. Activation of cardiac gene expression by myocardin, a transcriptional cofactor for serum response factor. Cell. 2001;105:851–862. doi: 10.1016/s0092-8674(01)00404-4. [DOI] [PubMed] [Google Scholar]
- 17.Wang DZ, Li S, Hockemeyer D, Sutherland L, Wang Z, Schratt G, Richardson JA, Nordheim A, Olson EN. Potentiation of serum response factor activity by a family of myocardin-related transcription factors. Proc Natl Acad Sci U S A. 2002;99:14855–14860. doi: 10.1073/pnas.222561499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cen B, Selvaraj A, Burgess RC, Hitzler JK, Ma Z, Morris SW, Prywes R. Megakaryoblastic leukemia 1, a potent transcriptional coactivator for serum response factor (SRF), is required for serum induction of SRF target genes. Mol Cell Biol. 2003;23:6597–6608. doi: 10.1128/MCB.23.18.6597-6608.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li S, Wang DZ, Wang Z, Richardson JA, Olson EN. The serum response factor coactivator myocardin is required for vascular smooth muscle development. Proc Natl Acad Sci U S A. 2003;100:9366–9370. doi: 10.1073/pnas.1233635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Du KL, Chen M, Li J, Lepore JJ, Mericko P, Parmacek MS. Megakaryoblastic leukemia factor-1 transduces cytoskeletal signals and induces smooth muscle cell differentiation from undifferentiated embryonic stem cells. J Biol Chem. 2004;279:17578–17586. doi: 10.1074/jbc.M400961200. [DOI] [PubMed] [Google Scholar]
- 21.Hinson JS, Medlin MD, Lockman K, Taylor JM, Mack CP. Smooth muscle cell-specific transcription is regulated by nuclear localization of the myocardin-related transcription factors. Am J Physiol Heart Circ Physiol. 2007;292:H1170–1180. doi: 10.1152/ajpheart.00864.2006. [DOI] [PubMed] [Google Scholar]
- 22.Chen J, Kitchen CM, Streb JW, Miano JM. Myocardin: a component of a molecular switch for smooth muscle differentiation. J Mol Cell Cardiol. 2002;34:1345–1356. doi: 10.1006/jmcc.2002.2086. [DOI] [PubMed] [Google Scholar]
- 23.Layne MD, Yet S-F, Maemura K, Hsieh C-M, Liu X, Ith B, Lee M-E, Perrella MA. Characterization of the mouse aortic carboxypeptidase-like protein promoter reveals activity in differentiated and dedifferentiated vascular smooth muscle cells. Circ Res. 2002;90:728–736. doi: 10.1161/01.res.0000013289.97650.c8. [DOI] [PubMed] [Google Scholar]
- 24.Sayers RL, Sundberg-Smith LJ, Rojas M, Hayasaka H, Parsons JT, Mack CP, Taylor JM. FRNK expression promotes smooth muscle cell maturation during vascular development and after vascular injury. Arterioscler Thromb Vasc Biol. 2008;28:2115–2122. doi: 10.1161/ATVBAHA.108.175455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schlaeger TM, Bartunkova S, Lawitts JA, Teichmann G, Risau W, Deutsch U, Sato TN. Uniform vascular-endothelial-cell-specific gene expression in both embryonic and adult transgenic mice. Proc Natl Acad Sci U S A. 1997;94:3058–3063. doi: 10.1073/pnas.94.7.3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li L, Miano JM, Mercer B, Olson EN. Expression of the SM22α promoter in transgenic mice provides evidence for distinct transcriptional regulatory programs in vascular and visceral smooth muscle cells. J Cell Biol. 1996;132:849–859. doi: 10.1083/jcb.132.5.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith AF, Bigsby RM, Word RA, Herring BP. A 310-bp minimal promoter mediates smooth muscle cell-specific expression of telokin. Am J Physiol. 1998;274:C1188–1195. doi: 10.1152/ajpcell.1998.274.5.C1188. [DOI] [PubMed] [Google Scholar]
- 28.Lilly B, Olson EN, Beckerle MC. Identification of a CArG box-dependent enhancer within the cysteine-rich protein 1 gene that directs expression in arterial but not venous or visceral smooth muscle cells. Dev Biol. 2001;240:531–547. doi: 10.1006/dbio.2001.0507. [DOI] [PubMed] [Google Scholar]
- 29.Manabe I, Owens GK. The smooth muscle myosin heavy chain gene exhibits smooth muscle subtype selective modular regulation in vivo. J Biol Chem. 2001;276:39076–39087. doi: 10.1074/jbc.M105402200. [DOI] [PubMed] [Google Scholar]
- 30.Miano JM, Carlson MJ, Spencer JA, Misra RP. Serum response factor-dependent regulation of the smooth muscle calponin gene. J Biol Chem. 2000;275:9814–9822. doi: 10.1074/jbc.275.13.9814. [DOI] [PubMed] [Google Scholar]
- 31.Manabe I, Owens GK. CArG elements control smooth muscle subtype-specific expression of smooth muscle myosin in vivo. J Clin Invest. 2001;107:823–834. doi: 10.1172/JCI11385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balza RO, Jr., Misra RP. Role of the serum response factor in regulating contractile apparatus gene expression and sarcomeric integrity in cardiomyocytes. J Biol Chem. 2006;281:6498–6510. doi: 10.1074/jbc.M509487200. [DOI] [PubMed] [Google Scholar]
- 33.Sun Q, Taurin S, Sethakorn N, Long X, Imamura M, Wang DZ, Zimmer WE, Dulin NO, Miano JM. Myocardin-dependent activation of the CArG box-rich smooth muscle γ-actin gene: preferential utilization of a single CArG element through functional association with the NKX3.1 homeodomain protein. J Biol Chem. 2009;284:32582–32590. doi: 10.1074/jbc.M109.033910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hendrix JA, Wamhoff BR, McDonald OG, Sinha S, Yoshida T, Owens GK. 5' CArG degeneracy in smooth muscle α-actin is required for injury-induced gene suppression in vivo. J Clin Invest. 2005;115:418–427. doi: 10.1172/JCI22648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Z, Wang DZ, Hockemeyer D, McAnally J, Nordheim A, Olson EN. Myocardin and ternary complex factors compete for SRF to control smooth muscle gene expression. Nature. 2004;428:185–189. doi: 10.1038/nature02382. [DOI] [PubMed] [Google Scholar]
- 36.Weiskirchen R, Erdel M, Utermann G, Bister K. Cloning, structural analysis, and chromosomal localization of the human CSRP2 gene encoding the LIM domain protein CRP2. Genomics. 1997;44:83–93. doi: 10.1006/geno.1997.4855. [DOI] [PubMed] [Google Scholar]
- 37.Selvaraj A, Prywes R. Expression profiling of serum inducible genes identifies a subset of SRF target genes that are MKL dependent. BMC Mol Biol. 2004;5:13. doi: 10.1186/1471-2199-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


