Summary
The survival of mammalian cells exposed to adverse environmental conditions, requires a radical reprogramming of protein translation. Stress-activated kinases target components of the initiation machinery (e.g., eIF2α, eIF4E-BP, eIF4B, and ribosomal protein S6) to inhibit the translation of “housekeeping” proteins and promote the translation of repair enzymes. Accumulating untranslated mRNA is concentrated at stress granules where it is sorted and triaged to sites of storage, reinitiation, or decay. At the same time, the translation of mRNAs encoding repair enzymes is selectively preserved by both internal ribosome entry site-dependent and independent mechanisms. In combination, these stress-activated processes coordinately reprogram mRNA translation and decay in a way that conserves anabolic energy, preserves essential mRNAs, and promotes the repair of stress-induced molecular damage.
Introduction
The environmental conditions under which our cellular ancestors evolved from single cells to multicellular organisms were decidedly inhospitable [1]. Cellular survival required the efficient repair of molecular damage caused by unfiltered radiation, temperature extremes, and high levels of atmospheric oxygen. This adaptive pressure left all cells will the ability to respond to adverse environmental conditions by activating an integrated stress response that reprograms cell metabolism and diverts anabolic energy to the repair of stress-induced molecular damage. The need to dynamically reprogram on-going protein synthesis required that a major component of this stress response program target the translational machinery [2]. In mammalian cells, this is accomplished by modifying protein and RNA components of the translation machinery and by directing specific mRNAs to sites of translation, storage or decay [3–5]. These mechanisms redirect mRNAs encoding non-essential “housekeeping” proteins from polysomes to stress granules (SGs) and/or P-bodies to allow the selective translation of mRNAs encoding repair enzymes [3,5]. Defects in this stress response have been implicated in diverse disease processes, including cancer, microbial infection, diabetes and inflammatory disease [4]. Here we discuss the molecular mechanisms responsible for reprogramming protein translation in cells exposed to environmental stress.
Inhibition of General Translation
Protein translation is regulated at the levels of initiation, elongation and termination [6]. Although stress influences each step in translation, the majority of stress-induced translational silencing is a consequence of translation initiation [7]. Stress-induced translational arrest results from the modification of components of the translational machinery. In the absence of stress, translation is initiated when the preinitiation complex (composed of the 40S ribosomal subunit, eukaryotic initiation factor 3 (eIF3), eIF1A, and eukaryotic initiation factor 2 (eIF2)/GTP/methionyl initiator tRNA (Met-tRNAimet)) binds to capped mRNA in association with the eIF4E cap-binding protein, the eIF4A RNA helicase, and the eIF4G scaffold protein. The resulting 48S preinitiation complex scans the 5′ untranslated region until the tRNAimet anticodon recognizes the initation codon to trigger the release of early initiation factors, recruit the large ribosomal subunit, and activate the peptidyltransferase reaction [7]. Several stress-activated signaling pathways inhibit protein synthesis by disabling this initiation program. Proximal events in these pathways include: 1) phosphorylation of eIF2α, 2) phosphorylation of eIF4E binding proteins, 3) phosphorylation of ribosomal protein S6, 4) inosine modification of double-stranded RNA, and 5) endonucleolytic cleavage of ribosomal RNA (Figure 1). Combinations of these stress-activated inhibitory pathways play a major role in reprogramming protein translation in stressed cells.
Figure 1.
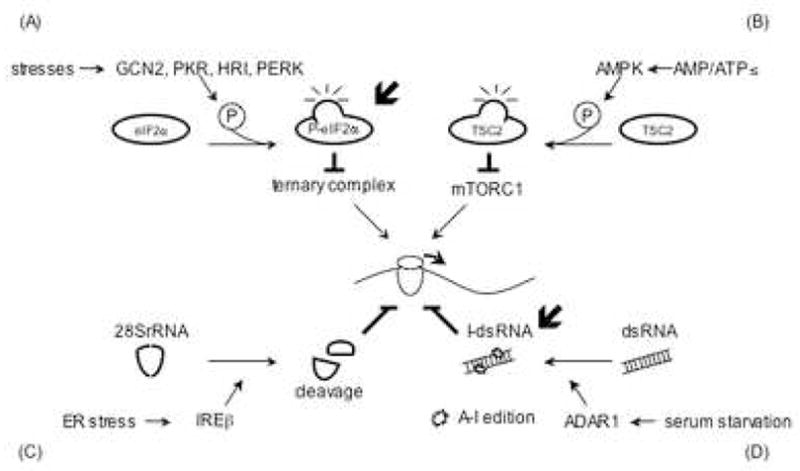
General translation inhibition by stress. (A) Phosphorylation of eIF2α (P-eIF2α) is induced by GCN2 (amino acid starvation, UV), PKR (dsRNA), HRI (low heme, arsenite, osmotic stress, heat stress) or PERK (ER stress). P-eIF2α blocks translation initiation by inhibiting the eIF2/GTP/tRNAMet ternary complex. (B) Energy starvation elevates the AMP/ATP ratio activating the AMPK-TSC pathway. This in turn attenuates mTORC1 activity to inhibit phosphorylation of 4E-BP and S6K. (C) 28S rRNA cleaved by IREβ. The resulting structural alteration inhibits translation by an unknown mechanism. (D) Hyper-edited inosine containing dsRNA (I-dsRNA) is produced by ADAR1 whose protein expression is up-regulated by serum starvation. I-dsRNA inhibits translation at initiation probably by nucleating the assembly of stress granules (SGs). The localization of ADAR1 in SGs has not been confirmed, however, APOBEC3G, a C to U RNA editing enzyme, is found in SGs.  indicates the possible SG-related mechanism.
indicates the possible SG-related mechanism.
Stress induced phosphorylation of eIF2α and assembly of stress granules
The one of the most potent translation inhibitory pathways is mediated by phosphorylated eIF2α [7]. Phosphorylation of eIF2α at serine 51 converts the eIF2/GTP/tRNAiMet ternary complex into a competitive inhibitor of the GDP/GTP exchange factor eIF2B [8]. The resulting depletion of active ternary complex prevents the assembly of 48S preinitiation complexes. Phosphorylation of eIF2α is mediated by a family of kinases that are activated by different types of environmental stress [7]. General control non-depressible-2 (GCN2) is activated by the accumulation of uncharged tRNAs in cells experiencing nutrient deprivation. Protein kinase RNA (PKR) is activated by double-stranded RNA replication intermediates produced during virus infection. Heme-regulated inhibitor kinase (HRI) is activated by hemin during erythrocyte maturation and by arsenite, an inducer of oxidative stress. PKR-like endoplasmic reticulum (ER) kinase (PERK) is activated when unfolded proteins accumulate in the ER, a consequence of secretory or membrane protein overload. By inhibiting translation initiation, phospho-eIF2α promotes polysome disassembly resulting in the accumulation of untranslated mRNPs. These mRNPs contain aggregation-prone proteins (e.g., TIA-1, TIAR, G3BP, Lsm4) that nucleate the assembly of SGs [3,9,10]. SGs play an important role in reprogramming mRNA metabolism by directing incoming transcripts to sites of storage, reinitiation or decay. Cells that cannot phosphorylate eIF2α are resistant to oxidative stress-induced translational arrest and SG assembly [11]. As a consequence, these cells are hypersensitive to the toxic effects of stress, suggesting that SG-mediated reprogramming of mRNA metabolism contributes to cell survival. Interestingly, stress-induced translational arrest is not completely abrogated in these cells, suggesting that phospho-eIF2α independent mechanisms of translational arrest are also induced by environmental stress [11,12].
Stress and the mTOR pathway
mTOR (mammalian target of rapamycin), a member of phosphatidylinositol kinase-related kinase (PIKK) family, is a serine/threonine kinase that forms two different complexes. mTOR complex I (mTORC1) includes raptor and G protein β-subunit-like protein (mLST8/GβL), whereas mTOR complex 2 (mTORC2) contains Rictor and mLST8/GβL. mTORC1 is known to phosphorylate eIF4E-binding proteins (4E-BPs) and the ribosomal kinases S6K1/S6K2. Phosphorylation of 4E-BP results in the release of active eIF4E to promote protein translation. S6K-mediated phosphorylation of ribosomal protein S6 and eIF4B also promotes protein translation [13,14]. Thus, active mTOR is required for the efficient translation of capped transcripts.
Adverse environmental conditions, including nutrient depletion, hypoxia, metabolic stress, osmotic stress, DNA damage, and heat shock can reduce the activity of mTORC1 [13–15]. In cells subjected to metabolic stress, the AMP-activated protein kinase (AMPK) is activated in response to the elevated AMP:ATP ratio [16,17]. AMPK phosphorylates tuberous sclerosis complex 2 (TSC2) allowing it to complex with TSC1. The TSC1/2 complex converts Ras-homolog enriched in brain (Rheb) from an active GTP-bound form to an inactive GDP-bound form. Since Rheb positively regulates mTORC1, the activation of AMPK-TSC1 kinase signaling by metabolic-stress results in inhibition of protein translation [13–15, 17,18]. AMPK is also activated by hypoxia, osmotic stress, low glucose, and impaired mitochondrial function [17]. Thus, the AMPK/mTOR pathway contributes to translational arrest in cells exposed to a variety of environmental stresses.
Stress and hyperedited dsRNA
RNA editing is a post-transcriptional modification that converts cytosine to uridine or adenosine to inosine [19]. RNA editing may contribute to host defense against viruses that assemble a dsRNA replicative intermediate [20]. Endogenous dsRNAs derived from inverted repeat sequences found in untranslated RNAs are also subject to RNA editing. Hyperedited dsRNAs can assemble an RNP complex that is retained in the nucleus and/or degraded by a selective riboendonuclease [21]. These chemical conversions have the potential to alter the coding capacity of mRNA or the target specificity of miRNA [22].
Surprisingly, hyperedited dsRNA has recently been shown to inhibit protein synthesis by nucleating the assembly of SGs [23]. Because adenosine deaminase, the enzyme that converts adenosine to inosine (A to I RNA editing) within dsRNA targets, is induced by interferon and serum starvation, inosine-modified dsRNAs (I-dsRNA) may trigger stress-induced translational arrest [24,25]. Pull-down experiments using mammalian cell lysates suggest that I-dsRNA nucleates the assembly of an RNP complex containing multiple SG components, including TIAR, HuR eIF4G, and G3BP [23]. Since these lysates were prepared from unstressed cells, it is likely that I-dsRNA-induced assembly of SGs is independent of phospho-eIF2α. Transfection of I-dsRNA into HeLa cells inhibits global protein synthesis, a possible consequence of SG assembly. The finding that embryonic fibroblasts from ADAR−/− mice are more susceptible to serum starvation-induced apoptosis than wild type controls [25] suggests that I-dsRNA-induced SGs may potentiate cell survival. Thus I-dsRNA may provide a novel stimulus for stress-induced translational arrest. Interestingly, APOBEC3G (apolipoprotein B mRNA editing enzyme catalytic polypeptide 1-like), an RNA-editing enzyme that is important in host mediated anti-viral defense, is localized in P-bodies and SGs [26].
Cleavage of ribosomal RNA during stress
ER stress dramatically induces activation of IREβ, an ER resident transmembrane kinase/ribonuclease. IREβ is the endoribonuclease that cleaves XBP1 transcripts at intron/exon junctions to initiate a cytoplasmic splicing reaction required for the production of mature XBP1 transcripts [27]. Remarkably, IREβ also cleaves 28S ribosomal RNA, a modification that inhibits global protein synthesis [28]. A dominant negative IREβ mutant partially restores ER-stress-induced translational silencing, suggesting that this regulatory pathway contributes to stress-induced translational arrest. This process is reminiscent of the cleavage of 28S ribosomal RNA by α-sarcin, a fungal toxin that is a potent inhibitor of protein synthesis [29].
Since the synthesis of protein is a highly energy consuming process [30], the activity of translation must be tightly regulated, especially in cells dealing with adverse environmental conditions [1,2]. Moreover, reprogramming of protein synthesis by some combination of these stress-induced pathways can promote cell survival by selectively translating repair enzymes. In the following section, we will discuss how some transcripts are selected for preferred translation during stress.
Inhibition of Transcript Specific Translation
Capped mRNAs encoding heat shock proteins are selectively excluded from SGs, a phenomenon that likely contributes to their preferred translation during stress [31]. Although the molecular mechanism is unknown, it is possible that stress-induced transcripts acquire a distinctive protein “mark” that prevents their recruitment to SGs. This would ensure that stress-induced repair enzymes are efficiently translated during stress. Additional mechanisms that promote the translation of selected mRNAs in response to stress include IRES- and miRNA-mediated translation control
IRES-mediated translation and stress
Internal ribosome-entry sites (IRES) allow the cap-independent translation of selected transcripts involved in proliferation, differentiation and apoptosis, as well as viral transcripts [32]. The IRES allows these mRNAs to escape stress-activated global translation attenuation programs [32]. Whereas cap-dependent translation requires components of the 48S preinitiation complex, IRES-mediated translation requires an idiosyncratic set of IRES trans-acting factors (ITAFs) that recruit ribosomal subunits and initiate protein synthesis [33]. Some IRES-containing transcripts are resistant to the inhibitory effects of phospho-eIF2α, allowing their selective translation in stressed cells. Resistance to phospho-eIF2α can be achieved by: 1) the eIF2-independent recruitment of tRNAiMet (e.g., simian picornavirus) [34], 2) ternary complex independent recruitment of eIF2α and tRNAiMet (e.g., hepatitis C virus) [35,36], 3) upstream open-reading frame mediated ribosome stalling (e.g., cat-1 transporter) [37], and 4) unknown mechanisms (e.g., PITSLRE kinase) [38]. These findings reveal the central importance of phospho-eIF2α in reprogramming translation during stress. The relative contribution of stress granule assembly to IRES-mediated translational control remains to be determined.
miRNA and stress (editing)
MicroRNAs (miRNAs) are small noncoding RNAs that regulate gene expression post-transcriptionally by inhibiting translation or destabilizing target transcripts [39]. It is estimated that approximately 25% of mammalian genes are regulated by miRNAs. Recent results have implicated miRNAs in the reprogramming of gene expression observed in stressed cells. Stress-activated transcription factors have been shown to induce the expression of specific miRNAs [40]. Examples include the p53-induced transcription of a miR-34a precursor that regulates the cell cycle in response to genotoxic stress [41] and the HIF1-induced transcription of miRNA precursors that regulate cell survival [42]. In addition to the transcription of specific miRNAs, cellular stress can directly modulate the miRNA machinery. Examples include the HSP90-mediated stabilization of Argonaute, an essential component of the RNA-induced silencing complex [40], and the stress-induced override of miRNA-mediated translational silencing of CAT-1 transcripts, a consequence of HuR-mediated rescue from P-bodies [43]. SGs may be the location for modulation of miRNA-mediated translational silencing in stress because the Argonaute localizes at SGs in a miRNA dependent manner in response to stress [44]. Finally, stress-activated dsRNA editing mechanisms have been shown to target miRNA precursors to alter the stability, processing, nuclear export, or target specificity of selected miRNAs [21–23]. There results reveal an important role for miRNA in the reprogramming of mRNA translation during stress.
Conclusions
Stress-induced reprogramming of mRNA translation is essential for the survival of cells exposed to adverse environmental conditions. The evolution of multiple, parallel mechanisms that modulate translation during stress points out the fundamental importance of this process. This is underscored by the finding that viruses target these pathways to subvert the translation machinery and favor the translation of viral proteins. Although much remains to be learned, identification of key components of stress-induced translational control pathways provides a framework for future research focused on how these diverse mechanisms regulate the survival of stressed cells.
Figure 2.
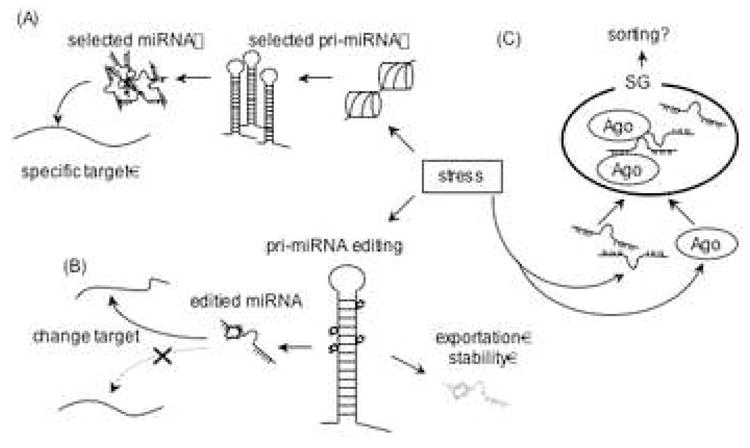
Transcript specific translation inhibition by stress. (A) In stressed cells, some transcription factors (e.g. p53, HIF1α) promote the expression of miRNAs that reduce the translation of selected targeted mRNAs. (B) Stress induces the expression of RNA editing enzymes to alter the processing of primary miRNA transcripts. Moreover, editing of pre-miRNA, can alter its target specificity. (C) SGs may regulate miRNA function by sequestering argonaute, miRNAs and RNA editing enzymes. It is possible that miRNPs are sorted, modified, and delivered to target mRNAs at the SG.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sorensen JG, Loeschcke V. Studying stress responses in the post-genomic era: its ecological and evolutionary role. J Biosci. 2007;32:447–456. doi: 10.1007/s12038-007-0044-x. [DOI] [PubMed] [Google Scholar]
- 2.Brostrom CO, Brostrom MA. Regulation of translational initiation during cellular responses to stress. Prog Nucleic Acid Res Mol Biol. 1998;58:79–125. doi: 10.1016/s0079-6603(08)60034-3. [DOI] [PubMed] [Google Scholar]
- 3.Anderson P, Kedersha N. RNA granules. J Cell Biol. 2006;172:803–808. doi: 10.1083/jcb.200512082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keene JD. RNA regulons: coordination of post-transcriptional events. Nat Rev Genet. 2007;8:533–543. doi: 10.1038/nrg2111. A comprehensive review about mRNA regulation by RNA binding proteins. The author describes their roles in splicing, nuclear export, stability, localization and translation of mRNA. [DOI] [PubMed] [Google Scholar]
- 5.Moore MJ. From birth to death: the complex lives of eukaryotic mRNAs. Science. 2005;309:1514–1518. doi: 10.1126/science.1111443. [DOI] [PubMed] [Google Scholar]
- 6.Scheper GC, van der Knaap MS, Proud CG. Translation matters: protein synthesis defects in inherited disease. Nat Rev Genet. 2007;8:711–723. doi: 10.1038/nrg2142. [DOI] [PubMed] [Google Scholar]
- 7.Holcik M, Sonenberg N. Translational control in stress and apoptosis. Nat Rev Mol Cell Biol. 2005;6:318–327. doi: 10.1038/nrm1618. [DOI] [PubMed] [Google Scholar]
- 8.Krishnamoorthy T, Pavitt GD, Zhang F, Dever TE, Hinnebusch AG. Tight binding of the phosphorylated alpha subunit of initiation factor 2 (eIF2alpha) to the regulatory subunits of guanine nucleotide exchange factor eIF2B is required for inhibition of translation initiation. Mol Cell Biol. 2001;21:5018–5030. doi: 10.1128/MCB.21.15.5018-5030.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kedersha N, Stoecklin G, Ayodele M, Yacono P, Lykke-Andersen J, Fritzler MJ, Scheuner D, Kaufman RJ, Golan DE, Anderson P. Stress granules and processing bodies are dynamically linked sites of mRNP remodeling. J Cell Biol. 2005;169:871–884. doi: 10.1083/jcb.200502088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilks N, Kedersha N, Ayodele M, Shen L, Stoecklin G, Dember LM, Anderson P. Stress granule assembly is mediated by prion-like aggregation of TIA-1. Mol Biol Cell. 2004;15:5383–5398. doi: 10.1091/mbc.E04-08-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McEwen E, Kedersha N, Song B, Scheuner D, Gilks N, Han A, Chen JJ, Anderson P, Kaufman RJ. Heme-regulated inhibitor kinase-mediated phosphorylation of eukaryotic translation initiation factor 2 inhibits translation, induces stress granule formation, and mediates survival upon arsenite exposure. J Biol Chem. 2005;280:16925–16933. doi: 10.1074/jbc.M412882200. [DOI] [PubMed] [Google Scholar]
- 12.Scheuner D, Song B, McEwen E, Liu C, Laybutt R, Gillespie P, Saunders T, Bonner-Weir S, Kaufman RJ. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell. 2001;7:1165–1176. doi: 10.1016/s1097-2765(01)00265-9. [DOI] [PubMed] [Google Scholar]
- 13.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 14.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 15.Reiling JH, Sabatini DM. Stress and mTORture signaling. Oncogene. 2006;25:6373–6383. doi: 10.1038/sj.onc.1209889. [DOI] [PubMed] [Google Scholar]
- 16.Hardie DG, Hawley SA, Scott JW. AMP-activated protein kinase--development of the energy sensor concept. J Physiol. 2006;574:7–15. doi: 10.1113/jphysiol.2006.108944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Corradetti MN, Inoki K, Guan KL. TSC2: filling the GAP in the mTOR signaling pathway. Trends Biochem Sci. 2004;29:32–38. doi: 10.1016/j.tibs.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 19.Keegan LP, Gallo A, O’Connell MA. The many roles of an RNA editor. Nat Rev Genet. 2001;2:869–878. doi: 10.1038/35098584. [DOI] [PubMed] [Google Scholar]
- 20.Conticello SG, Langlois MA, Yang Z, Neuberger MS. DNA deamination in immunity: AID in the context of its APOBEC relatives. Adv Immunol. 2007;94:37–73. doi: 10.1016/S0065-2776(06)94002-4. [DOI] [PubMed] [Google Scholar]
- 21.Nishikura K. Editor meets silencer: crosstalk between RNA editing and RNA interference. Nat Rev Mol Cell Biol. 2006;7:919–931. doi: 10.1038/nrm2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawahara Y, Zinshteyn B, Sethupathy P, Iizasa H, Hatzigeorgiou AG, Nishikura K. Redirection of silencing targets by adenosine-to-inosine editing of miRNAs. Science. 2007;315:1137–1140. doi: 10.1126/science.1138050. The first report showing that edited miRNA can alter target recognition. The authors show that edited miR376 represses expression of an enzyme involved in uric acid synthesis. The phonotype is not observed in ADAR knockout mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scadden AD. Inosine-containing dsRNA binds a stress-granule-like complex and downregulates gene expression in trans. Mol Cell. 2007;28:491–500. doi: 10.1016/j.molcel.2007.09.005. The first report of the translational inhibitory effect of hyper edited dsRNA. The authors clearly demonstrate the in vivo and in vitro translation inhibition by synthetic inosine containing dsRNA. Furthermore, the dsRNA complexes with SG components. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samuel CE. Antiviral actions of interferons. Clin Microbiol Rev. 2001;14:778–809. doi: 10.1128/CMR.14.4.778-809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Q, Miyakoda M, Yang W, Khillan J, Stachura DL, Weiss MJ, Nishikura K. Stress-induced apoptosis associated with null mutation of ADAR1 RNA editing deaminase gene. J Biol Chem. 2004;279:4952–4961. doi: 10.1074/jbc.M310162200. [DOI] [PubMed] [Google Scholar]
- 26.Gallois-Montbrun S, Kramer B, Swanson CM, Byers H, Lynham S, Ward M, Malim MH. Antiviral protein APOBEC3G localizes to ribonucleoprotein complexes found in P bodies and stress granules. J Virol. 2007;81:2165–2178. doi: 10.1128/JVI.02287-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881–891. doi: 10.1016/s0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- 28.Iwawaki T, Hosoda A, Okuda T, Kamigori Y, Nomura-Furuwatari C, Kimata Y, Tsuru A, Kohno K. Translational control by the ER transmembrane kinase/ribonuclease IRE1 under ER stress. Nat Cell Biol. 2001;3:158–164. doi: 10.1038/35055065. [DOI] [PubMed] [Google Scholar]
- 29.Yang X, Gerczei T, Glover LT, Correll CC. Crystal structures of restrictocin-inhibitor complexes with implications for RNA recognition and base flipping. Nat Struct Biol. 2001;8:968–973. doi: 10.1038/nsb1101-968. [DOI] [PubMed] [Google Scholar]
- 30.Rudra D, Warner JR. What better measure than ribosome synthesis? Genes Dev. 2004;18:2431–2436. doi: 10.1101/gad.1256704. [DOI] [PubMed] [Google Scholar]
- 31.Kedersha N, Anderson P. Stress granules: sites of mRNA triage that regulate mRNA stability and translatability. Biochem Soc Trans. 2002;30:963–969. doi: 10.1042/bst0300963. [DOI] [PubMed] [Google Scholar]
- 32.Baird SD, Turcotte M, Korneluk RG, Holcik M. Searching for IRES. RNA. 2006;12:1755–1785. doi: 10.1261/rna.157806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spriggs KA, Bushell M, Mitchell SA, Willis AE. Internal ribosome entry segment-mediated translation during apoptosis: the role of IRES-trans-acting factors. Cell Death Differ. 2005;12:585–591. doi: 10.1038/sj.cdd.4401642. [DOI] [PubMed] [Google Scholar]
- 34.de Breyne S, Yu Y, Pestova TV, Hellen CU. Factor requirements for translation initiation on the Simian picornavirus internal ribosomal entry site. RNA. 2007 doi: 10.1261/rna.696508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lancaster AM, Jan E, Sarnow P. Initiation factor-independent translation mediated by the hepatitis C virus internal ribosome entry site. RNA. 2006;12:894–902. doi: 10.1261/rna.2342306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robert F, Kapp LD, Khan SN, Acker MG, Kolitz S, Kazemi S, Kaufman RJ, Merrick WC, Koromilas AE, Lorsch JR, Pelletier J. Initiation of protein synthesis by hepatitis C virus is refractory to reduced eIF2. GTP. Met-tRNA(i)(Met) ternary complex availability. Mol Biol Cell. 2006;17:4632–4644. doi: 10.1091/mbc.E06-06-0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fernandez J, Yaman I, Huang C, Liu H, Lopez AB, Komar AA, Caprara MG, Merrick WC, Snider MD, Kaufman RJ, Lamers WH, Hatzoglou M. Ribosome stalling regulates IRES-mediated translation in eukaryotes, a parallel to prokaryotic attenuation. Mol Cell. 2005;17:405–416. doi: 10.1016/j.molcel.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 38.Tinton SA, Schepens B, Bruynooghe Y, Beyaert R, Cornelis S. Regulation of the cell-cycle-dependent internal ribosome entry site of the PITSLRE protein kinase: roles of Unr (upstream of N-ras) protein and phosphorylated translation initiation factor eIF-2alpha. Biochem J. 2005;385:155–163. doi: 10.1042/BJ20040963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bushati N, Cohen SM. microRNA functions. Annu Rev Cell Dev Biol. 2007;23:175–205. doi: 10.1146/annurev.cellbio.23.090506.123406. [DOI] [PubMed] [Google Scholar]
- 40.Leung AK, Sharp PA. microRNAs: a safeguard against turmoil? Cell. 2007;130:581–585. doi: 10.1016/j.cell.2007.08.010. This review concisely describes how stress influences the generation, regulation and localization of miRNAs. [DOI] [PubMed] [Google Scholar]
- 41.Bommer GT, Gerin I, Feng Y, Kaczorowski AJ, Kuick R, Love RE, Zhai Y, Giordano TJ, Qin ZS, Moore BB, MacDougald OA, Cho KR, Fearon ER. p53-mediated activation of miRNA34 candidate tumor-suppressor genes. Curr Biol. 2007;17:1298–1307. doi: 10.1016/j.cub.2007.06.068. [DOI] [PubMed] [Google Scholar]
- 42.Kulshreshtha R, Ferracin M, Wojcik SE, Garzon R, Alder H, Agosto-Perez FJ, Davuluri R, Liu CG, Croce CM, Negrini M, Calin GA, Ivan M. A microRNA signature of hypoxia. Mol Cell Biol. 2007;27:1859–1867. doi: 10.1128/MCB.01395-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bhattacharyya SN, Habermacher R, Martine U, Closs EI, Filipowicz W. Relief of microRNA-mediated translational repression in human cells subjected to stress. Cell. 2006;125:1111–1124. doi: 10.1016/j.cell.2006.04.031. Derepression of cationic amino acid transporter 1 (CAT-1) translation under stress is due to relief of microRNA-mediated suppression by miR-122 in human hepatic cell lines. [DOI] [PubMed] [Google Scholar]
- 44.Leung AK, Calabrese JM, Sharp PA. Quantitative analysis of Argonaute protein reveals microRNA-dependent localization to stress granules. Proc Natl Acad Sci U S A. 2006;103:18125–18130. doi: 10.1073/pnas.0608845103. [DOI] [PMC free article] [PubMed] [Google Scholar]


