Abstract
Objective
To examine the impact of low density lipoprotein (LDL), an established mediator of atherosclerosis, on the transcription factor, cAMP Response Element Binding Protein (CREB), a regulator of vascular smooth muscle cell (VSMC) quiescence.
Methods and Results
VSMC CREB content is diminished in rodent models of diabetes and pulmonary hypertension. We examined aortic CREB content in rodent models of aging, hypertension and insulin resistance, and determined nuclear CREB protein in the medial VSMC of high fat-fed LDL receptor null mice. There was significant loss of CREB protein in all models. In vitro, primary culture rat aortic VSMC exposed to LDL and oxidized LDL (oxLDL) exhibited a rapid, transient increase in CREB phosphorylation and transient phosphorylation/activation of Akt and P38-, ERK- and JNK-MAPK. Exposure to oxLDL, but not LDL, for 24 – 48 h decreased CREB protein in a dose-dependent fashion and led to nuclear exclusion of CREB. Pharmacological reactive oxygen species (ROS) scavengers and inhibition of ERK activation blocked oxLDL-mediated CREB downregulation.
Conclusion
These data support a model wherein loss of VSMC CREB protein, which renders these cells more susceptible to activation and apoptosis, is a common pathological response to vascular injury and potentially contributes to plaque progression.
Atherosclerosis is the leading cause of death in the Western world [http://www.cdc.gov/nchs-National]. In the past few decades much has been learned about cardiovascular risk factors and their impact on the development of atherosclerotic plaque. The current theoretical model of atherogenesis includes a complex interplay between endothelial cells (EC), vascular smooth muscle cells (VSMC), circulating inflammatory cells and the vascular adventitia 1, 2. The end result is a proinflammatory microenvironment that promotes a vicious cycle of plaque progression. Interruption of this cycle is necessary to slow atherosclerosis progression and stabilize existing plaque. Medications that lower low density lipoprotein (LDL) cholesterol have revolutionized therapy for cardiovascular disease (CVD) but do not fully normalize CV risk 3.
Our laboratory has identified the transcription factor, cAMP Response Element Binding protein (CREB), as a modulator of smooth muscle cell phenotype. CREB blunts mitogen stimulated VSMC proliferation, migration, matrix protein expression and protects smooth muscle cells from apoptosis 4–6. In previous reports, we observed decreased levels of CREB protein and the active form of CREB, (phosphoserine 133 CREB, PCREB), in medial VSMC in rodent models of insulin-resistant and insulin-deficient diabetes 6. Loss of VSMC CREB protein in this model was, in part, secondary to oxidant stress and could be modeled in vitro by exposure of aortic VSMC to H2O2 or glucose oxidase 6. Similarly, in a model of pulmonary vascular injury, hypoxia-induced pulmonary hypertension, we reported loss of CREB function concurrent with pulmonary artery hypertrophy 5. This could be modeled in vitro by exposing pulmonary VSMC to platelet derived growth factor (PDGF) which induced CREB nuclear export and degradation by a pathway downstream of Akt and casein kinase 2 (CK2).
In this manuscript we report on the response of VSMC in vivo, to rodent models of traditional cardiovascular risk factors such as hyperlipidemia, hypertension and obesity, and in vitro in the context of hyperlipidemia. We present data demonstrating loss of aortic CREB content across rodent models of elevated cardiovascular risk factors. For example, in an established model of atherosclerosis, LDL receptor null (LDLR −/−) mice exposed to high fat diet, we observe a significant loss of CREB protein in the vascular media. These observations suggest that loss of CREB content and function is a common, pathogenic VSMC response to cardiovascular risk factors. In additional studies in vitro, 24–48 hour exposure of VSMC to LDL and oxLDL revealed CREB downregulation by only oxLDL through a mechanism involving generation of ROS and ERK activation, but independent of Akt, leading to nuclear exclusion. This suggests that CREB downregulation in VSMC may be induced by several mechanisms, including generation of ROS, that are likely to be important for the consistent loss of CREB observed in rodent models of elevated CVD risk.
Methods
All materials and methods employed standard techniques and commercially available reagents. Animal studies were conducted under AAALAC guidelines and were approved by individual Institutional Animal Care and Use Committees. Methods and statistical analysis were done as previously reported and are outlined in detail in the supplemental material and methods file.
Results
Loss of CREB protein in aging and hypertension
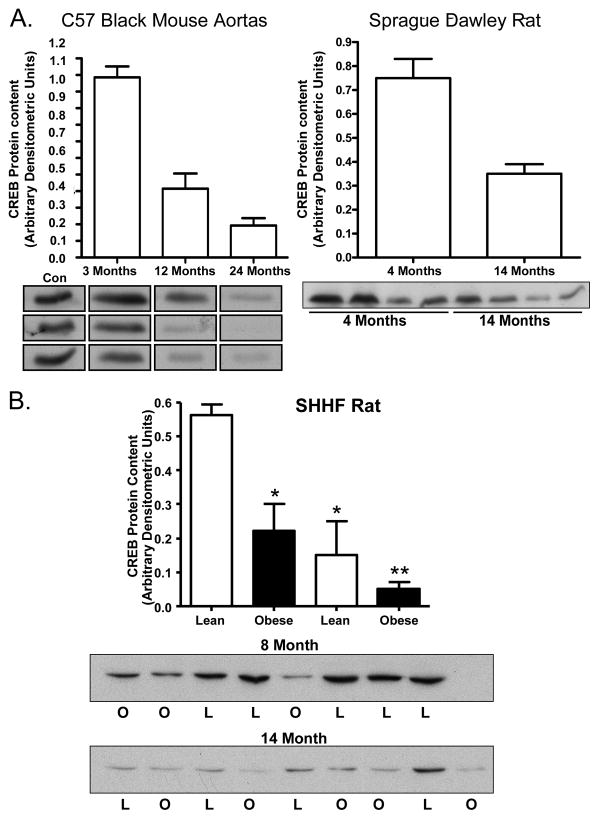
We previously reported decreased vascular CREB protein content in animal models of diabetes and pulmonary hypertension 5, 6. We queried whether loss of vascular CREB was also observed across the spectrum of other conditions known to induce vascular pathology including aging, hypertension, and dyslipidemia. Figure 1 demonstrates significantly decreased CREB content in aortic lysates with aging in C57/B6 mice and Sprague-Dawley rats (Figure 1A). We next examined the spontaneously hypertensive heart failure (SHHF) lean and obese rat. This rat model has a hypertension phenotype in the lean line and hypertension, insulin resistance, obesity and glucose intolerance in the obese line. We observed a significant age-dependent loss of CREB protein which was more pronounced in the obese rats (Figure 1B) as observed in the insulin-resistant Zucker rat 6.
Figure 1.
CREB protein content in aortic tissue extracts from rodent models of aging, obesity, and hypertension: (A) C57/B6 mice at 3,12 and 24 months of age (p< 0.001; n= 5–7) and Sprague-Dawley rats at 4 and 14 months of age (p< 0.001; n=6). (B) SHHF rats (lean and obese strains) at 8 and 14 months of age. * P<0.001 vs. young lean animals; **P=0.013 vs. young obese animals. Data are mean +/− SEM with n=5–8 animals per group.
CREB content is decreased in the vasculature of high-fat fed LDL receptor knockout mice
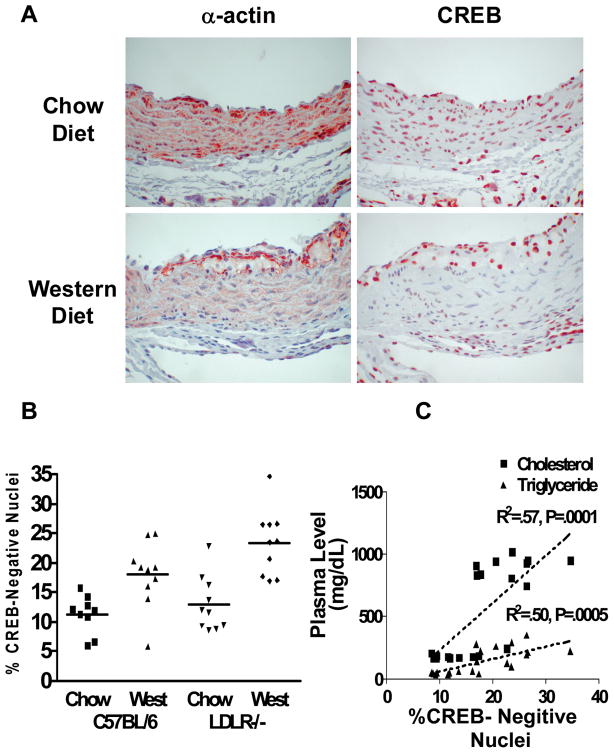
One of the best established models of rodent atherosclerosis is the LDL receptor null (LDLR−/−) mouse which develops a human pattern dyslipidemia on a Western diet. Aortic root sections were examined from 4 groups of animals (other results from this set of studies reported in 7): a) C57BL/6 mice fed a chow diet, b) C57BL/6 mice fed the high fat, Western diet, c) genetically-hypercholesterolemic, LDLR−/− mice fed a chow diet and d) LDLR−/− mice fed a Western diet. Specimens were analyzed from 10 animals in each group. The data are presented as representative photos (Figure 2A) and graphically as percent CREB-negative nuclei (Figure 2B). In the chow-fed animals there was CREB staining in the majority of the nuclei of the medial VSMCs in the aorta in both control and LDLR−/− mice. The percentage of CREB-negative nuclei increased significantly in response to high fat feeding in both control and LDLR−/− mice (Figure 2A and 2B). Significant cholesterol elevation was only observed in the LDLR−/− mice 7. In the LDLR −/− mice, cholesterol levels were 5-fold higher in the Western diet group than in the chow group, and lipids correlated significantly with loss of nuclear CREB staining (Figure 2C). These data suggest that both high fat diet and elevated cholesterol contribute to decreased nuclear CREB expression in aortic VSMC.
Figure 2.
Immunohistochemical analysis of aortic VSMC CREB content: (A) representative micrographs of α-actin and CREB staining (red stain) in aortic sections from LDLR−/− mice on a chow diet or a high fat “Western” diet. (B) quantification of nuclear CREB content in C57BL/6 and LDLR−/− mice after 10 weeks of Western diet. (C) correlation of %CREB-negative nuclei with serum cholesterol in LDLR−/− mice (p<0.005) and C57BL/6 mice (NS).
Oxidized LDL induces a time- and dose-dependent downregulation of nuclear CREB protein
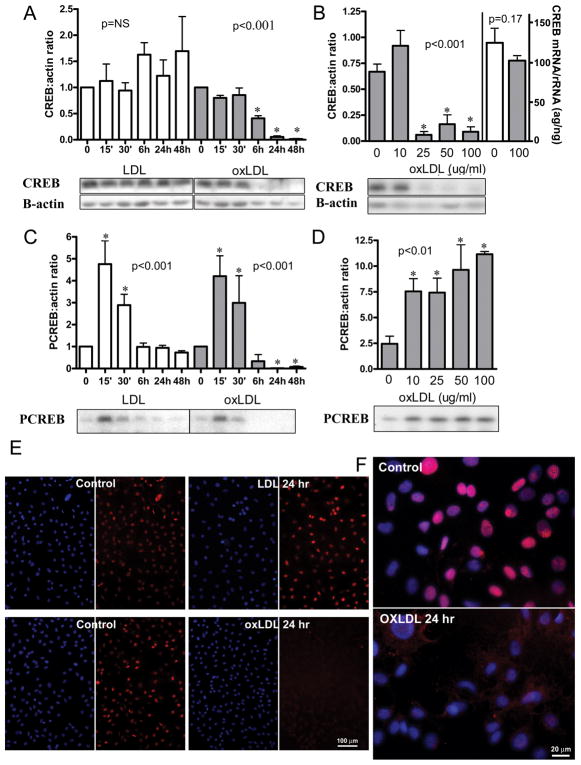
Previous work from our group indicates that oxidative stress is a potent stimulus for CREB downregulation 6, 8, 9. Published reports using LDL and modified LDL suggest that both directly increase VSMC oxidative stress 10. We explored the effects of both LDL and oxLDL on VSMC CREB expression in vitro. Rat aortic VSMC were serum-depleted for 48 hours and then exposed to either LDL or oxLDL for 0–48 hours. We observed a significant reduction in CREB protein level within 24–48 hours of exposure to oxLDL, whereas native LDL did not affect CREB protein content (Figure 3A). Downregulation of CREB was dose-dependent, occurring at oxLDL doses of 25 μg/ml or higher after 24 hours of exposure (Figure 3B) but occurred as early as 6 hours with higher oxLDL concentrations (data not shown). There was no significant change in CREB RNA (Figure 3B). Exposure of cells to oxLDL led to exclusion of CREB from the nucleus at 24 hours, whereas LDL-treated cells had preserved nuclear CREB content (figure 3E, 3F). Nuclear condensation was also observed at 24 hours with oxLDL only (data not shown).
Figure 3.
Effect of LDL and oxLDL on CREB content, activation, and mRNA in primary culture rat VSMC: CREB and PCREB protein were quantified by densitometric analysis of Western blots. CREB mRNA was quantified by Quantitative RT PCR and normalized to 18S rRNA. (A) Time course for CREB content with exposure to 100μg/ml LDL or oxLDL. (Error bars represent SD from two separate experiments with total n=4, except for duplicate samples at 6H; LDL: NS; oxLDL: p value for trend <0.001, *p<0.05 vs. 0 time). (B) dose response for CREB protein (first 5 bars) and CREB mRNA (last 2 bars) content at 24 hours of exposure to oxLDL (Error bars represent SD from two separate experiments with total n=3–5 for protein levels and n=5 for mRNA levels; p value for protein trend <0.001, *p<0.05 vs. 0 μg/ml; p value for mRNA difference = 0.17); (C) time course for CREB activation with 100μg/ml LDL or ox LDL (error bars and sample number as in A; ox LDL and LDL: p value for trends <0.001, *p<0.05 vs. 0 time); (D) dose response for CREB activation at 15 minutes of exposure to oxLDL (error bars and sample number as in B; p value for trend <0.01, *p<0.05 vs. 0 μg/ml); (E and F): CREB localization after 24 hour exposure to LDL or oxLDL. Cy3 (red)=CREB; DAPI (blue) =nuclei. Representative western blots are shown. Results shown are representative of >10 separate experiments. Values are mean ± SD, P values for trends are by one way ANOVA, p values for comparison to control are by ANOVA with Dunnett’s post test.
LDL and OxLDL activate CREB
Reports from our group and others have demonstrated that an acute burst of oxidant stress activates numerous signaling pathways that activate CREB 4, 6, 11. We recently reported that free fatty acids acutely activate CREB via a PKC dependent, ROS/ERK independent pathway 12. Here we examined CREB activation (PCREB) by LDL. In contrast to the differential effects of LDL and oxLDL on CREB content, both LDL and oxLDL led to an acute, transient activation of CREB (Figure 3C). CREB activation is dose-dependent, and doses as low as 10 μg/ml of both LDL and oxLDL significantly and transiently increase CREB phosphorylation as early as 15 minutes (figure 3D). Return of PCREB levels to normal after oxLDL treatment was somewhat variable and dependent on cell growth conditions and oxLDL potency (data not shown). CREB activation also correlates with induction of the known CREB-dependent gene, hemeoxygenase-1 (HO-1) (Figure 5).
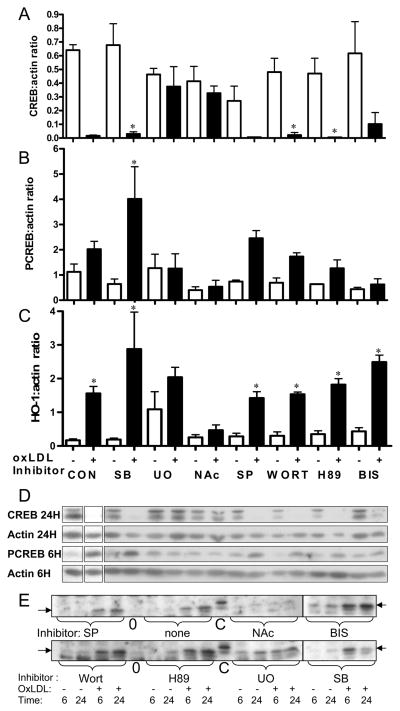
Figure 5.
Involvement of signaling pathways in the effects of oxLDL on primary culture rat VSMC. Cells were pretreated for 30 minutes with or without (CON) inhibitors of signaling pathways: SB-203580 (SB, 20 μM, p38 MAPK), SP-600125 (SP, 20μM, JNK MAPK), U-0126 (U0, 10 μM, ERK MAPK), Wortmannin (WORT, 100nM, PI3K), H89 (10 μM, PKA), Bisindolyl maleimide (BIS, 4μM, PKC), N-acetyl cysteine (NAC, 30mM, ROS), and then incubated with (+) or without (−) oxLDL (40 ug/ml) as indicated. CREB at 24 hours (A), PCREB at 6 hours (B) and HO-1 at 6 hours (C) were quantified and normalized to β-actin by densitometric analysis of Western blots. (D) Representative western blots are shown for CREB, PCREB, and β-actin at 6 and 24 hours. Individual portions of western blots were from two gels from a single experiment. A control extract was loaded on all gels to allow gel to gel comparison, and blots of comparable exposure are shown. (E) Representative western blots are shown for HO-1. Blot portions shown were from the same experiment, but multiple gels as in D. Note that inhibitor order is different from that shown in the graphs. Graphs represent data combined from three experiments, each with duplicate or triplicate independent samples. Because some experiments did not include all inhibitors, 3–7 independent samples are represented in each data point. Values are mean ± SD; p values for comparison to corresponding control are by ANOVA with Bonferroni correction for multiple comparisons. * p<0.05 vs. corresponding no oxLDL control.
ROS and ERK1/2 MAPK contribute to CREB downregulation by oxLDL
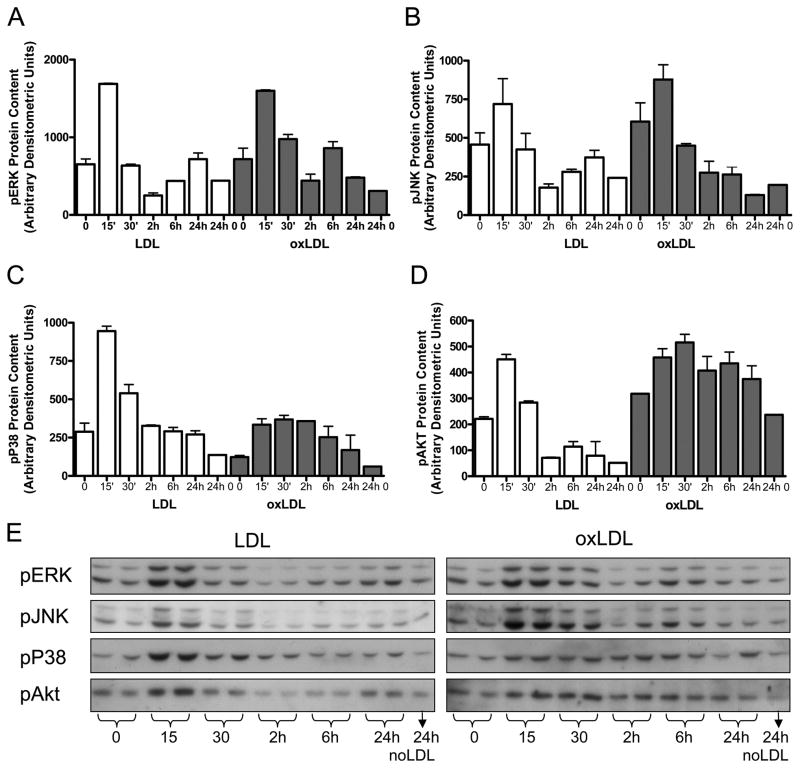
To characterize the signaling events critical for oxLDL-mediated CREB downregulation, we first assessed the signaling pathways activated in response to oxLDL. Consistent with previous reports in VSMC exposed to oxLDL 10, we observed acute transient activation of two stress MAP kinases (ERK and p38 MAPK) as detected by Western blot analysis (Figure 4) as well as a trend towards acute activation of another stress MSP kinase (JNK). In addition we observe a modest activation of Akt. Both p38 and Akt activation are more persistent after oxLDL treatment than after LDL treatment. Our group recently reported that the mitogen/cytokine PDGF induces CREB nuclear export and degradation in pulmonary VSMC through persistent activation of Akt/CK2 and site-specific phosphorylation of CREB S103 and S107 4. In earlier studies, we also reported ROS-mediated CREB downregulation 6, 8, 9. To assess the importance of ROS activation of stress MAPK versus Akt/CK2 for CREB downregulation, in studies described here, primary rat VSMC were pretreated with a panel of pharmacological inhibitors or the ROS scavenger, N-acetylcysteine (NAc) prior to oxLDL exposure (Figure 5A). Pharmacological inhibition of PI3K, Akt, p38 MAPK and JNK had no significant effect on oxLDL-mediated CREB downregulation (Figure 5A). Furthermore, experiments examining the Akt/CK2 pathway do not demonstrate induction of CK2 expression by either oxLDL or LDL (data not shown). Of interest, pharmacological inhibition of PKA (H89), the original physiological activator of CREB, and PKC (BIS), the mediator of fatty acid-induced CREB activation12, also did not block oxLDL-mediated CREB downregulation. Only scavenging of ROS (NAc) and inhibition of the ERK MAPK pathway (UO126) blocked CREB down-regulation by oxLDL. In Figure 5B and C we demonstrate that oxLDL-mediated CREB activation is also blocked by ROS scavenging and ERK MAPK inhibition while induction of the CREB target gene HO-1 is inhibited only by the ROS scavenger NAc. In contrast, inhibition of ERK MAPK (UO126) increases basal HO-1 content, suggesting that the protective effect of ERK inhibition may occur through a preemptive induction of antioxidant pathways.
Figure 4.
Activation of MAPkinase and Akt pathways in primary culture rat VSMC after 15 minutes exposure to LDL or oxLDL (50 ug/ml). Phosphoprotein concentrations were quantified by densitometric analysis of Western blots with antibodies to (A) pERK, B) pJNK, (C) pP38, and D) pAkt. (E) Representative western blots with duplicate loading are shown. Values are mean of independent duplicate experiments and error bars represent the maximum value. Similar results were obtained in 2–4 separate experiments, each in duplicate or triplicate, for individual pathways.
Antagonism of LDL receptor does not interfere with oxLDL mediated CREB degradation
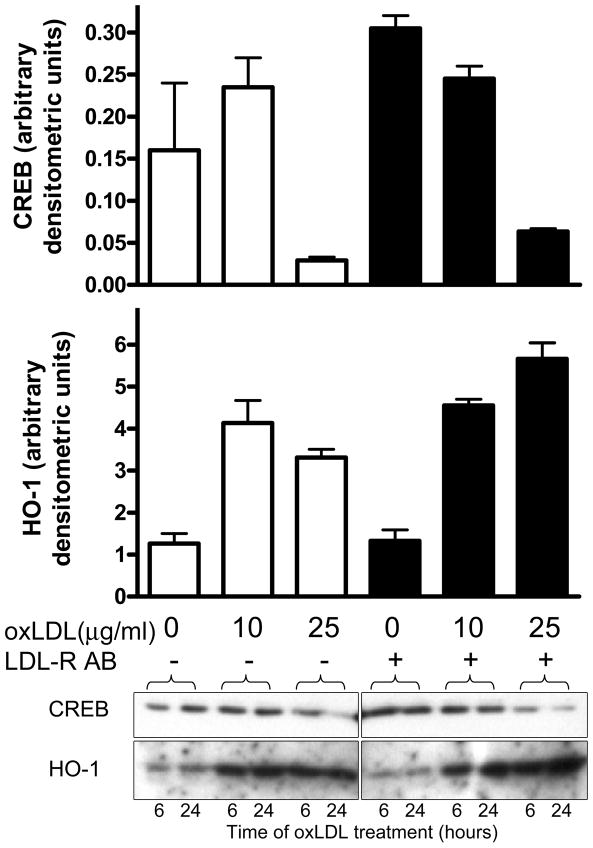
To determine whether the oxLDL-mediated CREB degradation required the LDL receptor, cells were pretreated with antibodies to the LDL receptor (R&D Systems, Minneapolis, MN) for 30 minutes as outlined in the figure legend. Acute CREB activation and later CREB downregulation and HO-1 induction by oxLDL were not prevented by pretreatment with anti-LDLR antibodies (Figure 6 and data not shown) suggesting that these events do not require interaction with LDL receptor consistent with a recent report by Hansen-Hagge 13.
Figure 6.
OxLDL-mediated CREB downregulation and HO-1 induction are not LDL-receptor-dependent: rat VSMC cells pretreated with or without LDL-receptor-blocking antibody (5 μg/ml) for 30 minutes, and then exposed to oxLDL for 24 hours at the indicated doses. CREB (top graph) and HO-1 (bottom graph). Bar heights represent means for independent duplicate samples with error bars illustrating maximum values. Data shown are representative of two separate experiments, each performed with independent duplicate samples. One of the corresponding western blots is shown below the graphs with a normalization control lane spliced out centrally.
Discussion
Atherosclerosis is a complex process in which long-term exposure of the vessel wall to vascular insults induces the vicious cycle of vascular pathology (reviewed in 1, 2). Regardless of the etiological factor or factors, most atherosclerotic lesions share overlapping features including endothelial dysfunction, neointimal proliferation, lipid accumulation, inflammation and necrosis 1, 2. Risk factors such as dyslipidemia, hypertension, diabetes, smoking and age likely have both common and unique effects on the cellular and extracellular components of the vessel wall. We previously have reported that oxidant stress, mitogens and cytokines lead to CREB downregulation in pulmonary and aortic VSMC as well as neurons and beta cells 4–6, 8, 9. Here, we present evidence that diminished aortic medial CREB expression is a common deleterious response to a variety of atherosclerotic risk factors. This series of experiments demonstrates significant age, hypertension, insulin resistance and obesity -induced aortic VSMC CREB downregulation. Importantly, we directly demonstrate decreased aortic nuclear CREB expression in response to high fat feeding in both C57BL/6 and LDLR −/− mice. Furthermore, we demonstrate that in vitro, exposure of rat primary VSMC to oxLDL leads to decreased CREB protein expression specifically via a ROS/ERK-dependent pathway. These data support a model wherein loss of VSMC CREB expression is a common pathological response to vascular injury, likely due to oxidant stress, which renders the vessel wall more susceptible to proliferation, migration, inflammation and apoptosis and potentially contributes to plaque progression.
Others and we have reported that CREB is critical for oxidant defense and survival of VSMC, cardiac myocytes, neurons and adipocytes 4, 14–17,18. Cardiac selective expression of dominant negative CREB leads to accelerated heart failure and blocks the adaptive cardiac response to exercise training 14, 19. In this context cardiac CREB appears to regulate not only myocardial apoptosis but also metabolic adaptation by affecting mitochondrial biogenesis and efficiency 17. We have further reported that overexpression of CREB in cultured neurons and beta cells can protect these cell types from oxidant and cytokine mediated apoptosis 8, 9. It seems likely that CREB plays a similar protective role in the vessel wall where inflammation and oxidant stress accelerate atherosclerosis.
CREB protects cells from oxidant injury through transcriptional regulation of numerous targets including genes involved in anti-apoptotic mechanisms, upregulation of antioxidant defense, and augmentation of growth factor signaling. First, CREB inhibits apoptosis by stabilizing mitochondria through induction of bcl-2 and by augmenting mitochondrial biogenesis via PGC-1. In addition, oxidative stress leads to the induction of CREB-mediated antioxidant defenses on two levels: by inducing expression of antioxidant enzymes such as manganese superoxide dismutase (MnSOD), thioredoxin (Trx) and heme-oxygenase-1 (HO-1)20,21, 22 and by increasing mitochondrial biogenesis via upregulation of PGC-1. Finally, CREB enhances appropriate growth factor signaling through regulation of BDNF and IRS-2 23, 24. Thus, loss of VSMC CREB function may contribute to disruption of vascular growth regulation, antioxidant and anti-apoptotic defenses.
In this report we demonstrate dramatic upregulation at the protein level of one CREB target anti-oxidant, HO-1, in response to oxLDL treatment. We hypothesize that this upregulation of HO-1 is at least in part a response to CREB activation and is one beneficial effect of CREB activation that could be lost with CREB downregulation. The persistence of HO-1 protein after CREB downregulation is not inconsistent with a CREB role in HO-1 activation, as the half-life of HO-1 mRNA is increased to up to 11 hours under certain types of oxidant stress25. However, HO-1 regulation is complex and multifactorial, involving many signaling pathways and transcription factors other than CREB, in addition to non-transcriptional regulatory mechanisms26, 27. Overall, the HO-1 activation we demonstrate is a marker of severe oxidant stress imposed by oxLDL treatment, and our studies do not rule out a role for other regulatory pathways. In fact, the oxLDL resistance inferred by ERK inhibition with UO126 may be the result of early HO-1 activation and represent a preconditioning that allows cells to cope with the oxidant stress imposed by oxLDL.
The hypothesis argued above, that CREB downregulation contributes to vascular pathology in atherosclerosis, implies that CREB activity is important for maintaining normal vascular physiology. CREB is a differentiation factor in VSMC, but CREB’s function in normal physiology is not as clearly delineated in the vasculature as it is in the nervous system 28, 29. Acute CREB activation by a broad array of toxins may be a cytoprotective response to injury 18. Recently, a number of observations have been published demonstrating acute CREB activation by established mediators of vascular injury such as fatty acids, LDL cholesterol, VLDL cholesterol, thrombin, endothelin 1, angiotensin II and tumor necrosis factor-α in endothelial cells and VSMC, both in vitro and in vivo 11, 30–34,35. We postulate that acute CREB activation by vascular toxins is a healthy response that is intended to decrease oxidant injury and inflammation and maintain VSMC survival and function. However, we acknowledge that there are conflicting data on the role of CREB in acute vascular injury. CREB activity was found to be increased in carotid arteries following wire injury 36. Introduction of a dominant negative CREB adenovirus at the time of wire injury blunted neointimal lesion formation and led to increased VSMC apoptosis and decreased proliferation 36. The explanation for these differences from our studies on CREB and VSMC phenotype remains unclear. They are likely dependent on intracellular pathway activation profiles and co-adaptor/co-repressor recruitment to CREB target genes which are unique depending upon the external stimulus and cell context 37, 38,39, 40.
The mechanism of CREB down-regulation in the vasculature also remains unclear. Numerous mechanisms have been shown to contribute to the CREB dysfunction observed in Alzheimer’s disease, HIV, cocaine addiction and pulmonary hypertension 4, 34, 41–47. It is likely that decreased vascular CREB content across the different rodent models presented in this manuscript is also the consequence of more than one mechanism. First, CREB is a self-regulating protein, such that loss of upstream signaling to CREB can result in decreased CREB expression. For example, depletion of cyclic nucleotides and decreased PKA/PKG activation decreases CREB expression 48, 49. The loss of eNOS stimulated cyclic nucleotide accumulation in endothelial dysfunction results could contribute to decreased vascular CREB expression. Expression of PDGF and its receptor are increased in models of atherosclerosis 50, 2, 51, as such PDGR stimulation of AKT/CK2 and CREB ubiquination could also occur in the systemic vasculature 4. It is probable that generation of ROS is the major underlying contributor to CREB downregulation across vascular disease models. ROS have been shown to disrupt signaling to CREB 9 and to promote CREB degradation in Alzheimer’s disease 47. We have reported downregulation of CREB in aortic VSMC, neurons, and beta cells in response to direct oxidant stress 4, 6, 8, 9. In further support of this hypothesis we now report that the potent CV risk factor, oxLDL, directly induces CREB downregulation in VSMC in vitro. In cell culture, simple screening with pharmacological inhibitors indicates that generation of ROS and activation of ERK, but not PKA or Akt, are critical for oxLDL-mediated loss of CREB expression. Another recent report has also suggested that induction of oxidant defense can block effects of LDL on VSMC 10. A more exhaustive series of studies will be needed to define fully the oxidant-induced signaling pathways important for CREB downregulation.
Loss of vascular medial CREB expression appears to be an early response of the vessel wall to cardiovascular risk exposure; as such, interventions aimed at restoring or maintaining vascular CREB are an appealing goal. Lifestyle interventions demonstrated to improve longevity and decrease cardiovascular risk also increase CREB function 52, 53. In the CNS, exercise training enhances nNOS expression leading to induction of CREB 54, 18. Furthermore, exercise increases active CREB and contributes to improved recovery from ischemia, stroke, and memory tasks post-seizure 52, 55. Preliminary observations indicate that age-related decrease in aortic CREB protein content can be abrogated by caloric restriction (unpublished 2009 JEBR, PAW, JG). Watson et al also recently reported increased cardiac CREB content with modest exercise training in a rodent model of heart failure 17. Unfortunately, the compensation for VSMC CREB deficiency is not as easy as adding CREB to the vessel wall because inflammatory cell differentiation also employs CREB (see the lumen –Figure 2). Thus, interventions aimed at restoring CREB will likely need to be directed at the underlying mechanism(s) of CREB loss and will require a better understanding of these mechanism(s). Interestingly, thiazolidinediones, agents which increase insulin sensitivity, have also been found to increase CREB content in the heart and blood vessels of the insulin resistant ob/ob mouse model (6 and unpublished observations). In an Alzheimer’s disease model, blocking CREB degradation with an inhibitor of ubiquitin 1 ligase led to increased CREB expression and improved cognition 43. Overall, clarification of the mechanism(s) of CREB loss in vascular disease may suggest novel therapeutic target(s) for vascular intervention and augmentation of CREB dependent vascular defenses.
In summary, we report loss of CREB expression across a spectrum of rodent models of vascular risk including insulin resistance, aging, and high fat diet. The relevance of oxLDL to the in vivo vascular CREB depletion model is borne out by the loss of CREB upon direct challenge of VSMC with oxLDL in vitro. Simple analysis of critical pathways reveals that blockade of ROS generation or ERK activation protects cells from CREB depletion and from oxLDL toxicity. This in vitro model will allow further analysis of the mechanism of oxLDL-mediated CREB loss in VSMC. These experiments provide new information on a common response to vascular injury that may be an early event in atherosclerosis and is a potential new target for CVD intervention.
Materials and Methods
Chemicals and Reagents
Fetal bovine serum, L-glutamine, and penicillin/streptomycin were purchased from Gemini Bio-Products (Cabassas, CA). Dulbecco’s Modified Eagle’s media, Hams F-12 nutrient mixture and sodium pyruvate were purchased from Invitrogen (Carlsbad, CA). The trypsin/EDTA was purchased from Life Technologies (Grand Island, NY). LDL and oxLDL were purchased from Intracel Resources (Fredrick, MD). A non-essential amino acid solution, DAPI, bovine serum albumin (BSA), fatty acid free low endotoxin BSA, β-Actin antibody and N-Acetyl-L-cysteine (NAc) were purchased from Sigma-Aldrich (St. Louis, MO). Antibodies to CREB, phospho-CREB, phospho-ERK, phospho-p38, phospho-JNK, phospho-Akt and anti-rabbit and anti-mouse IgG alkaline phosphatase-linked antibodies were from Cell Signaling Technology (Danvers, MA). SB-203580 (SB), SP-600125 (SP), U-0126 (U0), Wortmannin (WORT) and H-89 were purchased from BIOMOL International (Plymouth Meeting, PA). Bisindolyl maleimide (BIS) is from Calbiochem (San Diego, CA). N-acetyl cysteine (NAC) is from Sigma (St. Louis, MO). The Cy3 AffiniPur Donkey Anti-Mouse IgG was purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). CDP-Star reagent kit was purchased from New England BioLabs (Ipswitch, MA).
Animals
Archived aorta samples were procured through collaborations in order to assess an array of disease states. Aortas from C57/B6 (3,12,24 months) were generated in collaboration with Dr. M. Levi (University of Colorado, Denver, CO); aortas from Sprague-Dawley rats (4-month and 14-month old females) were harvested in collaboration with Dr. M. Levi (University of Colorado, Denver, CO); aortas from spontaneous hypertensive heart failure (SHHF) rats were donated by Dr. S. McCune (University of Colorado, Boulder, CO).
Six- to 8-week old female LDL-receptor-null (LDLR−/−) mice bred onto a C57BL/6 background (Jackson Laboratories, Bar Harbor, ME) and wild-type C57BL/6 mice were fed ad libitum chow diets (Wayne Rodent BLOX, 4% fat (wt/wt), 0.04% cholesterol) or “Western” diets high in saturated fat (21%), with added cholesterol (0.15%) for 10 weeks. All diets were purchased from Harlan-Teklad, Madison, WI, and all percentages are weight/weight. Aortas were collected from LDLR−/− mice and controls as previously described 1.
All animals were housed in temperature controlled rooms, allowed unrestricted access to food and water and maintained on a 12-hour light/dark cycle. Animal studies were conducted under Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) guidelines and were approved by individual Institutional Animal Care and Use Committees.
Tissue processing of aortic lysates
Following termination, aortic tissue was taken from animals and immediately frozen in liquid nitrogen. Samples were homogenized in Mammalian Protein Extraction Reagent (M-PER) lysis buffer (Thermo-Scientific, Rockford, IL) and cellular debris was pelleted by centrifugation. Protein concentrations in these extracts were quantified by Bradford protein assay.
Cell Culture
Primary culture rat vascular smooth muscle cells (VSMC) were isolated from aortas of Sprague-Dawley rats as previously described 2. All experiments used cells within passages 4 – 10. Cells were expanded in a 10% fetal bovine serum (FBS) media that consisted of high glucose (4.5 g/L) DMEM:Hams F-12 nutrient solution (1:1) plus L-glutamine, non-essential amino acids, penicillin and streptomycin. When cells reached 50–75% confluency, they were serum-deprived in 1:1 DMEM:Ham’s F-12 with 0.1% FBS for 48 hours before each experiment. After serum deprivation the cells were treated with reagents and pharmacological inhibitors as noted in the text or figure legends. Cells were either fixed and stained for immunocytochemistry or harvested in 1× LSB containing BME or 1× mammalian lysis buffer (MLB,: 150mM NaCl, 1mM EDTA, 1mM EGTA, 5mM sodium pyrophosphate, 1mM sodium orthovandadate, 20mM sodium fluoride, 500mM okadaic acid, protease inhibitor cocktail p8340 (Sigma-Aldrich), M-PER mammalian protein extraction reagent (Thermo Scientific, Rockford, IL)) for Western blot analysis.
Tissue processing for immunohistochemistry
Aortas were collected from LDLR−/− mice and controls as previously described 1. After 10 weeks of diet treatment, mice were euthanized by cervical dislocation, and the heart and aorta were removed and perfusion-fixed with 10% formalin. All data regarding lipids and atherosclerosis burden are as reported previously 1.
Immunohistochemistry
A. Aortic sinus immunohistochemistry
Single-label immunohistochemistry was performed on murine aortic sinuses using procedures described in detail previously 3. VSMC were detected using a mouse monoclonal antibody against α-actin (titer 1:1,000; Sigma St Louis, MO). CREB was detected using a mouse monoclonal Ab (titer 1:50; Cell Signaling, Danvers, MA, State). Nova Red (Vector Laboratories) was used as the peroxidase substrate to yield a red-brown reaction product; cell nuclei were identified by counterstaining with hematoxylin. Quantification of medial VSMC nuclear CREB expression was performed by manual counting of positively- and negatively-stained nuclei from an average of 798 nuclei for C57BL/6 and an average of 677 nuclei for LDLR −/− by an operator (TM) blinded to treatment group.
B. Immunohistochemistry of cultured VSMC
Cells were grown on 8-well Poly-D-lysine/laminin treated glass slides (BD Biosciences, Bedford, MA), serum starved for 48 hours and treated with LDL or oxLDL. After treatment cells were fixed with 4% paraformaldehyde and permeabilized (5% BSA/0.2% Triton X-100/PBS). They were then incubated with an anti-CREB antibody (mouse monoclonal, 1:500) followed by a Cy3-tagged anti-mouse IgG antibody (1:500). Cells were also incubated with DAPI (1:500) in order to visualize the nuclei. Photographs were taken using a Zeiss Axioplan 2 EPI Fluorescence up right microscope (Carl Zeiss, Maple Grove, MN). All images were digitally captured with a Cooke Sensicam QE high resolution (1376 × 1024 resolution) black and white super-cooled CCD camera. The digital images were then assigned colors by the software interface, SlideBook (Intelligent Imaging Innovations, Inc, Denver, CO).
LDL/oxLDL treatment
Each individual lot of oxLDL was assessed for potency. Cell cultures were serum-starved with DMEM:Ham’s F-12 + 0.1% fetal bovine serum for 48 hours and then treated with 10–100 μg/ml LDL or oxLDL Intracel Resources (Fredrick, MD) in the same medium. After 24 hours of exposure, the health of the cells was visually ascertained by shape, adherence, and granularity. The concentration that caused morphological change, without significant cell or protein loss and induced a 70% reduction in CREB protein at 24 hours was used during all experiments treated with each characterized lot of oxLDL. LDL treatment was always with 100 μg/ml LDL.
Western Blot Analysis
Protein content was assessed using a Bradford analysis protocol (3). Equal protein or equal volume samples were run on SDS 12%-polyacrylamide gels. In large experiments where more than one gel was required, a sample of a large volume control extract was run on each gel to allow normalization to a common control band. This allowed quantitation and comparison between gels for any gels loaded with the same batch of control extract. Following electrophoresis, proteins were transferred from the gels to PVDF membranes (Millipore, Billerica, MA). Membranes were blocked in a 5%milk/TBST solution and incubated with primary antibodies diluted in 5% BSA in TBST. The membranes were then incubated with anti-rabbit or anti-mouse IgG alkaline phosphatase-linked antibodies. An alkaline phosphatase-based chemiluminescent detection assay (CDP Star, New England BioLabs, Ipswitch, MA) was used, and membranes were exposed to Kodak scientific imaging film. Films were quantitatively evaluated by densitometry with Quantity One software by Bio-Rad (Hercules, CA). For probing with multiple antibodies with targets in the same MW range, blots were stripped between antibodies for 15 minutes at 55°C in TBST. To control for actual protein loading, blots were last probed with anti-β-actin antibody. If β-actin signal varied. experimental signals were normalized to β-actin. If between gel comparisons or averages were to be performed experimental signals were normalized to corresponding to control extract signals.
CREB mRNA Quantification
Cells were grown in 6-well plates to 50–60% confluency and then serum starved for 24 hrs. After 24 hours of exposure to 0.1 mg/ml oxLDL, media was removed and cells were washed with ice cold PBS. 1ml of Trizol reagent (Invitrogen) was applied directly to each well and RNA extraction was followed according to manufacturer’s protocol up to chloroform extraction. RNA cleanup was done using a Qiagen RNeasy Mini Kit. RT-PCR was run by the University of Colorado Cancer Center PT-PCR core for CREB mRNA and normalized to 18S rRNA using primers and probes from ABI on an ABI 7900. Control and exposed samples were done in quintuplicate.
Statistical Analysis
Values shown are mean ± SEM or SD as indicated in figure legends. Significance of differences between means was assessed by Student’s t test or one way ANOVA with post test for multiple comparisons (Dunnett’s test for comparison to one control or Bonferroni correction for pairwise comparisons). Significant relationships were analyzed by multiple linear regression analyses. Probability values with p<0.05 were considered statistically significant.
Acknowledgments
a. Source(s) of support: JEBR- VA Merit; DK64741;JEBR/DJK- HL56481; JEBR/KDO-Merck Investigator initiated research support; AC/TOM/KDO P30 DK035816; IES-BIRCWH K12; b. I would like to thank Mandeep Brar for technical assistance and Sylvia McCune for tissues. c. Disclosure-the manuscript was supported in part JEBR/KDO-Merck Investigator initiated research support.
Footnotes
Disclaimers: none
Literature Cited
- 1.Eckel RH, Wassef M, Chait A, Sobel B, Barrett E, King G, Lopes-Virella M, Reusch J, Ruderman N, Steiner G, Vlassara H. Prevention Conference VI: Diabetes and Cardiovascular Disease: Writing Group II: pathogenesis of atherosclerosis in diabetes. Circulation. 2002;105:e138–143. doi: 10.1161/01.cir.0000013954.65303.c5. [DOI] [PubMed] [Google Scholar]
- 2.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 3.Freeman MW. Statins, cholesterol, and the prevention of coronary heart disease. Faseb J. 2006;20:200–201. doi: 10.1096/fj.06-0202ufm. [DOI] [PubMed] [Google Scholar]
- 4.Garat CV, Fankell D, Erickson PF, Reusch JE, Bauer NN, McMurtry IF, Klemm DJ. Platelet-derived growth factor BB induces nuclear export and proteasomal degradation of CREB via phosphatidylinositol 3-kinase/Akt signaling in pulmonary artery smooth muscle cells. Mol Cell Biol. 2006;26:4934–4948. doi: 10.1128/MCB.02477-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klemm DJ, Watson PA, Frid MG, Dempsey EC, Schaack J, Colton LA, Nesterova A, Stenmark KR, Reusch JE. cAMP response element-binding protein content is a molecular determinant of smooth muscle cell proliferation and migration. J Biol Chem. 2001;276:46132–46141. doi: 10.1074/jbc.M104769200. [DOI] [PubMed] [Google Scholar]
- 6.Watson PA, Nesterova A, Burant CF, Klemm DJ, Reusch JE. Diabetes-related changes in cAMP response element-binding protein content enhance smooth muscle cell proliferation and migration. J Biol Chem. 2001;276:46142–46150. doi: 10.1074/jbc.M104770200. [DOI] [PubMed] [Google Scholar]
- 7.Lewis KE, Kirk EA, McDonald TO, Wang S, Wight TN, O’Brien KD, Chait A. Increase in serum amyloid a evoked by dietary cholesterol is associated with increased atherosclerosis in mice. Circulation. 2004;110:540–545. doi: 10.1161/01.CIR.0000136819.93989.E1. [DOI] [PubMed] [Google Scholar]
- 8.Jambal P, Masterson S, Nesterova A, Bouchard R, Bergman B, Hutton JC, Boxer LM, Reusch JE, Pugazhenthi S. Cytokine-mediated down-regulation of the transcription factor cAMP-response element-binding protein in pancreatic beta-cells. J Biol Chem. 2003;278:23055–23065. doi: 10.1074/jbc.M212450200. [DOI] [PubMed] [Google Scholar]
- 9.Pugazhenthi S, Nesterova A, Jambal P, Audesirk G, Kern M, Cabell L, Eves E, Rosner MR, Boxer LM, Reusch JE. Oxidative stress-mediated down-regulation of bcl-2 promoter in hippocampal neurons. J Neurochem. 2003;84:982–996. doi: 10.1046/j.1471-4159.2003.01606.x. [DOI] [PubMed] [Google Scholar]
- 10.Lin SJ, Shyue SK, Shih MC, Chu TH, Chen YH, Ku HH, Chen JW, Tam KB, Chen YL. Superoxide dismutase and catalase inhibit oxidized low-density lipoprotein-induced human aortic smooth muscle cell proliferation: role of cell-cycle regulation, mitogen-activated protein kinases, and transcription factors. Atherosclerosis. 2007;190:124–134. doi: 10.1016/j.atherosclerosis.2006.02.044. [DOI] [PubMed] [Google Scholar]
- 11.Yang SN, Yang CH, Huang LT, Wu YT, Wang CL. Enhancement of CREBSerine-133 phosphorylation through nitric oxide-mediated signaling induced by bacterial lipopolysaccharide in vascular smooth muscle cells from rats. Chin J Physiol. 2002;45:69–74. [PubMed] [Google Scholar]
- 12.Schauer IERJ. Nonesterified fatty acid exposure activates protective and mitogenic pathways in vascular smooth muscle cells by alternate signaling pathways. Metabolism Clinical and Experimental. 2009;58:319–327. doi: 10.1016/j.metabol.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansen-Hagge TE, Baumeister E, Bauer T, Schmiedeke D, Renne T, Wanner C, Galle J. Transmission of oxLDL-derived lipid peroxide radicals into membranes of vascular cells is the main inducer of oxLDL-mediated oxidative stress. Atherosclerosis. 2008;197:602–611. doi: 10.1016/j.atherosclerosis.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 14.Fentzke RC, Korcarz CE, Lang RM, Lin H, Leiden JM. Dilated cardiomyopathy in transgenic mice expressing a dominant-negative CREB transcription factor in the heart. J Clin Invest. 1998;101:2415–2426. doi: 10.1172/JCI2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pugazhenthi S, Miller E, Sable C, Young P, Heidenreich KA, Boxer LM, Reusch JE. Insulin-like growth factor-I induces bcl-2 promoter through the transcription factor cAMP-response element-binding protein. J Biol Chem. 1999;274:27529–27535. doi: 10.1074/jbc.274.39.27529. [DOI] [PubMed] [Google Scholar]
- 16.Pugazhenthi S, Nesterova A, Sable C, Heidenreich KA, Boxer LM, Heasley LE, Reusch JE. Akt/protein kinase B up-regulates Bcl-2 expression through cAMP-response element-binding protein. J Biol Chem. 2000;275:10761–10766. doi: 10.1074/jbc.275.15.10761. [DOI] [PubMed] [Google Scholar]
- 17.Watson PA, Reusch JE, McCune SA, Leinwand LA, Luckey SW, Konhilas JP, Brown DA, Chicco AJ, Sparagna GC, Long CS, Moore RL. Restoration of CREB function is linked to completion and stabilization of adaptive cardiac hypertrophy in response to exercise. Am J Physiol Heart Circ Physiol. 2007;293:H246–259. doi: 10.1152/ajpheart.00734.2006. [DOI] [PubMed] [Google Scholar]
- 18.Finkbeiner S. CREB couples neurotrophin signals to survival messages. Neuron. 2000;25:11–14. doi: 10.1016/s0896-6273(00)80866-1. [DOI] [PubMed] [Google Scholar]
- 19.Spencer KT, Collins K, Korcarz C, Fentzke R, Lang RM, Leiden JM. Effects of exercise training on LV performance and mortality in a murine model of dilated cardiomyopathy. Am J Physiol Heart Circ Physiol. 2000;279:H210–215. doi: 10.1152/ajpheart.2000.279.1.H210. [DOI] [PubMed] [Google Scholar]
- 20.Kronke G, Bochkov VN, Huber J, Gruber F, Bluml S, Furnkranz A, Kadl A, Binder BR, Leitinger N. Oxidized phospholipids induce expression of human heme oxygenase-1 involving activation of cAMP-responsive element-binding protein. J Biol Chem. 2003;278:51006–51014. doi: 10.1074/jbc.M304103200. [DOI] [PubMed] [Google Scholar]
- 21.Chiueh CC, Andoh T, Chock PB. Induction of thioredoxin and mitochondrial survival proteins mediates preconditioning-induced cardioprotection and neuroprotection. Ann N Y Acad Sci. 2005;1042:403–418. doi: 10.1196/annals.1338.034. [DOI] [PubMed] [Google Scholar]
- 22.St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jager S, Handschin C, Zheng K, Lin J, Yang W, Simon DK, Bachoo R, Spiegelman BM. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 23.Tseng YH, Ueki K, Kriauciunas KM, Kahn CR. Differential roles of insulin receptor substrates in the anti-apoptotic function of insulin-like growth factor-1 and insulin. J Biol Chem. 2002;277:31601–31611. doi: 10.1074/jbc.M202932200. [DOI] [PubMed] [Google Scholar]
- 24.Ying Z, Roy RR, Zhong H, Zdunowski S, Edgerton VR, Gomez-Pinilla F. BDNF-exercise interactions in the recovery of symmetrical stepping after a cervical hemisection in rats. Neuroscience. 2008;155:1070–1078. doi: 10.1016/j.neuroscience.2008.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leautaud V, Demple B. Regulation of heme oxygenase-1 mRNA deadenylation and turnover in NIH3T3 cells by nitrosative or alkylation stress. BMC Mol Biol. 2007;8:116. doi: 10.1186/1471-2199-8-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin D, Rojo AI, Salinas M, Diaz R, Gallardo G, Alam J, De Galarreta CM, Cuadrado A. Regulation of heme oxygenase-1 expression through the phosphatidylinositol 3-kinase/Akt pathway and the Nrf2 transcription factor in response to the antioxidant phytochemical carnosol. J Biol Chem. 2004;279:8919–8929. doi: 10.1074/jbc.M309660200. [DOI] [PubMed] [Google Scholar]
- 27.Hartsfield CL, Alam J, Choi AM. Transcriptional regulation of the heme oxygenase 1 gene by pyrrolidine dithiocarbamate. Faseb J. 1998;12:1675–1682. doi: 10.1096/fasebj.12.15.1675. [DOI] [PubMed] [Google Scholar]
- 28.Ichiki T. Role of cAMP response element binding protein in cardiovascular remodeling: good, bad, or both? Arterioscler Thromb Vasc Biol. 2006;26:449–455. doi: 10.1161/01.ATV.0000196747.79349.d1. [DOI] [PubMed] [Google Scholar]
- 29.Brandenburg SL, Lindenfeld J, Reusch JE, Regensteiner JG. Cardiovascular risk in women with type 2 diabetes. Med Clin North Am. 2003;87:955–969. doi: 10.1016/s0025-7125(03)00113-5. [DOI] [PubMed] [Google Scholar]
- 30.Martorell L, Rodriguez C, Calvayrac O, Gentile M, Badimon L, Martinez-Gonzalez J. Vascular effects of thrombin: involvement of NOR-1 in thrombin-induced mitogenic stimulus in vascular cells. Front Biosci. 2008;13:2909–2915. doi: 10.2741/2895. [DOI] [PubMed] [Google Scholar]
- 31.Harrison JG, Sugden PH, Clerk A. Endothelin-1 promotes phosphorylation of CREB transcription factor in primary cultures of neonatal rat cardiac myocytes: implications for the regulation of c-jun expression. Biochim Biophys Acta. 2004;1644:17–25. doi: 10.1016/j.bbamcr.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 32.Inoue K, Zama T, Kamimoto T, Aoki R, Ikeda Y, Kimura H, Hagiwara M. TNFalpha-induced ATF3 expression is bidirectionally regulated by the JNK and ERK pathways in vascular endothelial cells. Genes Cells. 2004;9:59–70. doi: 10.1111/j.1356-9597.2004.00707.x. [DOI] [PubMed] [Google Scholar]
- 33.Norata GD, Pirillo A, Callegari E, Hamsten A, Catapano AL, Eriksson P. Gene expression and intracellular pathways involved in endothelial dysfunction induced by VLDL and oxidised VLDL. Cardiovasc Res. 2003;59:169–180. doi: 10.1016/s0008-6363(03)00335-3. [DOI] [PubMed] [Google Scholar]
- 34.Lipskaia L, Pourci ML, Delomenie C, Combettes L, Goudouneche D, Paul JL, Capiod T, Lompre AM. Phosphatidylinositol 3-kinase and calcium-activated transcription pathways are required for VLDL-induced smooth muscle cell proliferation. Circ Res. 2003;92:1115–1122. doi: 10.1161/01.RES.0000074880.25540.D0. [DOI] [PubMed] [Google Scholar]
- 35.Illi A, Kampman O, Anttila S, Roivas M, Mattila KM, Lehtimaki T, Leinonen E. Interaction between angiotensin-converting enzyme and catechol-O-methyltransferase genotypes in schizophrenics with poor response to conventional neuroleptics. Eur Neuropsychopharmacol. 2003;13:147–151. doi: 10.1016/s0924-977x(02)00176-1. [DOI] [PubMed] [Google Scholar]
- 36.Tokunou T, Shibata R, Kai H, Ichiki T, Morisaki T, Fukuyama K, Ono H, Iino N, Masuda S, Shimokawa H, Egashira K, Imaizumi T, Takeshita A. Apoptosis induced by inhibition of cyclic AMP response element-binding protein in vascular smooth muscle cells. Circulation. 2003;108:1246–1252. doi: 10.1161/01.CIR.0000085164.13439.89. [DOI] [PubMed] [Google Scholar]
- 37.Ravnskjaer K, Kester H, Liu Y, Zhang X, Lee D, Yates JR, 3rd, Montminy M. Cooperative interactions between CBP and TORC2 confer selectivity to CREB target gene expression. Embo J. 2007;26:2880–2889. doi: 10.1038/sj.emboj.7601715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang X, Odom DT, Koo SH, Conkright MD, Canettieri G, Best J, Chen H, Jenner R, Herbolsheimer E, Jacobsen E, Kadam S, Ecker JR, Emerson B, Hogenesch JB, Unterman T, Young RA, Montminy M. Genome-wide analysis of cAMP-response element binding protein occupancy, phosphorylation, and target gene activation in human tissues. Proc Natl Acad Sci U S A. 2005;102:4459–4464. doi: 10.1073/pnas.0501076102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cha-Molstad H, Keller DM, Yochum GS, Impey S, Goodman RH. Cell-type-specific binding of the transcription factor CREB to the cAMP-response element. Proc Natl Acad Sci U S A. 2004;101:13572–13577. doi: 10.1073/pnas.0405587101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sawka-Verhelle D, Escoubet-Lozach L, Fong AL, Hester KD, Herzig S, Lebrun P, Glass CK. PE-1/METS, an antiproliferative Ets repressor factor, is induced by CREB-1/CREM-1 during macrophage differentiation. J Biol Chem. 2004;279:17772–17784. doi: 10.1074/jbc.M311991200. [DOI] [PubMed] [Google Scholar]
- 41.Featherby T, van den Buuse M, Lubman DI, Lawrence AJ. Persistent downregulation of hippocampal CREB mRNA parallels a Y-maze deficit in adolescent rats following semi-chronic amphetamine administration. Br J Pharmacol. 2008;154:417–428. doi: 10.1038/bjp.2008.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fumagalli F, Di Pasquale L, Caffino L, Racagni G, Riva MA. Repeated exposure to cocaine differently modulates BDNF mRNA and protein levels in rat striatum and prefrontal cortex. Eur J Neurosci. 2007;26:2756–2763. doi: 10.1111/j.1460-9568.2007.05918.x. [DOI] [PubMed] [Google Scholar]
- 43.Gong B, Cao Z, Zheng P, Vitolo OV, Liu S, Staniszewski A, Moolman D, Zhang H, Shelanski M, Arancio O. Ubiquitin hydrolase Uch-L1 rescues beta-amyloid-induced decreases in synaptic function and contextual memory. Cell. 2006;126:775–788. doi: 10.1016/j.cell.2006.06.046. [DOI] [PubMed] [Google Scholar]
- 44.Li J, Li YH, Yuan XR. Changes of phosphorylation of cAMP response element binding protein in rat nucleus accumbens after chronic ethanol intake: naloxone reversal. Acta Pharmacol Sin. 2003;24:930–936. [PubMed] [Google Scholar]
- 45.Tardito D, Perez J, Tiraboschi E, Musazzi L, Racagni G, Popoli M. Signaling pathways regulating gene expression, neuroplasticity, and neurotrophic mechanisms in the action of antidepressants: a critical overview. Pharmacol Rev. 2006;58:115–134. doi: 10.1124/pr.58.1.7. [DOI] [PubMed] [Google Scholar]
- 46.Feng MJ, Yan SE, Yan QS. Cocaine exposure at a sublethal concentration downregulates CREB functions in cultured neuroblastoma cells. Brain Res. 2006;1077:59–66. doi: 10.1016/j.brainres.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 47.Liang Z, Liu F, Grundke-Iqbal I, Iqbal K, Gong CX. Down-regulation of cAMP-dependent protein kinase by over-activated calpain in Alzheimer disease brain. J Neurochem. 2007;103:2462–2470. doi: 10.1111/j.1471-4159.2007.04942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosethorne EM, Nahorski SR, Challiss RA. Regulation of cyclic AMP response-element binding-protein (CREB) by Gq/11-protein-coupled receptors in human SH-SY5Y neuroblastoma cells. Biochem Pharmacol. 2008;75:942–955. doi: 10.1016/j.bcp.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pilz RB, Casteel DE. Regulation of gene expression by cyclic GMP. Circ Res. 2003;93:1034–1046. doi: 10.1161/01.RES.0000103311.52853.48. [DOI] [PubMed] [Google Scholar]
- 50.Thomas JA, Deaton RA, Hastings NE, Shang Y, Moehle CW, Eriksson U, Topouzis S, Wamhoff BR, Blackman BR, Owens GK. PDGF-DD, a novel mediator of smooth muscle cell phenotypic modulation, is upregulated in endothelial cells exposed to atherosclerosis-prone flow patterns. Am J Physiol Heart Circ Physiol. 2009;296:H442–452. doi: 10.1152/ajpheart.00165.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22:1276–1312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luo CX, Jiang J, Zhou QG, Zhu XJ, Wang W, Zhang ZJ, Han X, Zhu DY. Voluntary exercise-induced neurogenesis in the postischemic dentate gyrus is associated with spatial memory recovery from stroke. J Neurosci Res. 2007;85:1637–1646. doi: 10.1002/jnr.21317. [DOI] [PubMed] [Google Scholar]
- 53.Leung FP, Yung LM, Laher I, Yao X, Chen ZY, Huang Y. Exercise, vascular wall and cardiovascular diseases: an update (part 1) Sports Med. 2008;38:1009–1024. doi: 10.2165/00007256-200838120-00005. [DOI] [PubMed] [Google Scholar]
- 54.Chen MJ, Russo-Neustadt AA. Exercise activates the phosphatidylinositol 3-kinase pathway. Brain Res Mol Brain Res. 2005;135:181–193. doi: 10.1016/j.molbrainres.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 55.Ploughman M, Granter-Button S, Chernenko G, Attwood Z, Tucker BA, Mearow KM, Corbett D. Exercise intensity influences the temporal profile of growth factors involved in neuronal plasticity following focal ischemia. Brain Res. 2007;1150:207–216. doi: 10.1016/j.brainres.2007.02.065. [DOI] [PubMed] [Google Scholar]
- 1.Lewis KE, Kirk EA, McDonald TO, Wang S, Wight TN, O’Brien KD, Chait A. Increase in serum amyloid a evoked by dietary cholesterol is associated with increased atherosclerosis in mice. Circulation. 2004;110:540–545. doi: 10.1161/01.CIR.0000136819.93989.E1. [DOI] [PubMed] [Google Scholar]
- 2.Klemm DJ, Watson PA, Frid MG, Dempsey EC, Schaack J, Colton LA, Nesterova A, Stenmark KR, Reusch JE. cAMP response element-binding protein content is a molecular determinant of smooth muscle cell proliferation and migration. J Biol Chem. 2001;276:46132–46141. doi: 10.1074/jbc.M104769200. [DOI] [PubMed] [Google Scholar]
- 3.O’Brien KD, McDonald TO, Kunjathoor V, Eng K, Knopp EA, Lewis K, Lopez R, Kirk EA, Chait A, Wight TN, deBeer FC, LeBoeuf RC. Serum amyloid A and lipoprotein retention in murine models of atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25:785–790. doi: 10.1161/01.ATV.0000158383.65277.2b. [DOI] [PubMed] [Google Scholar]