Abstract
Animals that depend on smell for communication and survival extract multiple pieces of information from a single complex odor. Mice can collect information on sex, genotype, health and dietary status from scent marks of urine, a stimulus made up of hundreds of molecules. This ability is all the more remarkable considering that natural odors are encountered in varying olfactory backgrounds, so the olfactory system must provide some mechanism for extracting the most relevant information. Here we discuss recent data indicating that the readout of olfactory input by mitral cells in the olfactory bulb can be modified by behavioral context. We speculate that the olfactory cortex plays a key role in tuning the readout of olfactory information from the olfactory bulb.
The means by which odorous volatile molecules detected in the periphery are transformed into an odor object representation in the cortex remains to be fully understood. According to the combinatorial coding hypothesis1-3, odors detected in the nose are deconstructed into molecular features represented in a topographical pattern of glomerular activity called an “odor map” (Box 1). This representation is then processed and ultimately reconstructed into an odor “object” in the olfactory cortex4,5. While this hypothesis for feedforward flow of information is often used to interpret experimental findings, it does not necessarily incorporate the influence of odor associations or meaning on odor signal processing. An alternative hypothesis presented by Kay and Sherman6 postulated that the olfactory bulb (OB) acts as a transiently modifiable (active) filter that can shape odor representations at the level of olfactory bulb output. The olfactory bulb/cortex circuit does not simply deconstruct, sharpen, and reconstruct complex odors. Instead, the circuitry in the olfactory bulb could extract the most relevant odor information, while filtering out parts of the signal that are not as important for the animal's current needs. Ramón y Cajal predicted more than a century ago7 that the process of feature extraction by the olfactory bulb is modulated by what he termed centrifugal fibers originating in olfactory cortex and neuromodulatory centers in the brain (Fig. 1). We speculate that odor associations or meaning affect the feedback circuit to the olfactory bulb from the olfactory cortex (Fig. 2A). By incorporating meaning into the feedback circuit, the cortex can then dynamically tune the readout of the odor map by principal neurons of the bulb (tufted, T, and mitral, MT, cells) in behaviorally relevant ways.
Box 1. Processing in the Olfactory Bulb.
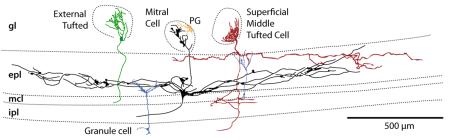 Each olfactory sensory neuron (OSN) in the main olfactory epithelium expresses one of ~1000 odorant receptors. Axons from OSNs synapse onto their second order targets in discrete ovoid neuropil called glomeruli. Each glomerulus receives only axons that express the same odorant receptor51, so the pattern of glomerular activation on the surface of the bulb, called an odor map, is unique for each odor. Each glomerulus and its associated cells can be considered a functional “column” since the input to these groups of cells is derived from a single odorant receptor51,52. The principal output neurons of the glomerulus, the tufted (T) and mitral (MT) cells, project to only one glomerulus (in mammals). The external tufted (ET) cells receive direct monosynaptic input from OSNs and drive synchronous activity in other cells innervating the glomerulus (including MT cells). In slices, these cells respond to electrical stimulation at a lower threshold and with a faster onset compared to MT cells53-55. The ET and MT cells could convey different information to olfactory cortex to be used for different purposes, including feedback to the olfactory bulb.
Each olfactory sensory neuron (OSN) in the main olfactory epithelium expresses one of ~1000 odorant receptors. Axons from OSNs synapse onto their second order targets in discrete ovoid neuropil called glomeruli. Each glomerulus receives only axons that express the same odorant receptor51, so the pattern of glomerular activation on the surface of the bulb, called an odor map, is unique for each odor. Each glomerulus and its associated cells can be considered a functional “column” since the input to these groups of cells is derived from a single odorant receptor51,52. The principal output neurons of the glomerulus, the tufted (T) and mitral (MT) cells, project to only one glomerulus (in mammals). The external tufted (ET) cells receive direct monosynaptic input from OSNs and drive synchronous activity in other cells innervating the glomerulus (including MT cells). In slices, these cells respond to electrical stimulation at a lower threshold and with a faster onset compared to MT cells53-55. The ET and MT cells could convey different information to olfactory cortex to be used for different purposes, including feedback to the olfactory bulb.
Activity in each glomerular column is regulated by inhibitory interneurons, the periglomerular (PG) and granule cells (GCs). PG cells influence both intra- and inter-glomerular modulation of column activation12 while granule cells extend dendrites to the EPL and make reciprocal dendrodendritic synapses on the lengthy lateral dendrites of the MT and superficial middle tufted cells (also see Fig. 2).
Dynamically inhibiting columns by activating GCs may create meaningful spatial and temporal patterns56 or synchronization between columns57 thereby encoding a stronger signal for downstream targets34,35. There is some evidence that proximal glomeruli are able to laterally inhibit each other and synchronize58,59. However, a recent survey of MT cell responsiveness to stimulation of multiple glomeruli in the dorsal olfactory bulb favors sparse glomerular column inputs to MT cells60, consistent with the sparse columnar connections seen in viral tracings52.
The figure shows neural elements of the olfactory bulb (using nomenclature from Ezeh and co-workers61) rendered from published data with permission62-64. gl, glomerular layer; epl, external plexiform layer; mcl, mitral cell layer; ipl, internal plexiform layer.
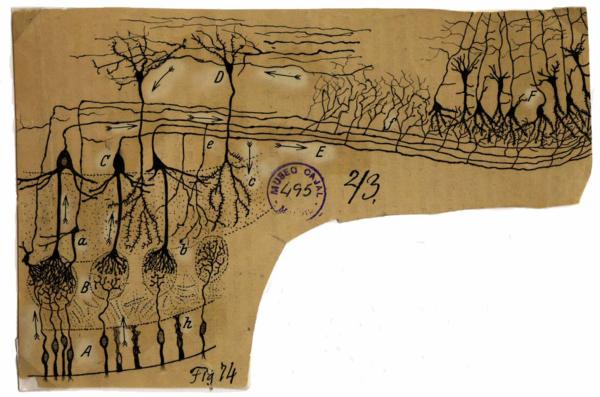
Figure 1.
Drawing by Ramón y Cajal showing the olfactory system from olfactory epithelium to olfactory cortex. He labeled the olfactory sensory neurons (A) and sustentacular cells (h) in the olfactory epithelium; glomeruli (B), mitral cells (C), tufted cells (a), granule cells (D), the lateral olfactory tract (E) in the olfactory bulb; and the olfactory cortex (F). Note the arrows that he drew implying the flow of information through the circuit. The fibers at the top of the drawing (what he called centrifugal fibers) have arrows that imply information flow in the direction of the olfactory bulb. These centrifugal fibers are now known to be centrifugal with respect to the olfactory cortex and neuromodulatory centers where they originate. Reproduced with permission from the original at the Cajal Institute CSIC, Madrid.
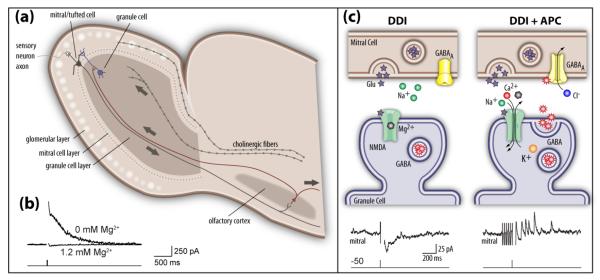
Figure 2.
Near coincident activation of centrifugal fibers from olfactory cortex and depolarization of MT cells elicits enhanced dendrodendritic inhibition. (a). Diagram showing the arrangement of olfactory bulb and olfactory cortex where MT cells send information to cortex where principal neurons in turn feed back onto granule cells. In addition to centrifugal collaterals from olfactory cortex that innervate the proximal dendrites of granule cells, the diagram shows centrifugal feedback from one of the neuromodulatory brain areas (the cholinergic basal forebrain). Note that the lateral dendrites of the MT cells contact the distal dendrites of the granule cells where they form the reciprocal synapse shown schematically in figure (c). (b). Data from Balu and co-workers45 show that removal of Mg2+ from the extracellular solution releases block of the NMDA receptors thereby allowing large dendrodendritic inhibitory currents (outward currents) to a 20 mV depolarization of the mitral cell. These dendrodendritic responses were blocked by the NMDAR blocker D-APV. (c). Top panels: Schematic representation of the function of reciprocal synapses where MT cells release glutamate to excite distal dendrites of granule cells. The bottom panels display data from Balu and co-workers45 showing that near coincident mitral cell depolarization (dendrodendritic inhibition-DDI) and anterior piriform cortex (APC) stimulation evokes outward inhibitory currents in mitral cells. Example responses to voltage-clamp depolarization alone (DDI, to +20 mV, 2 ms duration; left) and both intracellular depolarization and APC stimulation (DDI + APC, right) are shown. The diagram at the top shows that APC stimulation releases Mg2+ block of NMDA receptors in granule cells thereby allowing synaptic activation of the granule cell distal synapse and release of GABA onto the mitral cell, in turn eliciting outward inhibitory currents in the mitral cell.
Lateral Interactions Could Allow Flexible Readout of the Fragmented Chemotopic Odor Map
Sensory systems must optimize the processing of input to allow timely and efficient extraction of information. One elegant solution to this problem is to organize information into a spatial map. In vision, for instance, the cornea focuses a spatial representation of an image onto the retinal surface in the eye, and in hearing the representation of sound is organized as a frequency map in cochlea. Odor maps also appear to have a gross chemotopic arrangement. For example, carboxylic acids, methyl and ethyl esters stimulate a dorsal anterior domain in the olfactory bulb, while aromatic compounds stimulate a dorsal posterior domain8,9.
Nevertheless, a detailed comparison of the chemotopic odor map with other sensory maps reveals fundamental differences. For one thing, auditory and visual systems have clear relationships along one or two dimensional space between neighboring neural elements and the stimuli being processed (e.g. the frequency scale or the visual field). In these systems, dense short range interactions play an important role in the processing of signals. Objects analyzed by the auditory and visual systems can be very complex, but local processing of the stimulus in one or two dimensions respectively is an advantageous first step in analysis of the sensory input. In contrast, each chemical bond of a molecule is a dimension in the chemical structure, so there is no two-dimensional arrangement that would allow a glomerulus to have all “similar” glomeruli nearby. Indeed mathematical analysis shows that collapsing multidimensional maps onto two dimensions inevitably fragments contiguous representation of an object10. Accordingly the map is fragmented as adjacent glomeruli are often not related in terms of stimulus tuning and frequently respond to structurally disparate sets of odors3,11. In fact, a recent functional survey of approximately 30 unique glomeruli on the dorsal surface of the bulb in response to a large bank of odors revealed only a weak correlation between response and interglomerular distance11. Thus, the chemotopic structure of the map is “loosely organized”12 likely reflecting the fact that behaviorally significant odors are complex mixtures of molecules that are often unrelated to previously encountered odors. For example, even if two adjacent glomeruli are functionally similar, the optimal processing of an incoming signal may abruptly change if a novel and behaviorally relevant odor appears that contains the odor feature identified by one of the glomeruli but not the other. As such, a major problem for the olfactory system is the inherent unpredictability of potentially relevant stimuli. The problem lies not only in the large number of potential molecular features but also in their near endless combinatorial possibilities13.
Given that not all relationships between molecular features are represented by neighboring placement of glomeruli in a two dimensional map, it is not surprising that processing in the olfactory bulb takes place through long distance interactions mediated by lateral dendrites of MT cells or by long range inter-glomerular interactions (Box 1). These lateral interactions could provide the flexibility needed for the processing of novel stimuli whose molecular features are represented by a different subset of distant glomeruli. Modulation of lateral interactions between glomeruli12 or through MT cell lateral dendrites14 may provide a mechanism for amplifying signals from some activated glomeruli and suppressing others. The combination of a loose chemotopic map and extensive lateral interactions provides a flexible circuit that could easily be modified through feedback from cortical or modulatory areas to allow optimal extraction of information from distinct subsets of glomeruli in different behavioral contexts.
Mitral Cell Odor Responses Are Influenced by Learning, Behavior, and Context
Evidence for top down regulation of processing in the olfactory bulb was first provided by Kerr and Hagbarth15 who showed that excitation of centrifugal fibers enhances the local field potential (LFP) activity of the olfactory bulb. The LFP—first described by Adrian— is a field potential recorded extracellularly in the olfactory bulb that reflects the oscillatory synchronous activity of neurons aligned on the average in the same direction16,17. Since 1955, other groups have shown that non-olfactory stimuli and olfactory learning tasks also alter the odor-evoked LFP signal recorded in the bulb18-21.
Substantial work has conclusively shown that there is a change in MT cell population responses to odors due to learning. Arguably, the most comprehensive work to date focused on early olfactory learning (EOL). These studies demonstrated the involvement of noradrenaline in a rodent's preference learning of a conditioned odor when paired with an unconditioned stimulus, such as stroking22. Moreover, Wilson and co-workers found an increased fraction of population MT cell responses that were suppressed in response to the conditioned odor23. Importantly, the changes in MT cell activity in EOL do not reflect the valence of the odor; conditioning with either aversive or appetitive unconditioned stimulus results in similar changes.
While studies in adult animals are not as comprehensive, there are many studies that show changes in population MT activity due to learning. For example, Keverne and co-workers presented data suggesting that MT cells in ewes respond to lamb odors more strongly after parturition24. In elegant multi unit recordings in awake, behaving rodents, Pager showed that MT cells respond more strongly to odors associated with food in hungry rats25, and Moulton showed that multiunit M/T cell activity changed during learning in an odor discrimination task in rabbits26
A key question is how individual MT cells change responsiveness during olfactory learning. The pioneering work of Kay and Laurent27 described changes in MT cell odor responses during learning in an odor discrimination task, but the sparseness of the responses in the awake, behaving animal28 limited the strength of their conclusion. In a recent study, Fuentes and co-workers showed that the response of MT cells to odors differs markedly in terms of the percent of cells responding and whether the responses are excitatory or inhibitory depending on the behavioral task29. Finally, a recent study of MT cell activity during associative learning showed that responses of MT cells to odors change dramatically during the course of an odor discrimination task (Fig. 3)30. While prescreening ensured a large number of MT cells (~20%) was sensitive to the presented odors, most cells did not initially respond differentially to the two odors presented in the discrimination task. Yet, as the animal learned to discriminate between the two odors, MT cells started to respond differently to the rewarded and unrewarded odors. Divergence in the response to the two odors was transient, subsiding by the end of the learning session. These experiments demonstrated a profound change in MT cell responsiveness to odor during learning.
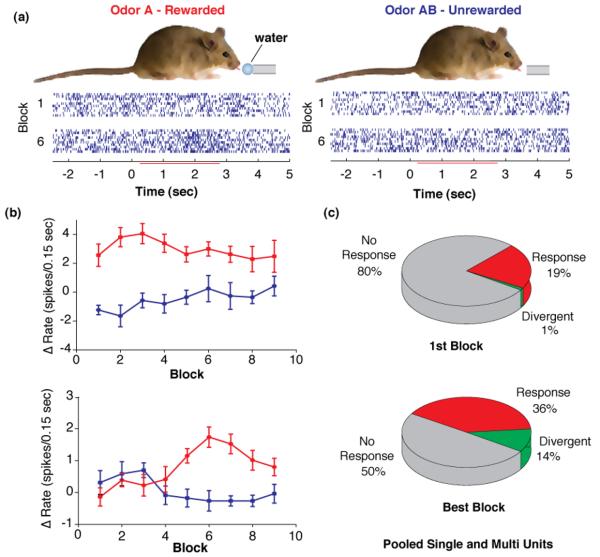
Figure 3.
Divergence of MT cell responses during learning to discriminate between two novel odors. The data reproduced from Doucette and Restrepo30 show that MT cells undergo a profound change in odor responsiveness during a session where animals learn to associate one odor with reward (rewarded) and another with no reward (unrewarded). (a). A thirsty mouse learns to associate the reinforced odor with a water reward and the unreinforced odor with no reward. The mouse must lick on a metal tube for two seconds when presented with the rewarded odor to obtain the water reward. Rasters below the mouse show the responsiveness of a suspected mitral cell to the reinforced odor and unreinforced odor during the first block of 20 trials (10 reinforced and 10 unreinforced) and for block 6 (trials 100 to 120). During the first block the mouse responds randomly to the two odors while in block 6 the mouse is responding correctly ~80% of the time. (b). Examples of changes in odor responsiveness throughout the learning session. Red denotes rewarded odor and blue denotes unrewarded odor. The ordinate shows the change in the number of spikes fired in a 0.15 sec interval elicited by addition of odor. The top panel shows odor responsiveness of a unit that responded differentially to the two odors from the onset of the session. This was rare (observed in 2 of 660 units). The bottom panel shows odor responsiveness of the cell whose responses are shown in (a). This cell developed a transient differential response to the two odors. This is representative of 93 of 660 units. (c). Pie charts showing the percent of units that responded to odors (red), and those that responded differently to the two odors (green). The first block is the first 20 trials in the session and the best block is the 20 trials during the block where the unit displayed the largest difference in odor-evoked firing between reinforced and unreinforced odors.
There are several possible explanations for the changes in MT cell odor responses described above. First, input to the olfactory bulb could be modified by changes in sniffing (Box 2). Active sniffing is a way for animals to directly control the input to the olfactory system, and fast sniffing has been shown to produce a different odor map compared to slow sniffing31. The intrinsic bulb circuitry could also contribute to changes in MT cell responses through lateral interactions that could amplify, attenuate, or increase the contrast between activated glomeruli12,14. Finally, central modulation of the intrinsic OB network could elicit these behaviorally relevant changes. Below we discuss changes in processing that occur intrinsically to the bulb, and those that are triggered by top down regulation.
Box 2. The role of sniffing in olfactory processing.
The activation of olfactory sensory areas is intimately linked to respiration (breathing and/or sniffing). MT/T cells fire bursts of action potentials locked to respiration in both anesthetized and, to a lesser degree, awake animals65. Similarly, oscillating field potentials in the olfactory bulb appear to be driven by input to olfactory sensory neurons65. These respiration-driven oscillations can promote synchrony within a given cluster of MT/T cells corresponding to a single glomerulus66,67 and have important implications for olfactory coding68 (see Box 1). Whereas studies from anesthetized animals and tissue slices have provided most of the evidence for tight oscillatory coupling between olfactory bulb activity and respiration, recordings from MT/T cells in awake animals indicate a higher baseline firing rate with a variable degree of phase-locking to respiratory oscillations27,28. In addition, sniff frequencies above 4 Hz dramatically alter both olfactory nerve input and glomerular activation patterns31 and decouple MT/T cell firing from respiration27 in rats. Therefore, changes in respiration frequency cause dramatic changes in both input to the olfactory bulb and the response of cells in the circuit.
Animals commonly display fast sniffing during many natural behaviors69, and often alternate between slow respiration and fast sniffing. By alternating their respiratory patterns, animals potentially extract different information from the same odorants. For example, the slow respiratory oscillations could promote synchronous firing, whereas fast sniffing could favor tonic input. In this fashion, the active modulation of sniff frequency represents yet another potential mechanism for the dynamic control of olfactory bulb circuit processing.
Local Processing in the Olfactory Bulb is Intrinsically Dynamic
As discussed in Box 1, the spatio-temporal information contained in odor maps is processed by the interplay between the principal neurons of the bulb (tufted, T, and mitral, MT, cells) and interneurons in the glomerular and external plexiform layers (EPL). This interplay gives rise to lateral inhibition12,14, which could synchronize firing of MT cells. The synchronization of MT cells through their reciprocal connections to granule cells has been demonstrated in OB slices32, and synchronized firing of MT cells has also been observed in anesthetized animals33. Local OB circuits can dynamically regulate the synchronization among MT cells, and synchronized firing of output cells leads to a more robust activation of principal cells in the olfactory cortex34,35. Thus, a change in the strength of lateral inhibition would be expected to affect the degree of synchronization, and hence the reliability of information transfer to the olfactory cortex.
Factors intrinsic to the bulb, such as a change in the basal level of MT cell excitation, can cause changes in OB interneuron circuits modifying MT cell activity. Arevian and co-workers studied how the magnitude of lateral inhibition through the granule cell circuit by neighboring MT cells depended on the basal firing rate36 in olfactory bulb slices. They found that the magnitude of granule cell lateral inhibition is entirely dependent on the activity level of the MT cell. The magnitude of lateral inhibition ranges parabolically from virtually no inhibition at low MT cell basal firing rates, to a maximum inhibition at intermediate firing rates then back down to no inhibition at high levels of activity. This trend results in a tendency for the circuit to optimize contrast among the active MT cells, an action that is advantageous in a system where the relationship between neighboring glomeruli may change unexpectedly.
Animals can directly regulate input to the olfactory bulb by modifying their sniffing, so alterations in sniffing patterns could underlie a change in the basal level of OB activation. Sniffing is affected by behavioral context37,38, although it is not known whether changes in sniffing strategies (such as increased sniff frequency) affect information transfer at the level of the MT cells. Indeed, a recent study concluded that changes in sniffing do not influence low-level processing of the neural process underlying odor perception39. However, the differences in MT cell odor responses depending on behavioral context reported by Fuentes et al 29 could be due to differences in sniffing patterns between the two tasks.
While intrinsic bulb circuitry and modulation of sniffing can both alter MT cell odor responses, these mechanisms are unlikely to completely account for learning-induced changes in MT cell firing. Sniffing controls input to the entire olfactory bulb, so it can cause changes on a global scale, such as an overall increase in excitation or enhanced lateral inhibition31. But it would be more difficult for sniffing to account for the differential firing patterns of MT cells observed during learning30, especially since trials are shuffled randomly and animals don't know when they start sniffing whether the trial will be rewarded or not (Fig 3). Unfortunately, no study of MT single unit odor responses during learning27-30 included recordings of sniffing patterns, so this remains an open question. Similarly, the intrinsic bulb circuitry, while optimally suited for contrast enhancement, would require input from a higher brain region to modify MT cell output in a behaviorally relevant way.
Mechanisms for Top Down Regulation of Mitral Cell Responsiveness
The influence of neuromodulatory systems from adrenergic, cholinergic and serotonergic fibers on MT cell responsiveness is fairly well established40. In a recent study, Shea and co-workers found that odor-evoked increases in MT cell firing are suppressed in anesthetized mice when odor stimulation is paired with activation of the locus coeruleus (LC), the brainstem nucleus that houses the adrenergic neurons that innervate the olfactory bulb41. In another study, bulbar acetylcholine was shown to enhance learning to discriminate structurally related odors, and the effects on learning were correlated with cholinergic sharpening of the odorant receptive field of MT cells42. It also stands to reason that divergent firing of cells in neuromodulatory centers between rewarded and unrewarded odors43 would provide differential modulation and therefore contribute to a differential output by the olfactory bulb. Even neuromodulatory systems that are thought to affect the entire olfactory bulb simultaneously, such as the adrenergic system where all locus coreuleous neurons fire similarly 43, could result in modulation of subsets of glomerular columns through mechanisms such as nearly coincident odor responses and (slightly delayed) neuromodulatory input. Therefore, although the precise mechanisms remain unknown, intrinsic processing of contextual information conveyed by the neuromodulatory systems undoubtedly alters processing of odor information in the olfactory bulb. It is also important to note that sniffing is controlled by a subcortical motor control circuit that receives input from neuromodulatory systems (e.g. cholinergic and serotonergic)44.
Alternately, or in conjunction with neuromodulatory input, the cortical centrifugal input to the bulb could gate MT cells on or off as in thalamocortical circuits6. Indeed, increasing evidence implicates the cortex in feedback regulation of the olfactory bulb circuit. Balu and co-workers45 found that the stimulation of cortical centrifugal fibers relieves the tonic Mg2+ block of NMDA receptors at the MT/granule cell dendrodendritic synapses located at the distal end of the granule cell dendrites (Fig. 2). These experiments effectively demonstrate that the centrifugal fibers originating from olfactory cortex gate dendrodendritic inhibition onto MT cells. The massive cortical centrifugal innervation of the olfactory bulb through the anterior commissure terminates mainly, but not exclusively, on the proximal synapses of granule cell dendrites46.
Recent intriguing, albeit inconclusive, data suggests that unlike neuromodulatory fibers in the olfactory bulb, cortical centrifugal fibers do not terminate across all areas in the granule cell layer, but rather in small patches, presumably on individual glomerular columns47. If such a situation is indeed the case, then the cortical centrifugal fibers could gate the response of different glomerular columns. Therefore, changes in the responsiveness of MT cells to odors during a learning task could be mediated by centrifugal feedback from the olfactory cortex. Such a feedback mechanism would be particularly effective in an odor discrimination task for MCs that send their primary dendrite to a single glomerulus that is activated by two similar odors . Cortical feedback could specifically relieve inhibition to these cells for one odor, but not the other, thereby increasing the difference between the MT cell readout of the odor map for the two odors. Alternatively, this same type of feedback could increase the differential response of cells that initially responded differently to the two odors, maximizing a difference that was always present.
Finally, recent evidence in olfactory bulb slices indicates that the input from cortical centrifugal fibers through proximal synapses in granule cells undergoes long-term potentiation (LTP, although there is some controversy on whether LTP occurs in mature vs. newly incorporated granule cells)48,49. This indicates that cortical centrifugal modulation of MT cell responsiveness can flip a switch on (and presumably off) for sustained periods of time.
The olfactory cortex receives information not only from the olfactory bulb, but also from other areas of the brain, and performs associative processing of the signal50. As such, it is conceivable that the olfactory cortex does not passively reconstruct the olfactory signal into an odor object. Rather, the olfactory cortex could serve as an active player that tunes the processing of glomerular columns in the olfactory bulb to optimize the readout of the odor-evoked olfactory glomerular map. In such a scenario, the glomerular layer of the olfactory bulb would be analogous to an orchestra whose instruments (the glomeruli) are being activated by odor features, and the olfactory cortex and/or neuromodulatory systems permit attention to be drawn to discrete voices or ensembles within the orchestra. We can hear a single violin, concentrate on the cello section, or listen to the complete orchestra. Analogously, an odor object is like one of these timbres that can be actively filtered from the orchestra. In our opinion, cortical modulation of the readout of the odor map has the potential to allow exquisite context-dependent exploration of odor space.
Statement from Ramón y Cajal7 to be included as a quote.
(Translated by Diego Restrepo)
“It is fitting, in the current state of science, to conjecture that through them [thick centrifugal fibers] the sphenoidal region of the brain [olfactory cortex], or another undetermined cortical territory, sends nervous currents to the bulb, currents that flow primarily through the granules [granule cells] and flows into the cells with tufts [mitral and tufted cells]. These centrifugal impulses that Duval and Manoumelian ingeniously used to formulate their hypothesis of the nervi nervorum, could produce in the glomeruli some action indispensable for the normal interplay of the mechanism of transmission.”
Acknowledgements
We would like to thank Drs. Peter Brunjes, Tom Finger, Kurt Illig, Laura López-Mascaraque, Nathan Schoppa, Michael Shipley, John Scott and Ben Strowbridge and Mr. David Gire for enlightening discussion.
Footnotes
Disclosure Statement
The authors declare no conflict of interest
Reference List
- 1.Axel R. Scents and sensibility: a molecular logic of olfactory perception (Nobel lecture) Angew. Chem. Int. Ed Engl. 2005;44:6110–6127. doi: 10.1002/anie.200501726. [DOI] [PubMed] [Google Scholar]
- 2.Malnic B, et al. Combinatorial receptor codes for odors. Cell. 1999;96:713–723. doi: 10.1016/s0092-8674(00)80581-4. [DOI] [PubMed] [Google Scholar]
- 3.Friedrich RW, Korsching SI. Combinatorial and chemotopic odorant coding in the zebrafish olfactory bulb visualized by optical imaging. Neuron. 1997;18:737–752. doi: 10.1016/s0896-6273(00)80314-1. [DOI] [PubMed] [Google Scholar]
- 4.Yaksi E, et al. Transformation of odor representations in target areas of the olfactory bulb. Nat. Neurosci. 2009;12:474–482. doi: 10.1038/nn.2288. [DOI] [PubMed] [Google Scholar]
- 5.Zou Z, Buck LB. Combinatorial effects of odorant mixes in olfactory cortex. Science. 2006;311:1477–1481. doi: 10.1126/science.1124755. [DOI] [PubMed] [Google Scholar]
- 6.Kay LM, Sherman SM. An argument for an olfactory thalamus. Trends Neurosci. 2006;30:47–53. doi: 10.1016/j.tins.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Ramón y Cajal S. La textura del sistema nervioso del hombre y los vertebrados, Moya. 1904 [Google Scholar]
- 8.Johnson BA, Leon M. Chemotopic odorant coding in a mammalian olfactory system. J. Comp Neurol. 2007;503:1–34. doi: 10.1002/cne.21396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mori K, et al. Maps of odorant molecular features in the Mammalian olfactory bulb. Physiol Rev. 2006;86:409–433. doi: 10.1152/physrev.00021.2005. [DOI] [PubMed] [Google Scholar]
- 10.Cleland TA, Sethupathy P. Non-topographical contrast enhancement in the olfactory bulb. BMC. Neurosci. 2006;7:7. doi: 10.1186/1471-2202-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soucy ER, et al. Precision and diversity in an odor map on the olfactory bulb. Nat. Neurosci. 2009;12:210–220. doi: 10.1038/nn.2262. [DOI] [PubMed] [Google Scholar]
- 12.Wachowiak M, Shipley MT. Coding and synaptic processing of sensory information in the glomerular layer of the olfactory bulb. Semin. Cell Dev. Biol. 2006;17:411–423. doi: 10.1016/j.semcdb.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 13.Hudson R. From molecule to mind: the role of experience in shaping olfactory function. J. Comp Physiol [A] 1999;185:297–304. doi: 10.1007/s003590050390. [DOI] [PubMed] [Google Scholar]
- 14.Mori K, et al. The olfactory bulb: coding and processing of odor molecule information. Science. 1999;286:711–715. doi: 10.1126/science.286.5440.711. [DOI] [PubMed] [Google Scholar]
- 15.Kerr DI, Hagbarth KE. An investigation of olfactory centrifugal fiber system. J. Neurophysiol. 1955;18:362–374. doi: 10.1152/jn.1955.18.4.362. [DOI] [PubMed] [Google Scholar]
- 16.Kay LM, et al. Olfactory oscillations: the what, how and what for. Trends Neurosci. 2009 doi: 10.1016/j.tins.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adrian ED. The electrical activity of the mammalian olfactory bulb. Electroencephalogr. Clin. Neurophysiol. 1950;2:377–388. doi: 10.1016/0013-4694(50)90075-7. [DOI] [PubMed] [Google Scholar]
- 18.Di Prisco GV, Freeman WJ. Odor-related bulbar EEG spatial pattern analysis during appetitive conditioning in rabbits. Behav. Neurosci. 1985;99:964–978. doi: 10.1037//0735-7044.99.5.964. [DOI] [PubMed] [Google Scholar]
- 19.Gray CM, et al. Chemical dependencies of learning in the rabbit olfactory bulb: acquisition of the transient spatial pattern change depends on norepinephrine. Behav. Neurosci. 1986;100:585–596. doi: 10.1037//0735-7044.100.4.585. [DOI] [PubMed] [Google Scholar]
- 20.Martin C, et al. Learning modulation of odor-induced oscillatory responses in the rat olfactory bulb: a correlate of odor recognition? J. Neurosci. 2004;24:389–397. doi: 10.1523/JNEUROSCI.3433-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ravel N, et al. Olfactory learning modifies the expression of odour-induced oscillatory responses in the gamma (60-90 Hz) and beta (15-40 Hz) bands in the rat olfactory bulb. Eur. J. Neurosci. 2003;17:350–358. doi: 10.1046/j.1460-9568.2003.02445.x. [DOI] [PubMed] [Google Scholar]
- 22.Wilson DA, et al. Norepinephrine and posttraining memory consolidation in neonatal rats. Behav. Neurosci. 1994;108:1053–1058. doi: 10.1037//0735-7044.108.6.1053. [DOI] [PubMed] [Google Scholar]
- 23.Wilson DA, Leon M. Spatial patterns of olfactory bulb single-unit responses to learned olfactory cues in young rats. J. Neurophysiol. 1988;59:1770–1782. doi: 10.1152/jn.1988.59.6.1770. [DOI] [PubMed] [Google Scholar]
- 24.Kendrick KM, et al. Changes in the sensory processing of olfactory signals induced by birth in sheep. Science. 1992;256:833–836. doi: 10.1126/science.1589766. [DOI] [PubMed] [Google Scholar]
- 25.Pager J. A selective modulation of the olfactory bulb electrical activity in relation to the learning of palatability in hungry and satiated rats. Physiol Behav. 1974;12:189–195. doi: 10.1016/0031-9384(74)90172-3. [DOI] [PubMed] [Google Scholar]
- 26.Goldberg SJ, Moulton DG. Olfactory bulb responses telemetered during an odor discrimination task in rats. Exp. Neurol. 1987;96:430–442. doi: 10.1016/0014-4886(87)90060-4. [DOI] [PubMed] [Google Scholar]
- 27.Kay LM, Laurent G. Odor- and context-dependent modulation of mitral cell activity in behaving rats. Nat. Neurosci. 1999;2:1003–1009. doi: 10.1038/14801. [DOI] [PubMed] [Google Scholar]
- 28.Rinberg D, et al. Sparse odor coding in awake behaving mice. J. Neurosci. 2006;26:8857–8865. doi: 10.1523/JNEUROSCI.0884-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fuentes RA, et al. Neuronal activity of mitral-tufted cells in awake rats during passive and active odorant stimulation. J. Neurophysiol. 2008;100:422–430. doi: 10.1152/jn.00095.2008. [DOI] [PubMed] [Google Scholar]
- 30.Doucette W, Restrepo D. Profound context-dependent plasticity of mitral cell responses in olfactory bulb. PLoS. Biol. 2008;6:e258. doi: 10.1371/journal.pbio.0060258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verhagen JV, et al. Sniffing controls an adaptive filter of sensory input to the olfactory bulb. Nat. Neurosci. 2007;10:631–639. doi: 10.1038/nn1892. [DOI] [PubMed] [Google Scholar]
- 32.Schoppa NE. Synchronization of olfactory bulb mitral cells by precisely timed inhibitory inputs. Neuron. 2006;49:271–283. doi: 10.1016/j.neuron.2005.11.038. [DOI] [PubMed] [Google Scholar]
- 33.Kashiwadani H, et al. Synchronized oscillatory discharges of mitral/tufted cells with different molecular receptive ranges in the rabbit olfactory bulb. J. Neurophysiol. 1999;82:1786–1792. doi: 10.1152/jn.1999.82.4.1786. [DOI] [PubMed] [Google Scholar]
- 34.Franks KM, Isaacson JS. Strong single-fiber sensory inputs to olfactory cortex: implications for olfactory coding. Neuron. 2006;49:357–363. doi: 10.1016/j.neuron.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 35.Luna VM, Schoppa NE. GABAergic circuits control input-spike coupling in the piriform cortex. J. Neurosci. 2008;28:8851–8859. doi: 10.1523/JNEUROSCI.2385-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arevian AC, et al. Activity-dependent gating of lateral inhibition in the mouse olfactory bulb. Nat. Neurosci. 2008;11:80–87. doi: 10.1038/nn2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Youngentob SL, et al. A quantitative analysis of sniffing strategies in rats performing odor detection tasks. Physiol Behav. 1987;41:59–69. doi: 10.1016/0031-9384(87)90131-4. [DOI] [PubMed] [Google Scholar]
- 38.Wesson DW, et al. Sniffing behavior of mice during performance in odor-guided tasks. Chem. Senses. 2008;33:581–596. doi: 10.1093/chemse/bjn029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wesson DW, et al. Why sniff fast? The relationship between sniff frequency, odor discrimination, and receptor neuron activation in the rat. J. Neurophysiol. 2009;101:1089–1102. doi: 10.1152/jn.90981.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mandairon N, Linster C. Odor perception and olfactory bulb plasticity in adult mammals. J. Neurophysiol. 2009 doi: 10.1152/jn.00076.2009. [DOI] [PubMed] [Google Scholar]
- 41.Shea SD, et al. Noradrenergic induction of odor-specific neural habituation and olfactory memories. J. Neurosci. 2008;28:10711–10719. doi: 10.1523/JNEUROSCI.3853-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chaudhury D, et al. Bulbar acetylcholine enhances neural and perceptual odor discrimination. J. Neurosci. 2009;29:52–60. doi: 10.1523/JNEUROSCI.4036-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bouret S, Sara SJ. Reward expectation, orientation of attention and locus coeruleus-medial frontal cortex interplay during learning. Eur. J. Neurosci. 2004;20:791–802. doi: 10.1111/j.1460-9568.2004.03526.x. [DOI] [PubMed] [Google Scholar]
- 44.Bianchi AL, et al. Central control of breathing in mammals: neuronal circuitry, membrane properties, and neurotransmitters. Physiol Rev. 1995;75:1–45. doi: 10.1152/physrev.1995.75.1.1. [DOI] [PubMed] [Google Scholar]
- 45.Balu R, et al. Multiple modes of synaptic excitation of olfactory bulb granule cells. J. Neurosci. 2007;27:5621–5632. doi: 10.1523/JNEUROSCI.4630-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Olmos J, et al. The afferent connections of the main and the accessory olfactory bulb formations in the rat: an experimental HRP-study. J. Comp Neurol. 1978;181:213–244. doi: 10.1002/cne.901810202. [DOI] [PubMed] [Google Scholar]
- 47.Matsutani S, Yamamoto N. Centrifugal innervation of the mammalian olfactory bulb. Anat. Sci. Int. 2008;83:218–227. doi: 10.1111/j.1447-073X.2007.00223.x. [DOI] [PubMed] [Google Scholar]
- 48.Gao Y, Strowbridge BW. Long-term plasticity of excitatory inputs to granule cells in the rat olfactory bulb. Nat. Neurosci. 2009;12:731–733. doi: 10.1038/nn.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nissant A, et al. Adult neurogenesis promotes synaptic plasticity in the olfactory bulb. Nat. Neurosci. 2009;12:728–730. doi: 10.1038/nn.2298. [DOI] [PubMed] [Google Scholar]
- 50.Roesch MR, et al. Associative Encoding in Anterior Piriform Cortex versus Orbitofrontal Cortex during Odor Discrimination and Reversal Learning. Cereb. Cortex. 2006 doi: 10.1093/cercor/bhk009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shepherd GM, et al. olfactory bulb. In: Shepherd GM, editor. The Synaptic Organization of the Brain. 5th edn Oxford University Press; 2004. pp. 159–204. [Google Scholar]
- 52.Willhite DC, et al. Viral tracing identifies distributed columnar organization in the olfactory bulb. Proc. Natl. Acad. Sci. U. S. A. 2006;103:12592–12597. doi: 10.1073/pnas.0602032103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De Saint Jan D, et al. External tufted cells drive the output of olfactory bulb glomeruli. J. Neurosci. 2009;29:2043–2052. doi: 10.1523/JNEUROSCI.5317-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nagayama S, et al. Mitral and tufted cells differ in the decoding manner of odor maps in the rat olfactory bulb. J. Neurophysiol. 2004;91:2532–2540. doi: 10.1152/jn.01266.2003. [DOI] [PubMed] [Google Scholar]
- 55.Hayar A, et al. External tufted cells: a major excitatory element that coordinates glomerular activity. J. Neurosci. 2004;24:6676–6685. doi: 10.1523/JNEUROSCI.1367-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spors H, et al. Temporal dynamics and latency patterns of receptor neuron input to the olfactory bulb. J. Neurosci. 2006;26:1247–1259. doi: 10.1523/JNEUROSCI.3100-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schoppa NE, Urban NN. Dendritic processing within olfactory bulb circuits. Trends Neurosci. 2003;26:501–506. doi: 10.1016/S0166-2236(03)00228-5. [DOI] [PubMed] [Google Scholar]
- 58.Luo M, Katz LC. Response correlation maps of neurons in the mammalian olfactory bulb. Neuron. 2001;32:1165–1179. doi: 10.1016/s0896-6273(01)00537-2. [DOI] [PubMed] [Google Scholar]
- 59.Yokoi M, et al. Refinement of odor molecule tuning by dendrodendritic synaptic inhibition in the olfactory bulb. Proc. Natl. Acad. Sci. U. S. A. 1995;92:3371–3375. doi: 10.1073/pnas.92.8.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fantana AL, et al. Rat olfactory bulb mitral cells receive sparse glomerular inputs. Neuron. 2008;59:802–814. doi: 10.1016/j.neuron.2008.07.039. [DOI] [PubMed] [Google Scholar]
- 61.Ezeh PI, et al. Organization of inhibition in the rat olfactory bulb external plexiform layer. J. Neurophysiol. 1993;70:263–274. doi: 10.1152/jn.1993.70.1.263. [DOI] [PubMed] [Google Scholar]
- 62.Orona E, et al. Dendritic and axonal organization of mitral and tufted cells in the rat olfactory bulb. J. Comp Neurol. 1984;226:346–356. doi: 10.1002/cne.902260305. [DOI] [PubMed] [Google Scholar]
- 63.Orona E, et al. Different granule cell populations innervate superficial and deep regions of the external plexiform layer in rat olfactory bulb. J. Comp Neurol. 1983;217:227–237. doi: 10.1002/cne.902170209. [DOI] [PubMed] [Google Scholar]
- 64.Antal M, et al. External tufted cells in the main olfactory bulb form two distinct subpopulations. Eur. J. Neurosci. 2006;24:1124–1136. doi: 10.1111/j.1460-9568.2006.04988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Buonviso N, et al. Respiratory modulation of olfactory neurons in the rodent brain. Chem. Senses. 2006;31:145–154. doi: 10.1093/chemse/bjj010. [DOI] [PubMed] [Google Scholar]
- 66.Schoppa NE, Westbrook GL. Glomerulus-specific synchronization of mitral cells in the olfactory bulb. Neuron. 2001;31:639–651. doi: 10.1016/s0896-6273(01)00389-0. [DOI] [PubMed] [Google Scholar]
- 67.Carlson GC, et al. Long-lasting depolarizations in mitral cells of the rat olfactory bulb. J. Neurosci. 2000;20:2011–2021. doi: 10.1523/JNEUROSCI.20-05-02011.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ermentrout GB, et al. Reliability, synchrony and noise. Trends Neurosci. 2008;31:428–434. doi: 10.1016/j.tins.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kepecs A, et al. The sniff as a unit of olfactory processing. Chem. Senses. 2006;31:167–179. doi: 10.1093/chemse/bjj016. [DOI] [PubMed] [Google Scholar]