Abstract
A deletion polymorphism in the serotonin transporter-linked polymorphic region (5-HTTLPR) has been associated with vulnerability to affective disorders, yet the mechanism by which this gene confers vulnerability remains unclear. Two studies examined associations between the 5-HTTLPR polymorphism and attentional bias for emotional stimuli among non-depressed adults. Biased attention, attention engagement, and difficulty with attention disengagement were assessed with a spatial cueing task using emotional stimuli. Results from Study 1 (N = 38) indicated that short 5-HTTLPR allele carriers experienced greater difficulty disengaging their attention from sad and happy stimuli compared to long allele homozygotes. Study 2 participants (N = 144) were genotyped for the 5-HTTLPR polymorphism, including single nucleotide polymorphism (SNP) rs25531 in the long allele of the 5-HTTLPR. Consistent with Study 1, individuals homozygous for the low expressing 5-HTTLPR alleles (i.e., S and LG) experienced greater difficulty disengaging attention from sad, happy, and fear stimuli than high expressing 5-HTTLPR homozygotes. Since this association exists in healthy adults, it may represent a susceptibility factor for affective disorders that becomes problematic during stressful life experiences.
Keywords: genetic association, depression vulnerability, serotonin transporter, information processing
Individuals who inherit the low expressing variant of the serotonin transporter-linked polymorphic region (5-HTTLPR) of the serotonin transporter gene (SLC6A4) and experience significant life stress appear to be at greater risk for depression than people who inherit two copies of the high expressing 5-HTTLPR allele (e.g., Caspi et al., 2003; Kendler, Kuhn, Vittum, Prescott, & Riley, 2005). These landmark studies have stimulated research aimed at understanding why the low expressing 5-HTTLPR variant putatively increases sensitivity to life stress and, in turn, increases vulnerability to depression. One possibility is that the 5-HTTLPR polymorphism biases the processing of emotional information. Therefore, we sought to examine whether healthy adult carriers of the low expressing 5-HTTLPR allele display more pronounced attentional biases for emotional stimuli than individuals homozygous for the high expressing 5-HTTLPR allele.
The serotonin transporter (5-HTT) regulates the reuptake of serotonin to the presynaptic neuron for recycling or degradation after serotonin has been released. It thus plays a critical role in determining the duration and intensity of serotonin communication with post-synaptic receptors and targets, such as those in limbic regions involved in the regulation of emotional information (for a review, see Hariri & Holmes, 2006). Importantly, the efficiency with which the 5-HTT returns serotonin to the presynaptic neuron appears to be influenced by the 5-HTTLPR polymorphism. A common deletion polymorphism in the promoter region of the 5-HTT gene results in 2 variants: a short (S) allele and a long (L) allele. The presence of one or two short alleles, rather than two copies of the long allele, is associated with reduced transcriptional efficiency that putatively results in significant decreases (approximately 50%) in serotonin reuptake (Heinz et al., 2000; Lesch et al., 1996). Short 5-HTTLPR allele carriers should thus have increased levels of extracellular serotonin and increased serotonin signaling compared to long allele homozygotes.
This difference in how serotonin is regulated appears to impact a cortical-limbic circuit that is critical for regulating emotional information. For instance, among healthy participants, Pezawas et al. (2005) found that short 5-HTTLPR allele carriers had significant reduction in gray matter volume in the perigenual anterior cingulate (pACC) and the rostral anterior cingulated—regions of the prefrontal cortex previously implicated in affect regulation (Drevets, 2000)—compared to long allele homozygotes. Further, using fMRI analyses to assess relative activation of the pACC and amygdala in response to negative stimuli (e.g., angry and scared facial expressions), short 5-HTTLPR allele carriers had less functional coupling between the pACC and the amygdala. The “uncoupling” of this emotion circuit may explain why short-allele carriers have greater amygdala responses to emotional stimuli (Bertolino et al., 2005; Hariri et al., 2005; Hariri et al., 2002).
Other research has similarly found that reduced functional coupling between the prefrontal cortex and limbic system is associated with biased processing of emotional information at a neural level. Heinz et al. (2005) reported that a region of the prefrontal cortex more dorsal and rostral to the pACC was over-activated in short 5-HTTLPR allele carriers than long 5-HTTLPR homozygotes when presented with emotional images. Activation of the ventromedial prefrontal cortex region was positively correlated with amygdala activation, suggesting a compensatory effort to regulate exaggerated amygdala responses of the short allele carriers. This heightened amygdala response in short 5-HTTLPR allele carriers likely applies to a variety of emotional stimuli, as the amygdala also responds to positive stimuli (Cunningham, Van Bavel, & Johnsen, 2008), as well as novel, salient, and ambiguous stimuli (Whalen, 2007).
Difficulty regulating emotional information at a neural level may have important implications for the cognitive processing of emotional information. The ability to allocate attention to emotional cues in the environment is a crucial element of adaptive self-regulation (Posner & Rothbart, 2000). Although it is adaptive for salient stimuli to capture attention, successful behavioral regulation requires some flexibility and control over attention. This may include strategic filtering of certain stimuli, timely disengagement from stimuli, and being appropriately vigilant for meaningful emotional cues. In line with this conclusion, Hasler et al. (2004) argued that biased processing of emotional stimuli is a highly plausible and important putative intermediate phenotype for MDD. Further, they specifically identified the 5-HTTLPR as a highly promising candidate gene that may be associated with biased processing of emotional information (see Figure 1, pg. 1766).
Figure 1.
Trial sequence for valid and invalid trials. Fixation cross and probe are not to scale.
Despite this promising neuroimaging research, few studies have examined associations between the 5-HTTLPR polymorphism and behavioral assessments of biased processing of emotional information. Roiser et al. (2005) reported that among Ecstasy users, a drug that causes long term reductions in synaptic 5-HT release, short 5-HTTLPR allele carriers showed abnormal emotional processing (e.g., more likely to make errors of commission) than long 5-HTTLPR allele homozygotes on an affective Go/No-Go test. Similarly, Hayden et al. (2008) reported that children who were homozygous for the short 5-HTTLPR allele showed greater self-referent encoding of negative stimuli than the other 5-HTTLPR allele groups. Consistent with these analyses, work by Fallgatter and colleagues using event-related potentials indicates that short 5-HTTLPR allele carriers experience greater brain electrical activity during evaluation of an erroneous behavioral response than age and gender-matched controls homozygous for the long 5-HTTLPR allele (Fallgatter et al., 2004).
The current study builds upon this research and provides an additional test of whether the 5-HTTLPR polymorphism is associated with biased processing of emotional stimuli. In our previous work, using a standard dot-probe task, Beevers et al. (2007) reported that short 5-HTTLPR allele carriers displayed biased attention for anxious-relevant words when paired with neutral words compared to long 5-HTTLPR allele homozygotes. Although those findings were intriguing, that study had a number of limitations. Participants were a heterogeneous mix of psychiatric inpatients experiecing a variety of emotional states and pharmacological treatments, which may have influenced the serotonin system and emotion processing. Further, there are concerns that the dot-probe task, especially when used with longer stimulus durations, may not directly assess attention engagement but might instead assess difficulty with the disengagement of attention. As a result, researchers have suggested using tasks that separately measure attention engagement and disengagement (e.g., Koster, De Raedt, Goeleven, Franck, & Crombez, 2005; Posner & Petersen, 1990).
We therefore used a spatial cueing task that allowed us to assess general attentional bias, attentional engagement, and difficulty with attentional disengagement from emotional stimuli (Koster et al., 2005). For this version of the task, a cue is presented to the left or right side of visual field. Cues are faces expressing an emotion (e.g., happiness, sadness) or no emotion (e.g., neutral). After the offset of the cue, a target appears either in the same location of the cue (e.g., valid cue trial) or in the opposite side of visual field (e.g., invalid cue trial). The dependent measure is latency to identify the location of the cue. Typically, reaction times are shorter for valid cue trials than invalid cue trials, although this pattern can reverse when cues are presented for longer durations (i.e., inhibition of return effect). The difference in mean reaction time for invalid and valid trials is referred to as the cue validity effect. A positive cue validity effect is interpreted as maintained attention to the cue.
With the emotional modification of the spatial cuing task it is possible to determine whether the cue validity effect is influenced by cue valence (e.g., happy, sad, neutral facial expressions). Three different but related outcomes can be examined: (a) general measure of biased attention, (b) attentional engagment, and (c) difficulty with attentional disengagment. Each of these outcomes compares reaction time to identify targets following emotional cues relative to neutral cues. For instance, if the cue validity effect was stronger for emotional cues than neutral cues, we would observe a positive biased attention score for emotional stimuli. This would indicate attention was maintained by emotional cues to a greater extent than neutral cues (Mogg, Holmes, Garner, & Bradley, 2008). Similarly, faster latencies following emotional valid cues than neutral valid cues suggests that emotional stimuli facilitated attentional engagement, whereas longer latencies following invalid emotional cues compared to invalid neutral cues suggests difficulty disengaging attention from emotional cues. Together, these outcomes provide a general assessment of maintained attention and the specific attentional processes related to maintained attention (Koster et al., 2005).
Using the spatial cueing task, we conducted two studies designed to address the limitations of our previous study and provide a more precise test of the hypothesis that the 5-HTTLPR polymophism is associated with attentional biases for emotional stimuli. Specifically, in Study 1, we recruited a sample of healthy adults who did not meet criteria for a current psychiatric disorder or a past history of a mood disorder and who were not currently taking any psychiatric medications. These individuals were genotyped for the 5-HTTLPR polymorphism and then completed a spatial cueing task that used sad, happy, and neutral facial expressions as cues. For Study 2, we recruited a large sample of unmedicated, non-depressed undergraduate students. We administered the same spatial cueing task as in Study 1 but also included fear facial expressions as cues.
We expected to observe an interaction between cue validity, stimuli valence, and 5-HTTLPR allele group when predicting response time latencies on the spatial cueing task. We hypothesized that carriers of the low expressing 5-HTTLPR allele would display biased attention for emotional stimuli in general; however, given the lack of specificity with which we measured biased attention in our previous work, it was unclear whether we should expect enhanced attentional engagement or increased difficulty with attentional disengagement among the low expressing 5-HTTLPR allele carriers. Therefore, we did not make specific hypotheses regarding each component of biased attention.
Study 1
Participants
Participants were thirty-eight adults recruited from the Austin, Texas community (see Table 1 for demographic information). On average, the sample was in their early 30s, Caucasian, female, and educated. Participants were recruited using flyers posted in the community and with ads posted on electronic websites. Participants received $15/hour for their participation. The average laboratory session was 2 hours. Interviewers used structured clinical interviews to determine presence and history of psychopathology. Inclusion criteria included normal or corrected to normal vision, fluency in the English language, and age between 22 and 60. Exclusion criteria included the presence of any current Axis I disorder. Further, participants with a history of Major Depressive Disorder, Dysthymia, or were currently prescribed psychiatric medication were also excluded.
Table 1.
Study 1 demographics as a function of 5-HTTLPR allele status.
| LL (n = 14) | SL (n = 17) | SS (n = 7) | |
|---|---|---|---|
| Age (years) | 28.79 (7.77) | 30.07 (6.65) | 32.86 (6.59) |
| Gender (M/F) | 21%/79% | 18%/82% | 43%/57% |
| Hispanic (Yes/No) | 7%/93% | 11%/89% | 28%/72% |
| Race (Caucasian/Other) | 93%/7% | 77%/23% | 72%/28% |
| Married (yes/no) | 36%/64% | 24%/76% | 28%/72% |
| College degree (yes/no) | 64%/36% | 59%/41% | 85%/15% |
| Income (mean) | $38,500 | $50,400 | $39,142 |
| Depressive symptoms (BDI-II) | 2.64 (4.19) | 6.07 (8.82) | 4.86 (6.07) |
Assessments
Mini International Neuropsychiatric Interview (MINI)
The electronic version of the MINI was used as part of a screening interview to determine whether participants met criteria for study entry. The MINI is a short, structured screening interview that was developed for DSM-IV and ICD-10 psychiatric disorders (Kendler et al., 2005; Sheehan et al., 1998). The MINI assesses diagnostic criteria for the following disorders: MDD, Dysthymia, Panic Disorder, Agoraphobia, Social Phobia, Obsessive Compulsive Disorder, Post-traumatic Stress Disorder, Psychotic Disorder, Anorexia Nervosa, Bulimia Nervosa, Generalized Anxiety Disorder, and Antisocial Personality Disorder. It also assesses for suicidal ideation and behavior, mania, and hypomania. The MINI has been validated against the Structured Clinical Interview for DSM-IV diagnoses and against the Composite International Diagnostic Interview for ICD-10 (Lecrubier et al., 1997; Sheehan et al., 1998).
Interviewers were undergraduate research assistants who received at least 10 hours of training, wherein they learned interview skills, reviewed diagnostic criteria, and role-played interviews. Because this was a screening interview, brevity was important. Interviewers could terminate the interview as soon as the participant did not meet study criteria. Therefore, the entire MINI was typically only completed for participants who met criteria for study entry. The average length of MINI screening interview was approximately 15 minutes.
Structured Clinical Interview for DSM-IV Diagnoses (SCID)
To confirm key inclusion/exclusion criteria from the screening interview, the Mood Disorders and the Anxiety Disorders Modules of the Structured Clinical Interview for the DSM-IV Diagnoses – Patient Version (SCID; First, Spitzer, Gibbon, & Williams, 1998) were administered during an in-person interview at the time of study participation. Three assessors with at least a bachelor’s degree in psychology conducted all interviews. Assessors participated in 15 hours of training, wherein they learned interview skills, reviewed diagnostic criteria for relevant Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV; American Psychiatric Association, 1994), observed mock interviews, and role-played interviews. Twenty percent of all interviews were rated by an independent assessor. Agreement between study interviewer and independent assessor diagnosis of MDD was excellent (kappa [k] = 1.0).
Beck Depression Inventory – II (BDI-II; Beck, Brown, & Steer, 1996)
The BDI-II is a widely used self-report questionnaire to assess depression severity. The BDI-II consists of 21 items and measures the presence and severity of cognitive, motivational, affective, and somatic symptoms of depression. Past reports indicate test-retest reliability is adequate (Beck, Steer, & Garbin, 1988). The BDI has been found to be valid among psychiatric inpatient and outpatient samples (Beck et al., 1988). Internal consistency reliability in the current study was good (α = .81).
Genotyping
Genomic DNA were isolated from buccal cells using a modification of published methods (Freeman et al., 1997; Lench, Stanier, & Williamson, 1988; Meulenbelt, Droog, Trommelen, Boomsma, & Slagboom, 1995; Spitz et al., 1996). The cheeks and gums are rubbed for 20 s with three sterile, cotton-tipped wooden swabs. The swabs are placed in a 50-ml capped polypropylene tube containing lysis buffer (500 μl of 1 M Tris-HCl; 200 mM disodium ethylene diaminetetracetic acid (EDTA), pH 8.0; 500 μl of 10% sodium docecyl sulfate; and 100 μl of 5 M sodium chloride). The subjects then rinse out the mouth vigorously with 10 ml of distilled water for 20 seconds and this was added to the 50-ml tube. The tubes were stored at 4°C until the DNA was extracted.
To extract the DNA, proteinase K (0.2mg/ml) was added to the tubes and the tubes were incubated at 65°C for 60 min. The swabs were removed and residual lysis buffer was extracted by centrifugation (using a 3-ml syringe barrel and sterile 15ml tube) for 5 minutes at 1,000 x g. The residual fluid was added back to the original sample. An equal volume of isopropyl alcohol was then added to each tube, the contents were mixed, and the DNA was collected by centrifugation at 3,500 x g for 10 min. The DNA pellet was rinsed once with 1ml of 50% isopropyl alcohol and allowed to air dry. The pellet was resuspended in 1 ml of 10μM TRIS-HCl, 10mM EDTA buffer (pH 8.0) and place in a 1.8-ml cryovial.
The 5-HTT gene (SLC6A4), which maps to 17q11.1–17q12, contains a 44 bp deletion in the 5′ regulatory region of the gene (Heils et al., 1996). The VNTR in the promoter appears to be associated with variations in transcriptional activity: the long (L) variant has approximately three times the basal activity of the shorter (S) promoter with the deletion (Lesch et al., 1996). The assay is a modification of the method of Lesch and colleagues (Lesch et al., 1996).
Amplification of 5-HTTLPR target sequences by polymerase chain reaction
Polymerase chain reactions contain 0.5 μl of genomic DNA (20 ng), 10% DMSO (Hybra-Max® grade; Sigma-Aldrich, St. Louis, MO), 1.8 mM MgCl2, 180 μM deoxynucleotides, with 7′-deaza-2′-deoxyGTP (Roche Applied Science, Indianapolis, IN) substituted for one half of the dGTP, forward and reverse primers (5HTT, 480 nM; obtained from IDT, Coralville, IA) and 1 U of AmpliTaq Gold® polymerase (Applied Biosystems, Foster City, CA), in a total volume of 20 μl. Amplification is performed using touchdown PCR (Don, Cox, Wainwright, Baker, & Mattick, 1991). A 95°C incubation for 10 min is followed by two cycles of 95°C for 30 s, 65°C for 30 s, and 72°C for 60 s. The annealing temperature is decreased every two cycles from 65°C to 57°C in 2°C increments (10 cycles total), and a final 30 cycles of 95°C for 30 s, 55°C for 30 s, and 72°C for 60 s and a final 30-min incubation at 72°C. After amplification, 1 μl of PCR product is combined with 2 μl of loading buffer containing size standard (Genescan 2500 TAMRA®; Applied Biosystems) and 0.8 μl is loaded into each of 48 lanes of a 12-cm gel. PCR products are electrophoresed through a 4.25% polyacrylamide gel under denaturing conditions (6 M urea) with an Applied Biosystems ABI Prism 377 DNA sequencer using protocols supplied by the company.
The primer sequences are: forward, 5′-GGCGTTGCCGCTCTGAATGC-3′ (fluorescently labeled), and reverse, 5′-GAGGGACTGAGCTGGACAACCAC-3′. These primer sequences yield products of 484 or 528 bp. Two investigators scored allele sizes independently and any inconsistencies were reviewed and rerun (less than 20% of sample). An additional 10% of the sample was randomly selected and rerun to ensure genotyping accuracy. There was 100% agreement for allele groupings between the initial and re-tested samples. The 5-HTTLPR allele frequency distribution was SS: n = 7 (18.4%), SL: n = 17 (44.7%), LL: n = 14 (36.8%). Genotype distribution was in Hardy-Weinberg equilibrium, (X2 = 0.207, p = 0.649), and is consistent with previous reports (Lesch et al., 1996).
Spatial cueing task
This task was developed by Posner (1980) and modified by others to incorporate emotional cues (e.g., Koster et al., 2005). Each trial sequence (shown in Figure 1) began by presenting a fixation cross in the center of the screen for 500ms. Then, a face cue was presented on either the left or right side of the visual field for 1500ms. After cue offset, a probe (either * or **) immediately appeared on the left or right side of visual field and remained on the screen until the participant responded by pressing an appropriate response box button. The participant’s task was to identify probe type as quickly and accurately as possible. Participants pressed a corresponding button on a response box to indicate the type of probe that appeared. After the participant responded, the screen was blank for 500ms before the next trial began. Seventy five percent of probes appeared on the same side of visual field as the cue (a valid trial). Twenty five percent of the probes appeared on the opposite side of visual field as the cue (an invalid trial). Both valid and invalid trials had a 50% chance of having either the single or double asterisk probe.
Cue stimuli were images of faces taken from the Pictures of Facial Affect (Ekman & Friesen, 1976) photo set. We selected human faces because facial expressions receive special processing priority (Farah, Wilson, Drain, & Tanaka, 1998), they have been used extensively in behavioral and imaging studies, and are arguably more ecologically valid than written words. We selected 12 faces from each of the following categories: happy, sad, and neutral. All stimuli were presented on a black background on a 17-inch color monitor. Stimuli were approximately 10.5 × 17 cm when presented on the screen. Participants completed 10 practice trials using neutral faces as cues. Anyone failing to respond accurately to at least 8 of the 10 trials repeated the practice trials until they achieved 80% accuracy. Participants then completed a total of 72 trials. They viewed each of the 36 stimuli twice. Order of stimulus presentation was randomized for each participant with the stipulation that each of the 36 stimuli were viewed once before stimuli were repeated.
We were interested in three primary outcomes from the spatial cueing task: general attentional bias, attentional engagement, and difficulty with attentional disengagement. As suggested by Mogg et al. (2008), a general measure of attentional bias can be derived from the spatial cueing task using the following formula:
| (1) |
This attentional bias score does not attempt to differentiate attention engagement versus disengagement. Positive values reflect an attentional bias for emotional cues relative to neutral cues. Negative values reflect an attentional bias for neutral cues relative to emotional cues.
Attentional engagement and difficulty with attention disengagement can be calculated for each of the emotion cue categories with the following formulae:
| (2) |
| (3) |
Positive attentional engagement scores indicate that participants identify the target more quickly when it follows an emotion cue from that category than when it follows a neutral cue. Negative scores indicate the reverse. Positive attentional disengagement scores indicate greater difficulty disengaging attention from an emotional cue than a neutral cue. That is, positive scores indicate a slower shift of attention away from an emotional cue than a neutral cue. Negative scores indicate less difficulty disengaging attention from emotional cues than a neutral cue. Mean reaction time scores are used to compute each of these outcomes.
Procedure
Participants contacted the Mood Disorders Laboratory at the University of Texas at Austin expressing an interest in study participation. The MINI screening interview was then conducted by a trained interviewer. If the participant passed the screening assessment, they were scheduled for a laboratory appointment. Upon arrival to the laboratory, participants were oriented to the laboratory, provided informed consent, and then completed a demographics form.
They next completed the SCID interview to confirm the absence of mood and anxiety disorders. Participants then completed several self-report questionnaires, and provided buccal cells via a cheek swab/mouthwash procedure for genotyping. Next, participants completed the spatial cueing task (and other tasks not included in this report). Upon completion of study procedures, participants were debriefed and paid $15/hour (up to a maximum of $50) for their participation. The Internal Review Board at the University of Texas at Austin approved all study procedures.
Results
Sample Characteristics
Descriptive statistics for the sample are presented in Table 1 stratified by 5-HTTLPR allele status. There were no significant differences as a function of allele grouping for age, F(2, 37) = 0.58, p = .57, gender, χ2 (2, N = 38) = 4.26, p = .12, ethnicity, χ2 (2, N = 38) = 1.93, p = .38, race, χ2 (2, N = 38) = 1.93, p = .38, marital status, χ2 (2, N = 38) = 0.55, p = .76, education,χ2 (2, N = 38) = 1.61, p = .44, income, F(2, 37) = 0.58, p = .57, and depressive symptoms, F(2, 37) = 0.65, p = .53.
Data Reduction
Trials with incorrect responses (0.4% of all trials) were deleted and not used for analyses. Further, reaction times that were at least three standard deviations beyond the individual reaction time mean were deleted (1.4%). Together, these procedures resulted in the exclusion of less than 1.9% of the data.
Main results
A 2 (cue validity: valid, invalid) × 3 (stimulus valence: sad, happy, neutral) × 3 (allele status: SS, SL, LL) mixed-plot analysis of variance (ANOVA) examined whether the 5-HTTLPR polymorphism was associated with latency to identify target (see Table 2 for descriptive statistics). Results indicated a significant cue validity main effect, F(1, 35) = 26.39, p < .001, η2 = .43, and a marginally significant stimuli valence × allele status interaction, F(4, 70) = 2.15, p = .08, η2 = .11. The cue validity × allele status interaction, F(2, 35) = 0.95, p = .40, η2 = .05, stimuli valence main effect, F(2, 70) = 1.70, p = .19, η2 = .05, cue validity × stimuli valence, F(2, 70) = 0.02, p = .98, η2 = .00, and the cue validity × stimuli valence × allele status interaction, F(4, 70) = 1.11, p = .36, η2 = .06, did not reach statistical significance. However, given the preliminary nature of Study 1, and that conducting a replication study (Study 2) with a larger sample will help to tease apart Type I errors from true effects, we examined whether 5-HTTLPR allele status was associated with biased attention, attention engagement, and attention disengagement.
Table 2.
Mean (standard deviations) latencies to identify target (ms) presented by cue validity, stimuli valence, and 5-HTTLPR allele status for Study 1.
| 5-HTTLPR allele group | ||||||
|---|---|---|---|---|---|---|
| LL | SL | SS | ||||
| Stimuli valence | Valid Cue | Invalid Cue | Valid Cue | Invalid Cue | Valid Cue | Invalid Cue |
| Happy | 645 (97) | 662 (110) | 702 (205) | 766 (231) | 616 (118) | 667 (146) |
| Sad | 634 (117) | 661 (92) | 696 (199) | 750 (212) | 619 (111) | 672 (135) |
| Neutral | 633 (94) | 684 (120) | 679 (197) | 728 (201) | 617 (126) | 643 (127) |
Attentional bias
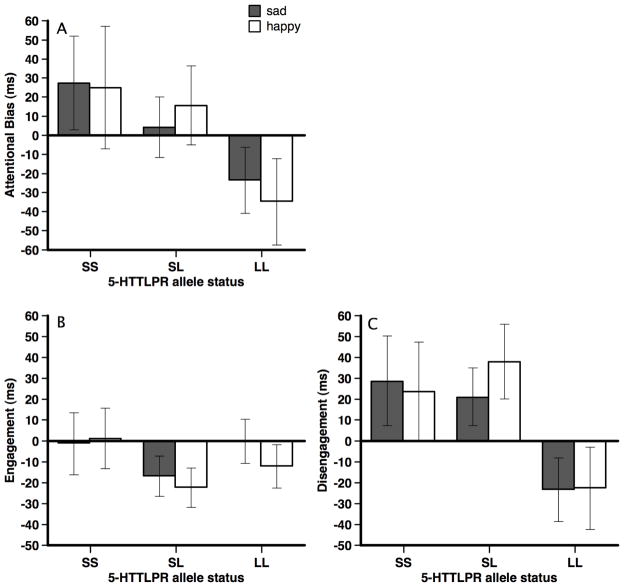
A 2 (stimulus valence: sad, happy) × 2 (allele status: SS, SL, LL) mixed-plot ANOVA examined whether 5-HTTLPR allele status predicted attentional bias scores. Results indicated a non-significant main effect for valence, F(1, 35) = .002, p = .96, η2 = .00, and a non-significant valence × allele status interaction, F(2, 35) = 0.26, p = .78, η2 = .01. However, the main effect for allele status approached significance, F(2, 35) = 2.50, p = .09, η2 = .12. Pairwise LSD comparisons indicated significant group differences between the LL and SS groups (p = .05), marginal difference between the LL and SL groups (p = .09), and no group differences between the SL and SS groups (p = .56). Mean attentional bias scores (and standard errors) collapsing across stimuli valence were LL: −29.23 (19.59), SL: 9.97 (12.20), SS: 26.28 (22.28). Figure 2A presents attentional bias scores for each stimulus valence.
Figure 2.
Mean attentional bias (panel A), attentional engagement (panel B), and difficulty with attentional disengagement (panel C) with standard errors presented as a function of stimuli valence and 5-HTTLPR allele status for Study 1.
Attention engagement and disengagement
A 2 (bias: engagement, disengagement) × 2 (stimulus valence: sad, happy) × 3 (allele status: S, SL, LL) mixed-plot analysis of variance (ANOVA) examined whether the 5-HTTLPR polymorphism was associated with attentional engagement and disengagement for emotional stimuli. Results indicated a significant interaction between bias and allele status, F(2, 35) = 3.63, p < .05, η2 = .17. The bias main effect, F(1, 35) = 2.71, p = .11, η2 = .07, stimulus valence main effect, F(1, 35) = 0.02, p = .96, η2 = .00, stimulus valence × allele status interaction, F(2, 35) = 0.26, p = .78, η2 = .01, bias × stimulus valence interaction, F(1, 35) = 0.53, p = .47, η2 = .02, and the three-way interaction, F(2, 35) = 0.66, p = .66, η2 = .02, did not reach statistical significance.
We followed up the significant interaction by examining attention engagement and disengagement scores across the genetic groups. As there were no significant effects involving stimuli valence, we collapsed across valence for these follow-up analyses. For attention engagement, results of a univariate ANVOA (allele status: S, SL, LL) revealed no significant differences between 5-HTTLPR allele groups, F(1, 35) = 1.17, p = .13, η2 = .06 (see Figure 2B). For attention disengagement, there was a significant main effect for allele status, F(2, 35) = 3.74, p < .05, η2 = .18. Pairwise LSD comparisons indicated that the LL group disengaged from emotional stimuli significantly faster than the SL group (p = .02) and marginally faster than the SS group (p = .06). There was no significant difference in disengagement between the SS and SL groups (p = .86; see Figure 2C).
Finally, we also examined whether attention engagement and disengagement scores (collapsing across valence) were significantly different from 0 for each of the genetic groups. For engagement scores, only the SL group was significantly different than 0, t(16) = −2.27, p < .05. For disengagement scores, the SL group was also significantly greater than 0, t(16) = 2.13, p < .05, whereas the SS, t(6) = 1.47, p = .17, and LL, t(13) = −1.47, p = .16, groups did not significantly differ from 0.
Discussion of Study 1
Findings from Study 1 provide tentative evidence that short 5-HTTLPR allele carriers exhibit biased attention for emotional stimuli compared to individuals homozygous for the long 5-HTTLPR allele. Further, the 5-HTTLPR polymorphsim was associated with difficulty disengaging attention from happy and sad facial expressions relative to neutral faces but was not associated with attentional engagement. This is among the first evidence that the 5-HTTLPR polymorphism influences attentional biases for emotional stimuli among healthy controls.
Nevertheless, we thought it was important to replicate and extend these initial findings for several reasons. First, initial behavioral genetics findings are often not replicated in subsequent studies, particularly when the initial studies involve small samples (Ioannidis, Ntzani, Trikalinos, & Contopoulos-Ioannidis, 2001). This concern is particularly relevant for the current study, as the predicted three-way interaction between cue validity, stimuli valence, and 5-HTTLPR allele status was not statistically significant. Thus, analyses of linkages between the 5-HTTLPR polymorphism and biased attention are likely associated with a higher Type I error rate than is typically tolerated in a single study. We therefore conducted a second test of the hypothesized three-way interaction in a larger sample of participants in Study 2.
Second, it has recently been discovered that the long 5-HTTLPR allele may have two variants (i.e., LA and LG). In the first of two extra 20 to 23 bp repeats in the L allele, a common single nucleotide polymorphism occurs at the sixth nucleotide (adenine to guanine; A to G). Importantly, the LG variant and the S allele appear to be very similar in terms of transcriptional activity; therefore, only the LA variant is high expressing with regard to transcriptional activity (Hu et al., 2005). In Study 2, we used this more recent approach to genotype the 5-HTTLPR and more precisely examine the association of the 5-HTTLPR genotype with biased attention.
Third, we added a third emotional stimulus category to the spatial cueing task, faces depicting fear. We included this stimulus category to determine whether carriers of the low expressing 5-HTTLPR allele display greater biased attention and experience greater difficulty disengaging their attention from negative stimuli other than sad stimuli. We therefore conducted a second study with the modifications described above to replicate and extend our initial findings.
Study 2
For Study 2, we recruited a convenient sample of non-depressed undergraduate students who provided buccal cells for genetic analyses and then completed the spatial cueing task. Based on findings from Study 1, we expected carriers of the low expressing 5-HTTLPR allele to exhibit greater biased attention for emotional stimuli than individuals homozygous for the high expressing 5-HTTLPR allele. Further, we also expected 5-HTTLPR allele group differences for difficulty disengaging attention but none were expected for attention engagement.
Participants
Participants were 144 non-depressed individuals recruited from introductory to psychology classes at the University of Texas at Austin. All participants had BDI-II scores of 9 or less and were unmedicated at the time of testing (see Table 3 for descriptive statistics). Participants received course credit for their participation. This study took approximately 30 minutes to complete.
Table 3.
Study 2 demographics as a function of 5-HTTLPR allele status.
| L′L′ (n = 31) | S′L′ (n = 84) | S′S′ (n = 29) | |
|---|---|---|---|
| Age (years) | 19.31 (1.71) | 19.02 (1.11) | 18.94 (1.10) |
| Gender (M/F) | 57%/43% | 40%/60% | 47%/53% |
| Hispanic (Yes/No) | 15%/84% | 17%/83% | 26%/74% |
| Race (White/Other) | 82%/18% | 70%/30% | 60%/40% |
| Marital Status (married: yes/no) | 6%/94% | 4%/96% | 3%/97% |
| Depressive symptoms (BDI-II) | 3.77 (2.21) | 2.88 (2.66) | 1.78 (2.44) |
Note: S′S′ includes the SS, LGLG, and SLG allele groups, S′L′ includes the SLA, LGLA allele groups, and the L′L′ includes the LALA allele groups.
Genotyping
DNA samples were genotyped for the 5-HTTLPR deletion polymorphism using methods described in Study 1. The 5-HTTLPR allele frequency distribution was SS: n = 28 (19.4%), SL: n = 73 (50.6%), LL: n = 43 (29.9%). Genotype distribution was in Hardy-Weinberg equilibrium, χ2 = 0.09, p = .76). To distinguish between the S, LA, and LG fragments, the PCR fragment was digested with MspI according to the methods found in Wigg et al. (2006). The resulting polymorphic fragments were separated using an ABI 377 DNA sequencer (S: 297, 127, 62 bp, LA: 340, 127, and 62 bp, LG: 174, 166, 127, and 62 bp). Using this approach, allele frequencies were S: n = 129 (44.7%), LA: n = 146 (50.6%), LG: n = 13 (4.5%). Genotype distribution for the A/G SNP was in Hardy-Weinberg equilibrium, χ2 = 0.32, p = .57.
Consistent with previous research (Hu et al., 2005; Zalsman et al., 2006), the low expressing S and LG alleles were designated S′ and the higher expressing LA allele was designated L′. We therefore formed three groups: (a) S′S′ (i.e., SS: n = 28 (19.4%), SLG: n = 1 (0.7%), LGLG: n = 0 (0%)), (b) S′L′ (i.e., SLA: n = 72 (50.0%), LGLA: n = 12 (8.3%)), and (c) L′L′ (i.e., LALA: n = 31 (21.5%)).
Procedure
Undergraduate students who scored less than 4 on the short-form of the Beck Depression Inventory (BDI-SF) during mass pre-testing were invited to participate in this study. Upon arrival to the laboratory, depression severity was re-assessed using the BDI-II. Participants with BDI-II scores greater than 9 or anyone currently taking psychiatric medications were excluded from this study. In contrast to Study 1, participants did not complete a SCID interview; however, all participants were experiencing low levels of depressive symptoms. Participants who qualified were then oriented to the laboratory, provided informed consent, and completed a demographics form. Participants completed several self-report questionnaires, and provided buccal cells via a cheek swab/mouthwash for genotyping. Next, participants completed the spatial cueing task. The Internal Review Board at the University of Texas at Austin approved all study procedures.
Spatial cueing task
The task was the same as in Study 1, with two important exceptions. First, we included 12 fear stimuli in addition to the sad, happy, and neutral stimuli used in Study 1. These stimuli were also selected from the Ekman collection. Further, participants were instructed to identify the location of the probe (left or right side of visual field) rather than the probe type. We made this change because probe location instructions can lead to fewer participant errors and produce results that are highly similar to probe identification instructions (Mogg & Bradley, 1999). The current task thus required participants to complete 96 trials (12 stimuli * 4 emotion categories * 2 presentations). As before, 75% of the trials were validly cued trials and 25% were invalidly cued trials. The probe was equally likely to appear on the left or right side of visual field following a valid and invalid trial.
Results and Discussion
Sample Characteristics
Descriptive statistics for the sample are presented in Table 3 stratified by 5-HTTLPR allele status. There were no significant differences as a function of allele grouping for age, F(2, 143) = 1.57, p = .21, gender, χ2 (2, N = 143) = 2.93, p = .23, ethnicity, χ2 (2, N = 143) = 2.43, p = .29, marital status, χ2 (2, N = 143) = 1.80, p = .41, and depressive symptoms, F(2, 143) = 2.78, p = .11. There were significant allele frequency differences for race, χ2 (2, N = 143) = 13.48, p < .01. Therefore, race was entered as a covariate in all subsequent analyses; however, race was not significantly associated with any of the attentional bias outcomes (see pg. 22).
Data Reduction
Trials with incorrect responses (0.5% of all trials) were deleted and not used for analyses. Further, reaction times that were at least three standard deviations beyond the individual reaction time mean were deleted (0.9%). Together, these procedures resulted in the exclusion of less than 1.5% of the data.
Main Results
A 2 (cue validity: valid, invalid) × 4 (stimulus valence: sad, happy, fear, neutral) × 3 (5-HTTLPR allele type: S′S′, S′L′, L′L′) mixed-plot analysis of variance (ANOVA) examined whether the 5-HTTLPR polymorphism was associated with latency to identify the target (see Table 4 for descriptive statistics). Results indicated a significant three-way interaction between stimulus valence, cue validity, and 5-HTTLPR allele status, F(6, 420) = 2.32, p = .03, η2 = .03. The cue validity main effect, F(1, 140) = 0.12, p = .73, η2 = .00, cue validity × allele type interaction, F(2, 141) = 1.07, p = .35, η2 = .00, stimulus valence main effect, F(3, 420) = 1.39, p = .24, η2 = .01, stimulus valence × allele group interaction, F(6, 420) = 0.94, p = .46, η2 = .01, and the stimulus valence × cue validity interaction, F(3, 420) = 0.75, p = .94, η2 = .00, did not reach statistical significance. Further, the race main effect, F(1, 140) = 0.70, p = .98, η2 = .00, race × valence interaction, F(3, 420) = 1.21, p = .30, η2 = .01, cue validity × race interaction, F(1, 140) = 0.53, p = .47, η2 = .00, valence x cue validity × race interaction, F(3, 420) = 0.32, p = .81, η2 = .00, were all non-significant. We followed up the significant three-way interaction by examining attentional bias, attentional disengagement, and attentional disengagement computed as described in Study 1.
Table 4.
Mean (standard deviation) latencies in milliseconds to identify target presented by cue validity, stimuli valence, and 5-HTTLPR allele status for Study 2.
| 5-HTTLPR allele group | ||||||
|---|---|---|---|---|---|---|
| L′L′ | S′L′ | S′S′ | ||||
| Stimuli valence | Valid Cue | Invalid Cue | Valid Cue | Invalid Cue | Valid Cue | Invalid Cue |
| Happy | 419 (61) | 406 (59) | 420 (52) | 419 (59) | 431 (74) | 447 (83) |
| Sad | 416 (56) | 412 (59) | 420 (59) | 423 (72) | 437 (74) | 446 (86) |
| Fear | 418 (57) | 413 (55) | 423 (52) | 420 (58) | 441 (87) | 442 (85) |
| Neutral | 418 (59) | 419 (51) | 418 (54) | 421 (66) | 436 (85) | 431 (80) |
Attentional bias
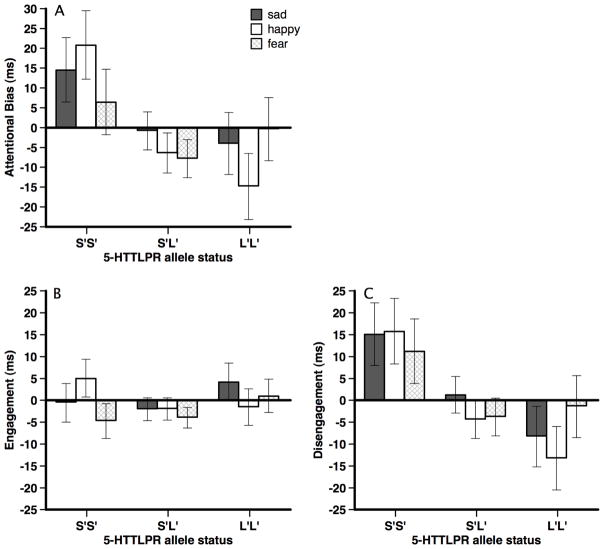
A 3 (stimulus valence: sad, happy, fear) × 2 (allele status: S′S′, S′L′, L′L′) mixed-plot ANOVA examined whether 5-HTTLPR allele status predicted attentional bias scores. Results indicated a non-significant main effect for stimuli valence, F(2, 282) = 0.60, p = .55, η2 = .00, and a marginally significant stimuli valence × allele status interaction, F(4, 282) = 2.02, p = .09, η2 = .03. However, the main effect for allele status was significant, F(2, 141) = 3.01, p = .05, η2 = .04. Pairwise LSD comparisons indicated significant group differences between the L′L′ and S′S′ groups (p = .04), no difference between the L′L′ and S′L′ groups (p = .86), and a significant group difference between the S′L′ and S′S′ groups (p = .02). Mean attentional bias scores (and standard errors) collapsing across stimuli valence were L′L′: −6.41 (6.84), S′L′: −5.01 (4.16), SS: 13.97 (7.08). Thus, the S′S′ group showed a significantly greater attentional bias than the L′L′ and S′L′ groups. Figure 3A presents attentional bias scores for each stimulus valence.
Figure 3.
Mean attentional bias (panel A), attention engagement (panel B), and difficulty with attention disengagement (panel C) with standard errors presented as a function of stimulus valence and 5-HTTLPR allele status for Study 2. Note that S′S′ includes the SS, LGLG, and SLG allele groups, S′L′ includes the SLA, LGLA allele groups, and L′L′ includes the LALA allele group.
Attention engagement and disengagement
A 2 (bias: engagement, disengagement) × 3 (stimulus valence: sad, happy, fear) × 3 (allele status: S′S′, S′L′, L′L′) mixed-plot analysis of variance (ANOVA) examined whether 5-HTTLPR polymorphism was associated with attentional engagement and disengagement for emotional stimuli. Results indicated a significant stimuli valence × allele status interaction, F(4, 282) = 2.67, p = .03, η2 = .04, and a significant bias × allele status interaction, F(2, 141) = 3.82, p < .05, η2 = .05. The stimulus valence main effect, F(2, 282) = 0.45, p = .77, η2 = .00, bias main effect, F(1, 140) = 0.42, p = .51, η2 = .00, stimulus valence × bias interaction, F(2, 282) = 1.18, p = .31, η2 = .01, and the three-way interaction were not significant, F(4, 282) = 0.45, p = .77, η2 = .01.
We followed up the significant bias × allele status interaction by examining reaction time for each attention bias type (collapsing across stimuli valence) for the 5-HTTLPR allele groups with a one-way ANOVA. For attention engagement, there was not a significant allele group effect, F(2, 141) = 0.56, p = .57 (see Figure 3B). In contrast, for attention disengagement, there was a significant allele group effect, F(2, 141) = 3.79, p = .03. Further, group comparisons indicated that the S′S′ allele group had significantly greater difficulty disengaging their attention from emotional stimuli than the S′L′ and the L′L′ groups (ps < .01). The S′L′ and the L′L′ groups did not differ from each other (p = .44; see Figure 3C). Mean disengagement scores (and standard errors) collapsing across stimuli valence were LL: −7.64 (5.99), SL: −2.32 (3.64), SS: 14.05 (5.27).
We also examined whether the engagement and disengagement scores were significantly different from 0 for each of the genetic groups. For engagement scores, none of the groups were significantly different from 0 (ts < |1.5|, ps > .13). However, for disengagement scores, the S′S′ group was significantly greater than 0, t(28) = 2.66, p = .01. The S′L′ and L′L′ groups were not significantly different from 0, (ts < |1.2|, ps > .21). People homozygous for the low expressing 5-HTTLPR allele had particular difficulty disengaging their attention from emotional cues relative to neutral cues.1
General Discussion
Results from these two studies suggest that the low-expressing 5-HTTLPR allele (i.e., S or LG) is significantly associated with attentional biases for emotional information among healthy, non-depressed individuals. Further, difficulty disengaging attention from emotional information appeared to be primarily responsible for these effects. More specifically, in Study 1, short 5-HTTLPR allele carriers took significantly longer than long allele homozygotes to disengage their attention from happy and sad stimuli relative to neutral stimuli. In Study 2, individuals homozygous for the low expressing 5-HTTLPR allele (i.e., S, LG) took significantly longer than individuals homozygous for the high expressing allele (i.e., LA) to disengage their attention from happy, sad, and fear stimuli relative to neutral stimuli. These studies are among the first to demonstrate that attentional biases for emotional stimuli may have a genetic component.
Identifying the genetic etiology of attentional biases for emotional information may have important implications for understanding vulnerability to emotional disorders. For instance, the ability to effectively regulate attention away from emotional stimuli has been associated with current depression (Koster et al., 2005), depression vulnerability (Beevers & Carver, 2003), and enhanced emotional reactivity (MacLeod, Rutherford, Campbell, Ebsworthy, & Holker, 2002). Further, Compton (2000) found that difficulty disengaging attention from an invalidly cued location was associated with experiencing heightened negative affect after viewing a distressing film. Future work should examine whether the 5-HTTLPR polymorphism hampers effective emotion regulation via its influence on the disengagement of attention from emotional stimuli. Additional research that measures genotype, biased attention, and emotion regulation is needed to test this possibility.
In contrast to the associations between 5-HTTLPR genotype and difficulty disengaging attention, we did not observe any attention engagement effects. This is likely due to duration of stimulus presentation. Indeed, as cue duration increases, attention for the location of the cue becomes inhibited in favor of new locations (Posner & Petersen, 1990). A 1500 millisecond cue duration used in the current study likely provides sufficient time to orient attention toward the cued location, and is therefore not likely to detect enhanced engagement of attention. Our previous work, however, has found significant genotype associations with biased attention for anxiety-related stimuli that were presented for much briefer durations (e.g., 14 and 750 milliseconds, Beevers et al., 2007). Future work should consider including a variety of cue durations to identify how the 5-HTTLPR polymorphism is associated with attentional engagement and disengagement over the time course of attentional processing. Methodologies that allow for more continuous assessment of attention processing (e.g., eye tracking, evoked response potential) may be particularly effective at disentangling these effects.
It was intriguing that individuals homozygous for the low expressing 5-HTTLPR alleles had difficulty disengaging their attention from negative and positive stimuli. It may be that the short 5-HTTLPR allele carriers have increased sensitivity to emotion in general. There is neurological evidence to suggest that this is the case. For instance, Canli et al. (2005) reported that the 5-HTTLPR polymorphism was associated with differential neural activation in response to negative and positive stimuli in limbic, striatal, and cortical regions. They suggested that genetically influenced serotonin transport efficiency may have a greater role in how emotional information in general is processed at a neural level than previously thought. The current findings are consistent with this conclusion and extend the Canli et al. (2005) findings to the psychological level.
Future research examining the 5-HTTLPR polymorphism should also consider simultaneously examining a second 5-HTT polymorphism, the SLC6A4 intron2 VNTR polymorphism. The 10-repeat SLC6A4 intron2 VNTR allele is associated with relatively decreased 5-HTT expression in comparison with the 12-repeat allele (Hranilovic et al., 2004). Thus, if relatively increased 5-HT signaling is partly responsible for the effects observed in this study, 10-repeat allele carriers should exhibit biased attention and greater difficulty disengaging attention from emotional stimuli than 12-repeat carriers. Convergence of findings across the 5-HTTLPR and SLC6A4 intron 2 VNTR polymorphisms would provide compelling evidence for the role of 5-HT signaling in biased attention for emotional stimuli. We hope future research will be able to address this issue.
Neuroimaging work may also provide important insights into the neural mediators of difficulty disengaging from emotional material. For instance, there is remarkably consistent evidence for the effect of the 5-HTTLPR polymorphism on amygdala reactivity (for a meta-analysis, see Munafo, Brown, & Hariri, 2008), which may be due to the effect of the 5-HTTLPR polymorphism on downstream targets such as the 5-HT1A autoreceptor (Hariri & Holmes, 2006). Imaging studies with adults suggest that the 5-HTTLPR polymorphism is associated with structural alterations and disrupted functional activation in cortical and limbic areas that are critical for the processing of emotional stimuli (Pezawas et al., 2005). It may be that short 5-HTTLPR allele carriers have poorer prefrontal cortical control over automatic neural responses in the amygdala, which in turn, contributes to greater difficulty disengaging their attention from emotional stimuli. Additional work is needed to test this hypothesis.
Several limitations of this study should be noted. Recent research with the spatial cueing task suggests that briefly presented emotional cues may impart greater slowing effects on motor responses than neutral cues. For instance, Mogg, Holmes, Garner, and Bradley (2008) demonstrated significant motor slowing effects on reaction time when emotional cues were presented for 200 milliseconds. Whether similar motor slowing effects are observed for cues that are presented for much longer durations, as in the current study (i.e., 1500 milliseconds), remain to be determined. Further, it is important to note that the assessment of general biased attention (see equation #1) in this paradigm is not influenced by motor slowing effects. Nevertheless, future work in this area would be well served to measure the impact of emotional cues on motor responses in order to control for any effects (for an example, see Mogg et al., 2008). Doing so could help to more precisely identify the mechanisms that produce increased reaction time responses to the invalid emotional cues relative to neutral cues among individuals homozygous for the low expressing 5-HTTLPR polymorphism.
We also only examined a relatively new single nucleotide polymorphism in the long version of the 5-HTTLPR promoter polymorphism (Hu et al., 2005) in Study 2. However, it is encouraging that the results were largely consistent across studies even though we only examined the deletion polymorphism in Study 1. Analyses from Study 2 also indicated that results obtained from the traditional bi-allelic classification were highly consistent with those using the tri-allelic classification (see Footnote 1). In addition, the spatial cueing task utilized in the current study was briefer than versions used in past research, in part because this task was presented in the context of a larger study and we wanted to minimize participant burden. Future research should consider using spatial cueing task with more trials.
As with any genetic association study, population stratification is a potential concern (Hutchison, Stallings, McGeary, & Bryan, 2004). Population stratification occurs when cases and controls differ with respect to their ethnic background or another variable that may have led to a pattern of non-random mating. In our study, this confound is unlikely as 5-HTTLPR allele frequencies did not differ across race or ethnicity in Study 1. Allele frequencies did differ across race in Study 2; however, race was unrelated to attentional bias and statistically controlling for race had no effect on our findings. Third variable explanations, such as the possibility that the 5-HTTLPR promoter polymorphism is in linkage disequilibrium with another functional genetic marker, should also be considered as explanations for the observed effects.
Despite these limitations, we believe this study makes an important and interesting contribution to understanding depression vulnerability. Individuals who inherit two copies of the low expressing variants of the 5-HTTLPR gene may be more sensitive to life stress and thus at greater risk for depression because they have greater difficulty disengaging their attention from emotional relative to neutral information. Additional work is now needed that examines complex etiological models of depression that link these various mechanisms of risk. By studying mechanisms of risk across levels of analyses (genetic, neural, cognitive, environmental), we may be able to develop more comprehensive models of depression vulnerability and further our understanding of this debilitating disorder.
Acknowledgments
We thank the research assistants from the Mood Disorders Laboratory at the University of Texas for their assistance with data collection.
Preparation of this article was facilitated by a grant (R01MH076897) from the National Institute of Mental Health to Christopher Beevers and shared equipment grants (1S10RR023457-01A1) from the National Center for Research Resources and the Department of Veteran Affairs to John McGeary.
Footnotes
We also conducted analyses using the traditional (or bi-allelic) 5-HTTLPR polymorphism groups (i.e., not accounting for the LA and LG variants of the L allele). The cue validity (valid, invalid) x stimulus valence (sad, happy, fear, neutral) x 5-HTTLPR allele type (SS, SL, LL) interaction approached significance for the prediction of latency to identify the target, F(6, 420) = 1.98, p = .07, η2 = .03. Further, the attentional bias (engagement, disengagement) x stimulus valence (sad, happy, fear) x allele status (SS, SL, LL) interaction was statistically significant, F(2, 140) = 3.77, p < .05, η2 = .05. Results were therefore largely consistent for the bi-allelic and tri-allelic 5-HTTLPR classifications in Study 2.
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/abn.
Contributor Information
Christopher G. Beevers, Department of Psychology, University of Texas at Austin
Tony T. Wells, Department of Psychology, University of Texas at Austin
Alissa J. Ellis, Department of Psychology, University of Texas at Austin
John E. McGeary, Providence Veterans Affairs Medical Center and Center for Alcohol and Addiction Studies, Brown University
References
- Beevers CG, Carver CS. Attentional bias and mood persistence as prospective predictors of dysphoria. Cognitive Therapy and Research. 2003;27:619–637. [Google Scholar]
- Beevers CG, Gibb BE, McGeary JE, Miller IW. Serotonin Transporter Genetic Variation and Biased Attention for Emotional Word Stimuli Among Psychiatric Inpatients. Journal of Abnormal Psychology. 2007;116(1):208. doi: 10.1037/0021-843X.116.1.208. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Arciero G, Rubino V, Latorre V, De Candia M, Mazzola V, et al. Variation of Human Amygdala Response During Threatening Stimuli as a Function of 5′HTTLPR Genotype and Personality Style. Biological Psychiatry. 2005;57(12):1517. doi: 10.1016/j.biopsych.2005.02.031. [DOI] [PubMed] [Google Scholar]
- Canli T, Omura K, Haas BW, Fallgatter A, Constable RT, Lesch KP. Beyond affect: A role for genetic variation of the serotonin transporter in neural activation during a cognitive attention task. PNAS. 2005;102(34):12224–12229. doi: 10.1073/pnas.0503880102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Compton RJ. Ability to disengage attention predicts negative affect. Cognition & Emotion. 2000;14(3):401–415. [Google Scholar]
- Cunningham WA, Van Bavel JJ, Johnsen IR. Affective Flexibility: Evaluative Processing Goals Shape Amygdala Activity. Psychological Science. 2008;19(2):152–160. doi: 10.1111/j.1467-9280.2008.02061.x. [DOI] [PubMed] [Google Scholar]
- Don RH, Cox PT, Wainwright BJ, Baker K, Mattick JS. ‘Touchdown’ PCR to circumvent spurious priming during gene amplification. Nucleic Acids Research. 1991;19(14):4008. doi: 10.1093/nar/19.14.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging studies of mood disorders. Biological Psychiatry. 2000;48(8):813. doi: 10.1016/s0006-3223(00)01020-9. [DOI] [PubMed] [Google Scholar]
- Fallgatter AJ, Herrmann MJ, Roemmler J, Ehlis AC, Wagener A, Heidrich A, et al. Allelic variation of serotonin transporter function modulates the brain electrical response for error processing. Neuropsychopharmacology. 2004;29(8):1506. doi: 10.1038/sj.npp.1300409. [DOI] [PubMed] [Google Scholar]
- Farah MJ, Wilson KD, Drain M, Tanaka JN. What is “special” about face perception? Psychological Review. 1998;105:482–498. doi: 10.1037/0033-295x.105.3.482. [DOI] [PubMed] [Google Scholar]
- Freeman B, Powell J, Ball D, Hill L, Graig I, Plomin R. DNA by mail: An inexpensive and noninvasive method for collecting DNA samples from widely dispersed populations. Behavioral Genetics. 1997;27:251–257. doi: 10.1023/a:1025614231190. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Drabant EM, Munoz KE, Kolachana BS, Mattay VS, Egan MF, et al. A susceptibility gene for affective disorders and the response of the human amygdala. Arch Gen Psychiatry. 2005;62(2):146–152. doi: 10.1001/archpsyc.62.2.146. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Holmes A. Genetics of emotional regulation: the role of the serotonin transporter in neural function. Trends in Cognitive Sciences. 2006;10(4):182. doi: 10.1016/j.tics.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297(5580):400. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Hasler G, Drevets WC, Manji HK, Charney DS. Discovering endophenotypes for major depression. Neuropsychopharmacology. 2004;29(10):1765. doi: 10.1038/sj.npp.1300506. [DOI] [PubMed] [Google Scholar]
- Hayden EP, Dougherty LR, Maloney B, Olino TM, Sheikh H, Durbin CE, et al. Early-emerging cognitive vulnerability to depression and the serotonin transporter promoter region polymorphism. Journal of Affective Disorders. 2008;107(1–3):227–230. doi: 10.1016/j.jad.2007.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heils A, Teufel A, Petri S, Stober G, Riederer P, Bengel D, et al. Allelic variation of the human serotonin transporter gene expression. Journal of Neurochemistry. 1996;66:2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- Heinz A, Braus DF, Smolka MN, Wrase J, Puls I, Hermann D, et al. Amygdala-prefrontal coupling depends on a genetic variation of the serotonin transporter. Nat Neurosci. 2005;8(1):20. doi: 10.1038/nn1366. [DOI] [PubMed] [Google Scholar]
- Heinz A, Jones DW, Mazzanti C, Goldman D, Ragan P, Hommer D, et al. A relationship between serotonin transporter genotype and in vivo protein expression and alcohol neurotoxicity. Biol Psychiatry. 2000;47(7):643–649. doi: 10.1016/s0006-3223(99)00171-7. [DOI] [PubMed] [Google Scholar]
- Hranilovic D, Stefulj J, Schwab S, Borrmann-Hassenbach M, Albus M, Jernej B, et al. Serotonin transporter promoter and intron 2 polymorphisms: relationship between allelic variants and gene expression. Biological Psychiatry. 2004;55(11):1090–1094. doi: 10.1016/j.biopsych.2004.01.029. [DOI] [PubMed] [Google Scholar]
- Hu X, Oroszi G, Chun J, Smith TL, Goldman D, Schuckit MA. An expanded evaluation of the relationship of four alleles to the level of response to alcohol and the alcoholism risk. Alcohol Clin Exp Res. 2005;29(1):8–16. doi: 10.1097/01.alc.0000150008.68473.62. [DOI] [PubMed] [Google Scholar]
- Hutchison KE, Stallings M, McGeary J, Bryan A. Population Stratification in the Candidate Gene Study: Fatal Threat or Red Herring? Psychological Bulletin. 2004;130(1):66. doi: 10.1037/0033-2909.130.1.66. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Kuhn JW, Vittum J, Prescott CA, Riley B. The Interaction of Stressful Life Events and a Serotonin Transporter Polymorphism in the Prediction of Episodes of Major Depression. Archives of General Psychiatry. 2005;62(5):529. doi: 10.1001/archpsyc.62.5.529. [DOI] [PubMed] [Google Scholar]
- Koster EHW, De Raedt R, Goeleven E, Franck E, Crombez G. Mood-Congruent Attentional Bias in Dysphoria: Maintained Attention to and Impaired Disengagement From Negative Information. Emotion. 2005;5(4):446. doi: 10.1037/1528-3542.5.4.446. [DOI] [PubMed] [Google Scholar]
- Lecrubier Y, Sheehan DV, Weiller E, Amorim P, Bonora I, Sheehan KH, et al. The Mini International Neuropsychiatric Interview (MINI): A short diagnostic structured interview: Reliability and validity according to the CIDI. European Psychiatry. 1997;12(5):224. [Google Scholar]
- Lench N, Stanier P, Williamson R. Simple non-invasive method to obtain DNA for gene analysis. Lancet. 1988;1(8599):1356–1358. doi: 10.1016/s0140-6736(88)92178-2. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274(5292):1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- MacLeod C, Rutherford E, Campbell L, Ebsworthy G, Holker L. Selective attention and emotional vulnerability: Assessing the causal basis of their association through the experimental manipulation of attentional bias. Journal of Abnormal Psychology. 2002;111(1):107–123. [PubMed] [Google Scholar]
- Meulenbelt I, Droog S, Trommelen GJ, Boomsma DI, Slagboom PE. High-yield noninvasive human genomic DNA isolation method for genetic studies in geographically dispersed families and populations. American Journal of Human Genetics. 1995;57:1252–1254. [PMC free article] [PubMed] [Google Scholar]
- Mogg K, Holmes A, Garner M, Bradley BP. Effects of threat cues on attentional shifting, disengagement and response slowing in anxious individuals. Behaviour Research and Therapy. 2008;46(5):656–667. doi: 10.1016/j.brat.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafo MR, Brown SM, Hariri AR. Serotonin Transporter (5-HTTLPR) Genotype and Amygdala Activation: A Meta-Analysis. Biological Psychiatry. 2008;63(9):852–857. doi: 10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nature Neuroscience. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The Attention System of the Human Brain. Annual Review of Neuroscience. 1990;13(1):25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK. Developing mechanisms of self-regulation. Development & Psychopathology. 2000;12:427–441. doi: 10.1017/s0954579400003096. [DOI] [PubMed] [Google Scholar]
- Roiser JP, Cook LJ, Cooper JD, Rubinsztein DC, Sahakian BJ. Association of a Functional Polymorphism in the Serotonin Transporter Gene With Abnormal Emotional Processing in Ecstasy Users. Am J Psychiatry. 2005;162(3):609–612. doi: 10.1176/appi.ajp.162.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998;59(20):22. [PubMed] [Google Scholar]
- Spitz E, Moutier R, Reed T, Busnel MC, Marchaland C, Roubertoux PL, et al. Comparative diagnoses of twin zygosity by SSLP variant analysis, questionnaire, and dermatoglyphic analysis. Behavior Genetics. 1996;26:55–64. doi: 10.1007/BF02361159. [DOI] [PubMed] [Google Scholar]
- Whalen PJ. The uncertainty of it all. Trends in Cognitive Sciences. 2007;11(12):499–500. doi: 10.1016/j.tics.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Wigg KG, Takhar A, Ickowicz A, Tannock R, Kennedy JL, Pathare T, et al. Gene for the serotonin transporter and ADHD: No association with two functional polymorphisms. American journal of medical genetics. Part B, Neuropsychiatric genetics. 2006;141(6):566–570. doi: 10.1002/ajmg.b.30247. [DOI] [PubMed] [Google Scholar]
- Zalsman G, Huang Y-y, Oquendo MA, Burke AK, Hu X-z, Brent DA, et al. Association of a Triallelic Serotonin Transporter Gene Promoter Region (5-HTTLPR) Polymorphism With Stressful Life Events and Severity of Depression. Am J Psychiatry. 2006;163(9):1588–1593. doi: 10.1176/ajp.2006.163.9.1588. [DOI] [PubMed] [Google Scholar]