Abstract
Mosquito-borne infections cause some of the most debilitating human diseases, including yellow fever and malaria, yet we lack an understanding of how disease risk scales with human-driven habitat changes. We present an approach to study variation in mosquito distribution and concomitant viral infections on the landscape level. In a pilot study we analyzed mosquito distribution along a 10-km transect of a West African rainforest area, which included primary forest, secondary forest, plantations, and human settlements. Variation was observed in the abundance of Anopheles, Aedes, Culex, and Uranotaenia mosquitoes between the different habitat types. Screening of trapped mosquitoes from the different habitats led to the isolation of five uncharacterized viruses of the families Bunyaviridae, Coronaviridae, Flaviviridae, and Rhabdoviridae, as well as an unclassified virus. Polymerase chain reaction screening for these five viruses in individual mosquitoes indicated a trend toward infection with specific viruses in specific mosquito genera that differed by habitat. Based on these initial analyses, we believe that further work is indicated to investigate the impact of anthropogenic landscape changes on mosquito distribution and accompanying arbovirus infection.
Key words: tropical rainforest, anthropogenic habitat change, flavivirus, bunyavirus, coronavirus, rhabdovirus
Introduction
Looking at the world from above, it is striking how once vast belts of tropical rainforest are being fragmented into ever smaller forest blocks (Myers, 1990; Myers et al., 2000; Fig. 1). Human encroachment on pristine ecosystems is driven by logging and agricultural conversion, resulting in sharp and rapidly moving gradients between the relatively cool and humid primary forest and the cultured land or villages, which show strong insolation, higher temperature, and lower humidity (Malhi and Wright, 2004). Simultaneously global warming is further modifying rainfall and temperature levels (Kerr, 2007). The consequences of these changes on the flora and fauna are frequently discussed (Trenberth, 2004; Vora, 2008; Ellis and Wilcox, 2009). In general, alterations to habitats and increasing proximity of humans to wild animals are creating new opportunities for infectious diseases to emerge in humans and wildlife, including vector-borne diseases, such as dengue fever, yellow fever, malaria, or Lyme disease, and also parasitic or respiratory infections (Daszak et al., 2000; Allan et al., 2003; Patz et al., 2005; Nunn and Altizer, 2006; Reiter and LaPointe, 2007; Gillespie and Chapman, 2008; Köndgen et al., 2008).
Figure 1.
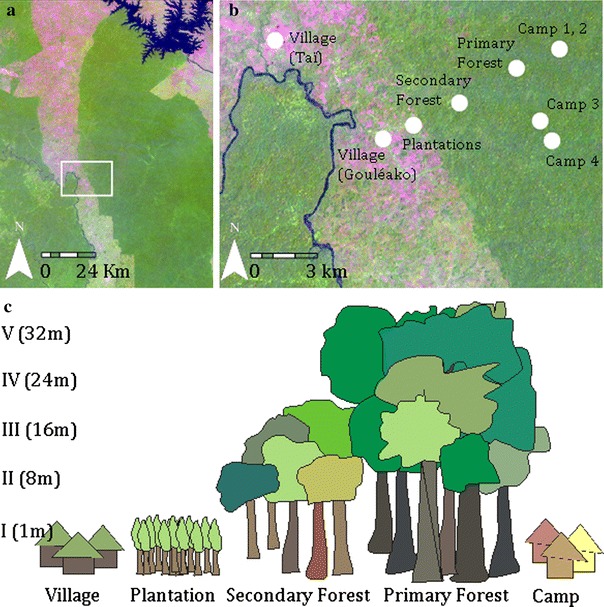
Habitats in which mosquitoes were sampled. a Satellite overview of the sampling region. b Detailed satellite picture with sampling locations (image was taken January 30, 2003, Sensor: Landsat ETM+, Source: Global Land Cover Facility, www.landcover.org). Dark green areas indicate rainforest, lighter areas are habitats with lower and not so dense vegetation; areas with almost no vegetation are marked in red. Red dots indicate sampling sites. The red dot camp I combine two camps that are next to each other. c Figure shows at which heights the traps were installed for each habitat type.
Nearly one-fourth of emerging infectious diseases (EIDs) in humans are vector-borne, and one-third of these have been identified during the last decade (Jones et al., 2008). Mosquitoes are common disease vectors because they provide an effective way to spread pathogens between humans and wildlife (Gubler, 2001; Mackenzie et al., 2004; Enserink, 2007). Infection risk with mosquito-borne diseases depends on several factors, including the abundance of competent vectors, the availability of vertebrate hosts, and the biting rate (number of mosquitoes biting their host per time unit). Mosquito prevalence, density, and distribution are in turn influenced by a number of other factors: notably, temperature, rainfall, humidity, vegetation, food supply, and hatchery resources (Kramer and Ebel, 2003).
Because mosquitoes are dependent on ecological conditions, it is likely that habitat degradation and climate change greatly impact the abundance and richness of mosquitoes (Daszak et al., 2000; Reiter and LaPointe, 2007). Indeed, deforestation was shown to have an impact on the biting rate of malaria vectors in South America (Vittor et al., 2006), and anthropogenic land use changes are associated with altered abundance of mosquito vectors for West Nile virus and malaria in France (Poncon et al., 2007). However, the studies focused on mosquito abundance rather than virus or parasite prevalence in the vectors.
Less widely appreciated is that mosquito richness and habitat conditions can impact the abundance and richness of mosquito-vectored pathogens. Thus, an understanding of the links between habitat variation, mosquito abundance, and pathogens carried by the mosquitoes is essential for building predictive models of disease emergence in humans and wildlife.
In this pilot study we developed a system for analyzing the mosquito species richness in disturbed and undisturbed areas of a tropical rainforest region and for investigating whether the observed diversity translates into variation in virus infection prevalence. Our study provides a basis for further in-depth studies to assess how anthropogenic changes can modulate vector and virus distribution, and potentially disease risks, for both humans and wildlife.
Materials and Methods
Study Area
At 435,000 hectares, Taï National Park in Côte d’Ivoire represents the largest remaining tropical rainforest in West Africa, yet it accounts for only a fraction of the original forest, which once covered approximately 40 million hectares (Boesch and Boesch-Achermann, 2000). The climate is characterized by two dry seasons: from December to March, and from August to September. The annual rainfall is approximately 1,800 mm in this region. In 1979 a research camp was constructed in the primary forest (geographic coordinates: lat 5.86767554/long −7.33968803) with the purpose of studying chimpanzees. Three additional research camps have since been built and included in this study (Fig. 1; geographic position: lat 5.87235332/long −7.33774074; lat 5.83327353/long −7.34220930; lat 5.84151328/long −7.34666177). Five sampling points were in the primary forest of Taï National Park (lat 5.86350739/long −7.35515901; lat 5.86298704/long −7.35587248; lat 5.86266518/long −7.35780903; lat 5.86294949/long −7.35530385; lat 5.86287975/long −7.35483178). The border of the park is lined by a secondary forest, which was partially logged for agricultural use but integrated into the park in 1977. Vegetation in the secondary forest is not as high and dense as in the primary forest (trees < 20 m). In the secondary forest mosquitoes were collected at three different sampling points (lat 5.84795058/long −7.38078483; lat 5.84979594/long −7.37809190; lat 5.84871769/long −7.38030204). Cultivated land, including coffee plantations (lat 5.83680868/long −7.40369090) and cocoa plantations (lat 5.83935142/long −7.39825674), abuts the park. Humans are present on a daily basis in these plantations. The human population around the park increased from 23,000 in 1965 to 375,000 in 1988 and with that more coffee and cocoa plantations were established around the park. Two villages (Gouléako lat 5.83436787/long −7.41086313 and Taï lat 5.87468348/long −7.45474125) were included as sampling points (Fig. 1). The number of inhabitants today is estimated at 600 for Gouléako and 8,000 for Taï. Settlements were founded during the French colonization at the end of the 19th century. Different expert opinions were used to describe primary and secondary forest habitats.
Mosquito Collection
Adult mosquitoes were collected with CDC miniature light and gravid traps (John W. Hock Company, USA) in the described habitats between February and June 2004. Alternating Octanol, carbon dioxide-producing hand heaters, and unwashed socks were used as attractants for the light traps, which operated for 12-h periods from 6 p.m. to 6 a.m. Water was mixed with sugar (5 spoons per liter) at least 24 h before use to act as an attractant in the gravid traps, which operated from 6 a.m. to 6 p.m. In the primary forest, traps were set up at five different heights above forest ground (1 m, 8 m, 16 m, 24 m, 32 m) and in the secondary forest at three different heights (1 m, 8 m, 16 m; Fig. 1). Traps were installed for 3 days at each sampling point. To cover the total height of each habitat and to sample a representative mosquito population, traps were set up to the canopy level for each habitat. Female mosquitoes were anesthetized with triethylamine and identified using the key of Gillies and De Meillon (1968) and Gillies and Coetzee (1987) for the Anophelinae and the key of Jupp (1996) and Edwards (1941) for the Culicidae. Species belonging to the Culex decens complex, which can only be differentiated by dissection of genitalia, were not differentiated and are marked as Culex decens complex. After identification, the mosquitoes were pooled according to species, sex, habitat, and altitude level, placed in sterile cryovials, and stored in liquid nitrogen.
Composition of Pools
Female mosquitoes take blood meals and contribute to virus transmission, whereas male mosquitoes nourish on nectar only. Therefore, only female mosquitoes were used for further analyses. Mosquito species from each sampling point were separated and divided into pools of 1–50 specimens (Table 1), such that pools were generated according to species, height, and sampling location. Mosquito species that were rarely found (less than 10 individuals per sampling point) were grouped with other rare species from the same sampling point to minimize number of pools. Although data were acquired by species across multiple canopy heights, due to limited numbers data were combined to genus level by habitat type for the statistical analyses.
Table 1.
Female Mosquitoes Trapped and Chosen for Virological Analyses (Number of Mosquitoes Captured/Mosquitoes Used for Virological Analyses/Number of Pools/Number of Positive Pools)
| Species | Camp sites | Primary Forest | Secondary Forest | Plantations | Villages |
|---|---|---|---|---|---|
| Aedes | |||||
| Ae africana | 1/1/1/0 | ||||
| Ae calceatus | 3/3/1/0 | ||||
| Ae fascipalpis | 1/1/1/1 | ||||
| Ae harrisoni | 11/11/3/0 | 10/10/4/0 | 52/48/4/1 | 3/3/2/1 | 12/9/1/1 |
| Ae mettalicus | 1/1/1/0 | 7/7/1/1 | |||
| Ae nd | 4/4/1/0 | 8/3/3/0 | 44/44/3/0 | 4/1/1a | 2/1/1/0 |
| Aedomyia | |||||
| Aed africana | 5/3/2/2 | 4/2/2/0 | 1/1/1/0 | ||
| Anopheles | |||||
| An azaniae | 3/3/1c | ||||
| An buxtoni | 2/2/1d | ||||
| An dancalicus | 5/5/1d | ||||
| An keniensis | 5/5/1d | ||||
| An gambiae | 1/1/1/0 | 68/67/4/4 | |||
| An obscurus | 1/1/1/0 | 7/7/1d | |||
| An rhodiensis | 9/9/1/0 | ||||
| An rhodiensis rubicolus | 4/4/1/0 | ||||
| An salbaii | 12/11/2/0 | 12/12/1/0 | |||
| An smithii | 1/1/1d | ||||
| An welcomei erepens | 1/1/1c | ||||
| An nd | 7/7/5/0 | 1/1/1/0 | 87/87/7/1 | 5/4/2/1 | 158/141/7/5 |
| Coquilletidia | |||||
| Coq metallica | 1/1/1/0 | 1/1/1/0 | |||
| Culiseta | |||||
| Cul nd | 1/1/1/0 | ||||
| Culicinae | |||||
| Cu nd | 39/10/1/1 | ||||
| Culex | |||||
| Cx annulioris | 230/182/12/0 | 13/10/1/1 | |||
| Cx antennatus | 51/35/3/0 | 29/29/2/2 | |||
| Cx argenteopunctatus | 1/1/1/0 | ||||
| Cx bitaeniorhynchus | 7/7/2/0 | ||||
| Cx cinerellus | 13/8/1/1 | 16/16/1/0 | |||
| Cx cinereus | 2/2/1e | ||||
| Cx decens complex | 4/2/2/0 | 14/7/4/0 | 399/324/20/9 | 210/208/11/1 | 4/4/1/0 |
| Cx horridus | 71/71/5/0 | 5/5/2/0 | |||
| Cx nebulosus | 40/40/4/2 | 12/12/5/1 | 134/134/14/6 | 94/78/6/2 | 339/329/17/11 |
| Cx pipiens | 9/8/1/2 | 2/1/1/0 | 6/5/1/0 | ||
| Cx quinquefasciatus | 4/4/2/0 | 2/2/1b | 85/85/5/2 | ||
| Cx rubinotus | 3/2/1b | 5/5/1/0 | |||
| Cx simpliforceps | 2/2/2/0 | ||||
| Cx telesilla | 12/12/2/1 | ||||
| Cx zombaensis | 1/1/1/0 | ||||
| Cx nd | 354/277/20/5 | 38/37/10/2 | 248/145/17/3 | 296/296/17/4 | 140/140/8/1 |
| Eretmapodites | |||||
| Er subsimplicipes | 1/1/1/0 | ||||
| Harpagomyia | |||||
| Ha nd | 10/10/1/0 | ||||
| Mansonia | |||||
| Ma africana | 1/1/1/0 | ||||
| Toxorhychitinae | |||||
| To nd | 6/6/1/0 | ||||
| Uranotaenia | |||||
| Ur chorleyi | 55/20/2/0 | 12/12/3/0 | 5/4/2/0 | 8/8/1/0 | |
| Ur mashoaensis | 7/7/2/0 | 535/163/11/4 | 35/31/5/5 | 21/18/2/0 | 19/19/2/0 |
| Ur ornata | 424/163/9/0 | ||||
| Ur nd | 157/125/8/1 | 108/38/5/0 | 6/5/1/0 | 5/4/2/0 | |
| Other females | |||||
| Female nd | 51/3/4/1 | 354/281/34/6 | 342/270/23/10 | 78/78/6/5 | 51/42/2/0 |
nd = not determined; pooled with other species from the same habitat: awith Ae calceatus; bwith bitaeniorhynchus; cwith An azaniae, erepens, obscurus; dwith An buxtoni, An dancalicus, An keniensis, An smithii; ewith Cx spp.
Virus Isolation and Characterization
Mosquitoes were homogenized in 1 ml of medium and after centrifugation the clarified supernatant was used for infection of insect cells (Aedes albopictus, C6/36) and vertebrate cells (African Green monkey, Vero) (Junglen et al., 2009). Cell culture supernatant was passaged two times on fresh cells. Induction of cytopathic effect (CPE) was scored as virus infection. Viruses in culture supernatants were identified morphologically by electron microscopy and genetically by using sequence-independent amplification methods (Junglen et al., 2009). Smaller and larger pools were random between habitats and thus not likely to bias the frequency of CPE-positive pools. We did not score the intensity of the CPE; all cell cultures that showed signs of morphological changes were further passaged and investigated. Positive pools were screened in retrospect by molecular means to ensure viral detection.
Specific Real-time Polymerase Chain Reaction Assays
RNA was extracted from supernatants of infected pools using the Viral RNA Mini Kit (Qiagen, Hilden, Germany) and eluted in 60 μl of AVE buffer. cDNA synthesis was performed using the Superscript Kit (Invitrogen, Karlsruhe, Germany) and random hexamer primers (TIB Molbiol, Berlin, Germany). Based on the viral sequence information obtained primers and probes for specific real-time polymerase chain reaction (PCR) systems for viral sequence fragments were designed as follows: Gouléako virus (GOUV F 5′ AACTGGAGGGAAGATGTGGAAGAG, GOUV R 5′ ATCTAGCGACCTCCACCCTCACA, GOUV TM 6-FAM-CCGTTCCAAGGACACTCCGAACAGCCq), Cavally virus (CAVV F 5′ CCACGTTAAAGACTCAAGCAGAGA, CAVV R 5′ TTTCCGTTGCATAGTATGGGTTT, CAVV TM 6-FAM-CCATATACAAATGTGCGCAACCTCGACA-TMR), Nounané virus (NOUV F 5′ CCAACAGCGTCACTCCTAATGAG, NOUV R 5′ GCTGCTCTGGATGATGATGCA, NOUV TM 6-FAM-CCACGCACCCATCAGCATCATCCq), Herbert virus (HERV F 5′ AGAATGCTTTGTCAGTGG, HERV R 5′ AGCAGCAACTTATAAAACAAATC, HERV TM 6-FAM-TTCTCCGCTAATAAAA-MGB) and Moussa virus (MOUV F 5′ TTTCTCAGGGCACTGTAAGTGACT, MOUV R 5′ GGAGACGGAGTTCCTGAATCAT, MOUV TM 6-FAM-TCCCTCTGCTCCTACCTCGGTCACC-TMR). Primers and probes were synthesized by TIB Molbiol (Berlin, Germany). PCR conditions were as follows: 1 cycle of 10 min at 95°C and 45 cycles of 15 sec at 95°C, 35 sec at 60°C, using the ABI Prism™ 7500 real-time PCR System. Products were sequenced using the ABI Big Dye Termination Kit. Pools were also screened using real-time PCR for YFV (Bae et al., 2003), WNV (Linke et al., 2007), and DENV 1–4 (details will be published elsewhere).
Estimating Mosquito Infection Probability
Infection rate could not be derived directly from the sampled mosquitoes, because CPE values represented sized mosquito pools that ranged from 2 to 100 mosquitoes. Thus, we derived the most likely infection rate using a maximum likelihood approach (Gu et al., 2003). We considered the trapping of uninfected mosquitoes to be a binomial random process. For each CPE/virus-positive pool, we estimated the cumulative binomial probability P (0 < k ≤ n) that at least one mosquito in the pool was infected (CPE/virus positive) as
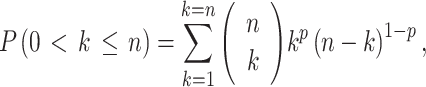 |
(n = pool size, k = number of infected mosquitoes, p = infection probability). For each negative pool, we estimated the probability that no mosquito in the pool was infected as
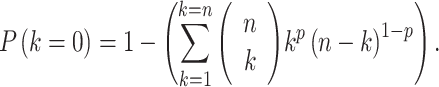 |
The proportion of captured infected mosquitoes per trapping period was calculated as the average number of trapped mosquitoes per night and habitat times the infection probability. To exclude differences reflecting different collection height, comparative analyses were performed only for traps set at the same height. Results did not significantly differ when all heights were considered.
Testing for Habitat Differences
We compared mosquito abundance, CPE, and virus prevalence in different habitats using permutation tests (Manly, 1997). For mosquito abundance, we chose as the test statistic the absolute difference between trapping rates derived for secondary forest and the other habitats. We permuted the data by first randomly choosing a number of values from the other habitats, with the number of values chosen equal to the number of trap sites in the secondary forest. We then exchanged these randomly chosen values with randomly chosen values observed in secondary forest. To reflect that under the null hypothesis any observation could happen in each habitat, an actual exchange of two values (between secondary forest and any other habitat) took place with a probability equaling the proportion of other habitats in our sample. All random selections were performed with replacement, and a total of 1,000 permutations were conducted. For CPE and virus prevalence, we permuted the mosquito pools 100 times to reestimate infection probability. The multiple testing for the analysis on the induction of CPE in cell culture according to mosquito genera in each habitat required error-level correction. We did this using Fisher’s omnibus test. This procedure combines a number of P values into a single chi-square distributed variable with its degrees of freedom equaling twice the number of P values (Haccou and Meelis, 1994). All statistical analyses were run in R.
Results
Analyses of mosquito distribution were performed at genus level (Aedes n = 169, Anopheles n = 357, Culex n = 2958, Uranotaenia n = 1492, and others n = 919 (composed of Aedomyia n = 14, Coquilletidia n = 2, Culiseta n = 1, Culicinae n = 39, Eretmapodites n = 1, Harpagomyia n = 10, Mansonia n = 10, Toxorhychitinae, and genus not determined n = 845); Table 1). We recorded significant variation in the distribution of mosquito genera in different habitats in and around a tropical rainforest region (Fig. 2). Although Uranotaenia mosquitoes were dominant in the primary forest, they were rarely found in the other surveyed habitats (permutation test, P = 0.032). Conversely, Culex mosquitoes were most common in disturbed habitats, including the research camps, but were least abundant in the primary forest (permutation test, P = 0.002). Anopheles mosquitoes were found at significant numbers only in the villages (permutation test, P = 0.029), whereas Aedes mosquitoes were mainly trapped in the secondary forest (permutation test, P = 0.019). Among these mosquito genera, Aedes, Anopheles, and Culex are known to transmit human pathogens. Because variance among species within these genera in host preference and vector competence due to differences in vectorial capacity amongst species for specific viruses is great, comparison on species level by habitat type was performed for ten species that were caught in sufficient numbers (Ae harrisoni n = 85, An gambiae complex n = 71, Cx annulioris n = 274, Cx decens complex n = 631, Cx nebulosus n = 619, Cx quinquefasciatus n = 85, Ur chorleyi n = 93, Ur mashonaensis n = 630, Ur ornata n = 424). Aedes harrisoni was the most abundant Aedes species and encountered predominantly in the secondary forest. Although mosquitoes of the Anopheles gambiae complex, the primary vectors of Plasmodium, were not found in the primary forest as previously reported (Doucet et al., 1960), one mosquito of the Anopheles gambiae complex was trapped at a camp site. The majority of mosquitoes belonging to the Anopheles gambiae complex were found in the villages, indicating a potential risk for malaria infection. Culex species were prominent in human-altered habitats but differed between habitat types: Cx annulioris showed a higher prevalence only in the plantations, mosquitoes belonging to the Culex decens complex in the secondary forest and the plantations; Cx nebulosus was highest in the villages followed by secondary forest and plantations; Cx quinquefasciatus was highest in the villages. Ur chorleyi, Ur mashonaensis, and Ur ornata were almost exclusively found in the primary forest.
Figure 2.
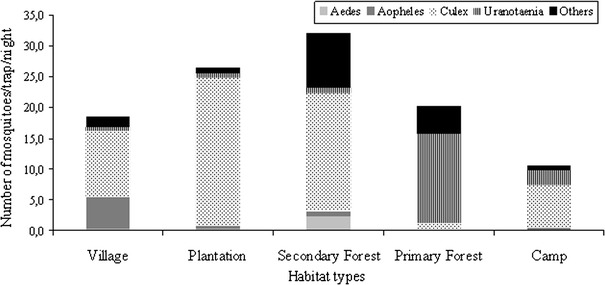
Relative frequency of mosquitoes of the genera Aedes, Anopheles, Culex, Uranotaenia, and others in each habitat per trapping night. Number of trapped mosquitoes divided by number of trapping nights (mosquito abundance per trap night). Habitat types: Village, Taï and Gouléako village; Plantation, coffee and cocoa plantation; Secondary Forest, sampling sites I–III; Primary Forest, Sampling sites I–V, Camp, research camps I–IV.
To control for trap efficacy among habitat types, trapping counts (all mosquitoes per trap per night) were compared using the permutation test. Trap counts did not significantly differ between habitats (permutation test, P = 0.229), suggesting that the distribution of mosquito genera is not related to trap efficacy but rather to general habitat preferences for each mosquito genus. To exclude an influence of different trap heights, comparative analyzes were performed using the same statistics but only for trappings at the same height.
To investigate the presence of viruses in trapped mosquitoes, pools of female mosquitoes were generated as indicated in Table 1 and used for virus isolation in an insect and primate cell line (C6/36 and Vero E6/7). For statistical analyses genus and habitat specific pools were compared (4,839 mosquitoes in 432 pools). The induction CPE was scored as an indication of virus replication. Ninety-eight of the pools (22.7%) induced CPE; the proportion of CPE-positive pools was significantly higher in the samples originating from the villages (44%, permutation test, P = 0.002) and the secondary forest (30%, permutation test, P = 0.017) than in those originating from camp sites and the primary forest (14%, permutation test, P = 0.012; Fig. 3). Estimated statistical infection rates for a single mosquito did not differ significantly for the primary and secondary forest and for the plantations, yet this rate was four-fold higher for the villages and three-fold higher for the camp sites. Analysis of CPE by mosquito genus in each habitat indicated Culex mosquitoes as the predominant source for CPE-positive pools (permutation test, P < 0.001; Fig. 3). Pools of Aedes and Anopheles mosquitoes from the villages also caused CPE at higher level compared with other habitats. In contrast, mosquitoes of the genus Uranotaenia are estimated to account for less than 5% CPE-positive mosquitoes per trapping night across all habitats (Fig. 3).
Figure 3.
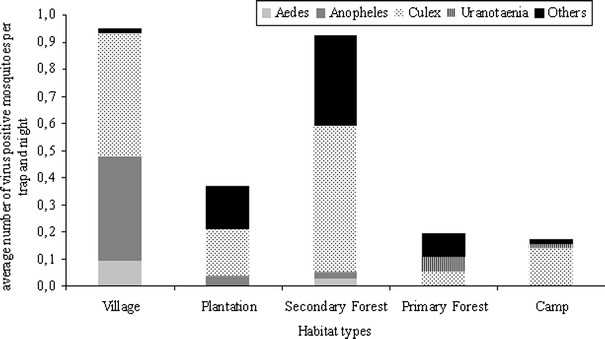
Relative frequency of CPE causing mosquito homogenates in different habitats by genus. Mosquito homogenates (432 pools) were inoculated into C6/36 cells and observed for CPE. The proportion of captured CPE inducing mosquitoes per pool and per trapping period was calculated (see statistics). Habitat types: Village, Taï and Gouléako village; Plantation, coffee and cocoa plantation; Secondary Forest, sampling sites I–III; Primary Forest, Sampling sites I–V, Camp, research camps I–IV.
Next, we investigated the distribution of five viruses that were isolated from the mosquitoes trapped in disturbed and undisturbed habitats. Real-time PCR assays were established for Nounané virus (NOUV, a flavivirus; Junglen et al., 2009), Moussa virus (MOUV, a rhabdovirus; Quan et al., 2009), Cavally virus (CAVV, coronavirus-like), Gouléako virus (GOUV, a bunyavirus), and Herbert virus (HERV, unclassified isolate). All pools that induced a CPE were tested for the presence of these five viruses; in addition all 432 pools tested negative for yellow fever virus, dengue virus, and West Nile virus, three common viruses of the region. As summarized in Fig. 4, NOUV was only found in two mosquito pools of the same species (Ur mashonaensis) trapped in the primary forest. In contrast, MOUV was found in seven pools of Culex and other mosquitoes from all habitat types except the villages, with highest prevalence in the secondary forest. CAVV was found in 39 pools and in all habitats, with highest prevalence in the villages. The virus was most frequently isolated from Culex species; however, Anopheles (three pools) and Aedes (one pool) mosquitoes also were infected. GOUV and HERV were detected in 28 pools and in all habitats, and in three different mosquito genera: Anopheles, Culex, and Uranotaenia. In most pools, both viruses were present, but infection with only one of the viruses was evident. The prevalence of these two viruses was lowest in the primary forest and highest in the villages. With the exception of NOUV, which was detected exclusively in primary forest, we found higher virus prevalence in mosquito pools from disturbed habitats, the camp sites, secondary forest, plantations, and villages compared with the primary forest (permutation test, P = 0.001).
Figure 4.
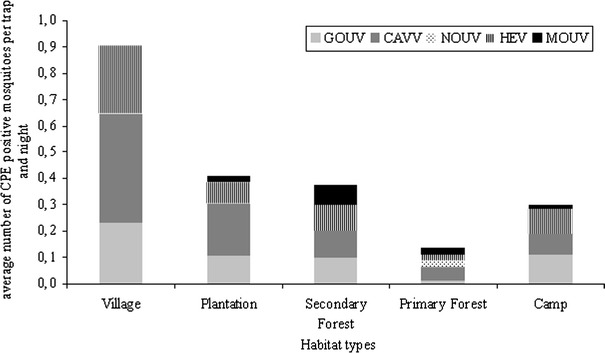
Distribution of virus isolates. RNA was extracted from all pools that induced CPE and cDNA was synthesized using random hexamer priming. Pools were tested by specific real-time PCR assays for the presence of Nounané virus (NOUV), Moussa virus (MOUV), Cavally virus (CAVV), Gouléako virus (GOUV), and Herbert Virus (HERV). The proportion of mosquitoes infected with these viruses per pool and per trapping period was calculated (see statistics). Habitat types: Village, Taï and Gouléako village; Plantation, coffee and cocoa plantation; Secondary Forest, sampling sites I–III; Primary Forest, Sampling sites I–V, Camp, research camps I–IV
Discussion
Our study design combined systematic mosquito sampling in different habitat types, including pristine rainforest, forest border zones, agricultural crop land, and human settlements, with screening for viral infections followed by specific testing of mosquitoes from all habitats for any of the isolated viruses. This approach to vector and virus surveillance can provide important insights into changes within mosquito populations, enable early detection of viral emergence, and provide insights into human driven habitat changes on the risk of mosquito-borne infections. However, our statistical analyses showed that broader sampling approaches are needed to accurately estimate the factors driving the differences observed.
The results of this pilot study point to differences in mosquito and virus prevalence in disturbed and undisturbed habitats. Our data for five virus isolates from widely differing taxonomic groups are consistent with the notion that disturbed habitats, and especially human settlements, seem to support proliferation of endemic viruses more efficiently than the pristine primary forest. Aedes, Anopheles, and Culex mosquitoes were most common in disturbed habitats and likely to carry pathogens. Species belonging to the genera Aedes, Anopheles, and Culex differ greatly in their feeding preferences and further investigation of the distribution of individual species is important to identify potential bridge vectors that can introduce viruses to new habitats or hosts. If we assume that mosquito abundance and the presence of virus-positive mosquitoes represent a human disease risk, the highest risk exists in the villages, followed by plantations and the secondary forest. The risk in the primary forest is lowest, but seems to increase in association with clearings and the presence of humans at camp sites. Thus, our data are in concert with others who have proposed that changes in habitat may have a marked impact on the distribution of mosquito genera and species, influencing virus distribution and risk for human disease (Vasconcelos et al., 1992; Patz et al., 2004; Obsomer et al., 2007).
Although the shifts that we have documented are associated with anthropogenic habitat degradation of the forest border zones and a gradient of human presence, other factors may be implicated. Differences in temperature, rainfall, and humidity are known to influence the life cycles of different mosquito species, thus contributing to the success of some species compared with others (Vasconcelos et al., 1992). Variation in vegetation—especially height of the forest—also can have major impact on mosquito abundance in general and on specific genera in particular, as can the density of suitable hosts for blood meals (Wolfe et al., 2007). We were unable to investigate differences along a forest canopy gradient due to insufficient numbers of mosquitoes collected. Furthermore, little is known about environmental effects on the survival of viruses within mosquito hosts. In this respect, assessment of male mosquitoes as an index for horizontal transmission will be an interesting aspect for future studies.
PCR is a sensitive and rapid method for routine detection of known arboviruses (Jupp et al., 2000; Kramer et al., 2002). We used PCR to test all mosquito pools and all CPE-positive cell cultures for the presence of WNV, DENV 1–4, and YFV. None of these viruses were detected in the pools by PCR. YFV and DENV infections most often occur at the beginning and in the second half of the rainy season (Germain et al., 1977; Cordellier, 1978; Cornet et al., 1978; Cornet et al., 1979; Germain et al., 1981). Because mosquitoes were collected in the dry and at the beginning of the rainy season from February to June, YFV and DENV may not have been circulating in detectable levels in the mosquito population. Furthermore, there were no human infections with YFV, WNV, or DENV reported during this interval.
With the exception of NOUV, viruses were most prevalent in disturbed habitats, and Culex mosquitoes were the most frequent vector. The majority of species belonging to this genus prefer habitats warmer and drier than the forest. Other studies have shown that mosquitoes, such as Cx tarsalis, Cx pipiens, and Cx nigripalpus, may serve as potential bridge vectors because they change their feeding preferences according to the seasons from birds to mammals (Edman and Taylor, 1968; Kilpatrick et al., 2006; Kyoko et al., 2006; Kent et al., 2009). At present we have no data that indicate mammal or human infection with the viruses reported here. However, human serum collections are under investigation to address this point.
Conclusions
Our data indicate that future research must include an even broader mosquito sampling approach than presented here to allow sufficient sample sizes that support statistical analyses at the species level. In addition, sampling for 1 year or multiple years is desirable to address seasonality, as well as validation of the trends revealed in our study through analyses of other rainforest edge areas. Although additional research is needed to place these real-world data into a firmer theoretical framework for generating predictive models of vector/virus dynamics, our data show the validity of such an approach and provide a basis for the design of further studies.
Acknowledgements
We thank the Ministry of the Environment and Forests and the Ministry of Research, the directorship of the Taï National Park, and the Swiss Research Center in Côte d’Ivoire. For helping with the mosquito catching, we thank Germain Gagné. We thank Dr. Kampen from the University of Bonn and the Naturkundemuseum, Berlin, for supporting help with the mosquito determination and Y. Sim-Brandenburg for skillful support. We thank Roger Mundry for statistical advice. Fieldwork in Côte d’Ivoire has been financially supported by the Max Planck Society, Cummings School of Veterinary Medicine at Tufts; the analyses were supported by the Robert Koch-Institute, the National Institutes of Health (GM070077; AI57158, Northeast Biodefense Center-Lipkin) and Google.org. For the study, S. Junglen was awarded a grant from NaföG (Nachwuchsförderungsgesellschaft) and Aventis Ilab.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Allan BF, Keesing F, Ostfeld RS. Effects of habitat fragmentation on Lyme disease risk. Conservation Biology. 2003;17:267–272. doi: 10.1046/j.1523-1739.2003.01260.x. [DOI] [Google Scholar]
- Bae HG, Nitsche A, Teichmann A, Biel SS, Niedrig M. Detection of yellow fever virus: a comparison of quantitative real-time PCR and plaque assay. Journal of Virological Methods. 2003;110:185–191. doi: 10.1016/S0166-0934(03)00129-0. [DOI] [PubMed] [Google Scholar]
- Boesch C, Boesch-Achermann H. The Chimpanzees of the Taϊ Forest: Behavioural Ecology and Evolution. Oxford/New York: Oxford University Press; 2000. [Google Scholar]
- Cornet M, Robin Y, Heme G, Valade M. Isolation in east Senegal of a yellow fever virus strain from a pool of Aedes belonging to the subgenus Diceromyia. Comptes Rendus Hebdomadaires des Séances de l'Académie des Sciences. Série D: Sciences Naturelles. 1978;287:1449–1451. [PubMed] [Google Scholar]
- Cornet M, Robin Y, Château R, Hème G, Adam C, Valade M. Isolements d’arbovirus au Sénégal oriental à partir de moustiques (1972–1977) et notes sur l’épidémiologie des virus transmis par les Aedes, en particulier du virus amaril. Ent méd et Parasitol ORSTOM. 1979;17:149–163. [Google Scholar]
- Cordellier R. Les vecteurs potentiels sauvages dans l’épidémiologie de la fièvre jaune en Afrique de l’Ouest. Travaux et Documents de l’ORSTOM. Bondy: Bib; 1978. [Google Scholar]
- Daszak P, Cunningham AA, Hyatt AD. Emerging infectious diseases of wildlife -threats to biodiversity and human health. Science. 2000;287:443–449. doi: 10.1126/science.287.5452.443. [DOI] [PubMed] [Google Scholar]
- Doucet J, Dam JP, Binson G. Culicidae of the Ivory Coast. Annales de Parasitologie Humaine et Comparée. 1960;35:391–408. [PubMed] [Google Scholar]
- Edman JD, Taylor DJ. Culex nigripalpus: seasonal shift in the bird-mammal feeding ratio in a mosquito vector of human encephalitis. Science. 1968;161:67–68. doi: 10.1126/science.161.3836.67. [DOI] [PubMed] [Google Scholar]
- Edwards FW. Mosquitoes of the Ethiopian region: III Culicine adults and pupae. London: British Museum (Natural History); 1941. [Google Scholar]
- Ellis BR, Wilcox BA. The ecological dimensions of vector-borne disease research and control. Cad Saúde Pública. 2009;25:155–167. doi: 10.1590/S0102-311X2009001300015. [DOI] [PubMed] [Google Scholar]
- Enserink M. Infectious diseases. Chikungunya: no longer a third world disease. Science. 2007;318:1860–1861. doi: 10.1126/science.318.5858.1860. [DOI] [PubMed] [Google Scholar]
- Germain M, Hervé JP, Geoffroy B. Variations du taux de survie des femelles d’Aedes africanus (Theo.) dans une galarie forestière du sud de l’Empire Centrafrican. Ent méd et Parasitol ORSTOM. 1977;15:291–299. [Google Scholar]
- Germain M, Cornet M, Mouchet J, Herve JP, Robert V, Camicas JL, Cordellier R, Hervy JP, Digoutte JP, Monath TP, Salaun JJ, Deubel V, Robin Y, Coz J, Taufflieb R, Saluzzo JF, Gonzales JP. Sylvatic yellow fever in Africa recent advances and present approach. Médecine Tropicale: Revue du Corps de Santé Colonial. 1981;41:31–43. [PubMed] [Google Scholar]
- Gillespie TR, Chapman CA. Prediction of parasite infection dynamics in primate metapopulations based on attributes of forest fragments. Conservation Biology. 2008;20:441–448. doi: 10.1111/j.1523-1739.2006.00290.x. [DOI] [PubMed] [Google Scholar]
- Gillies MT, De Meillon B. The Anophelinae of Africa South of the Sahara (Ethiopian Zoogeographical Region) Johannesburg, South Africa: The South African Institute for Medical Research; 1968. [Google Scholar]
- Gillies MT, Coetzee M. A Supplement to the Anophelinae of Africa South of the Sahara (Afrotropical Region) Johannesburg, South Africa: The South African Institute for Medical Research; 1987. [Google Scholar]
- Gu W, Lampman R, Novak RJ. Problems in estimating mosquito infection rates using minimum infection rates. Journal of Medical Entomology. 2003;40:595–596. doi: 10.1603/0022-2585-40.5.595. [DOI] [PubMed] [Google Scholar]
- Gubler DJ. Human arbovirus infections worldwide. Annals of the New York Academy of Sciences. 2001;951:13–24. doi: 10.1111/j.1749-6632.2001.tb02681.x. [DOI] [PubMed] [Google Scholar]
- Haccou P, Meelis E. Statistical Analyses of Behavioural Data. Oxford: University Press; 1994. [Google Scholar]
- Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, Daszak P. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junglen S, Kopp A, Kurth A, Pauli G, Ellerbrok H, Leendertz FH. A new flavivirus and a new vector: characterization of a novel flavivirus isolated from Uranotaenia mashonaensis mosquitoes from a tropical rainforest. Journal of Virology. 2009;83:4462–4468. doi: 10.1128/JVI.00014-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jupp P. Mosquitoes of Southern Africa. Hartebeespoort, South Africa: Ekoglide Publishers; 1996. [Google Scholar]
- Jupp PG, Grobbelaar AA, Leman PA, Kemp A, Dunton RF, Burkot TR, Ksiazek TG, Swanepoel R. Experimental detection of Rift Valley fever virus by reverse transcription-polymerase chain reaction assay in large samples of mosquitoes. Journal of Medical Entomology. 2000;37:467–471. doi: 10.1603/0022-2585(2000)037[0467:EDORVF]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Kent R, Juliusson L, Weissmann M, Evans S, Komar N. Seasonal blood-feeding behavior of Culex tarsalis (Diptera: Culicidae) in Weld County, Colorado, 2007. Journal of Medical Entomology. 2009;46:380–390. doi: 10.1603/033.046.0226. [DOI] [PubMed] [Google Scholar]
- Kilpatrick AM, Kramer LD, Jones MJ, Marra PP, Daszak P. West Nile virus epidemics in North America are driven by shifts in mosquito feeding behavior. PLoS Biology. 2006;4:e82. doi: 10.1371/journal.pbio.0040082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köndgen S, Kühl H, N’Goran PK, Walsh PD, Schenk S, Ernst N, Biek R, Formenty P, Mätz-Rensing K, Schweiger B, Junglen S, Ellerbrok H, Nitsche A, Briese T, Lipkin IW, Pauli G, Boesch C, Leendertz FH. Pandemic human viruses cause decline of endangered great apes. Current Biology. 2008;18:260–264. doi: 10.1016/j.cub.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Kramer LD, Wolfe TM, Green EN, Chiles RE, Fallah H, Fang Y, Reisen WK. Detection of encephalitis viruses in mosquitoes (Diptera: Culicidae) and avian tissues. Journal of Medical Entomology. 2002;39:312–323. doi: 10.1603/0022-2585-39.2.312. [DOI] [PubMed] [Google Scholar]
- Kerr RA. Climate change. Global warming is changing the world. Science. 2007;316:188–190. doi: 10.1126/science.316.5822.188. [DOI] [PubMed] [Google Scholar]
- Kramer L, Ebel GD. Dynamics of flavivirus infection in mosquitoes. Advances in Virus Research. 2003;60:187–232. doi: 10.1016/S0065-3527(03)60006-0. [DOI] [PubMed] [Google Scholar]
- Kyoko S, Haruhiko I, Keita H, Toshinori S, Yukiko H, Shinji K, Yoshio T, Isao N, Nobuo H, Shoji H. Research on infection vector and its control. Feeding preference of disease-bearing mosquitoes in Japan. Kansensho Baikai Bekuta no Jittai, Seisoku Boshi Taisaku ni kansuru Kenkyu Heisei. 2006;16:111–116. [Google Scholar]
- Linke S, Ellerbrok H, Niedrig M, Nitsche A, Pauli G. Detection of West Nile virus lineages 1 and 2 by real-time PCR. Journal of Virological Methods. 2007;146:355–358. doi: 10.1016/j.jviromet.2007.05.021. [DOI] [PubMed] [Google Scholar]
- Mackenzie JS, Gubler DJ, Petersen LR. Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nature Medicine. 2004;10:98–109. doi: 10.1038/nm1144. [DOI] [PubMed] [Google Scholar]
- Malhi Y, Wright J. Spatial patterns and recent trends in the climate of tropical rainforest regions. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2004;359:311–329. doi: 10.1098/rstb.2003.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manly B. Randomization, Bootstrap and Monte Carlo Methods in Biology. New York: Chapman & Hall; 1997. [Google Scholar]
- Myers N. The biodiversity challenge: expanded hot-spots analysis. The Environmentalist. 1990;10:243–256. doi: 10.1007/BF02239720. [DOI] [PubMed] [Google Scholar]
- Myers N, Mittermeier RA, Mittermeier CG, DaFonseca GA, Kent J. Biodiversity hotspots for conservation priorities. Nature. 2000;403:853–858. doi: 10.1038/35002501. [DOI] [PubMed] [Google Scholar]
- Nunn C, Altizer S. Infectious Diseases in Primates. Behavior, Ecology and Evolution. New York: Oxford University Press; 2006. [Google Scholar]
- Obsomer V, Defourny P, Coosemans M. The Anopheles dirus complex: spatial distribution and environmental drivers. Malaria Journal. 2007;6:26–42. doi: 10.1186/1475-2875-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patz JA, Daszak P, Tabor GM, Aguirre AA, Pearl M, Epstein J, Wolfe ND, Kilpatrick AM, Foufopoulos J, Molyneux D, Bradley DJ. Unhealthy landscapes: Policy recommendations on land use change and infectious disease emergence. Environmental Health Perspectives. 2004;112:1092–1098. doi: 10.1289/ehp.6877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patz JA, Campbell-Lendrum D, Holloway T, Foley JA. Impact of regional climate change on human health. Nature. 2005;438:310–317. doi: 10.1038/nature04188. [DOI] [PubMed] [Google Scholar]
- Poncon N, Balenghien T, Toty C, Baptiste FJ, Thomas C, Dervieux A, Lambert G, Schaffner F, Bardin O, Fontenille D. Effects of local anthropogenic changes on potential malaria vector Anopheles hyrcanus and West Nile virus vector Culex modestus, Camargue, France. Emerging Infectious Diseases. 2007;13:1810–1815. doi: 10.3201/eid1312.070730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan PL, Junglen S, Tashmukhamedova A, Conlan S, Hutchison SK, Kurth A, Ellerbrok H, Egholm M, Briese T, Leendertz FH, Lipkin WI (2009) Moussa virus: a new member of the Rhabdoviridae family isolated from Culex decens mosquitoes in Côte d’Ivoire. Virus Research [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- Reiter ME, LaPointe DA. Landscape factors influencing the spatial distribution and abundance of mosquito vector Culex quinquefasciatus (Diptera: Culicidae) in a mixed residential-agricultural community in Hawaii. Journal of Medical Entomology. 2007;44:861–868. doi: 10.1603/0022-2585(2007)44[861:LFITSD]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Trenberth KE. Climatology (communication arising): rural land-use change and climate. Nature. 2004;427:213. doi: 10.1038/427213a. [DOI] [PubMed] [Google Scholar]
- Vasconcelos PFC, Travassos da Rosa APA, Dégallier N. Clinical and ecoepidemiological situation of human arboviruses in Brazilian Amazonia. Journal of the Brazilian Association for the Advancement of Science. 1992;44:117–124. [Google Scholar]
- Vittor AY, Gilman RH, Tielsch J, Glass G, Shields T, Lozano WS, Pinedo-Cancino V, Patz JA. The effect of deforestation on the human-biting rate of Anopheles darlingi, the primary vector of Falciparum malaria in the Peruvian Amazon. The American Journal of Tropical Medicine and Hygiene. 2006;74:3–11. [PubMed] [Google Scholar]
- Vora N. Impact of anthropogenic environmental alterations on vector-borne diseases. Medscape Journal of Medicine. 2008;10:238. [PMC free article] [PubMed] [Google Scholar]
- Wolfe ND, Dunavan CP, Diamond J. Origins of major human infectious diseases. Nature. 2007;447:279–283. doi: 10.1038/nature05775. [DOI] [PMC free article] [PubMed] [Google Scholar]


