TO THE EDITOR
The desmosomal cadherins desmoglein (Dsg) 3 and Dsg1 are targets of autoantibodies in the potentially fatal blistering disease pemphigus vulgaris (PV) (Stanley and Amagai, 2006). Dsgs are synthesized as preproproteins, which are processed first in the endoplasmic reticulum to remove the signal sequence and subsequently by Golgi proprotein convertases to remove the propeptide prior to transport to the cell surface. The cadherin propeptide is thought to modulate the conformation of the extracellular domains to prevent intracellular aggregation due to interaction with other cadherins within the secretory pathway. Propeptide cleavage occurs upstream of the conserved tryptophan residue at position 2 that is responsible for cadherin strand dimer formation, suggesting that propeptide removal may unmask residues important in intermolecular adhesion. The proprotein convertase furin processes recombinant Dsgs in baculoviral overexpression systems (Posthaus et al., 2003), which are widely used for pemphigus research and clinical diagnostic purposes. Commercial Dsg ELISA kits use baculovirally-produced recombinant Dsg antigen and have been shown to be a sensitive and specific diagnostic tool for pemphigus (Ishii et al., 1997).
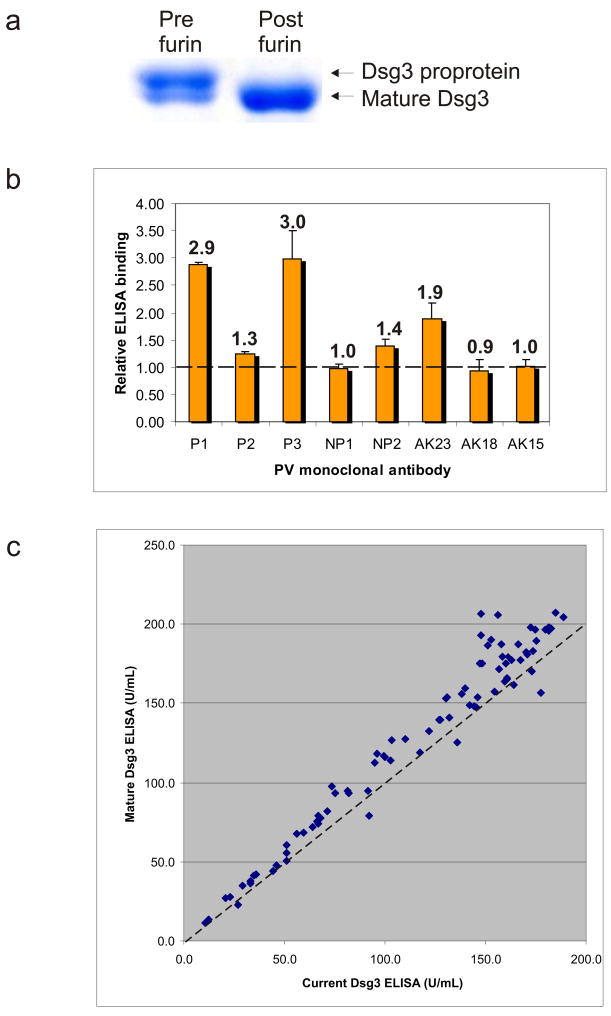
Previously, pathogenic anti-Dsg3 monoclonal antibodies (mAbs) were isolated from human patients and PV model mice (Payne et al., 2005;Amagai et al., 2000). We have recently observed decreased ELISA binding by some pathogenic PV mAbs, despite consistent pathogenicity against endogenously expressed Dsg3 in human keratinocytes. We hypothesized that the variability in pathogenic PV mAb ELISA was due to differential binding of mature Dsg3 versus Dsg3 proprotein, as the proprotein is commonly observed in recombinant antigens purified from baculoviral overexpression systems. We requested the antigen data from Medical and Biological Laboratories Co., Ltd. (MBL International, Woburn, MA), the commercial distributor for Dsg ELISA. Interestingly, an increase in purified Dsg3 proprotein is observed when antigen production methods switched from stationary plate culture (Figure 1a, lot 012) to roller bottle culture (Figure 1a, lots 013 and 014). Baculoviral roller bottle or spinner culture often results in a higher yield of recombinant proteins than stationary plate systems. However, as suggested by Figure 1a, increased cell lysis associated with these cultures can cause release of immature Dsg3 proprotein into culture supernatants.
Figure 1. Pathogenic anti-Dsg mAbs recognizing conformational epitopes selectively immunoprecipitate mature Dsg3.
(a) SDS-PAGE and Coomassie staining of Dsg3 antigen for three sequential lots of baculovirally-produced recombinant Dsg3 (data provided by MBL). (b) Immunochemical properties of human and mouse anti-Dsg mAbs (Payne et al., 2005;Amagai et al., 2000). ND, not determined. (c) Immunoprecipitation of recombinant Dsg3 baculoviral supernatants by pathogenic and nonpathogenic human and mouse anti-Dsg3 mAbs. Control shows total recombinant Dsg3 input, harvested by metal affinity chromatography. Immunoprecipitates were separated by SDS-PAGE, followed by immunoblotting using a horseradish peroxidase-coupled anti-E tag secondary antibody.
We evaluated a panel of pathogenic and nonpathogenic human and mouse anti-Dsg mAbs (summarized in Figure 1b) for their ability to immunoprecipitate proprotein and mature Dsg3 isoforms from recombinant baculoviral culture supernatants. Human pathogenic PV mAbs P1 and P3 and mouse pathogenic mAb AK23 selectively immunoprecipitate mature Dsg3 (Figure 1c). In contrast, human nonpathogenic mAbs NP1 and NP2, mouse nonpathogenic mAbs AK15 and AK18, and one human pathogenic mAb P2 (which recognizes a non-conformational epitope) immunoprecipitate both mature and proprotein isoforms.
To confirm that furin proprotein convertase cleaves the Dsg3 propeptide, recombinant Dsg3 was purified from baculoviral supernatants by metal affinity chromatography and incubated with furin (20 units/mg) for 16 hours at room temperature in the manufacturer’s recommended buffer (New England BioLabs, Ipswich, MA). Figure 2a demonstrates that furin effectively processes Dsg3 proprotein to the mature Dsg3 isoform.
Figure 2. Furin treatment increases the ratio of mature Dsg3 versus proprotein, resulting in increased ELISA binding by pathogenic anti-Dsg mAbs and PV patient serum.
(a) Purified recombinant Dsg3 was treated with furin enzyme as described in the text, resulting in effective processing of Dsg3 proprotein to its mature isoform. (b) Furin treatment of commercial Dsg3 ELISA increases binding of pathogenic PV mAbs P1, P2, P3, and AK23, as well as one nonpathogenic PV mAb (NP2) that binds an amino-terminal epitope. (c) 85 independent PV sera were tested by ELISA using current kits (containing a mixture of mature and proprotein Dsg3) and custom kits produced with mature Dsg3 antigen. Dashed line, 45 degree concordance.
To evaluate whether altered ratios of Dsg3 isoforms affects ELISA binding by anti-Dsg3 mAbs, we treated current commercial Dsg3 ELISA wells with furin enzyme (2 units/well in TBS plus 1 mM CaCl2 for one hour at room temperature) prior to incubation with anti-Dsg3 mAbs. Furin treatment increases the ELISA binding of all human pathogenic mAbs (P1, P2, and P3), as well as mouse pathogenic mAb AK23. Furin treatment also modestly increases the binding of human nonpathogenic NP2 mAb, which recognizes a non-conformational epitope in the amino-terminal domain of Dsg3 (Figure 2b). Furin treatment demonstrates no significant effect on the binding of other nonpathogenic human and mouse mAbs.
Because increases in proprotein antigen levels appear to disproportionately decrease binding of pathogenic versus nonpathogenic PV mAbs, we sought to determine whether the clinical performance of the Dsg3 ELISA would be affected by the variability of antigen isoforms. MBL produced custom mature Dsg3 ELISA plates by furin treatment of Dsg3 prior to antigen adsorption (as shown in Figure 2a). A pilot study of 85 independent PV patient sera indicates that use of mature Dsg3 antigen does not change the diagnostic result compared to the current Dsg3 ELISA. However, in 30 of the 85 samples, use of mature Dsg3 ELISA increases the serum index value by 15% or more compared to the current Dsg3 ELISA (range 15–33%), while only 1 of 85 samples demonstrates a decrease in index value of 15% or greater (value=15%) (demarcated by the 45 degree dashed line in Figure 2c). The mean serum index value increased from 116 to 129 with use of the mature Dsg3 ELISA, which was statistically significant by paired t-test analysis (p=1×10−14). Similar binding of Dsg3 isoforms between the two kits was confirmed by anti-E tag ELISA (unpublished data).
In summary, our results indicate that pathogenic PV mAbs preferentially bind epitopes in mature Dsg3 that are masked in the proprotein isoform. In contrast, non-pathogenic anti-Dsg3 mAbs recognize both mature and proprotein isoforms, correlating with binding of nonconformational Dsg epitopes. Previous studies have shown that pathogenic pemphigus antibodies more often bind conformational epitopes in the amino-terminal domain of desmogleins, while nonpathogenic antibodies bind non-conformational epitopes (Li et al., 2003;Sekiguchi et al., 2001;Payne et al., 2005;Ishii et al., 2008). Therefore, a predominance of proprotein in the Dsg3 ELISA might bias the test to the detection of non-pathogenic antibodies. Although the clinical diagnostic value of the ELISA is unaffected by variability in Dsg3 isoform (Figure 2c), we would predict that the mature Dsg3 ELISA would correlate better with disease activity. Our study does not directly evaluate this hypothesis, although a concurrent study supports this conclusion (Yokouchi et al., 2008). Commercial Dsg ELISA plates will utilize mature Dsg3 antigen, cleaved with furin prior to adsorption, beginning in December 2008 (lots 101 and up). Ongoing research studies may note changes in optical density values using the new ELISA kits. These findings are relevant for physicians and scientists using baculovirally-produced recombinant Dsg3 for clinical and basic research studies, including the use of ELISA to track disease activity and for evaluation of human and mouse anti-Dsg mAbs.
Acknowledgments
We thank John Stanley, Masa Amagai, and Shinsuke Tachi for helpful discussions on the manuscript. This work was supported by NIH AR053505 and the Jeannette Laws and Thomas McCabe Pilot Award (ASP).
Abbreviations
- Dsg3
desmoglein 3
- PV
pemphigus vulgaris
Footnotes
Conflict of Interest
Keiko Kuroda and Takahisa Hachiya are employees of Medical and Biological Laboratories Co., Ltd., the commercial distributor of the desmoglein ELISA. Preety M. Sharma, Eun Jung Choi, Ken Ishii, and Aimee S. Payne declare no conflict of interest.
References
- Amagai M, Tsunoda K, Suzuki H, Nishifuji K, Koyasu S, Nishikawa T. Use of autoantigen-knockout mice in developing an active autoimmune disease model for pemphigus. J Clin Invest. 2000;105:625–631. doi: 10.1172/JCI8748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii K, Amagai M, Hall RP, Hashimoto T, Takayanagi A, Gamou S, et al. Characterization of autoantibodies in pemphigus using antigen-specific enzyme-linked immunosorbent assays with baculovirus-expressed recombinant desmogleins. J Immunol. 1997;159:2010–2017. [PubMed] [Google Scholar]
- Ishii K, Lin CY, Siegel DL, Stanley JR. Isolation of pathogenic monoclonal anti-desmoglein 1 human antibodies by phage display of pemphigus foliaceus autoantibodies. J Invest Dermatol. 2008;128:939–948. doi: 10.1038/sj.jid.5701132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Aoki V, Hans-Filho G, Rivitti EA, Diaz LA. The role of intramolecular epitope spreading in the pathogenesis of endemic pemphigus foliaceus (fogo selvagem) J Exp Med. 2003;197:1501–1510. doi: 10.1084/jem.20022031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne AS, Ishii K, Kacir S, Lin C, Li H, Hanakawa Y, et al. Genetic and functional characterization of human pemphigus vulgaris monoclonal autoantibodies isolated by phage display. J Clin Invest. 2005;115:888–899. doi: 10.1172/JCI24185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posthaus H, Dubois CM, Muller E. Novel insights into cadherin processing by subtilisin-like convertases. FEBS Lett. 2003;536:203–208. doi: 10.1016/s0014-5793(02)03897-8. [DOI] [PubMed] [Google Scholar]
- Sekiguchi M, Futei Y, Fujii Y, Iwasaki T, Nishikawa T, Amagai M. Dominant autoimmune epitopes recognized by pemphigus antibodies map to the N-terminal adhesive region of desmogleins. J Immunol. 2001;167:5439–5448. doi: 10.4049/jimmunol.167.9.5439. [DOI] [PubMed] [Google Scholar]
- Stanley JR, Amagai M. Pemphigus, bullous impetigo, and the staphylococcal scalded-skin syndrome. New Engl J Med. 2006;355:1800–1810. doi: 10.1056/NEJMra061111. [DOI] [PubMed] [Google Scholar]
- Yokouchi M, Adly M, Kuroda K, Hachiya T, Stanley JR, Amagai M, et al. Pathogenic epitopes of autoantibodies in pemphigus reside in the amino-terminal adhesive region of desmogleins which are unmasked by proteolytic processing of prosequence. J Invest Dermatol. 2008 doi: 10.1038/jid.2009.61. JID 2008-0760: manuscript submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]