Abstract
Objective
To compare outcomes in older individuals receiving drug-eluting (DES) and bare metal stents (BMS).
Background
Comparative effectiveness of DES relative to BMS remains unclear.
Methods
Outcomes were evaluated in 262,700 patients from 650 National Cardiovascular Data Registry sites during 2004-2006 using procedural registry data linked to Medicare claims for follow-up. Outcomes including death, myocardial infarction (MI), revascularization, major bleeding, stroke, death or MI, death or MI or revascularization, and death or MI or stroke, were compared using estimated cumulative incidence rates with inverse probability weighted estimators and Cox proportional hazards ratios.
Results
DES were implanted in 217,675 patients and BMS in 45,025. At 30-months, DES patients had lower unadjusted rates of death (12.9% vs. 17.9%), MI (7.3 vs. 10.0/100 pts) and revascularization (23.0 vs. 24.5/100 pts) with no difference in stroke or bleeding. After adjustment, DES patients had lower rates of death (13.5% vs. 16.5%, HR=0.75, (95% CI: 0.72 ,0.79), p<0.001) and MI (7.5 vs. 8.9/100 pts, HR=0.77, (95% CI: 0.72,0.81), p<0.001), with minimal difference in revascularization (23.5 vs. 23.4/100 pts; HR=0.91, (95% CI: 0.87,0.96), stroke (3.1 vs. 2.7/100 pts, HR=0.97, (95% CI: 0.88,1.07) or bleeding (3.4 vs. 3.6/100 pts, HR=0.91, (95% CI: 0.84,1.00). The DES survival benefit was observed in all subgroups analyzed and persisted throughout 30-months’ follow-up.
Conclusion
In this largest ever real-world study, patients receiving DES had significantly better clinical outcomes than their BMS counterparts, without an associated increase in bleeding or stroke, throughout 30 months’ follow-up and across all prespecified subgroups.
Keywords: Coronary revascularization, Comparative effectiveness, Drug eluting stent
INTRODUCTION
The dramatic reductions in restenosis and repeat revascularization associated with drug-eluting coronary artery stents (DES) compared with their bare metal (BMS) counterparts(1), prompted swift adoption into clinical practice(2). However, reports of late stent thrombosis (3,4) and higher mortality(5,6) resulted in release of two special FDA advisories in 2006(7,8), as well as subsequent studies refining event rates(1,6,9-13). The rarity of late DES complications means that extremely large sample sizes are required to clarify their frequency. Furthermore, the ability to examine rates of lower frequency complications in important patient subgroups is limited in smaller sample sizes(14).
Accordingly, the Agency for Healthcare Research and Quality (AHRQ) and US Food and Drug Administration (FDA) commissioned the formation of a nationally-representative PCI database to determine the safety and effectiveness of DES and BMS among a contemporary ‘real world’ cohort. This was accomplished through linkage of the American College of Cardiology National Cardiovascular Data Registry (ACC-NCDR®) with the Centers for Medicare and Medicaid Services (CMS) national claims database. The resulting analyses will better inform national practice patterns overall and in important patient and lesion-level subgroups.
METHODS
Study Population
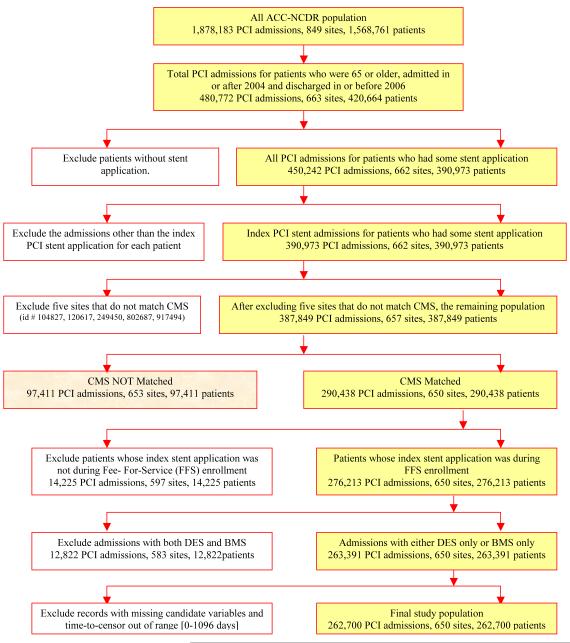
The national ACC-NCDR® CathPCI Registry collects information for patients undergoing percutaneous coronary intervention (PCI) procedures. We included all CathPCI patients ≥ 65 years undergoing an inpatient intracoronary stent procedure between January 1, 2004 and December 31, 2006. Patients receiving more than one stent type (ie both BMS and DES) were excluded. (Figure 1) The Duke University Medical Center Institutional Review Board granted a wavier of informed consent and authorization for this study.
Figure 1.
Population Selection – Flow Diagram
Follow-Up Information
Since ACC-NCDR® data are limited to a single episode of care we used the research-identifiable Medicare 100% inpatient fee-for-service claims file for longitudinal patient follow-up. PCI procedure codes (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] procedure codes 00.66, 36.0x, 37.22, 37.23, and 88.5x, except 88.59) were used to identify potential index procedure matches in the Medicare files which were then linked to NCDR using indirect identifiers (non-unique fields that when used in combination may identify unique hospitalizations) to create unidentified longitudinal profiles and obtain up to 3 years follow-up. Linking rules used a hierarchy of evidence approach such that rules with the most information were applied before those with less information. Once a match was achieved for a patient, no further rules were applied. Our linking rules contained combinations of information denoting the index PCI procedure site, patient date of birth (or components thereof) or age, admission date, discharge date, and sex. In the rare event that a single ACC-NCDR® record could be matched with multiple Medicare records using the same rule, no linking occurred. Sites that did not match to Medicare records were excluded as were patients whose index PCI procedure did not occur during a period of fee-for-service enrollment.
Clinical End Points
We evaluated 8 clinical endpoints: 5 events and 3 composites. Death was the only event defined both during the index PCI procedure (using ACC-NCDR® information) and post-discharge (using the Medicare denominator file). Clinical endpoints were defined using the Medicare claims file as the primary diagnosis for a hospital admission. The ICD-9-CM diagnosis codes used to identify events were: myocardial infarction (MI) (410.X1), stroke (430.X, 431.X, 432.X, 434.X), and bleeding (430-432, 578.X, 719.1X, 423.0, 599.7, 626.2, 626.6, 626.8, 627.0, 627.1, 786.3, 784.7, or 459.0). Revascularizations were identified using ICD-9-CM procedure codes (PCI, 36.00, 36.06, 36.07, 36.09, and coronary artery bypass graft surgery, 36.10-19). Only revascularizations occurring after discharge from the index hospitalization were included in the revascularization analysis. The composite events used in this study were: MI or death, MI or death or revascularization, and MI or death or stroke.
Statistical Analysis
Baseline and propensity matching characteristics were categorized by stent type (DES vs. BMS) and summarized as counts and percentages for categorical variables and means with standard deviations for continuous variables. Statistical significance was defined as p≤0.05, with no correction for multiple comparisons, using SAS statistical software (version 9.1; SAS Institute, Cary, NC) for all calculations.
Propensity Score Models
We used propensity scores to adjust for between-treatment group differences in baseline characteristics(15). Propensity scores represent the estimated probabilities of patients receiving drug-eluting vs. bare metal stents in our population(15), in this case conditioned upon 102 observed covariates. (Appendix)
Inverse probability weighted estimators incorporating propensity scores were used to compare treatment groups(16). The propensity score model had a c-index of 0.690. In addition, the distribution of propensity scores for DES patients closely match those for BMS patients as evidenced by the 5-number summaries (min, 25th, 50th, 75th, max) describing the curves for patients receiving each type of stent: (BMS: 14.5%, 70.7%, 79.6%, 85.9%, and 99.1%) and (DES: 16.0%, 79.7%, 86.1%, 90.7%, and 99.5%). The overlap between the groups is excellent and suggests that the propensity score approach is statistically appropriate.
Inverse probability weighted estimators with monthly data partitions were used to calculate cumulative incidence rates for clinical end points (adjusted and unadjusted)(17,18). Unadjusted estimates were based upon Kaplan-Meier estimates for treatment-specific censoring distributions; whereas adjusted estimates were based upon weights that were functions of Kaplan-Meier censoring estimates and propensity score estimates(17,18). Adjusted hazard ratios were calculated according to the inverse probability weighted (IPW) approach of Cole and Hernan.(19) In particular we calculated two IPW Cox proportional hazards models – one with an indicator for DES as the only covariate and one with DES plus a selected group of clinically important variables including: gender, age, diabetes, renal disease, prior revascularization, prior MI, multivessel CAD, year of procedures, and race. From these models, we estimated the adjusted hazard ratio for DES vs. BMS along with a 95% confidence intervals based on the sandwich estimated standard errors. To visually assess the proportional treatment effect assumptions we plotted the monthly cumulative incidence rates over the 30-month follow-up period. Additionally, we plotted the treatment-group specific cumulative incidence rates excluding events from the first 6 and 12 months to identify the long-term component of the treatment effect. We refer to these latter analyses as 6 and 12 month landmark analyses.
Cox Model
A Cox proportional hazards mortality model (without propensity score weighting) was developed using backward selection of the propensity score variables with a p=0.05 selection threshold. Forward selection was used in a sensitivity analysis for internal validation of the final model which contained 60 covariates. These models served to validate the adjusted hazard ratio estimates from the IPW Cox regression model method of Cole and Hernan.(19)
Subgroup Analyses
PCI status included STEMI [primary, rescue, or facilitated], urgent [non-STEMI or unstable], and elective subgroups. Within the DES group, off- vs. on-label use subgroups were examined. For patients enrolled in NCDR using version 2 of the data collection form (DCF), off-label use was defined as intervention on ACC/AHA Type C lesion, PCI status of urgent or STEMI, intervention in a previously treated lesion, use of more than two stents in a lesion, treatment of a left main or graft segment, or multi-vessel PCI. For those enrolled using DCF version 3, the off-label use definition was modified to also include device diameter ≤2.5 mm or >4mm, total stented or lesion length ≥30mm, and bifurcation lesions.
Sensitivity Analyses
We conducted two sensitivity analyses. For the first analysis, each of the five main outcomes were examined in a subgroup of patients fitting the inclusion and exclusion criterion from the Taxus IV and SIRIUS trials (n=49,355)(20,21) using a recalibrated propensity score including 76 clinical variables with a c-index of 0.71.
The second sensitivity analysis estimated ‘cause of death’ after stent implantation according to the primary diagnosis of a hospitalization during which the patient expired or the most recent hospitalization within 6-months of death. Using a previously validated list of ICD-9 codes(22) we examined the relative distribution of causes of death across DES and BMS patients.
RESULTS
Between January 2004 and December 2006, 390,973 NCDR patients ≥ 65 years underwent stent implantation, and 76% were linked to longitudinal Medicare records. After exclusions, the study population included 262,700 patients from 650 sites. (Figure 1) Comparison of NCDR® patients who did and did not match to Medicare records revealed non-match patients to be slightly younger (73 vs. 74 yrs), and more likely to be male (62% vs. 58%) and to have commercial insurance (15% vs. 3%).
Overall, 45,025 patients received one or more BMS and 217,675 received one or more DES (54% paclitaxel eluting, 46% sirolimus eluting). Unadjusted baseline characteristics show significant differences between DES and BMS, these differences were reduced following propensity score weighting (Table 1). Sixty-nine percent of DES implantations were for non-FDA-approved indications. Mean follow-up for BMS patients was slightly longer (496 ± 371 days) than for DES patients (456 ± 302 days) due to the trends in stent use over the time period studied.
Table 1.
Unadjusted baseline characteristics of patients in study population
| Patient Characteristics | Study Population | Study Population After Propensity Score Weighting |
|||||
|---|---|---|---|---|---|---|---|
| DES (217,675) |
BMS (45,025) |
p-value | DES* (217,675) |
BMS* (45,025) |
p-value | ||
| Age (mean ±SD years) | 74.5± 6.4 | 75.3± 6.7 | <0.001 | 74.7 | 74.8 | 0.03 | |
| Sex (Male) | 123209 (56.6 %) | 27024 (60.0) | <0.001 | 57.2 | 57.0 | NS | |
| Race | Caucasian | 196260 (90.2%) | 41029 (91.1%) | <0.001 | 90.3 | 90.2 | NS |
|
African
American |
9051 (4.2%) | 1984 (4.4%) | 0.017 | 4.2 | 4.3 | NS | |
| Asian | 1885 (0.9%) | 254 (0.6%) | <0.001 | 0.8 | 0.8 | NS | |
| Hispanic | 3843 (1.8%) | 578 (1.3%) | <0.001 | 1.7 | 1.7 | NS | |
| Other | 6258 (2.9) | 1092 (2.5%) | <0.001 | 2.8 | 2.8 | NS | |
|
Hospital
Region |
Northeast | 22642 (10.4%) | 5371 (11.9%) | <0.001 | 10.7 | 10.8 | NS |
| South | 80612 (37.0%) | 15614(34.7%) | <0.001 | 36.6 | 36.8 | NS | |
| Midwest | 83956 (38.6%) | 18901 (41.9%) | <0.001 | 39.1 | 39.0 | NS | |
| West | 29294 (13.5%) | 4874 (10.8 %) | <0.001 | 13.0 | 13.0 | NS | |
|
Hospital
Setting |
Rural | 29951 (13.8%) | 6756 (15.0%) | <0.001 | 14.0 | 13.9 | NS |
| Suburban | 55654 (25.6%) | 12658 (28.1%) | <0.001 | 26.0 | 26.4 | NS | |
| Urban | 132070 (60.7%) | 25611 (56.9%) | <0.001 | 60.0 | 59.8 | NS | |
|
Year Index
Procedure |
2004 | 58453 (26.9%) | 19515 (43.3%) | <0.001 | 29.7 | 30.3 | NS |
| 2005 | 74685 (34.3%) | 9437 (21.0%) | <0.001 | 32.0 | 31.2 | NS | |
| 2006 | 84537 (38.8%) | 16073 (35.7%) | <0.001 | 38.3 | 38.5 | NS | |
| Mean Follow-up (days ± SD) | 456.4 (302.5) | 495.8 (371.4) | <0.001 | 464.3 | 452.0 | NS | |
| Current smoking | 25955 (11.9 %) | 6108 (13.6%) | <0.001 | 12.2 | 12.3 | NS | |
| CHF | 124197 (57.1%) | 23112 (51.3%) | <0.001 | 56.1 | 55.7 | NS | |
| HTN | 175154 (80.5 %) | 35808 (79.5%) | <0.001 | 80.3 | 80.6 | NS | |
|
Renal
Failure |
No Dialysis | 10393 (4.8%) | 2701 (6.0%) | <0.001 | 5.0 | 5.2 | NS |
| Dialysis | 3380(1.6%) | 854(1.9%) | <0.001 | 1.6 | 1.7 | NS | |
| Diabetes |
Non-Insulin
Requiring |
49874 (22.9%) | 9930(22.1%) | <0.001 | 22.8 | 23.0 | NS |
| Insulin | 20430 (9.4%) | 4533 (10.1%) | <0.001 | 9.5 | 9.6 | NS | |
| Peripheral Vascular Disease | 32018 (14.7%) | 7776 (17.3%) | <0.001 | 15.2 | 15.5 | NS | |
| Stroke | 34067(15.7%) | 7908 (17.6%) | <0.001 | 16.0 | 16.5 | NS | |
| Chronic Lung Disease | 39611 (18.2%) | 9163 (20.4%) | <0.001 | 18.6 | 18.8 | NS | |
| Prior PCI | 61974 (28.5 %) | 11678(25.9 %) | <0.001 | 28.0 | 27.9 | NS | |
| Prior CABG | 47777 (22.0%) | 12735 (28.3%) | <0.001 | 23.1 | 23.4 | NS | |
| Prior MI | 57282 (26.2%) | 12941 (28.7%) | <0.001 | 26.8 | 26.9 | NS | |
| URGENCY | Elective | 111426 (51.2%) | 20511 (45.6%) | <0.001 | 50.2 | 49.6 | NS |
| Urgent | 82296 (37.8%) | 16048 (35.6%) | <0.001 | 37.4 | 37.8 | NS | |
| Emergent | 23616 (10.9%) | 8225 (18.3%) | <0.001 | 12.1 | 12.4 | NS | |
| Indication | Stable Angina | 38710 (17.8%) | 6129 (13.6%) | <0.001 | 17.1 | 17.0 | NS |
| UA/ NSTEMI | 34581 (15.9%) | 8413 (18.7%) | <0.001 | 51.3 | 51.0 | NS | |
| STEMI | 21170 (9.7%) | 7422 (16.5%) | <0.001 | 10.9 | 11.1 | NS | |
| Pre-Procedural Shock | 3675 (1.7%) | 1746 (3.9%) | <0.001 | 2.1 | 2.1 | NS | |
| Pre-Procedural Aspirin | 144296 (90.2%) | 22790(88.8%) | <0.001 | 64.3 | 62.6 | NS | |
| Pre-Procedural Clopidogrel | 173600 (80.2%) | 35821(80.2%) | 0.78 | 80.6 | 77.9 | <0.01 | |
| Pre-Procedural IABP | 637(0.2%) | 172(0.4%) | <0.001 | 0.3 | 0.3 | NS | |
| Gp IIb/IIIa inhibitors | 100043 (46.1%) | 21726 (48.6%) | <0.001 | 46.9 | 44.7 | <0.05 | |
| Multivessel PCI | 34185 (15.7%) | 5006 (11.1%) | <0.001 | 14.9 | 14.6 | NS | |
|
Intervened
Vessels |
LAD | 96278 (44.2%) | 15259 (33.9%) | <0.001 | 42.4 | 41.7 | NS |
| LCX | 66766 (30.7%) | 13725 (30.5%) | 0.43 | 30.6 | 30.7 | NS | |
| RCA | 83508 (38.4%) | 20062 (44.6%) | <0.001 | 39.5 | 39.8 | NS | |
| Graft | 17412 (8.0%) | 6997(15.5%) | <0.001 | 9.3 | 9.7 | NS | |
|
# stents per
patient |
1 | 135961 (62.5%) | 30833(68.5%) | <0.001 | 63.3 | 65.6 | <0.001 |
| ≥2 | 81714 (37.5%) | 14192 (31.5%) | 36.7 | 34.4 | |||
|
# of vessels
intervened |
1 | 183490 (84.3%) | 40019(88.9%) | <0.001 | 85.1 | 85.4 | NS |
| 2 | 32313(14.8%) | 4754(10.6%) | <0.001 | 14.1 | 13.8 | NS | |
| 3 | 1872(0.9%) | 252(0.6%) | <0.001 | 0.8 | 0.8 | NS | |
| ACC-AHA Type C Lesions | 83853 (38.5%) | 18127 (40.3%) | <0.001 | 38.8 | 39.2 | NS | |
| Sirolimus-Eluting (Cypher) | 100693 (46.3%) | 46.3 | |||||
| Paclitaxel-Eluting (Taxus) | 120320 (55.3%) | 55.2 | |||||
| Off-Label PCI | 112019 (69.2%) | 77.5 | |||||
Propensity matched comparisons reported as % of the matched population.
Death
During the 30-month study period, 21,254 deaths occurred. Thirty-month overall mortality was higher in patients who received BMS than DES both before (17.9% vs. 12.9%; p<0.0001), and after adjustment for population differences (16.5% vs. 13.5%, HR 0.75; 95% CI, 0.72 to 0.79). (Table 2) The adjusted mortality difference was statistically significant in the initial six months post-PCI, and continued to increase throughout the 30-month follow-up period.(Figure 2a) The estimated hazard ratio obtained using an unweighted Cox proportional hazards mortality model with backward variable selection was similar at 0.79 with a 95%CI (0.76 to 0.81). In addition to the use of DES, other factors favorably influencing 30-month post-PCI survival included female sex and prior PCI or CABG. As expected, mortality was higher in those with diabetes, renal failure, STEMI or CHF.
Table 2.
Unadjusted and adjusted results from time-to-event analyses for prespecified endpoints. Shown as Hazard Ratio and 95% confidence interval.
| Endpoint | Unadjusted | IPW Adjusted | IPW+covariates** |
|---|---|---|---|
| Death | 0.67 (0.65, 0.69) | 0.76 (0.72, 0.79) | 0.75 (0.72, 0.79) |
| Death or MI | 0.67 (0.65, 0.68) | 0.76 (0.72, 0.79) | 0.75 (0.72, 0.79) |
| Death or MI or Revasc | 0.78 (0.75, 0.80) | 0.84 (0.81, 0.87) | 0.84 (0.81, 0.87) |
| Death or MI or Stroke | 0.69 (0.67, 0.70) | 0.77 (0.74, 0.81) | 0.77 (0.74, 0.80) |
| MI* | 0.66 (0.64, 0.70) | 0.76 (0.72, 0.81) | 0.77 (0.72, 0.81) |
| Revascularization* | 0.89 (0.87, 0.92) | 0.92 (0.87, 0.96) | 0.91 (0.87, 0.96) |
| Stroke* | 0.90 (0.84, 0.98) | 0.98 (0.89, 1.07) | 0.97 (0.88, 1.07) |
| Bleed* | 0.87 (0.81, 0.93) | 0.92 (0.85, 1.00) | 0.91 (0.84, 1.00) |
| NSTEMI* | 0.69 (0.66, 0.73) | 0.79 (0.74, 0.85) | 0.79 (0.74, 0.84) |
| STEMI* | 0.63 (0.59, 0.68) | 0.74 (0.67, 0.81) | 0.74 (0.67, 0.82) |
Patients are censored after death in these analyses.
Additional covariates included in the IPW+covariates model were: DES, sex, age > 75, race, diabetes status, renal status, prior revascularizations, prior MI, multivessel CAD, procedure year, and off-label indications.
Figure 2a.
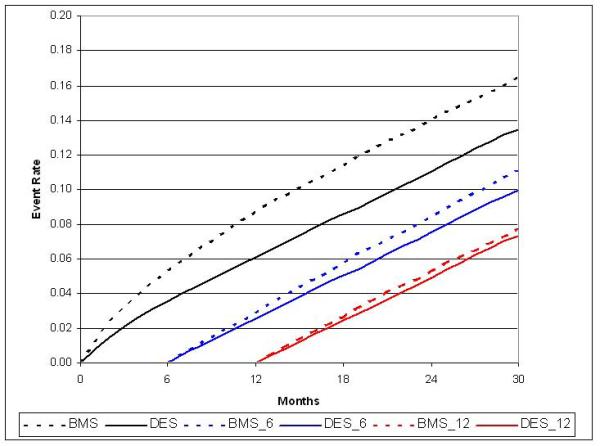
Adjusted cumulative incidence for death with 6- and 12-month landmark display
Myocardial Infarction
There were 10,528 MIs during the study period. Unadjusted MI rates at 30-months were 10.0 / 100 patients in BMS vs. 7.3 / 100 patients in DES (p<0.0001) with similar results following adjustment (8.9 / 100 patients vs. 7.5 / 100 patients, HR 0.77; 95% CI, 0.72 to 0.81).(Table 2) This result was driven by lower MI rates in DES patients during the first 12-months post-PCI,(Figure 2b) with no difference between 12 and 30-months of follow-up. In a secondary analysis, DES patients experienced a small increase in STEMI events beyond 12 months.(Figure 3)
Figure 2b.
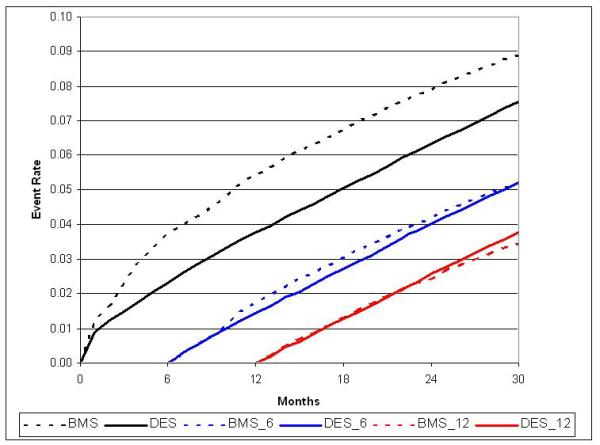
Adjusted cumulative incidence for MI with 6- and 12-month landmark display
Figure 3.
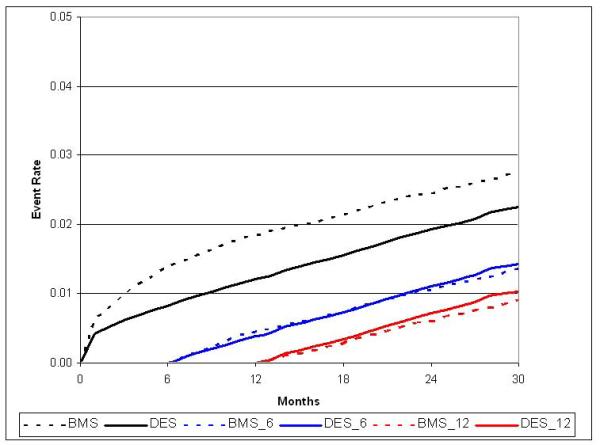
Adjusted cumulative incidence for STEMI with 6- and 12-month landmark display
Revascularization
Revascularization (PCI or CABG) was performed in 34,751 patients with a total of 40,427 revascularizations; 30-month unadjusted revascularization rates for BMS and DES populations were 24.5 / 100 patients and 23.0 / 100 patients (p=0.007). With risk-adjustment, no difference in overall revascularization was observed in DES versus BMS patients at 30-months (23.5 / 100 patients vs. 23.4 / 100 patients, HR 0.91; 95% CI, 0.87 to 0.96).(Table 2; Figure 2c) However, revascularization rates were lower in DES patients to twelve-months post-PCI (13.3 / 100 patients vs. 15.2 / 100 patients) followed by a late rebound in revascularization procedures in the DES group between 12 and 30-months (10.2 / 100 patients vs. 8.2 / 100 patients). When CABG and PCI revascularizations were examined separately, CABG was more common in BMS than DES over the 30-month follow up period (3.7 / 100 patients vs. 2.5 / 100 patients), while the rate of PCI was similar.
Figure 2c.
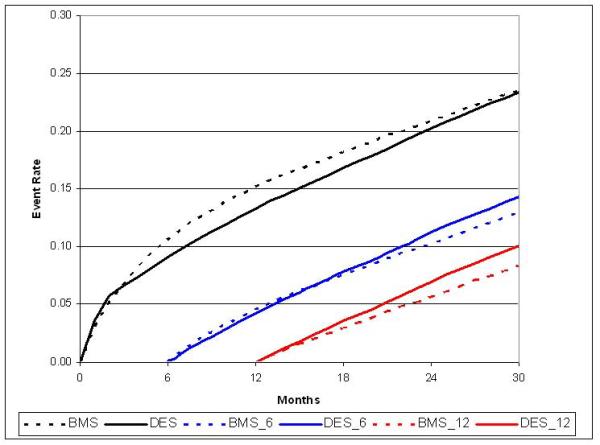
Adjusted cumulative incidence for revascularization with 6- and 12-month landmark display
Stroke and Major Bleeding
During follow-up, 4,010 strokes and 5,120 major bleeding events required hospitalization, with 59% of strokes and 49% of bleeds occurring within 6-months following PCI. Unadjusted and adjusted stroke rates were roughly 3 / 100 patients at 30-months in each group (HR 0.97; 95% CI, 0.88 to 1.07) and only a minimal difference was noted in bleeding (3.6 / 100 patients BMS vs. 3.4 / 100 patients DES, HR 0.91; 95% CI, 0.84 to 1.00). (Table 2; Figures 2d and 2e)
Figure 2d.
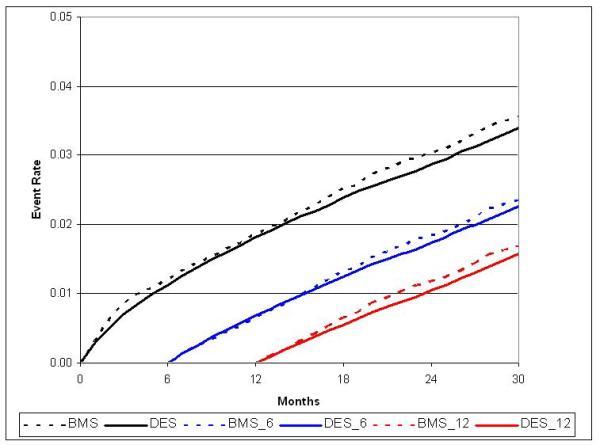
Adjusted cumulative incidence for bleeding with 6- and 12-month landmark display
Figure 2e.
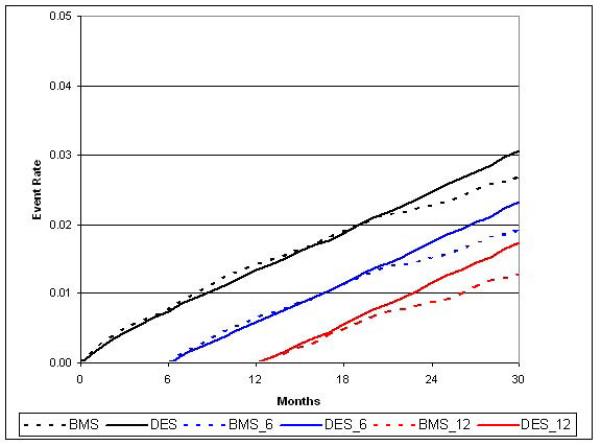
Adjusted cumulative incidence for stroke with 6- and 12-month landmark display
Composite Endpoints
Each of the composite endpoints tracked closely with its individual components, favoring DES over BMS treated patients both before and after statistical adjustment.(Table 2) The unadjusted 30-month rates of death or MI (17% vs. 23%), death or MI or revascularization (32% vs. 38%), and death or MI or stroke (19% vs. 24%) were each lower in DES than BMS patients.
Subgroup Analyses
The 30-month DES survival advantage was present across all patient subgroups, independent of sex, age, comorbidities, and procedural indication or urgency.(Figure 4a) This effect was somewhat less pronounced in those with a prior history of CABG and renal failure, with or without dialysis. Notably, patients receiving DES in 2005 and 2006 had a greater relative survival benefit than those receiving DES in 2004. Similarly, the 30-month risk of MI was lower in all patient subgroups except those with renal failure and insulin-dependent diabetes. (Figure 4b)
Figure 4a.
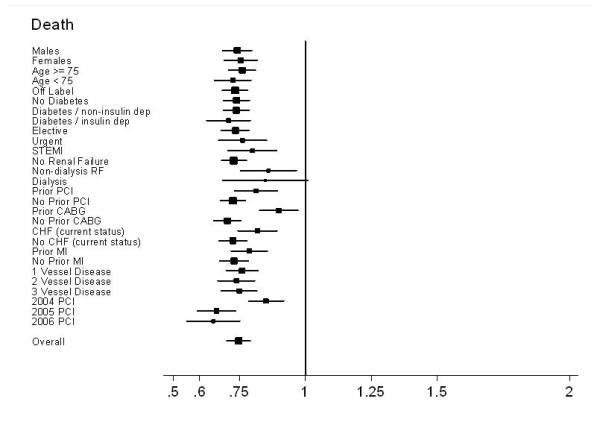
Subgroup results – Forest plot of Hazard Ratios for death
Figure 4b.
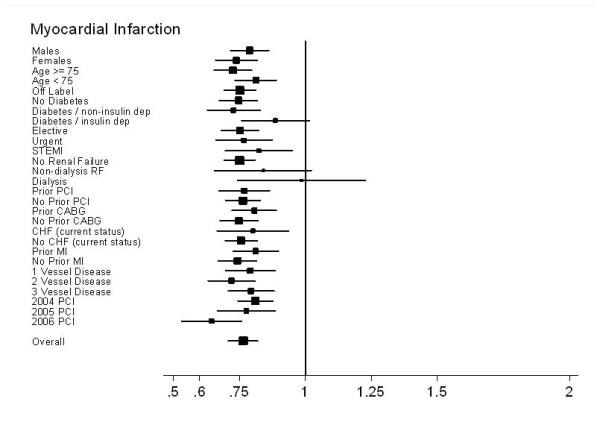
Subgroup results – Forest plot of Hazard Ratios for MI
Most patient subgroups experienced a slightly lower 30-month rate of revascularization with DES compared with BMS. (Figure 4c) However, no benefit was observed in patients >75 years, or with diabetes, renal failure, heart failure, or 3-vessel disease. Revascularization rates were similar in patients undergoing PCI in 2006, in contradistinction to the slightly lower DES revascularization rates from 2004 and 2005. (Figures 4d and 4e)
Figure 4c.
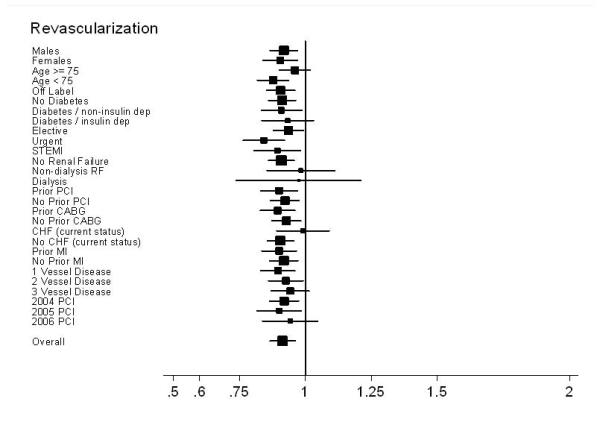
Subgroup results – Forest plot of Hazard Ratios for revascularization
Figure 4d.
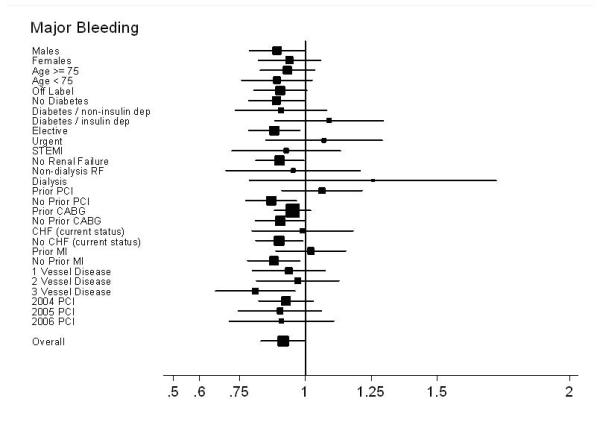
Subgroup results – Forest plot of Hazard Ratios for bleeding
Figure 4e.
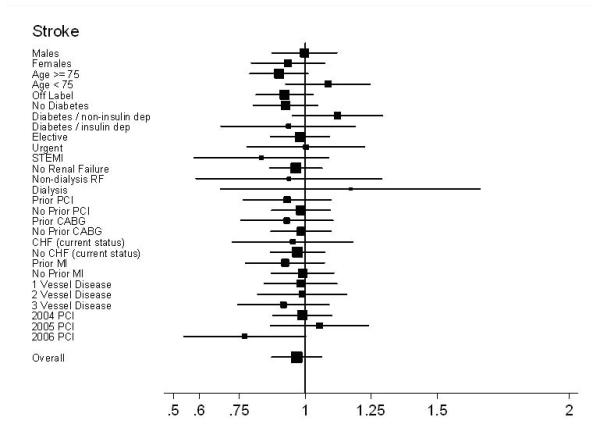
Subgroup results – Forest plot of Hazard Ratios for stroke
Sensitivity Analyses
Randomized trial cohort
The 49,355 NCDR® registry patients fitting the inclusion and exclusion criteria for the Taxus IV and SIRIUS DES randomized controlled trials had 30-month outcomes similar to those of the overall population such that those receiving DES had a lower 30-month risk of death (HR 0.62; 95% CI, 0.55 to 0.70), MI (HR 0.66; 95% CI, 0.55 to 0.80), death or MI (HR 0.64; 95% CI, 0.57 to 0.70) and revascularization (HR 0.87; 95% CI, 0.80 to 0.96) compared to BMS. No difference in stroke (HR 0.97; 95% CI, 0.74 to 1.28) or major bleeding (HR 0.87; 95% CI, 0.71 to 1.05) was noted between trial-eligible DES and BMS patients.
Cause of death
Presumed ‘cause’ was extrapolated in 19,132 (90%) deaths using the algorithm described above, and included 8451 inpatient and 10,591 outpatient deaths. Slightly more BMS deaths were attributable to MI (15.0% vs. 13.5%, p=0.01) and malignancy (6.7% vs. 5.5%, p=0.002) while more DES deaths were more attributable to chronic lung disease (2.5% vs. 1.9%, p=0.01) and cerebrovascular disease (5.3% vs. 4.2%, p=0.003). No significant differences were found for any of the remaining diagnoses. Overall, DES patients had a lower risk of CV-only (including CHF and MI) deaths compared with BMS patients (HR 0.80; 95% CI, 0.74 to 0.86), as well as non-CV death from all other causes (HR 0.74; 95%CI, 0.70 to 0.78).
DISCUSSION
Our study is the largest-ever observational comparison of long-term outcomes in older patients receiving BMS or DES. DES implantation was associated with lower risk of death and MI at 30-months as compared to BMS, while there were minor, if any, differences in bleeding, stroke, and overall revascularization. Our methodology allowed determination of comparative effectiveness in unselected individuals, in contemporaneous DES and BMS cohorts, with device selection and subsequent management of patients reflecting real-life, community practice.
Prespecified Outcomes
Death
Prior analyses comparing survival in DES and BMS treated patients from randomized controlled trials (RCTs) and smaller registries have produced conflicting results with relatively low precision. While no difference in late survival was demonstrated in some RCTs(1,23), registries and meta-analyses(9-11,23-28), other more recent studies have demonstrated a DES survival advantage with a point-estimate similar to that observed in our population(13,29-32). The higher annualized mortality rates for patients in our population receiving either DES or BMS (5.4%/year vs. 6.6%/year) than previously reported in some registries (range=1.3%/year to 4.3%/year)(23-27,33,34) is likely due to higher risk in our elderly, inpatient population, and are comparable to other Medicare cohorts(11,35).
Myocardial infarction
Patients receiving DES experienced a 23% relative reduction in subsequent MI with no late increase in combined NSTEMI/STEMI risk, a result similar to several other analyses(1,9,11). Angiographic assessment of stent thrombosis was not possible in our data set; however, isolated analysis of STEMI events revealed a slight increase in very late (>12-months) STEMI risk in DES patients, consistent with prior literature on late stent thrombosis(6) and the expected time-course of clopidogrel discontinuation(36,37).
Revascularization
Although DES have been associated with low revascularization rates (6,9,11,13,29-32,34), recent registry reports suggest that they may actually be as high as 15-19% over a 2-3 year follow-up(6,11,26), with little difference between DES and BMS patients(6). The higher rate of repeat revascularization in our population (24%) may be due to not censoring patients after an event, and to the inability to differentiate target lesion revascularization (TLR) from non-TLR follow-up procedures using claims data. For example, a recent report from the Duke database identified a 2-fold higher rate of overall revascularization versus target vessel revascularization in DES patients at 2 years (12.0% versus 6.6%)(26). Thus, the lack of anatomic data makes this database less than ideal for the comparison of revascularization between DES and BMS. An additional concern is the higher rate of late revascularization in DES compared to BMS, which tends to obscure the early benefit when examining overall DES-BMS hazard ratio. Our revascularization results should be interpreted cautiously.
Stroke and Major Bleeding
Few differences in stroke or major bleeding rates requiring re-hospitalization were observed between the overall DES and BMS populations. The anticipated greater use of clopidogrel in the DES group might have conferred a bleeding disadvantage, as has been seen in other studies (38,39); however, no statistically significant difference was observed in our population. Unexpectedly, although a slightly higher unadjusted rate of anemia-associated deaths was observed in DES patients, no significant adjusted or unadjusted difference in GI hemorrhage-associated deaths was evident at 30-months.
Registry versus RCT Results: Sensitivity analyses
The differences in outcomes between registry and RCT analyses have been previously attributed to possible differences in DES performance in a real world (registry) population as compared to a restricted RCT population; with the lack of a survival difference in RCTs being an artifact of their restricted patient populations. Since creation of a population subset fitting the inclusion and exclusion criteria of the Taxus IV and SIRIUS DES RCTs(20,21) only sharpened the precision for each endpoint, differences in age, acuity, lesion characteristics, and off label use in the registry and RCT populations are unlikely explanations of the observed differences in results.
Incomplete risk-adjustment following biased real-world stent selection may also contribute to the survival advantage noted in this and other registry analyses. Although we used both propensity analyses and Cox proportional hazards models to adjust for differences in baseline characteristics, it is possible that unmeasured baseline population differences remained. In fact, our ‘cause of death’ sensitivity analysis did show slightly higher rates of death due to malignancy in BMS patients, suggesting that biased patient selection may have contributed to the overall mortality result, such that ‘sicker’ patients with more comorbid disease received BMS. While it is possible that the observed differences between DES and BMS patients are the sole product of unmeasured patient selection biases not reflected by this analysis, this explanation is less likely given the large number of covariates used in our propensity matching.
Our study has several important strengths. This represents a novel large-scale linkage between a national procedural registry and a robust claims database, demonstrating that nationally representative analyses are feasible using clinically rich, procedural registries and a claims-based structure for follow-up. In combination, these two resources provide a powerful mechanism for tracking the post-marketing use and outcomes of novel devices and procedural innovations, at minimal cost. Importantly, the project was financed by the Agency for Healthcare Research and Quality, Cardiovascular Consortium of Effective Healthcare Program, and was independent of industry.
Data entered in NCDR® are intended to be used as a quality improvement tool and undergo rigorous quality control(40), while the Medicare claims database captures all inpatient care episodes. Despite these disparate intents and the size and complexity of these two databases, our linkage rate was over 75%, adding to the generalizability of our results. We analyzed these data by three methods, IPW alone, IPW with Cox proportional hazards modeling, and standard Cox proportional hazards modeling to compare results between the different approaches. The high level of agreement between these methods enhances confidence in our findings.
Our study has several important limitations, as well. Our data are observational and therefore dependent on the accuracy and completeness of the two matched data sets. Reliance on a claims database for outcomes may be fraught with underreporting or misclassification of events. Such misclassification and underreporting should be non-differential, however, and should bias our estimates toward the null value of no overall difference. Although differences in baseline characteristics were rigorously adjusted for using propensity weighting, it is possible that additional unadjusted differences between BMS and DES patients affected results, since our analyses are limited to the data collected by ACC-NCDR® and Medicare. Thus we are unable to directly address some important questions which have been raised regarding the safety of stents, including whether repeat revascularization represented target lesion or vessel restenosis, the incidence of late stent thrombosis, and the impact of variations in thienopyridine use. Although the slight excess of STEMIs in DES patients after 12-months fits the time course of late stent thrombosis, these events did not translate to increased late mortality.
While the linkage rate of 76% is incomplete, it is reflective of populations known to be absent from the Medicare data set such as patients treated at Veterans Administration facilities, with Medicare Advantage insurance coverage or undergoing outpatient procedures. Our findings are drawn from hospitalized patients over age 65, an age group which accounts for approximately half of all PCI’s nationally. While this cohort is likely sicker than the generally younger outpatient PCI population, the similarity in outcomes in those 65- 75 and those >75 years old suggests that these differences may have been accounted for in the risk adjustment.
CONCLUSIONS
In summary, in this large population of Medicare beneficiaries undergoing PCI at facilities participating in the ACC-NCDR® registry, patients who received DES had significantly lower mortality rates, including an early decrease in MI, than those who received BMS. No excess of major bleeding or stroke was noted. The survival advantage associated with DES was maintained across all subgroups analyzed and throughout the 30 months of follow-up. Drug eluting stents seem to be safe and effective in community practice in the elderly population. Longer follow up studies will need to be conducted to further support these results and to confirm the possible effects of antiplatelet agents.
Supplementary Material
Acknowledgments
FUNDING: This project was sponsored by the Agency for Healthcare Research and Quality, US Department of Health and Human Services, Rockville, MD as part of the Cardiovascular Consortium and funded under Project ID: 24-EHC-1 and Work Assignment Number: HHSAA290-2005-0032—TO4-WA1 as part of the Developing Evidence to Inform Decisions about Effectiveness (DEcIDE) program. The authors of this report are responsible for its content. Statements in the report should not be construed as endorsement by the Agency for Healthcare Research and Quality or the US Department of Health and Human Services.
Additional support was obtained from the National Cardiovascular Data Registry, American College of Cardiology, Washington, DC.
ABBREVIATIONS
- BMS
bare metal stent
- DES
drug-eluting stents
- ACC
American College of Cardiology
- NCDR®
National Cardiovascular Data Registry
- CMS
Centers for Medicare and Medicaid Services
- AHRQ
Agency for Healthcare Research and Quality
- PCI
percutaneous coronary intervention
- CABG
coronary artery bypass grafting
- IPW
inverse propensity weighted scoring
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stettler C, Wandel S, Allemann S, et al. Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. The Lancet. 370:937–948. doi: 10.1016/S0140-6736(07)61444-5. [DOI] [PubMed] [Google Scholar]
- 2.Rao SV, Shaw RE, Brindis RG, et al. Patterns and outcomes of drug-eluting coronary stent use in clinical practice. American Heart Journal. 2006;152:321–326. doi: 10.1016/j.ahj.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Virmani R, Guagliumi G, Farb A, et al. Localized Hypersensitivity and Late Coronary Thrombosis Secondary to a Sirolimus-Eluting Stent: Should We Be Cautious? Circulation. 2004;109:701–705. doi: 10.1161/01.CIR.0000116202.41966.D4. [DOI] [PubMed] [Google Scholar]
- 4.McFadden EP, Stabile E, Regar E, et al. Late thrombosis in drug-eluting coronary stents after discontinuation of antiplatelet therapy. The Lancet. 364:1519–1521. doi: 10.1016/S0140-6736(04)17275-9. [DOI] [PubMed] [Google Scholar]
- 5.Camenzind E, Steg PG, Wijns W. A Cause for Concern. Circulation. 2007;115:1440–1455. doi: 10.1161/CIRCULATIONAHA.106.666800. [DOI] [PubMed] [Google Scholar]
- 6.Lagerqvist B, James SK, Stenestrand U, et al. Long-Term Outcomes with Drug-Eluting Stents versus Bare-Metal Stents in Sweden. N Engl J Med. 2007;356:1009–1019. doi: 10.1056/NEJMoa067722. [DOI] [PubMed] [Google Scholar]
- 7.United States Food and Drug Administration [Accessed 9/15/08];FDA Statement on coronary drugeluting stents. http://www.fda.gov/cdrh/news/091406.html.
- 8.United States Food and Drug Administration [Accessed 9/15/08];Update to FDA statement on coronary drug-eluting stents. http://www.fda.gov/cdrh/news/010407.html.
- 9.Hannan EL, Racz M, Holmes DR, et al. Comparison of Coronary Artery Stenting Outcomes in the Eras Before and After the Introduction of Drug-Eluting Stents. Circulation. 2008;117:2071–2078. doi: 10.1161/CIRCULATIONAHA.107.725531. [DOI] [PubMed] [Google Scholar]
- 10.Jensen LO, Maeng M, Kaltoft A, et al. Stent Thrombosis, Myocardial Infarction, and Death After Drug-Eluting and Bare-Metal Stent Coronary Interventions. Journal of the American College of Cardiology. 2007;50:463–470. doi: 10.1016/j.jacc.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Malenka DJ, Kaplan AV, Lucas FL, Sharp SM, Skinner JS. Outcomes Following Coronary Stenting in the Era of Bare-Metal vs the Era of Drug-Eluting Stents. JAMA. 2008;299:2868–2876. doi: 10.1001/jama.299.24.2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daemen J, Tanimoto S, García-García HM, et al. Comparison of Three-Year Clinical Outcome of Sirolimus- and Paclitaxel-Eluting Stents Versus Bare Metal Stents in Patients With ST-Segment Elevation Myocardial Infarction (from the RESEARCH and T-SEARCH Registries) The American Journal of Cardiology. 2007;99:1027–1032. doi: 10.1016/j.amjcard.2006.11.070. [DOI] [PubMed] [Google Scholar]
- 13.Tu JV, Bowen J, Chiu M, et al. Effectiveness and Safety of Drug-Eluting Stents in Ontario. N Engl J Med. 2007;357:1393–1402. doi: 10.1056/NEJMoa071076. [DOI] [PubMed] [Google Scholar]
- 14.Jeremias A, Kirtane A. Balancing Efficacy and Safety of Drug-Eluting Stents in Patients Undergoing Percutaneous Coronary Intervention. Ann Intern Med. 2008;148:234–238. doi: 10.7326/0003-4819-148-3-200802050-00199. [DOI] [PubMed] [Google Scholar]
- 15.Rosenbaum P, Rubin D. The central role of propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 16.Rosenbaum P. Model-based adjustment. J Am Stat Assoc. 1987;82:387–394. [Google Scholar]
- 17.Anstrom KJ, Tsiatis AA. Utilizing propensity scores to estimate causal treatment effects with censored time-lagged data. Biometrics. 2001;57:1207–18. doi: 10.1111/j.0006-341x.2001.01207.x. [DOI] [PubMed] [Google Scholar]
- 18.Bang H, Tsiatis A. Estimating medial costs with censored data. Biometrika. 2000;82:387–394. [Google Scholar]
- 19.Cole SR, Hernan MA. Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed. 2004;75:45–9. doi: 10.1016/j.cmpb.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Stone GW, Ellis SG, Cox DA, et al. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N Engl J Med. 2004;350:221–31. doi: 10.1056/NEJMoa032441. [DOI] [PubMed] [Google Scholar]
- 21.Moses JW, Leon MB, Popma JJ, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003;349:1315–23. doi: 10.1056/NEJMoa035071. [DOI] [PubMed] [Google Scholar]
- 22.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 23.Spaulding C, Daemen J, Boersma E, Cutlip DE, Serruys PW. A Pooled Analysis of Data Comparing Sirolimus-Eluting Stents with Bare-Metal Stents. N Engl J Med. 2007;356:989–997. doi: 10.1056/NEJMoa066633. [DOI] [PubMed] [Google Scholar]
- 24.Schömig A, Dibra A, Windecker S, et al. A Meta-Analysis of 16 Randomized Trials of Sirolimus-Eluting Stents Versus Paclitaxel-Eluting Stents in Patients With Coronary Artery Disease. Journal of the American College of Cardiology. 2007;50:1373–1380. doi: 10.1016/j.jacc.2007.06.047. [DOI] [PubMed] [Google Scholar]
- 25.Stone GW, Moses JW, Ellis SG, et al. Safety and Efficacy of Sirolimus- and Paclitaxel-Eluting Coronary Stents. N Engl J Med. 2007;356:998–1008. doi: 10.1056/NEJMoa067193. [DOI] [PubMed] [Google Scholar]
- 26.Anstrom KJ, Kong DF, Shaw LK, et al. Long-term Clinical Outcomes Following Coronary Stenting. Arch Intern Med. 2008;168:1647–1655. doi: 10.1001/archinte.168.15.1647. [DOI] [PubMed] [Google Scholar]
- 27.Marzocchi A, Saia F, Piovaccari G, et al. Long-Term Safety and Efficacy of Drug-Eluting Stents: Two-Year Results of the REAL (REgistro AngiopLastiche dell’Emilia Romagna) Multicenter Registry. Circulation. 2007;115:3181–3188. doi: 10.1161/CIRCULATIONAHA.106.667592. [DOI] [PubMed] [Google Scholar]
- 28.Ajani AE, Reid CM, Duffy SJ, et al. Outcomes after percutaneous coronary intervention in contemporary Australian practice: insights from a large multicentre registry. Med J Aust. 2008;189:423–8. doi: 10.5694/j.1326-5377.2008.tb02113.x. [DOI] [PubMed] [Google Scholar]
- 29.Mauri L, Hsieh W-h, Massaro JM, Ho KKL, D’Agostino R, Cutlip DE. Stent Thrombosis in Randomized Clinical Trials of Drug-Eluting Stents. N Engl J Med. 2007;356:1020–1029. doi: 10.1056/NEJMoa067731. [DOI] [PubMed] [Google Scholar]
- 30.Shishehbor MH, Goel SS, Kapadia SR, et al. Long-Term Impact of Drug-Eluting Stents Versus Bare-Metal Stents on All-Cause Mortality. Journal of the American College of Cardiology. 2008;52:1041–1048. doi: 10.1016/j.jacc.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 31.Groeneveld PW, Matta MA, Greenhut AP, Yang F. Drug-Eluting Compared With Bare-Metal Coronary Stents Among Elderly Patients. Journal of the American College of Cardiology. 2008;51:2017–2024. doi: 10.1016/j.jacc.2008.01.057. [DOI] [PubMed] [Google Scholar]
- 32.Austin D, Oldroyd KG, McConnachie A, et al. Drug-Eluting Stents Versus Bare-Metal Stents for Off-Label Indications: A Propensity Score-Matched Outcome Study. Circ Cardiovasc Intervent. 2008;1:45–52. doi: 10.1161/CIRCINTERVENTIONS.108.769042. [DOI] [PubMed] [Google Scholar]
- 33.Kastrati A, Mehilli J, Pache J, et al. Analysis of 14 Trials Comparing Sirolimus-Eluting Stents with Bare-Metal Stents. N Engl J Med. 2007;356:1030–1039. doi: 10.1056/NEJMoa067484. [DOI] [PubMed] [Google Scholar]
- 34.Pfisterer M, Brunner-La Rocca HP, Buser PT, et al. Late Clinical Events After Clopidogrel Discontinuation May Limit the Benefit of Drug-Eluting Stents: An Observational Study of Drug-Eluting Versus Bare-Metal Stents. Journal of the American College of Cardiology. 2006;48:2584–2591. doi: 10.1016/j.jacc.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 35.Groeneveld PW, Matta MA, Greenhut AP, Yang F. Drug-eluting compared with bare-metal coronary stents among elderly patients. J Am Coll Cardiol. 2008;51:2017–24. doi: 10.1016/j.jacc.2008.01.057. [DOI] [PubMed] [Google Scholar]
- 36.Banerjee S, Varghese C, Samuel J, et al. Comparison of the impact of short (<1 year) and long-term (> or =1 year) clopidogrel use following percutaneous coronary intervention on mortality. Am J Cardiol. 2008;102:1159–62. doi: 10.1016/j.amjcard.2008.06.058. [DOI] [PubMed] [Google Scholar]
- 37.Eisenstein EL, Anstrom KJ, Kong DF, et al. Clopidogrel Use and Long-term Clinical Outcomes After Drug-Eluting Stent Implantation. JAMA. 2007;297:159–168. doi: 10.1001/jama.297.2.joc60179. [DOI] [PubMed] [Google Scholar]
- 38.Steinhubl SR, Berger PB, Mann Iii JT, et al. Early and Sustained Dual Oral Antiplatelet Therapy Following Percutaneous Coronary Intervention: A Randomized Controlled Trial. JAMA. 2002;288:2411–2420. doi: 10.1001/jama.288.19.2411. [DOI] [PubMed] [Google Scholar]
- 39.Bhatt DL, Flather MD, Hacke W, et al. Patients With Prior Myocardial Infarction, Stroke, or Symptomatic Peripheral Arterial Disease in the CHARISMA Trial. Journal of the American College of Cardiology. 2007;49:1982–1988. doi: 10.1016/j.jacc.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 40.Brindis RG, Fitzgerald S, Anderson HV, Shaw RE, Weintraub WS, Williams JF. The American College of Cardiology-National Cardiovascular Data Registry (ACC-NCDR): building a national clinical data repository. J Am Coll Cardiol. 2001;37:2240–5. doi: 10.1016/s0735-1097(01)01372-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.