Abstract
Ovarian cancer is the most lethal gynecologic malignancy. Efforts at early detection and new therapeutic approaches to reduce mortality have been largely unsuccessful because the origin and pathogenesis of epithelial ovarian cancer are poorly understood. Despite numerous studies that have carefully scrutinized the ovaries for precursor lesions, none have been found. This has led to the proposal that ovarian cancer develops de novo. Studies have shown that epithelial ovarian cancer is not a single disease but is composed of a diverse group of tumors that can be classified based on distinctive morphologic and molecular genetic features. One group of tumors, designated type I, is composed of low-grade serous, low-grade endometrioid, clear cell, mucinous and transitional (Brenner) carcinomas. These tumors generally behave in an indolent fashion, are confined to the ovary at presentation and, as a group, are relatively genetically stable. They lack mutations of TP53 but each histologic type exhibits a distinctive molecular genetic profile. Moreover, the carcinomas exhibit a shared lineage with the corresponding benign cystic neoplasm often through an intermediate (borderline tumor) step, supporting the morphologic continuum of tumor progression. In contrast, another group of tumors, designated type II, are highly aggressive, evolve rapidly and almost always present in advanced stage. Type II tumors include conventional high-grade serous carcinoma, undifferentiated carcinoma and malignant mixed mesodermal tumors (carcinosarcoma). They displayTP53 mutations in over 80% of cases and rarely harbor the mutations that are found in the type I tumors. Recent studies have also provided cogent evidence that what have been traditionally thought to be primary ovarian tumors actually originate in other pelvic organs and involve the ovary secondarily. Thus, it has been proposed that serous tumors arise from the implantation of epithelium (benign or malignant) from the fallopian tube. Endometrioid and clear cell tumors have been associated with endometriosis, which is regarded as the precursor of these tumors. Since it is generally accepted that endometriosis develops from endometrial tissue by retrograde menstruation it is reasonable to assume that the endometrium is the source of these ovarian neoplasms. Finally, preliminary data suggest that mucinous and transitional (Brenner) tumors arise from transitional-type epithelial nests at the tubal-mesothelial junction by a process of metaplasia. Appreciation of these new concepts will allow for a more rationale approach to screening, treatment and prevention which potentially can have a significant impact on reducing the mortality of this devastating disease.
The origin and pathogenesis of epithelial ovarian cancer has perplexed investigators for decades. Despite numerous studies that have carefully scrutinized the ovaries for precursor lesions, none have been found. This has led to the proposal that ovarian cancer develops de novo2. “Nothing will come from nothing,” but each year in the United States approximately 21,550 women develop ovarian cancer “de novo” and 14,600 women die from this disease18. Ovarian cancer is, in fact, the most lethal gynecologic malignancy. It is clear that de novo reflects our ignorance about the early events of ovarian carcinogenesis rather than our insight into its perplexing origin. The time honored concepts that have forged our views of ovarian carcinogenesis can be summarized as follows: 1) although it is recognized that there are profound differences among the various histologic types, the vast majority of ovarian carcinomas are high-grade serous carcinomas and therefore ovarian cancer is regarded as a single disease; 2) ovarian cancer originates from the ovarian surface epithelium (mesothelium) which invaginates into the underlying stroma resulting in inclusion cysts that eventually undergo malignant transformation; 3) ovarian cancer spreads from the ovary to the pelvis, abdomen and distant sites. Based on these views of ovarian carcinogenesis, efforts at improving survival have focused on early detection of ovarian cancer, when it is still confined to the ovary, and on the development of new chemotherapeutic drugs and routes of delivery irrespective of the histologic type. Unfortunately, these efforts have not been successful as evidenced by the fact that the overall survival for women with ovarian cancer has not changed over the last 50 years. The reasons for this are that the concepts of histogenesis on which these approaches are based, are flawed.
Recent morphologic and molecular genetic studies have illuminated our understanding of ovarian carcinogenesis in ways that have been quite unexpected and have challenged the conventional wisdom regarding their origin and development. Indeed, they have resulted in a paradigm shift that has important implications for research and for radically changing our approaches to early detection, prevention and treatment.
The Morphologic and Molecular Heterogeneity of Epithelial Ovarian Cancer
One of the major problems in elucidating the pathogenesis of ovarian cancer is that it is a heterogeneous disease composed of different types of tumors with widely differing clinicopathologic features and behavior. Based on a series of morphologic and molecular genetic studies, we have proposed a dualistic model that categorizes various types of ovarian cancer into two groups designated type I and type II44. Type I tumors are clinically indolent and usually present at a low stage. They exhibit a shared lineage between benign cystic neoplasms and the corresponding carcinomas often through an intermediate (borderline tumor) step, supporting the morphological continuum of tumor progression in these neoplasms. This stepwise sequence of events parallels the adenoma-carcinoma sequence that occurs in colorectal carcinoma. Type I tumors include low- grade serous, low-grade endometrioid, clear cell and mucinous carcinomas. In contrast to the clear-cut and distinctive morphologic differences among type I tumors, the morphologic differences among the type II tumors are more subtle and as a result there is considerable overlap in the diagnosis of these tumors by different pathologists. Type II tumors exhibit papillary, glandular, and solid patterns and are diagnosed as high-grade serous, high-grade endometrioid and undifferentiated carcinomas depending on the dominant pattern. Generally, most pathologists classify them as high-grade serous carcinomas even though they bear little resemblance to tubal-type epithelium (the basis for typing a tumor as serous); arguably many of those lacking distinctive serous or endometrioid features could be classified as “high-grade adenocarcinoma”. In addition to these neoplasms, malignant mixed mesodermal tumors (carcinosarcomas) are included in the type II category because they have epithelial components identical to the pure type II carcinomas. Type II tumors are highly aggressive and almost always present in advanced stage. Since they account for approximately 75% of all epithelial ovarian carcinomas and have relatively similar morphologic features and a uniformly poor outcome, ovarian cancer has been erroneously regarded as a single disease. The morphologic differences between type I and type II tumors are mirrored by marked differences in their molecular genetic features7. As a group, type I tumors are genetically more stable than type II tumors and display specific mutations in the different histologic cell types21. Thus, KRAS, BRAF, and ERBB2 mutations occur in approximately two thirds of low-grade serous carcinomas whereas TP53 mutations are rare in these tumors. Low-grade endometrioid carcinomas have aberrations in the Wnt signaling pathway involving somatic mutations of CTNNB1 (encoding β-catenin), PTEN and PIK3CA7. Mucinous carcinomas have KRAS mutations in more than 50% of specimens1, 28. Clear cell carcinoma is unique in that it has a high percentage of PIK3CA activating mutations when purified tumor samples and cell lines are analyzed22. There is little available molecular genetic data on transitional cell (Brenner) tumors. High-grade serous carcinoma, the prototypic type II tumor, is characterized by very frequent TP53 mutations (>80% of cases) and CCNE1 (endcoding cyclin E1) amplification but rarely mutations that characterize most type I tumors such as KRAS, BRAF, ERBB2, PTEN, CTNNB1 and PIK3CA7. Although only a small number of malignant mixed mesodermal tumors have been analyzed molecularly, the few that have been display a similar molecular genetic profile. In summary, type I tumors, as a group, are genetically more stable than type II tumors and display a distinctive pattern of mutations that occur in specific cell types (low-grade serous, low-grade endometrioid, clear cell and mucinous). In contrast, the type II tumors (high-grade serous, high-grade endometrioid, malignant mixed mesodermal tumors and undifferentiated carcinomas) show greater morphologic and molecular homogeneity, are genetically unstable and have a very high frequency of TP53 mutations. These findings suggest that different types of ovarian carcinomas develop along different molecular pathways.
The Cell of Origin of Most Epithelial Ovarian Cancer is not Ovarian
The cell of origin of ovarian cancer and the mechanisms by which cancer develops have been long debated. The traditional view of ovarian carcinogenesis has been that the various different tumors are all derived from the ovarian surface epithelium (mesothelium) and that subsequent metaplastic changes lead to the development of the different cell types (serous, endometrioid, clear cell, mucinous and transitional cell [Brenner]) which morphologically resemble the epithelia of the fallopian tube, endometrium, gastrointestinal tract or endocervix and urinary bladder, respectively. The normal ovary, however, has no constituents that resemble these tumors. Moreover, the cervix, endometrium and fallopian tubes are derived from the müllerian ducts whereas the ovaries develop from mesodermal epithelium on the urogenital ridge separate from the müllerian ducts. Therefore, an alternate theory proposes that tumors with a müllerian phenotype (serous, endometrioid and clear cell) are derived from müllerian-type tissue not mesothelium11. This müllerian-type tissue (columnar epithelium, often ciliated) lines cysts located in paratubal and paraovarian locations that have been referred to collectively as the “secondary müllerian system”23. According to this theory, ovarian tumors develop from these cysts. As the tumor enlarges, it compresses and eventually obliterates ovarian tissue resulting in an adenxal tumor that appears to have arisen in the ovary. More recently another theory has been advanced which argues that the majority of ovarian carcinomas, which are high-grade serous carcinomas, arise from high-grade intraepithelial serous carcinomas in the fallopian tube which then spread to the ovary. These conflicting views led us to undertake a review of the literature in an effort to determine which of the theories is best able to explain the various aspects of ovarian carcinogenesis.
Evaluating these theories is problematic because it is difficult to construct experimental systems, to test their validity. Accordingly, our evaluation is based on critical analysis of these studies in light of observations we have made in the course of pathologic examination of ovarian tumors. The discussion that follows is an attempt to distill the most plausible components from the various theories of cellular origin and integrate them with the clinicopathologic and molecular genetic data from the dualistic model in order to construct a unifying theory of ovarian carcinogenesis.
The theory of origin from ovarian surface epithelium (mesothelium) has a number of limitations. Histologically, the single layer of generally attenuated mesothelium overlying the ovaries bears no resemblance to serous, endometrioid, mucinous, clear cell or transitional (Brenner) carcinomas. As noted above in order to account for this apparent contradiction it was proposed that the mesothelium overlying the ovary invaginates into the underlying stroma to form so-called “cortical inclusion cysts”. These cysts under the influence of local factors, possibly steroid hormones, undergo a metaplastic change, which results in the mesothelium being converted to müllerian-type epithelium. These inclusion cysts, with their newly acquired müllerian phenotype, can then undergo malignant transformation resulting in carcinomas corresponding to the different cell types (serous, endometrioid and clear cell carcinomas)6. Although cortical inclusion cysts lined by ciliated (müllerian-type epithelium) are frequently observed in the ovarian cortex, well documented examples of what can be interpreted as a transition from these cysts to carcinoma have not been reported. Moreover, cortical inclusion cysts lined by intestinal-type epithelium to account for the development of mucinous carcinomas are distinctly rare. The same can be said for the absence of transitional-type epithelium lining cortical inclusion cysts to account for the development of Brenner tumors.
The limitations of the secondary müllerian system theory are that precursor lesions resembling serous, endometrioid and clear cell carcinomas have rarely, if ever, been reported in paratubal and paraovarian cysts. Moreover, the vast majority of mucinous tumors display intestinal rather than endocervical-type mucinous differentiation and therefore do not qualify as müllerian-type tumors. A similar problem exists for transitional cell (Brenner) tumors which resemble urothelium which is not müllerian in origin.
The most compelling evidence suggests that the vast majority of what appear to be, primary ovarian cancers, namely serous, endometrioid and clear cell carcinomas, are derived from the fallopian tube and endometrium, not directly from the ovary. Sporadic reports of tubal carcinoma and “dysplasia” had been reported in the past15 but in 2001 a group of Dutch investigators described these lesions, which closely resemble high-grade ovarian serous carcinoma, in women with a genetic predisposition to ovarian cancer33. This was a surprising finding, since numerous studies over the past two decades that carefully examined the ovaries of women with a genetic predisposition to ovarian cancer never reported similar lesions. In addition, other studies of normal appearing ovaries contralateral to sporadic (nonhereditary) unilateral ovarian carcinomas had never identified a convincing precursor lesion. These latter studies reported a number of morphologic changes in grossly normal appearing ovaries, such as an increased number of inclusion cysts, surface papillae, cortical inclusions, including some displaying minor degrees of atypia. The data, however, have been conflicting, some studies reporting a significant difference of these changes in cases versus controls and other studies reporting no difference. In any event, none of these changes, even remotely, resemble high-grade serous carcinoma. It was precisely because of a lack of convincing precursor lesions that the de novo hypothesis was invoked.
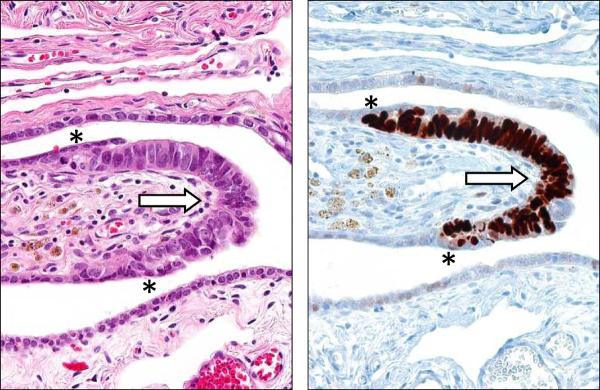
In hindsight, because it was assumed that precursors of ovarian carcinoma would logically be in the ovaries, the fallopian tubes were not carefully examined42, 10. Subsequent studies in which fallopian tubes were more carefully examined confirmed that in situ and small, early invasive tubal carcinomas occurred in women with a genetic predisposition for the development of ovarian cancer4, 5, 8, 12, 27, 29, 41. This led to fallopian tube carcinoma being included as part of the cancer spectrum associated with inherited BRCA mutations. It was subsequently proposed that a proportion of ovarian carcinomas might develop as a result of implantation of malignant cells from the tubal carcinoma to the ovary34–35. The next important step linking what had been termed “tubal intraepithelial carcinoma” (TIC) and subsequently “serous tubal intraepithelial carcinoma” (STIC) with ovarian carcinoma was the observation that over 70% of sporadic (nonhereditary) ovarian and peritoneal high-grade serous carcinomas demonstrated mucosal tubal involvement including STICs19. This observation gave substantial support to the proposal that STICs, which almost always are detected in the fimbria, may be the source of ovarian high-grade serous carcinoma in both women with hereditary mutations in BRCA as well as women who did not have a known genetic predisposition for ovarian cancer. Although it can be argued that mucosal tubal involvement could represent secondary spread from an ovarian carcinoma present in the same specimen, the presence of focal noncontiguous intraepithelial lesions (STICs), would be an unusual manifestation of metastasis. Furthermore, the identification of STICs in prophylactic specimens from women with a hereditary predisposition to ovarian cancer, in which complete microscopic evaluation of the fallopian tubes and ovaries failed to identify invasive carcinoma in these organs, lends additional support to the concept that the serous neoplastic process may well begin in the fallopian tube rather than the ovary. Further support for this argument is the finding that nearly all STICs overexpress p53 similar to high-grade serous carcinoma (Figure 1). Laser capture microdissection studies of these lesions have demonstrated that they harbor mutated TP5319. In addition, STICs associated with a concomitant ovarian carcinoma share not only morphologic features but also identical TP53 mutations indicating a clonal relationship between them. Adnexal malignant mixed mesodermal tumors (another type II tumor) have also been associated with STICs supporting the existence of a common precursor lesion for type II tumors14. Further evidence implicating the fallopian tube rather than ovarian surface epithelium as the site of origin of serous neoplasms comes from a gene profiling study showing that the gene expression profile of high-grade serous carcinoma is more closely related to the fallopian tube than to ovarian surface epithelium25. In addition high-grade serous carcinomas express PAX8, a müllerian marker, but not calretinin, a mesothelial marker43.
Figure 1.
Serous tubal intraepithelial carcinoma (STIC). A. High magnification. Hematoxylin and eosin stain. B. Immunohistochemical stain for p53. An asterisk defines the boundary of the lesion.
A recent finding has been the identification of benign tubal epithelium, specifically secretory as opposed to ciliated cells, that express p53 and in which laser capture microdissection studies have reported TP53 mutations in 57% of cases24. These lesions termed “p53 signatures” are found in association with STICs and in normal appearing fallopian tubes of women without STICs or carcinoma; they have been observed in approximately one third of women with and without BRCA mutations13, 17, 41. Like STICs, p53 signatures express γ-H2AX which localizes to areas of DNA damage in nuclei24. When associated with STICs and ovarian carcinoma, the p53 signature has had the identical TP53 mutation as the STIC and the carcinoma in some cases but not in others. Based on these findings, a sequence of pathogenetic events has been proposed, beginning with genotoxic DNA damage, followed by TP53 mutation and progressive loss of cell cycle control, which then eventuates in the development of carcinoma24. There are a number of questions that must be resolved, however, before this hypothesis can be completely accepted. First, as noted in some instances, TP53 mutations, when present in the p53 signature, are not always identical with the mutations in the STICs and carcinomas in the same specimen. Second, women at high risk have the same frequency of p53 signatures as women who are not at high risk. Third, the high prevalence of p53 signatures (a third of all women) compared to the low prevalence of high-grade serous ovarian carcinoma suggests that either a small minority of p53 signatures progress or that they are not related to carcinoma. It is conceivable that p53 signatures reflect an appropriate and physiological upregulation of p53 in response to DNA damage, based on the observation that TP53 mutations are absent in nearly half of p53 signatures. Although the proposal that the p53 signature is a precursor lesion is intriguing, its role in the genesis of ovarian high-grade serous carcinoma is far from clear at this time. As fallopian tubes are more carefully examined and these lesions studied, the nature of p53 signatures and their relationship to STICs will become better defined.
Generally, before a carcinoma acquires the ability to metastasize it must first invade and gain access to blood vessels or lymphatics. We have observed that the fimbria contain a rich angiolymphatic vasculature. Moreover, they are in almost direct contact with the basement membrane of the tubal epithelium and therefore a tubal carcinoma may not need to attain a very large size in order to invade this highly accessible angiolymphatic network. In addition, invasion in the case of a STIC may not be a necessary prerequisite for dissemination. Tubal intraepithelial carcinomas are similar morphologically and immunohistochemically to endometrial intraepithelial carcinomas, which are regarded as precursors or early forms of uterine serous carcinoma. These lesions have also been termed “uterine surface serous carcinomas”. They have been shown to disseminate throughout the peritoneal cavity presumably by passage of malignant cells through the fallopian tube without requisite myometrial invasion46. The cells that comprise both endometrial and tubal intraepithelial carcinomas are highly anaplastic and identical morphologically to high-grade serous carcinoma. The lesions form papillary tufts and the constituent cells are loosely cohesive. Presumably these cells can shed and implant on the surface of the ovary and the peritoneum in the absence of invasive growth in the fallopian tube. Evidence supporting this possibility are reports of positive pelvic washings in women whose only lesion was a STIC4.
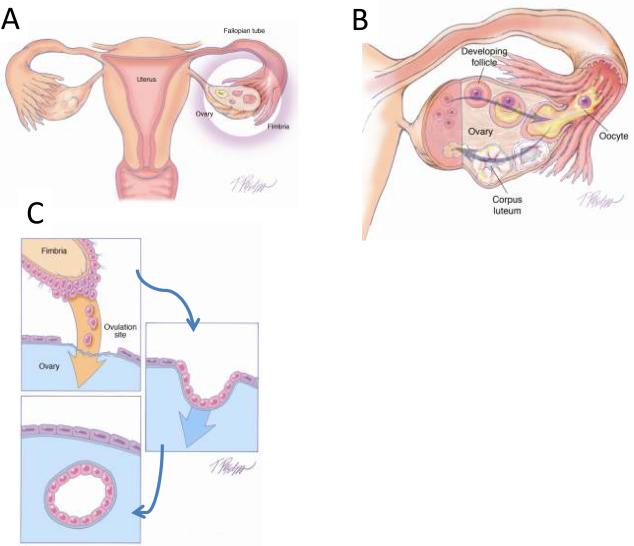
As previously noted, in studies of ovarian and primary peritoneal high-grade serous carcinomas in which the entire fallopian tubes were carefully sectioned, mucosal involvement of the tube, including STICs, were identified in approximately 70% of cases19. The question arises as to the source of the remaining ovarian carcinomas that lack evidence of tubal involvement. There are a number of possible explanations. First, despite thorough sectioning, a small STIC could have been missed (unpublished data). Second, on occasion high-grade serous carcinomas are intimately associated with serous borderline tumors and low-grade serous carcinomas. In these cases the high-grade tumors have had KRAS mutations identical to those in the serous borderline tumors and lacked TP53 mutations9. This finding suggests that some high-grade serous carcinomas arise from low-grade serous tumors and not by the usual (type II) pathway that begins with a TP53 mutation. Third, clear-cut mucosal tubal involvement could have been obscured by overgrowth of the pelvic carcinoma. Fourth, the fimbria of the fallopian tube normally is in intimate contact with the ovarian surface at the time of ovulation. It is conceivable that when the ovarian surface epithelium is disrupted at the time of ovulation, normal tubal epithelial cells from the fimbria may be dislodged and implant in the ovary to form an inclusion cyst (Figure 2) from which a high-grade serous carcinoma could develop (see below). Evidence to support this notion is the observation that fallopian tube epithelial cells are easily obtained for culture by flushing the fallopian tube34, 43. This mechanism could also explain the development of endosalpingiosis, a lesion composed of glands and papillary structures lined by tubal-type epithelium that is found on peritoneal surfaces in the pelvis, omentum and beneath the capsule of pelvic and para-aortic lymph nodes. Endosalpingiosis is frequently found in association with low-grade serous tumors and has been viewed as a possible precursor of these tumors. Finally, the possibility that some high-grade serous carcinomas arise in cortical inclusion cysts as a metaplastic process from the ovarian surface epithelium rather than from implantation of normal fallopian tube epithelium cannot be entirely dismissed.
Figure 2.
Transfer of normal tubal epithelium to the ovary. A. Anatomical relationship of fallopian tube to the ovary at the time of ovulation. The fimbria envelops the ovary. B. Ovulation. The ovarian surface ruptures with expulsion and transfer of the oocyte to the fimbria. The fimbria is in intimate contact with the ovary at the site of rupture. C. Tubal epithelial cells from the fimbria are dislodged and implant on the denuded surface of the ovary resulting in the formation of an inclusion cyst.
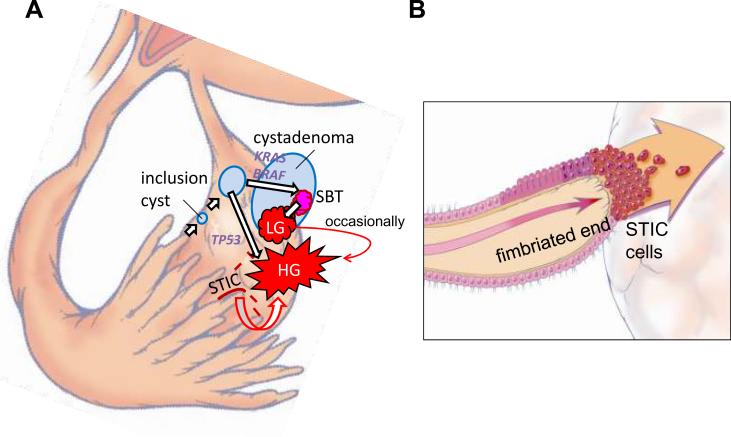
Direct implantation of tubal epithelium into the ovary to form an inclusion cyst, which in turn is the site of origin of ovarian serous carcinoma, although not yet demonstrated, is an attractive alternative theory to that of metaplasia from the surface epithelium (mesothelium). Implantation of fallopian tube epithelium from the fimbria at the time of ovulation when the surface epithelium is disrupted can explain the derivation of low- and high-grade serous carcinomas. In the case of a low-grade serous carcinoma the process develops slowly from a serous cystadenoma and then a serous borderline tumor after a KRAS or BRAF mutation whereas in the case of a high-grade serous carcinoma the process evolves rapidly, presumably from a cortical inclusion cyst after a TP53 mutation with the development of an intraepithelial carcinoma as an intermediate step. According to this view both low- and high-grade serous carcinomas are ultimately of tubal (müllerian) origin and in a sense the ovary is involved secondarily (Figure 3).
Figure 3.
Proposed development of low-grade (LG) and high-grade (HG) serous carcinoma. A. One mechanism involves normal tubal epithelium that is shed from the fimbria, which implants on the ovary to form an inclusion cyst. Depending on whether there is a mutation of KRAS/BRAF/ERRB2 or TP53 a low-grade or high-grade serous carcinoma develops respectively. Low-grade serous carcinoma often develops from a serous borderline tumor (SBT), which in turn arises from a serous cystadenoma. Another mechanism involves exfoliation of malignant cells from a serous tubal intraepithelial carcinoma (STIC) that implants on the ovarian surface resulting in the development of a high-grade serous carcinoma. B. A schematic representation of direct dissemination or shedding of STIC cells onto the ovarian surface where the carcinoma cells ultimately establish a tumor mass that is presumably arising from the ovary. Of note there may be stages of tumor progression that precede the formation of a STIC.
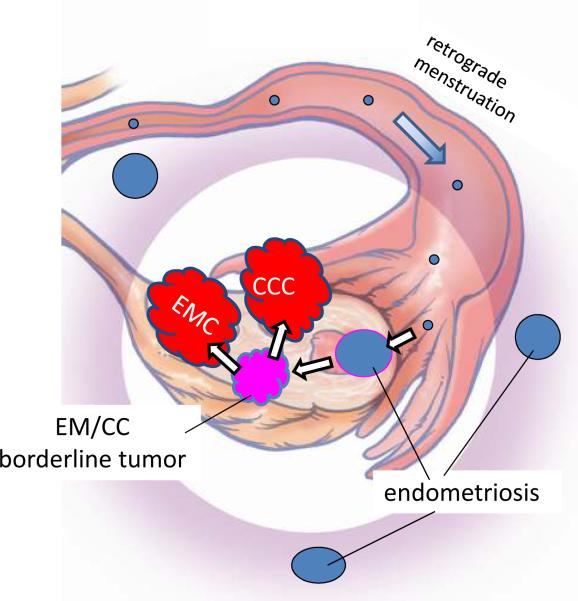
It has been well established both by morphologic and more recently molecular genetic studies that low-grade endometrioid and clear cell carcinomas develop from endometriotic cysts (endometriomas), which are frequently associated with implants of endometriosis elsewhere in the pelvis45. Although the precise origin of endometriosis has not been completely established, specifically, whether it develops in situ in the peritoneum through a process of metaplasia or from retrograde menstrual flow, the preponderance of data favor the latter mechanism3. Admittedly, the former theory is more difficult to prove experimentally. Thus, if retrograde menstruation accounts for most cases of endometriosis, it is logical to assume that endometrioid and clear cell tumors develop from endometrial tissue (müllerian derived) that implanted on the ovary and therefore the ovary is involved secondarily26 (Figure 4). Of further interest has been the observation that the eutopic endometrium in women with endometriosis exhibits intrinsic molecular abnormalities including activation of oncogenic pathways. Presumably, these changes permit the endometrial tissue to implant, survive and invade on ovarian and peritoneal surfaces3. This hypothesis by which endometrioid and clear cell carcinoma develop from endometrial tissue implanted on the ovary is supported by epidemiologic evidence showing that a protective effect for tubal ligation was seen only for endometrioid and clear cell carcinoma of the ovary37.
Figure 4.
Proposed development of low-grade endometrioid and clear cell carcinoma. Endometrial tissue, by a process of retrograde menstruation, implants on the ovarian surface to form an endometriotic cyst from which a low-grade endometrioid or clear cell carcinoma can develop. EMC: low-grade endometrioid carcinoma of the ovary; CCC: clear cell carcinoma of the ovary.
Finally, the derivation of mucinous tumors of gastrointestinal type and transitional cell (Brenner) tumors may also not involve the ovaries directly. The origin of these tumors is puzzling since unlike serous, endometrioid and clear cell tumors, they do not display a müllerian phenotype. Although it has been argued that these mucinous tumors bear some relationship to the endocervix, the mucinous epithelium that characterizes these neoplasms more closely resembles gastrointestinal mucosa. It seems most unlikely that they develop from cortical inclusion cysts since mucinous metaplasia involving cortical inclusion cysts is a very rare finding. On the other hand, the association of Brenner tumors and mucinous tumors has been recognized for many years. In a provocative study of mucinous cystadenomas and Brenner tumors it was reported that after extensive sectioning, mucinous cystadenomas contained foci of Brenner tumor in 18% of cases40. Interestingly, mucinous tumors were frequently associated with Walthard cell nests, which are composed of benign transitional-type epithelium, frequently found in paraovarian and paratubal locations. This raises the possibility that mucinous tumors and Brenner tumors have the same histogenesis, arising from these microscopic transitional cell nests at the tubal-mesothelial junction in keeping with their nonmüllerian appearance. The study reported that Brenner tumors are small (median size 0.5 cm, range 0.02–20 cm) whereas mucinous cystadenomas are large (median size 9 cm, range 1–30 cm). The investigators speculated that as a small Brenner tumor grows, the mucinous component becomes dominant resulting in the development of a mucinous cystadenoma, which as it enlarges, compresses and eventually obliterates the adjacent ovary giving the appearance that it arose in the ovary. The findings in this study are intriguing but must be regarded as preliminary. Additional morphologic and molecular genetic studies are necessary to determine whether this concept is valid.
In summary, none of the existing theories adequately reconciles all aspects of ovarian carcinogenesis. All of them have something to offer in explaining the development of ovarian carcinomas but none are all inclusive. It does appear that the vast majority of what have been thought to be primary epithelial ovarian and primary peritoneal carcinomas are, in fact, secondary. Thus, the most persuasive data support the view that serous tumors develop from the fimbriated portion of the fallopian tube, endometrioid and clear cell tumors from endometrial tissue passing through the fallopian tube resulting in endometriosis and mucinous and Brenner tumors from transitional-type epithelium located at the tubal-mesothelial junction where the fimbria makes contact with the peritoneum. The concept that the majority of epithelial ovarian carcinomas originates outside the ovary and involves it secondarily has emerged only recently because in the past the default diagnosis of carcinomas involving the pelvis and abdomen was that they were ovarian. A carcinoma was classified as tubal in origin only when the bulk of the tumor involved the fallopian tube rather than the ovary and there was evidence of an intraepithelial (in situ) tubal carcinoma39. A diagnosis of primary peritoneal carcinoma is even more restrictive. Even with extensive tumor involving the peritoneum, omentum and other abdominal organs, a carcinoma is classified as primary ovarian if there is as little as 5 mm of tumor involving the ovaries. Thus, there has been an inherent bias in classifying pelvic tumors as being ovarian in origin.
Although the data suggesting that epithelial ovarian carcinoma arises in extraovarian sites and involves the ovaries secondarily are compelling, serous neoplasms (low- and high-grade) involve the ovaries and other pelvic and abdominal organs, such as the omentum and mesentery, much more extensively than the fallopian tubes. Similarly, although endometrioid carcinomas develop from endometriosis, which frequently involves multiple sites in the pelvis, these neoplasms are almost always confined to the ovaries. It is likely that the propensity for growth in the ovary is mulifactorial but the precise reasons for this are unknown.
Implications for Research, Screening, Prevention and Treatment
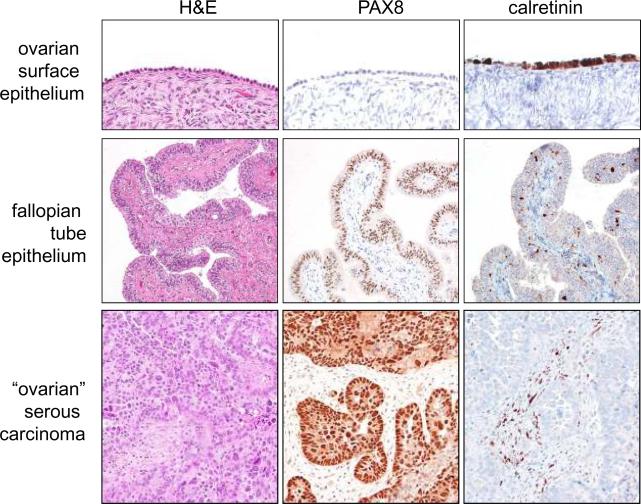
The implications of this new paradigm of ovarian carcinogenesis for investigators, clinicians, and women are significant. For researchers, the implication of tubal origin of ovarian serous carcinoma challenges many of the previous reports demonstrating “overexpressed” ovarian cancer associated genes, in which their expression levels in carcinoma are almost always compared to their “normal” counterparts, ovarian surface epithelium. As the gene expression profiles in ovarian surface epithelium, which is of mesothelial origin, are distinct from fallopian tube epithelium which is of müllerian origin, experiments in which ovarian surface epithelium (mesothelium) has been used as a control may not be valid. Whether the overexpressed genes that have been previously reported are indeed upregulated when they are compared to the more likely source of ovarian serous carcinoma, i.e., fallopian tube epithelium, needs to be revisited. In fact, a recent molecular genetic study showed that the different histologic types of ovarian cancer, do indeed, display distinct expression profiles that are concordant with the normal tissues they resemble and show little similarity to ovarian surface epithelium (mesothelium). Thus, the genes expressed in serous carcinoma were similar to those expressed in normal fallopian tube, whereas the expression profiles of endometrioid and clear cell carcinomas resembled endometrial epithelium. Interestingly, the expression profile of mucinous tumors resembled normal colonic epithelium25. We have also observed (unpublished data) that PAX8, a marker of müllerian-type epithelium, is expressed in ovarian serous carcinoma but not in ovarian surface epithelium (mesothelium) whereas calretinin, a mesothelial marker, reacts with ovarian surface epithelium and mesothelioma but not with tubal epithelium or ovarian serous carcinoma (Figure 5). In the future, analysis of overexpressed genes in ovarian cancer should take into account the histologic type of the tumors being studied and the data compared to the appropriate normal tissue.
Figure 5.
Comparison of the immunohistochemical staining pattern for ovarian surface epithelium (mesothelium), normal fallopian tube epithelium, and high-grade serous carcinoma. PAX8 is a marker of mullerian-type epithelium such as fallopian tube epithelium and calretinin is a marker of mesothelium.
From a clinical perspective the implications of this new paradigm are even more far reaching. For the last two decades numerous studies, including large clinical trials, have been performed in an effort to develop screening tests for ovarian cancer. The goal of these studies is to detect tumors when they are still confined to the ovaries, thereby increasing the likelihood of cure and reducing the mortality of the disease. The modalities that are currently being used to screen women are pelvic examination, transvaginal ultrasound and measurement of serum CA 125. An awareness of the dualistic model, which highlights the heterogeneity of ovarian carcinoma, clearly indicates that one screening test will not be effective in detecting all the different types of ovarian carcinomas. Type I tumors (low-grade serous, low-grade endometrioid, clear cell and mucinous) are slow growing and attain a large size while still confined to the ovary. They are easily detected by pelvic examination and/or transvaginal ultrasound. They constitute, however, only 25% of ovarian cancers and account for approximately 10% of ovarian cancer deaths16. Therefore, it can be argued that the development of a biomarker screening test is not urgently needed for type I tumors. More importantly, the recognition that the majority of type II tumors (high-grade serous and undifferentiated carcinomas and malignant mixed mesodermal tumors [carcinosarcomas]) originate outside the ovary illustrates the underlying flaws in screening approaches designed to detect these tumors while confined to the ovary. Moreover, type II tumors represent approximately 75% of all ovarian carcinomas and are responsible for 90% of ovarian cancer deaths16. It is the type II tumors that should be targeted for screening but unfortunately these tumors are rarely confined to the ovary, even at their inception. In a study of nearly 400 patients who were carefully staged from the Washington Center Hospital in Washington DC, which is largely a primary care hospital, less than 1.25% of high-grade serous carcinomas were confined to the ovary (Seidman et al, unpublished data). Similarly, the British Columbia Tumor Registry reported that only 0.5% of high-grade serous carcinomas were limited to the ovary38. The futility of detecting early stage ovarian cancer was recently underscored in a large multi-institutional prospective study (Prostate, Lung, Colorectal, and Ovarian [PLCO] Cancer Screening Trial) in which despite intensive annual screening of nearly 35,000 women with CA 125 and transvaginal ultrasound, 70% of the women presented with advanced stage disease. This was no different from unscreened populations31. For type II tumors, the goal in screening should be detection of low volume, not low stage disease. This can only be accomplished by developing a panel of sensitive and specific biomarkers that are expressed early in ovarian carcinogenesis.
As with early detection, the treatment of type I and type II tumors must be individualized. Type I tumors are generally low-grade, slow growing and localized to the ovary at diagnosis, spreading late in their evolution. Accordingly, when confined to the ovary, salpingo-oophorectomy may suffice. On the other hand, when they have spread beyond the ovary, chemotherapeutic agents that are effective against the more rapidly proliferating type II tumors are not as effective for type I tumors because the latter are slow growing. Therefore, new approaches for advanced stage type I tumors are needed. Deregulation of protein kinase activity as a result of somatic mutation in these genes occurs in many type I tumors. Mutations in these genes constitutively activate the signaling pathways they control, and tumor cells with mutations become dependent on those mutations for progression. Therefore, these genes could provide potential targets for therapeutic intervention. For example, in many type I carcinomas, there is constitutive activation of the MAPK signaling pathway because of mutations in ERBB2, KRAS or BRAF, the upstream regulators of MAPK. It is therefore conceivable that BRAF inhibitors and other MAPK kinase inhibitors could prolong disease-free interval and improve overall survival in patients with these types of advanced stage type I tumors when combined with conventional therapeutic modalities.
The approach to the treatment of type II tumors, should be completely different from that of the type I tumors. Treatment for type II tumors should be initiated based on detection of sensitive and specific biomarkers, before the appearance of morphologically recognizable disease, when therapy will likely be more effective. A precedent exists for this approach as women with hereditary BRCA mutations are treated based on that information only. Another important treatment issue that needs to be considered is whether patients found to have a STIC require adjuvant chemotherapy. The finding of positive pelvic washings in patients with only a STIC indicates that these microscopic lesions can shed malignant cells4. At present there is no consensus as to whether or not these women should be treated. This will have to be determined by a randomized clinical trial.
Finally, the mounting evidence that ovarian cancer does not develop in the ovary and the lack of success of ovarian cancer screening provides a strong rationale for directing efforts at primary prevention. It has been well established in epidemiological studies that the use of oral contraceptives reduces the risk of ovarian cancer substantially. The risk is reduced by about 50% for women using oral contraceptives for 5 or more years36. Parity has also been shown to be protective, conferring approximately a 50% decrease in risk compared to nulliparity32. Accordingly, the entire approach to prophylaxis, not only for women at high risk of developing ovarian cancer, but also for the general female population, needs to be reevaluated in light of the evolving new paradigm of ovarian carcinogenesis as discussed here. The traditional approach for reducing risk for women with a family history of ovarian carcinoma or who are found to have BRCA1/2 mutations has been hysterectomy and bilateral salpingo-oophorectomy. The ovarian tumors that develop are almost always high-grade serous carcinomas and there has been no convincing evidence that these women are at a higher risk of developing uterine serous carcinomas. If it can be unequivocally shown that the serous carcinomas in these women develop almost exclusively in the fimbria then salpingectomy alone would be sufficient to reduce the risk of ovarian cancer. This approach would have to be evaluated in a randomized clinical trial comparing it to the standard treatment of bilateral salpingo-oophorectomy. For women who are not considered to be at high risk but who undergo a hysterectomy for benign uterine disease, many gynecologists have argued that bilateral oophorectomy should be performed in order to reduce the risk of developing ovarian cancer. In a recent prospective study of nearly 30,000 women in the Nurses' Health Study, it was shown that compared with ovarian conservation, bilateral oophorectomy at the time of hysterectomy was associated with an increased risk of all-cause mortality, fatal and nonfatal coronary heart disease, and lung cancer30. Accordingly, for women undergoing a hysterectomy for benign uterine disease, removal of only the fallopian tubes with sparing of the ovaries would improve quality of life and overall survival while still reducing the risk of ovarian carcinoma. Such an approach has important public health implications as approximately 300,000 women in the United States undergo elective oophorectomy each year.
Conclusions
A new paradigm for the pathogenesis of ovarian cancer based on a dualistic model and the recognition that the majority of “ovarian” carcinomas originate outside the ovary assist in organizing this complex group of neoplasms and facilitates the development of new and novel approaches to prevention, screening and treatment. One group of tumors (type I) is generally indolent, presents in stage I (tumor confined to the ovary) and develops from well-established precursors, so-called borderline tumors. These tumors are characterized by specific mutations including KRAS, BRAF, ERBB2, CTNNB1, PTEN and PIK3CA but rarely TP53. They are relatively genetically stable. The other group (type II) is composed of tumors that are aggressive, present in advanced stage and develop from intraepithelial carcinomas in the fallopian tube. They have a very high frequency of TP53 mutations but rarely harbor the mutations detected in type I tumors. They are genetically highly unstable.
This proposed model is intended to serve as a framework for studying ovarian cancer. It is not complete and does not resolve all issues. For example, clear cell carcinoma is classified as a type I tumor based on having a characteristic PIKC3CA mutation, relative genetic stability, frequent presentation in stage I and association with endometriosis, a well established precursor lesion. But unlike other type I tumors clear cell carcinoma is high-grade at presentation. The inability to reconcile all of the many issues relating to ovarian pathogenesis does not invalidate or negate the utility of the paradigm. As pointed out by Thomas Kuhn, who introduced the concept of paradigms as a way of explaining how science progresses, “To be accepted as a paradigm, a theory must seem better than its competitors, but it need not, and in fact never does, explain all the facts with which it can be confronted”20.
Recent studies on the origin of ovarian cancer have directed attention to a putative precursor lesion in the fallopian tube that morphologically and molecularly resembles high-grade ovarian serous carcinoma and that has been designated “serous intraepithelial tubal carcinoma (STIC)”. Thus, rather than developing de novo from the ovary, as previously proposed, the majority of type II tumors appear to arise from a STIC in the fimbriated end of the fallopian tube that spreads to the ovary. Another possible mechanism for the development of “ovarian” carcinoma is dislodgement of normal tubal epithelium from the fimbria, which implants on the site of rupture where ovulation occurred resulting in the formation of an inclusion cyst that may then undergo malignant transformation. Thus, serous tumors may develop from inclusion cysts, as has been thought, but by a process of implantation of tubal (müllerian-type) tissue rather than by a process of metaplasia from ovarian surface epithelium (mesothelial). Endometrioid and clear cell carcinomas may also originate from nonovarian, müllerian-type tissue as it is widely accepted that these tumors develop from endometriosis, which is thought to develop as a result of retrograde menstruation. The origin of mucinous and transitional cell (Brenner) tumors is still not well established, although recent data suggest a possible origin from transitional epithelial nests located in paraovarian locations. Thus, there is mounting evidence that type I and type II ovarian tumors develop independently along different molecular pathways and that both types develop outside the ovary and involve it secondarily. This explains why current screening strategies designed to detect ovarian cancer, when it is confined to the ovary, are ineffective in accomplishing this goal.
Given the obstacles in early detection (screening) and the significant, but relatively limited success in treatment, attention should be directed to primary prevention. This takes on particular relevance with the recognition that the majority of ovarian carcinomas are derived from cells in the fallopian tube or from passage of endometrial tissue through the fallopian tubes and the important role of ovulation in ovarian carcinogenesis. Salpingectomy alone may be sufficient to accomplish this, as removal of the fallopian tubes would reduce the risk of ovarian cancer while preserving ovarian function. Ovarian conservation appears to be particularly important for a woman's health, as it has been shown that oophorectomy is associated with increased overall mortality and a higher frequency of nonfatal coronary heart disease. Other approaches should also be explored, as for example the use of oral contraceptives, which presumably by preventing ovulation, reduce the risk of ovarian cancer by as much as 50%. In any case, new diagnostic, prevention and therapeutic approaches must be developed based on our evolving understanding of ovarian carcinogenesis.
Acknowledgement
We wish to thank Drs. Kathleen Cho, Lora Hedrick Ellenson, Brigitte M. Ronnett, Jeffrey D. Seidman and Russell Vang for their critical review of the manuscript and helpful suggestions.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Auner V, Kriegshauser G, Tong D, et al. KRAS mutation analysis in ovarian samples using a high sensitivity biochip assay. BMC Cancer. 2009;9:111. doi: 10.1186/1471-2407-9-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bell DA, Scully RE. Early de novo ovarian carcinoma. A study of fourteen cases. Cancer. 1994;73:1859–64. doi: 10.1002/1097-0142(19940401)73:7<1859::aid-cncr2820730714>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 3.Bulun SE. Endometriosis. N Engl J Med. 2009;360:268–79. doi: 10.1056/NEJMra0804690. [DOI] [PubMed] [Google Scholar]
- 4.Callahan MJ, Crum CP, Medeiros F, et al. Primary fallopian tube malignancies in BRCA-positive women undergoing surgery for ovarian cancer risk reduction. J Clin Oncol. 2007;25:3985–90. doi: 10.1200/JCO.2007.12.2622. [DOI] [PubMed] [Google Scholar]
- 5.Carcangiu ML, Radice P, Manoukian S, et al. Atypical epithelial proliferation in fallopian tubes in prophylactic salpingo-oophorectomy specimens from BRCA1 and BRCA2 germline mutation carriers. Int J Gynecol Pathol. 2004;23:35–40. doi: 10.1097/01.pgp.0000101082.35393.84. [DOI] [PubMed] [Google Scholar]
- 6.Cheng W, Liu J, Yoshida H, et al. Lineage infidelity of epithelial ovarian cancers is controlled by HOX genes that specify regional identity in the reproductive tract. Nat Med. 2005;11:531–7. doi: 10.1038/nm1230. [DOI] [PubMed] [Google Scholar]
- 7.Cho KR, Shih I. Ovarian cancer. Annu Rev Pathol. 2009;4:287–313. doi: 10.1146/annurev.pathol.4.110807.092246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colgan TJ, Murphy J, Cole DE, et al. Occult carcinoma in prophylactic oophorectomy specimens: prevalence and association with BRCA germline mutation status. Am J Surg Pathol. 2001;25:1283–9. doi: 10.1097/00000478-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Dehari R, Kurman RJ, Logani S, et al. The development of high-grade serous carcinoma from atypical proliferative (borderline) serous tumors and low-grade micropapillary serous carcinoma: a morphologic and molecular genetic analysis. Am J Surg Pathol. 2007;31:1007–12. doi: 10.1097/PAS.0b013e31802cbbe9. [DOI] [PubMed] [Google Scholar]
- 10.Deligdisch L, Gil J, Kerner H, et al. Ovarian dysplasia in prophylactic oophorectomy specimens: cytogenetic and morphometric correlations. Cancer. 1999;86:1544–50. [PubMed] [Google Scholar]
- 11.Dubeau L. The cell of origin of ovarian epithelial tumours. Lancet Oncol. 2008;9:1191–7. doi: 10.1016/S1470-2045(08)70308-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finch A, Shaw P, Rosen B, et al. Clinical and pathologic findings of prophylactic salpingo-oophorectomies in 159 BRCA1 and BRCA2 carriers. Gynecol Oncol. 2006;100:58–64. doi: 10.1016/j.ygyno.2005.06.065. [DOI] [PubMed] [Google Scholar]
- 13.Folkins AK, Jarboe EA, Roh MH, et al. Precursors to pelvic serous carcinoma and their clinical implications. Gynecol Oncol. 2009;113:391–6. doi: 10.1016/j.ygyno.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 14.Gagner JP, Mittal K. Malignant mixed Mullerian tumor of the fimbriated end of the fallopian tube: origin as an intraepithelial carcinoma. Gynecol Oncol. 2005;97:219–22. doi: 10.1016/j.ygyno.2004.11.053. [DOI] [PubMed] [Google Scholar]
- 15.Gordts S, Campo R, Rombauts L, et al. Endoscopic visualization of the process of fimbrial ovum retrieval in the human. Hum Reprod. 1998;13:1425–8. doi: 10.1093/humrep/13.6.1425. [DOI] [PubMed] [Google Scholar]
- 16.Guth U, Huang DJ, Bauer G, et al. Metastatic patterns at autopsy in patients with ovarian carcinoma. Cancer. 2007;110:1272–80. doi: 10.1002/cncr.22919. [DOI] [PubMed] [Google Scholar]
- 17.Jarboe E, Folkins A, Nucci MR, et al. Serous carcinogenesis in the fallopian tube: a descriptive classification. Int J Gynecol Pathol. 2008;27:1–9. doi: 10.1097/pgp.0b013e31814b191f. [DOI] [PubMed] [Google Scholar]
- 18.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 19.Kindelberger DW, Lee Y, Miron A, et al. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: Evidence for a causal relationship. Am J Surg Pathol. 2007;31:161–9. doi: 10.1097/01.pas.0000213335.40358.47. [DOI] [PubMed] [Google Scholar]
- 20.Kuhn TS. The Structure of Scientific Revolutions. University of Chicago Press; 1996. [Google Scholar]
- 21.Kuo KT, Guan B, Feng Y, et al. Analysis of DNA copy number alterations in ovarian serous tumors identifies new molecular genetic changes in low-grade and high-grade carcinomas. Cancer Res. 2009;69:4036–42. doi: 10.1158/0008-5472.CAN-08-3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuo KT, Mao TL, Jones S, et al. Frequent activating mutations of PIK3CA in ovarian clear cell carcinoma. Am J Pathol. 2009;174:1597–601. doi: 10.2353/ajpath.2009.081000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lauchlan SC. The secondary Mullerian system. Obstet Gynecol Surv. 1972;27:133–46. doi: 10.1097/00006254-197203000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Lee Y, Miron A, Drapkin R, et al. A candidate precursor to serous carcinoma that originates in the distal fallopian tube. J Pathol. 2007;211:26–35. doi: 10.1002/path.2091. [DOI] [PubMed] [Google Scholar]
- 25.Marquez RT, Baggerly KA, Patterson AP, et al. Patterns of gene expression in different histotypes of epithelial ovarian cancer correlate with those in normal fallopian tube, endometrium, and colon. Clin Cancer Res. 2005;11:6116–26. doi: 10.1158/1078-0432.CCR-04-2509. [DOI] [PubMed] [Google Scholar]
- 26.Martin DC. Cancer and endometriosis: do we need to be concerned? Semin Reprod Endocrinol. 1997;15:319–24. doi: 10.1055/s-2008-1068762. [DOI] [PubMed] [Google Scholar]
- 27.Medeiros F, Muto MG, Lee Y, et al. The tubal fimbria is a preferred site for early adenocarcinoma in women with familial ovarian cancer syndrome. Am J Surg Pathol. 2006;30:230–6. doi: 10.1097/01.pas.0000180854.28831.77. [DOI] [PubMed] [Google Scholar]
- 28.Mok SC, Bell DA, Knapp RC, et al. Mutation of K-ras protooncogene in human ovarian epithelial tumors of borderline malignancy. Cancer Res. 1993;53:1489–92. [PubMed] [Google Scholar]
- 29.Paley PJ, Swisher EM, Garcia RL, et al. Occult cancer of the fallopian tube in BRCA1 germline mutation carriers at prophylactic oophorectomy: a case for recommending hysterectomy at surgical prophylaxis. Gynecol Oncol. 2001;80:176–80. doi: 10.1006/gyno.2000.6071. [DOI] [PubMed] [Google Scholar]
- 30.Parker WH, Broder MS, Chang E, et al. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the nurses' health study. Obstet Gynecol. 2009;113:1027–37. doi: 10.1097/AOG.0b013e3181a11c64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Partridge E, Kreimer AR, Greenlee RT, et al. Results from four rounds of ovarian cancer screening in a randomized trial. Obstet Gynecol. 2009;113:775–82. doi: 10.1097/AOG.0b013e31819cda77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Permuth-Wey J, Sellers TA. Epidemiology of ovarian cancer. Methods Mol Biol. 2009;472:413–37. doi: 10.1007/978-1-60327-492-0_20. [DOI] [PubMed] [Google Scholar]
- 33.Piek JM, van Diest PJ, Zweemer RP, et al. Dysplastic changes in prophylactically removed Fallopian tubes of women predisposed to developing ovarian cancer. J Pathol. 2001;195:451–6. doi: 10.1002/path.1000. [DOI] [PubMed] [Google Scholar]
- 34.Piek JM, van Diest PJ, Zweemer RP, et al. Tubal ligation and risk of ovarian cancer. Lancet. 2001;358:844. doi: 10.1016/S0140-6736(01)05992-X. [DOI] [PubMed] [Google Scholar]
- 35.Piek JM, Verheijen RH, Kenemans P, et al. BRCA1/2-related ovarian cancers are of tubal origin: a hypothesis. Gynecol Oncol. 2003;90:491. doi: 10.1016/s0090-8258(03)00365-2. [DOI] [PubMed] [Google Scholar]
- 36.Risch HA, Weiss NS, Lyon JL, et al. Events of reproductive life and the incidence of epithelial ovarian cancer. Am J Epidemiol. 1983;117:128–39. doi: 10.1093/oxfordjournals.aje.a113523. [DOI] [PubMed] [Google Scholar]
- 37.Rosenblatt KA, Thomas DB. Reduced risk of ovarian cancer in women with a tubal ligation or hysterectomy. The World Health Organization Collaborative Study of Neoplasia and Steroid Contraceptives. Cancer Epidemiol Biomarkers Prev. 1996;5:933–5. [PubMed] [Google Scholar]
- 38.Salvador S, Gilks B, Kobel M, et al. The fallopian tube: primary site of most pelvic high-grade serous carcinomas. Int J Gynecol Cancer. 2009;19:58–64. doi: 10.1111/IGC.0b013e318199009c. [DOI] [PubMed] [Google Scholar]
- 39.Sedlis A. Primary carcinoma of the fallopian tube. Obstet Gynecol Surv. 1961;16:209–26. doi: 10.1097/00006254-196104000-00022. [DOI] [PubMed] [Google Scholar]
- 40.Seidman JD, Khedmati F. Exploring the histogenesis of ovarian mucinous and transitional cell (Brenner) neoplasms and their relationship with Walthard cell nests: a study of 120 tumors. Arch Pathol Lab Med. 2008;132:1753–60. doi: 10.5858/132.11.1753. [DOI] [PubMed] [Google Scholar]
- 41.Shaw PA, Rouzbahman M, Pizer ES, et al. Candidate serous cancer precursors in fallopian tube epithelium of BRCA1/2 mutation carriers. Mod Pathol. 2009;22:1133–8. doi: 10.1038/modpathol.2009.89. [DOI] [PubMed] [Google Scholar]
- 42.Sherman ME, Lee JS, Burks RT, et al. Histopathologic features of ovaries at increased risk for carcinoma. A case-control analysis. Int J Gynecol Pathol. 1999;18:151–7. doi: 10.1097/00004347-199904000-00009. [DOI] [PubMed] [Google Scholar]
- 43.Shih I. 2009. Unpublished Data.
- 44.Shih I, Kurman RJ. Ovarian tumorigenesis: a proposed model based on morphological and molecular genetic analysis. Am J Pathol. 2004;164:1511–8. doi: 10.1016/s0002-9440(10)63708-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Veras E, Mao TL, Ayhan A, et al. Cystic and adenofibromatous clear cell carcinomas of the ovary: distinctive tumors that differ in their pathogenesis and behavior: a clinicopathologic analysis of 122 cases. Am J Surg Pathol. 2009;33:844–53. doi: 10.1097/PAS.0b013e31819c4271. [DOI] [PubMed] [Google Scholar]
- 46.Wheeler DT, Bell KA, Kurman RJ, et al. Minimal uterine serous carcinoma: diagnosis and clinicopathologic correlation. Am J Surg Pathol. 2000;24:797–806. doi: 10.1097/00000478-200006000-00004. [DOI] [PubMed] [Google Scholar]