Abstract
Blockade of the B7: CD28 costimulatory pathway has emerged as a promising therapy to prevent allograft rejection. However, this pathway has also been demonstrated to be important for the generation and maintenance of regulatory T cells. In this study, we investigated the role of the B7: CD28 pathway in the ‘bm12 into B6’ MHC class II-mismatched vascularized cardiac transplant model of chronic rejection. Allograft rejection was remarkably accelerated in B6 background B7DKO and CD28KO recipients compared with B6 wild-type (WT) recipients. Allograft rejection was associated with a significantly enhanced Th1/Th2 alloreactivity and marked reduction in the ratio of regulatory T cells to CD4+ effector/memory cells. We noted that administration of anti-B7-1 and anti-B7-2 mAb prior to transplantation also accelerated allograft rejection. Furthermore, depleting CD25+ cells in B6 WT recipients of bm12 hearts prior to transplant also precipitated rejection at a similar rate. Neither B7/CD28 deficiency nor CD25 depletion affected graft survival in single MHC class I-mismatched (bm1 into B6) recipients. This study highlights the paradoxical functions of B7: CD28 costimulation in a MHC class II-mismatched model, in which the B7: CD28 pathway is demonstrated to be important in preventing rejection through the generation and maintenance of Tregs.
Keywords: Costimulation, MHC class II, mice, rejection
Introduction
Although the role of B7: CD28 interactions in promoting initial T-cell activation is well established, a separate role for B7: CD28 interactions in promoting T-cell tolerance has only recently been acknowledged (1). Investigation of the severe and accelerated diabetes that develops in CD28-deficient (CD28KO) and B7.1/2-double-deficient (B7DKO) NOD (nonobese diabetic) mice (2) revealed that B7: CD28 interactions control regulatory T cells (Tregs). These NOD mice demonstrate a profound decrease of the CD4+CD25+ regulatory T-cell population in both CD28KO and B7DKO mice, and the transfer of this regulatory T-cell subset from control NOD animals into CD28-deficient animals can delay/prevent diabetes. The results suggest that the B7: CD28 costimulatory pathway is essential for the development and homeostasis of regulatory T cells. Recent research also reinforces that B7: CD28 interactions are needed for development and maintenance of CD4+CD25+ Tregs (3,4).
The B6.H-2bm12 mice have a spontaneous mutation of the I-Ab molecule, resulting in a 3-aa substitution in the third hypervariable region of the Aß chain. C57BL/6 (B6) mice do not acutely reject B6.H-2bm12 (bm12) heart allografts, although the grafts develop transplant-associated vasculopathy over time, characteristic of chronic rejection (5,6). However, skin grafts from bm12 are acutely rejected in B6 recipients, indicating that the antigenic mismatch between these strains can trigger a potent alloimmune response (7). In this single MHC class II-mismatched model, acute rejection of the bm12 cardiac allograft is inhibited by the emergence of CD25+ regulatory T cells that restrict the clonal expansion of alloreactive T cells (7). Therefore, lack of B7: CD28 costimulation in the ‘bm12 into B6’ transplant model may impair the generation of regulatory T cells and enhance the clonal expansion of effector T cells, consequently shortening allograft survival.
In this study, we use CD28-deficient and B7.1/2-double-deficient recipients of bm12 hearts to investigate the role of B7: CD28 blockade in a single MHC class II-mismatched cardiac transplantation model. This study may have important clinical implications for the application of Belatacept (competitive blocker of the B7: CD28 pathway), since regulatory T cells play an important role in the preservation of graft function.
Materials and Methods
Mice
C57BL/6 (H2b, B6), B6.C-H2bm12/KhEgJ (bm12), B6.C-H2bm1/ByJ (bm1), B6.129S2-Cd28tm1Mak/J (CD28KO), B6.129S4-Cd80tm1Shr/J (B7.1KO), B6.129S4-Cd86tm1Shr/J (B7.2KO) and B6.129S4-Cd80tm1ShrCd86tm2Shr/J (B7DKO) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). All mice were 8–12 weeks of age and housed in accordance with institutional and National Institutes of Health guidelines.
Heterotopic heart transplantation
Vascularized heart grafts were placed in an intra-abdominal location using microsurgical techniques as described by Corry et al. (8). Graft function was assessed by palpation of the heartbeat. Rejection was determined by complete cessation of palpable heartbeat and was confirmed by direct visualization after laparotomy. Graft survival is shown as the median survival time (MST) in days.
Antibodies and in vivo treatment protocol
Rat anti-mouse CD25 mAb (PC61), B7.1 mAb (1G10) and B7.2 mAb (ascites) were manufactured and purified from the original hybridomas by a commercial source, Bioexpress Cell Culture (West Lebanon, NH) (9). Cardiac allograft recipients were treated with PC61 250 μg on days 6 and 1 before transplantation. The 1G10 and ascites were administered i.p. 500 μg at day −10, and 250 μg at days −8, −6, −4, −2 and 0 before the transplantation.
ELISPOT and Luminex assay
Splenocytes harvested at 10 days after transplantation from WT B6, CD28KO and B7DKO recipients of bm12 heart allografts were restimulated by irradiated donor-type splenocytes. The ELISPOT assay (R&D Systems, Minneapolis, MN) was adapted to measure the frequency of alloreactive T cells producing interferon (IFN)-γ and interleukin (IL)-4 and IL-6, as described previously (5). The frequencies of cytokine-secreting alloreactive cells were expressed as the number of cytokine-producing cells per 0.5 × 106 responder cells. For Luminex assay, cell-free supernatants of individual wells were removed after 48 h of incubation and analyzed by a multiplexed cytokine bead-based immunoassay using a preconfigured 21-plex mouse cytokine detection kit (Millipore) according to the manufacturer’s instructions as described previously (6). All samples were tested in triplicate wells. The cytokine panel included IFN-γ , IL-1a, IL-1b, IL-2, IL-4, IL-5, IL-6, IL-7, IL-10, IL-12, IL-13, IL-15, IL-17, IP-10, MCP-1, MIG, RANTES and TNFα.
Flow cytometry
Splenocytes from B6 WT, B6 CD28KO and B6 B7 DKO recipients of bm 12 hearts at 10 days after transplantation were stained with fluorochrome-labeled monoclonal antibodies (mAbs) against CD4, CD8, CD62 ligand (CD62L), CD44, CD25 and FoxP3 (BD Biosciences, San Jose, CA). Intracellular FoxP3 staining was performed using the Cytofix/Cytoperm intracellular staining kit. Flow cytometry was performed with a FACSCalibur system (BD Biosciences) and analyzed using FlowJo software, assessing regulatory T cells (CD4+CD25+FoxP3+) as well as CD4+ and CD8+ effector memory (CD44high CD62Llow phenotype) and central memory (CD44high CD62Lhigh phenotype) cells.
Morphology
Cardiac graft samples from transplanted mice were harvested from rejecting (cessation of heartbeat by palpation) and long-term survivors (>56 days), then fixed in 10% formalin, embedded in paraffin, coronally sectioned and stained with hematoxylin and eosin for evaluation of the degree of rejection according to International Society of Heart and Lung Transplantation (ISHLT) guidelines (10) and for cellular infiltration and vasculopathy by light microscopy (11,12). An examiner blinded to the groups read all the samples.
In vitro suppression assays
To assess the regulatory function of Tregs in vitro, ELISPOT assays were set up in which CD4+ T cells from CD28KO recipients of bm12 cardiac allografts were used as responder cells to irradiated bm12 splenocytes. Subsequently, CD4+CD25+ T cells from naive B6 WT and CD28KO mice were added to each well as ‘modifying cells’ at various ratios. The regulatory function of Tregs was assessed by the suppressive effect on the frequency of IFN-γ -producing responder cells. CD4+ T cells and CD4+CD25+ T cells were isolated from splenocytes by magnetic activated sorting by a CD4+ T cell and CD4+CD25+ Regulatory T-cell isolation kits (130-090-860 and 130-091-041, respectively; Miltenyi Biotec). The purity of T cells was estimated to be greater than 90% by FACS.
Statistics
Graft survival was expressed graphically using the Kaplan–Meier method, and statistical differences in survival between the groups were assessed by the log-rank test. Student’s t-test was used for comparison of means. A p < 0.05 was considered statistically significant.
Results
B7/CD28 deficiency prolongs cardiac allograft survival in the fully MHC-mismatched transplant model, whereas B7/CD28 deficiency accelerates allograft loss in the single MHC class II-mismatched transplant model
Consistent with previously published data, B6 CD28KO recipients rejected BALB/c hearts at a delayed tempo (MST = 14 days vs. 7.5 days in WT recipients, n = 4, p = 0.0062) and B7.1/2-double-deficient recipients were unable to reject BALB/c hearts within 8 weeks (MST > 56, n = 5) (13,14).
The ‘bm12 into B6’ is a single MHC class II-mismatched transplant model in which cardiac allografts are devoid of acute rejection and survive long term, but develop severe transplant arteriosclerosis within 8 weeks of transplantation (5,6). Hearts from bm12 mice were transplanted into B6 WT and B6 background CD28KO, B7-1 knockout, B7-2 knockout and B7-1, B7-2 double KO (B7.1/2 DKO) mice. Bm12 cardiac grafts transplanted into B6 WT recipients had a MST of greater than 56 days. Bm12 cardiac grafts transplanted into single B7-1 (MST > 56 days, n = 4) or B7-2 KO (MST > 56 days, n = 4) recipients had comparable survival times to WT recipients. Allograft rejection was accelerated when bm12 hearts were transplanted into B7DKO recipients (MST = 7.5 days, n = 6, p < 0.0001). Allograft rejection was also significantly accelerated when bm12 hearts were transplanted into CD28KO recipients (MST = 13 days, n = 6, p < 0.0001) (Figure 1A). The histology of rejected allografts in CD28KO and B7DKO recipients demonstrated a typical acute rejection pattern with intensive cellular infiltration and necrosis, leading to allograft loss (Figure 1C, D). In comparison, B6 WT recipients had minimal cellular infiltration of the allograft (Figure 1B) at 2 weeks after transplantation.
Figure 1. B7/CD28 deficiency leads to accelerated cardiac allograft loss in the single MHC class II-mismatched transplant model.

The hearts from bm12 mice were transplanted into B6 wild-type, CD28-, B7–1-, B7–2- deficient and B7.1/2-double-deficient mice. Bm12 cardiac grafts transplanted into a B6 WT recipient had a median survival time (MST) of greater than 56 days. Bm12 cardiac grafts transplanted into single B7–1- or B7–2-deficient recipients had similar survival times to WT recipients; unexpectedly, allograft rejection was remarkably accelerated when bm12 hearts were transplanted into B7.1/2-double-deficient or CD28-deficient recipients (A). Representative photomicrographs of H&E staining demonstrate minimal parenchymal infiltrate in the control B6 WT recipient (B) and a typical acute cellular allograft rejection pattern in CD28-deficient (C) and B7.1/2-double-deficient (D) recipient, with an extensive cellular infiltration and necrosis that precipitated the allograft loss.
Analysis of the frequency of alloreactive cytokine-producing cells in B6 WT, CD28KO and B7DKO recipients of cardiac grafts from bm12 mice by ELISPOT at 10 days after transplantation showed that the frequency of alloreactive T cells producing IFN-γ in CD28KO and B7DKO recipients had a 5-fold and 45-fold increase, respectively, over the control B6 WT (Figure 2A). The same pattern of increase was seen in IL-4 and IL-6 producing cells (Figure 2A). The alloantigen-specific production of Th1 (IFN-γ), Th2 (IL-4, IL-5, IL-10 and IL-13) and proinflammatory cytokines (IL-2, TNF-α, IL-6, IL-17, IL-12p40 and IL-12p70) were also examined by Luminex in WT, CD28KO and B7DKO recipients of bm12 cardiac allografts. Consistent with the ELISPOT observations, CD28KO and B7DKO splenocytes produced significantly more Th1 cytokine IFN-γ, Th2 cytokines IL-4, IL-5, IL-10 and proinflammatory cytokines IL-2, TNF-α and IL-6 than WT splenocytes (Figure 2B). Finally, there was no evidence of significant IL-17 production, with only very low levels detected in all three groups (data not shown).
Figure 2. Upregulation of both Th1 and Th2 cytokine production in CD28-deficient and B7.1/2-double-deficient allograft recipients of bm12 hearts.

Th1, Th2 and proinflammatory cytokines production by the splenocytes of WT, CD28KO and B7DKO recipients of bm12 heart grafts assessed at 10 days post-Tx by ELISPOT (A) and Luminex assay (B). Data represent mean ± SEM of triplicate wells from three mice per group.
B7/CD28 deficiency impairs the generation of regulatory T cells in the B6 recipients of bm12 cardiac grafts, leading to acute allograft rejection instead of long-term survival
To investigate the mechanism of acceleration of allograft rejection, we measured the percentage of CD4+CD25+Foxp3+ regulatory T cells, CD4+ and CD8+ effector/memory T cells in the wild-type B6, CD28-deficient and B7-double-deficient recipients of bm12 cardiac grafts 10 days after transplantation. Although the percentage of CD4+ effector/memory phenotype (CD44highCD62Llow) was similar among all recipients (19.83 ± 1.908; 15.64 ± 2.12, p = 0.1968 and 15.15 ± 2.58, p = 0.1938, respectively), the number of CD4+CD25+Foxp3+ regulatory T cells was significantly reduced in CD28KO (0.5300 ± 0.06658, p = 0.0026) and B7DKO (0.1250 ± 0.0650, p = 0.0047) compared with WT mice (1.583 ± 0.1433), resulting in a significantly higher ratio of CD4+ effector/memory cells relative to Tregs in the CD28KO and B7DKO recipients (Figure 3A and B). Furthermore, the percentage of regulatory T cells in WT, CD28KO and B7DKO recipients was proportional to the length of allograft survival in these mice (Table 1). These data indicate that regulatory T cells are critical for maintaining the long-term survival of bm12 allografts in wild-type B6 recipients. This was confirmed by treating wild-type B6 recipients of bm12 heart allografts with 0.25mg rat anti-CD25 mAb at day −6, and −1 before transplantation. Recipients treated with anti-CD25 mAb had 100% allograft loss by day 40 (MST = 16 days, n = 8 vs. control MST>56 days, n = 10, p < 0.0001) (Figure 4A and B). Similarly, pretreatment of B6 WT recipients of bm12 hearts with anti-B7.1 and anti-B7.2 antibodies (0.5mg at day −10 and 0.25mg at day, and −8, −6, −4, −2, 0, i.p.) significantly reduced the percentage of CD4+CD25+Foxp3+ regulatory T cells and accelerated allograft rejection (MST = 19 days, n = 7 vs. control MST>56 days, n = 10, p < 0.0001) (Figure 4C and D). Consequently, we conclude that the survival time of the bm12 grafts is correlated to the number of regulatory T cells in the B6 recipient, suggesting a Treg-dependent transplant model (Table 1), which is consistent with the recent observation from Fairchild’s group (7).
Figure 3. CD4+CD25+Foxp3+ regulatory T cells remarkably decreased in CD28-deficient and B7.1/2-double-deficient allograft recipients of bm12 hearts.

FACS analysis of splenocytes of WT, CD28KO and B7DKO recipients of bm12 hearts at 10 days post-Tx showed that CD28KO and B7DKO recipients had a significant lower percentage of CD4+CD25+Foxp3+ regulatory T cells than wild-type recipients. (A). In addition, the ratio of CD4+ effector/memory cells relative to Tregs was significantly higher in CD28KO and B7DKO recipients when compared with WT recipients (B). Bar graphs show means ± SEM. All results are representative of at least three different sets of experiments.
Table 1.
The survival time of bm12 grafts is strictly correlated to the percentage of regulatory T cells in the B6 recipients’ spleens
| % CD4+Foxp3+Mean survival time | ||
|---|---|---|
| Bm12 into B6 | (Mean ± SEM) | (MST, Days) |
| B7.1/2 DKO | 2.67 ± 0.57 | 7.5 |
| CD28 KO | 3.63 ± 0.37 | 13 |
| Anti-CD25 (pre-Tx) | 4.99 ± 0.29 | 16 |
| Anti-B7.1+ Anti-B7.2 (pre-Tx) (pre-Tx) |
6.42 ± 1.13 | 19 |
| B6 WT | 13.18 ± 0.48 | >56 |
Figure 4. Treg cells depletion by anti-CD25 or combination of anti-B7.1 and B7.2 mAbs abrogated the long-term survival of bm12 cardiac grafts in B6 wild-type recipients.

B6 wild-type mice were treated with anti-CD25 or anti-B7.1/2 mAbs and received vascularized cardiac allografts from bm12 donors. MST was >56 days in recipients without treatment versus 16 days in anti-CD25 mAb-treated and 19 days in anti-B7.1/2 mAb-treated recipients (A, C). The percentage of CD4+Foxp3+ Tregs in spleens was determined by flow cytometry. Representative animals are indicated in the lower-right quadrant of the FACS plot graph (B, D).
Regulatory T cells from CD28-deficient mice are fully functional and able to suppress cytokine production of CD28-deficient alloreactive T cells in vitro when compared to naïveB6Tregs
In order to confirm that the accelerated rejection in CD28-deficient mice was primarily due to the low number of Tregs in the recipient, it was essential to exclude impaired function of Tregs in a CD28-deficient milieu. Consequently, an IFN-γ ELISPOT assay was set up to assess the regulatory function of CD4+CD25+ Tregs from naïve B6 wild-type and CD28 KO mice in vitro (Figure 5). CD4+CD25+ Tregs from naïve B6 wild-type (1:1, 259.7 ± 12.08 vs. 100.5 ± 9.500, p = 0.0004) and CD28 KO (1:1, 259.7 ± 12.08 vs. 95.50 ± 1.500, p = 0.0003) mice significantly suppressed IFN-γ production of CD28-deficient alloreactive T cells in a dose-dependent manner, clearly indicating that the CD4+CD25+ T-cell subset in naïve B6 and CD28KO mice functionally inhibits alloimmune responses.
Figure 5. CD4+CD25+ Tregs from naive B6 wild-type and CD28-deficient mice suppressed the alloimmune response in vitro.

ELISPOT assay was set up to assess the regulatory function of CD4+CD25+ Tregs from B6 wild-type and CD28KO in vitro. Isolated CD4+ T cells from CD28KO recipients of bm12 cardiac allografts were used as responder cells to irradiated bm12 splenocytes. CD4+CD25+ Tregs from naive B6 wild-type and CD28KO mice were added to each well as ‘modifying cells’ at different ratios. The regulatory function of Tregs was assessed by the suppressive effect on the frequency of IFN-γ -producing responder cells.
B7/CD28 deficiency did not significantly affect graft survival in single MHC class I-mismatched bm1 into B6 cardiac transplant model
To explore further the relevance of the B7/CD28 deficiency in another transplantation setting characterized by a weaker alloimmune response when compared with fully mismatched models, we evaluated the ‘bm1 into B6’ heart transplantation model. In this single MHC class I-mismatched model, CD8+ T cells primarily mediate allograft rejection. Similar to the ‘bm12 into B6’ cardiac model, all allografts survived long term (>56 days). However, B7/CD28-deficient B6 recipients did not alter the cardiac allograft survival of bm1 donors (Figure 6A), and depletion of CD25+ T cells did not accelerate bm1 allograft rejection in WT B6 recipients (Figure 6B). These combined data suggest that the B7/CD28 deficiency affects allograft survival exclusively in transplant models in which Tregs predominantly control the alloimmune response.
Figure 6. B7/CD28 deficiency did not significantly affect graft survival in single MHC class I-mismatched bm1 into B6 cardiac transplant model; accordingly, CD25+ T-cells depletion did not accelerate allograft rejection in B6 recipients of bm1 hearts.
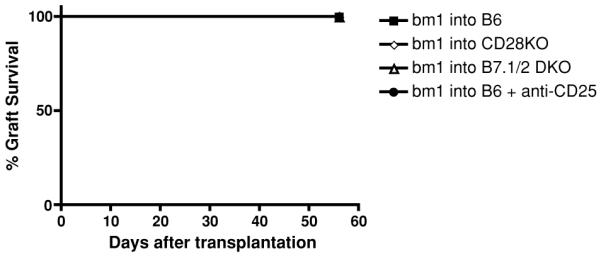
The bm1 hearts were transplanted into B6 wild-type, CD28-deficient, B7.1/2-double-deficient mice. All the bm1 allografts survived during the observation of 56 days in wild-type recipients. In contrast to ‘bm12 into B6’ transplant model, B7/CD28 deficiency in recipients did not alter bm1 graft survival (A). Moreover, anti-CD25 antibody treatment did not accelerate the bm1 allograft survival in B6 WT recipients (B).
Discussion
In this study, we report the intriguing finding that CD28- or B7.1/2-deficient recipients accelerated the cardiac allograft rejection in a single MHC class II-mismatched ‘bm12 into B6’ transplant model, in contrast to prolonging cardiac allograft survival in the fully MHC-mismatched ‘BALB/c into B6’ transplant model. We found that the acceleration of allograft rejection is associated with a marked reduction in the number of CD4+CD25+Foxp3+ regulatory T cells in the CD28-deficient or B7.1/2-double-deficient recipients of bm12 hearts. We also showed that depletion of CD25+ regulatory T cells in WT recipients accelerated allograft rejection at a comparable rate to CD28-deficient recipients. A similar observation has been reported previously in the ‘bm12 into B6’ cardiac transplant model, although a different treatment protocol of anti-CD25mAb was used (7). We note that the percentage of Tregs in CD28KO, B7DKO and WT recipients treated with anti-CD25 and anti-B7.1/2 mAbs correlated with the bm12 graft survival time (Table 1). Nevertheless, others have shown that B7DKO recipients of bm12 hearts did not experience an accelerated rejection when recipients were treated with immunosuppressives at the time of transplantation (15). This indicates that targeting effector cells with immunosuppression after transplantation, even when Tregs have been reduced as in the case of the B7DKO recipients, can still abrogate the alloimmune response.
It is well known that B7: CD28 costimulation lowers the activation threshold, enhances proliferation and increases cell survival by augmenting secretion of multiple cytokines (such as IL-2), and also promotes T-cell differentiation toward both T helper Th1 and Th2 (16). In an autoimmune model, it has been reported that both Th1 and Th2 cytokines are reduced in B7.1/2-double-deficient NOD mice (2) and CD28-deficient NOD mice have impaired Th2 differentiation while Th1 development is maintained (17). In contrast, the accelerated allograft rejection in CD28-deficient and B7.1/2-double-deficient recipients of bm12 hearts is associated with increased production of Th1, Th2 and proinflammatory cytokines. This increase in multiple cytokines probably does not directly result from the absence of B7: CD28 costimulation, but rather from the lack of suppression of effector T cells. This is supported by the observation that depletion of CD25+ Tregs significantly increases the frequency of IFN-γ producing cells in the ‘bm12 into B6’ cardiac transplantation model (7). The anti-CD25 antibody also targets activated effector T cells in addition to Tregs, however, it seems that in the setting of reduced alloreactive T-cell clone size, its dominant effect is related to the depletion of CD25+ regulatory T cells (7). Last, the absence of significant IL-17 production excludes the emergence of Th17 cells as potential mediators of this accelerated rejection (6).
Our findings indicate that B7: CD28 costimulation is essential for the development and homeostasis of regulatory T cells and protect bm12 cardiac allografts from acute rejection in WT B6 recipients, consistent with a recent study of B7: CD28 costimulation in the experimentally induced model of autoimmunity (2). The transfer of a regulatory T-cell subset from control NOD animals into CD28-deficient animals can delay/prevent diabetes. We were able to confirm the normal function of CD28-deficient Tregs in vitro, but an adoptive transfer of CD28-deficient Tregs into CD28KO recipients of bm12 allografts would have been ideal to confirm the importance of the T-effector/Tregs ratio in alloimmune regulation in this model. However, the limited number of Tregs present in CD28KO mice is a technical limitation (<0.6% of splenocytes) to this approach.
The B7: CD28 pathway has proven itself to be relevant in multiple models of transplantation and autoimmunity, and the blockade of this costimulatory pathway has been considered a good target for therapeutic intervention (18). Belatacept, a higher-affinity and more potent second-generation CTLA4-Ig, was developed specifically for use in organ transplantation, binding with greater affinity to B7.1 and B7.2 (CD80/CD86) than CD28 receptors (19). Our findings raise an important question about the long-term effects of this form of T-cell blockade on the allograft by affecting Tregs generation and maintenance, especially in well-matched recipients. However, extrapolation of our findings to humans cannot be made at this time and will require further investigation with the ongoing long-term trials with Belatacept. This will be of great interest as we attempt to develop new immunosuppressive targets that ultimately protect the allograft from chronic rejection.
Taken together, this study demonstrates for the first time the paradoxical role of B7: CD28 costimulation in fully allogeneic and single MHC class II-mismatched grafts and proposes a possible mechanism through the realignment of effector to regulatory T- cell ratio (Teff/Treg) in favor of a highly alloreactive immune response. These data suggest that B7 blockade may have a paradoxical effect depending on the timing of blockade and the balance of Tregs to T-effector cells within the recipient.
Acknowledgments
Funding sources: This study was supported by the following grants AST/Roche Basic Science Faculty Development Grant to JY; NIH AI-37691, AI-51559, PO1 AI-41521 to MHS; National Kidney Foundation Clinical Scientist Award to AC, and German Research Foundation (Deutsche Forschungsgemeinschaft, DFG) to OB.
References
- 1.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 2.Salomon B, Lenschow DJ, Rhee L, et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 3.Tang Q, Henriksen KJ, Boden EK, et al. Cutting edge: CD28 controls peripheral homeostasis of CD4+CD25+ regulatory T cells. J Immunol. 2003;171:3348–3352. doi: 10.4049/jimmunol.171.7.3348. [DOI] [PubMed] [Google Scholar]
- 4.Zeng M, Guinet E, Nouri-Shirazi M. B7-1 and B7-2 differentially control peripheral homeostasis of CD4+CD25+Foxp3+ regulatory T cells. Transplant Immunology. 2009;20:171–179. doi: 10.1016/j.trim.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 5.Yang J, Popoola J, Khandwala S, et al. Critical role of donor tissue expression of programmed death ligand-1 in regulating cardiac allograft rejection and vasculopathy. Circulation. 2008;117:660–669. doi: 10.1161/CIRCULATIONAHA.107.741025. [DOI] [PubMed] [Google Scholar]
- 6.Yuan X, Paez-Cortez J, Schmitt-Knosalla I, et al. A novel role of CD4 Th17 cells in mediating cardiac allograft rejection and vasculopathy. J Exp Med. 2008;205:3133–3144. doi: 10.1084/jem.20081937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schenk S, Kish DD, He C, et al. Alloreactive T cell responses and acute rejection of single class II MHC-disparate heart allografts are under strict regulation by CD4+ CD25+ T cells. J Immunol. 2005;174:3741–3748. doi: 10.4049/jimmunol.174.6.3741. [DOI] [PubMed] [Google Scholar]
- 8.Corry RJ, Winn HJ, Russell PS. Primarily vascularized allografts of hearts in mice. The role of H-2D, H-2K, and non-H-2 antigens in rejection. Transplantation. 1973;16:343–350. doi: 10.1097/00007890-197310000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Ueno T, Habicht A, Clarkson MR, et al. The emerging role of T cell Ig mucin 1 in alloimmune responses in an experimental mouse transplant model. J Clin Invest. 2008;118:742–751. doi: 10.1172/JCI32451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Billingham ME, Cary NR, Hammond ME, et al. A working formulation for the standardization of nomenclature in the diagnosis of heart and lung rejection: Heart Rejection Study Group The International Society for Heart Transplantation. J Heart Transplant. 1990;9:587–593. [PubMed] [Google Scholar]
- 11.Harada H, Salama AD, Sho M, et al. The role of the ICOS-B7h T cell costimulatory pathway in transplantation immunity. J Clin Invest. 2003;112:234–243. doi: 10.1172/JCI17008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimizu K, Schonbeck U, Mach F, Libby P, Mitchell RN. Host CD40 ligand deficiency induces long-term allograft survival and donor-specific tolerance in mouse cardiac transplantation but does not prevent graft arteriosclerosis. J Immunol. 2000;165:3506–3518. doi: 10.4049/jimmunol.165.6.3506. [DOI] [PubMed] [Google Scholar]
- 13.Lin H, Rathmell JC, Gray GS, Thompson CB, Leiden JM, Alegre ML. Cytotoxic T lymphocyte antigen 4 (CTLA4) blockade accelerates the acute rejection of cardiac allografts in CD28-deficient mice: CTLA4 can function independently of CD28. J Exp Med. 1998;188:199–204. doi: 10.1084/jem.188.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamada A, Kishimoto K, Dong VM, et al. CD28-independent costimulation of T cells in alloimmune responses. J Immunol. 2001;167:140–146. doi: 10.4049/jimmunol.167.1.140. [DOI] [PubMed] [Google Scholar]
- 15.Furukawa Y, Mandelbrot DA, Libby P, Sharpe AH, Mitchell RN. Association of B7-1 co-stimulation with the development of graft arterial disease. Studies using mice lacking B7-1, B7-2, or B7-1/B7-2. Am J Pathol. 2000;157:473–484. doi: 10.1016/S0002-9440(10)64559-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rothstein DM, Sayegh MH. T-cell costimulatory pathways in allograft rejection and tolerance. Immunol Rev. 2003;196:85–108. doi: 10.1046/j.1600-065x.2003.00088.x. [DOI] [PubMed] [Google Scholar]
- 17.Lenschow DJ, Herold KC, Rhee L, et al. CD28/B7 regulation of Th1 and Th2 subsets in the development of autoimmune diabetes. Immunity. 1996;5:285–293. doi: 10.1016/s1074-7613(00)80323-4. [DOI] [PubMed] [Google Scholar]
- 18.Sayegh MH, Turka LA. The Role of T-cell costimulatory activation pathways in transplant rejection. N Engl J Med. 1998;338:1813–1821. doi: 10.1056/NEJM199806183382506. [DOI] [PubMed] [Google Scholar]
- 19.Vincenti F, Larsen C, Durrbach A, et al. Costimulation blockade with belatacept in renal transplantation. N Engl J Med. 2005;353:770–781. doi: 10.1056/NEJMoa050085. [DOI] [PubMed] [Google Scholar]


