Abstract
Fifty years ago, Stern, Smith, van den Bosch and Hagen outlined a simple but sophisticated idea of pest control predicated on the complementary action of chemical and biological control. This integrated control concept has since been a driving force and conceptual foundation for all integrated pest management (IPM) programs. The four basic elements include thresholds for determining the need for control, sampling to determine critical densities, understanding and conserving the biological control capacity in the system and the use of selective insecticides or selective application methods, when needed, to augment biological control. Here we detail the development, evolution, validation and implementation of an integrated control (IC) program for whitefly, Bemisia tabaci (Genn.), in the Arizona cotton system that provides a rare example of the vision of Stern and his colleagues. Economic thresholds derived from research-based economic injury levels were developed and integrated with rapid and accurate sampling plans into validated decision tools widely adopted by consultants and growers. Extensive research that measured the interplay among pest population dynamics, biological control by indigenous natural enemies and selective insecticides using community ordination methods, predator:prey ratios, predator exclusion and demography validated the critical complementary roles played by chemical and biological control. The term ‘bioresidual’ was coined to describe the extended environmental resistance from biological control and other forces possible when selective insecticides are deployed. The tangible benefits have been a 70% reduction in foliar insecticides, a >$200 million saving in control costs and yield, along with enhanced utilization of ecosystem services over the last 14 years. Published in 2009 by John Wiley & Sons, Ltd.
Keywords: Bemisia tabaci, decision aids, conservation biological control, selective insecticides, bioresidual, ecosystem services
INTRODUCTION
Fifty years ago, four entomologists from California articulated a concept of pest management1 that has since become not only a seminal scientific contribution but also a driving force and conceptual foundation for all modern integrated pest management (IPM) programs. The integrated control concept (ICC), as it was coined,1 is ‘Applied pest control that combines and integrates biological and chemical control. Chemical control is used as necessary and in a manner that is least disruptive to biological control’.
This embodies what at first appears to be a simple set of requirements, but upon further reflection points to multiple key components that must be assembled strategically to produce the desired outcome. The four essential elements include thresholds for determining the need to control pest populations, sampling plans for measuring critical densities, an understanding of the impact of biological control on the pest and finally knowledge of selective insecticides or methods of deployment that complement rather than disrupt this biological control. The ICC was based around Stern, Smith, van den Bosch and Hagen's experiences in field crops in California, including alfalfa, cotton and safflower, but the incredible insights into the basic ecology that underpin control systems were then, and still are, boundless.
Integrated control (IC) has been an elusive goal for most modern-day integrated pest management (IPM) programs. There has always been an uneasy tension between biological control specialists and IPM scientists having to develop and deploy usable programs for client growers and pest management practitioners. Stern et al.1 had a depth of vision that still today we are trying to emulate through validated IC programs. Few examples exist in the literature (e.g. references 2 to 5), and even then specific IC elements may be missing or not fully addressed and validated. Still fewer examples of IC can be found in general grower practice. At some level, this represents neglect on the part of the scientific community which has not invested in the research and documentation needed to identify and promote IC. At another level, this underscores the difficulty of conducting systems-level research and development including elements of implementation and validation.
The implementation and validation of IC requires several postulates:
The biological control agents must be present and abundant in the untreated system.
The biological control agents must be able to survive, at some level, the application of selective controls in the system of interest.
Some functional assessment of conservation biological control must be conducted.
An interval of pest suppression (or degree of control) in excess of the chemical residual must be possible when a selective control is implemented.
The interval (or degree) of suppression is lost, or at least significantly reduced, when either control agent (conserved agent or selective control) is removed from the system.
Additionally, IC is predicated on efficient and validated sampling plans used in deployment of research-based economic injury levels (EILs) and economic thresholds (ETs).
In this review we will attempt to show how these multiple elements have been crafted, delivered and incorporated into an IC program for the whitefly, Bemisia tabaci (Genn.), in the Arizona cotton system. We will discuss development of decision tools based on economic thresholds and simple, but accurate, sampling plans. We will show that a rich beneficial arthropod fauna creates a flexible and resilient food web where 3–5 insect predator species dominate and remain viable control agents of B. tabaci even with well-timed applications of selective insecticides. Will we discuss multiple functional assessments of conserved biological control agents over many years will be discussed, including detailed life tables, analyses of marginal mortality rates, irreplaceable mortality and predator:prey ratios. We will show how these studies confirm the key role of predation in the cotton system, along with other natural abiotic sources of mortality such as weather. Furthermore, we will demonstrate the dynamic trade-off between the two key mortality factors, insecticides and predation, and show how irreplaceable mortality due to predation increases over time when selective, but not conventional broad-spectrum, chemistry is deployed. We demonstrate that the period of suppression can be measured in days for conventional chemistry versus weeks for selective chemistry, and the reintroduce concept of ‘bioresidual’, which was imparted to growers to communicate the extended suppressive interval made possible by selective insecticides and a functional IC program. The interdependence of chemical and biological control via predator exclusion studies will also be shown. Finally, we discuss the impact of our IC program as part of an overall IPM strategy and provide some perspective on the future.
THE SYSTEM
Cotton is cultivated in about 80 countries, with a total production of ≈23.6 billion kg of lint in 2008,6 is plagued by dozens of arthropod pests,7,8 and has historically been exposed to large volumes of insecticides.9,10 In Arizona, cotton has been an important agricultural commodity for many decades, with recent production exceeding 260 000 ha in the early 1980s. There are three key pests of cotton in Arizona and other low desert production areas in California and Mexico, the pink bollworm (Pectinophora gossypiella Saunders), Lygus bugs (primarily Lygus hesperus Knight) and the sweetpotato whitefly (B. tabaci), all of which can severely impact upon yield and lint quality in any given year. Several other pests can be occasionally problematic, depending on year, region and ongoing pest control tactics that might disrupt natural control processes. Some of the foundational elements of IC penetrated Arizona during the 1950s and continued into the 1970s with the implementation of supervised control.11 Stern et al.1 defined supervised control as ‘Control of insects … supervised by qualified entomologists and based on conclusions reached from periodically measured population densities of pests and beneficial species’. Supervised control was in effect a precursor to IC and introduced some of the same elements, including sampling and thresholds, but without the attendant theoretical basis found in the ICC. In the program discussed by Carruth and Moore,11 a cooperative, grower-sponsored, weekly scouting program was implemented on about 5000 ha of cotton in southeastern Arizona over a 3 year period in response to heavy infestations of P. gossypiella in prior years that resulted in six applications of insecticides per field, precipitated secondary outbreaks of cotton leaf perforator and caused heavy mortality of honey bees. In the end, the scouting program cost the growers $4.08 ha−1 and reduced insecticide use by 71–84%, testifying to the potential power of prescriptive pest control outlined by Stern et al.1 as part of the ICC.
The pest
Bemisia tabaci was first described as an agricultural pest in Greece 120 years ago12 and has since become one of the most devastating insect pests in the world, affecting multiple agronomic and horticultural crops. It is cosmopolitan in distribution, with outdoor populations limited to tropical and subtropical regions of the world, but through commercial trade it has established itself as a pest of many glasshouse production systems throughout temperate areas of Asia, Europe and North America. Unusual for aleyrodids, B. tabaci is highly polyphagous, with a historical host range of over 500 plants13,14 that continues to expand (e.g. reference 15). The pest causes direct feeding damage through the removal of phloem sap, vectors over 110 plant viruses,16 causes a number of feeding-induced plant disorders and can contaminate cotton lint and degrade produce through the deposition of honeydew.17,18 Bemisia tabaci has been known from Arizona since the 1920s19 but was associated with only sporadic pest issues until the early 1990s.20,21 This renewed pest status was due to the introduction of a new biotype (biotype B) that first invaded Florida in the mid-1980s, subsequently spread to the west coast by 1991 and rapidly displaced the indigenous biotype.22,23 The new B-biotype of B. tabaci shared many of the same general biological features of the indigenous biotype but in a more virulent form, including a broader host plant range, greater reproductive potential, enhanced mobility and a greater propensity to develop resistance to insecticides, among other qualities.22 The invasion of this new biotype wreaked havoc on existing pest management practices in Arizona and elsewhere and initially led to some of the costliest impacts on yield, lint quality and control measures ever recorded in the state.24
Like the 1954 episode with spotted alfalfa aphid threatening the California alfalfa industry1 (see Section 4.2), the Arizona whitefly outbreak of 1992, 40 years later, created serious concerns about the survival of the cotton industry in the short-term without effective chemical controls. Local research discovered that putative pyrethroid resistances in the B-biotype of B. tabaci could be overcome with an appropriate synergist like acephate or endosulfan,25–29 much as in other parts of the world.30 During the early stages of this crisis, 1993–1995, growers depended on key pyrethroid mixtures such as fenpropathrin plus acephate and bifenthrin plus endosulfan.29 The consequences of the use of such broad-spectrum biocides and the dependence on their repeated use caused much concern within scientific, governmental and industry circles, which all collaborated to exchange information within an annual USDA program-planning process.31,32 These severe outbreaks were met with a similarly acute response by the research, extension and industry community, which ultimately resulted in the development and continual evolution of a highly effective and efficient pest management program reflective of the forward vision embodied in the ICC.
THE ECOSYSTEM CONTEXT
A key underlying element of the ICC proposed by Stern et al.1 was ‘to recognize the “oneness” of any environment, natural or man-made’, and to understand that any control system imposed on a given pest in a given crop has consequences for the management of other pests and crops in the ecosystem. They also emphasized the multitactic nature of pest management, including consideration of factors such as plant nutrition, plant physiology and plant resistance and the economic considerations that bind them all. A proxy for this vision can be depicted in a pyramid representing the multiple tactics that can be brought to bear on a pest issue, as well as how these tactics interact, complement and build upon one another at different levels in an overall pest management strategy (Fig. 1). A large portion of the ecosystem as envisioned by Stern et al.1 is embodied in the foundational (avoidance) elements of the pyramid, such as area-wide impact, exploitation of pest biology and ecology and crop management. The pyramid also helps to demonstrate the simplicity, and at the same time the complexity, of the ICC, which calls primarily upon the interaction of four key elements: sampling, thresholds, chemical control and biological control, but as they are embedded in the overall ecosystem context.
Figure 1.
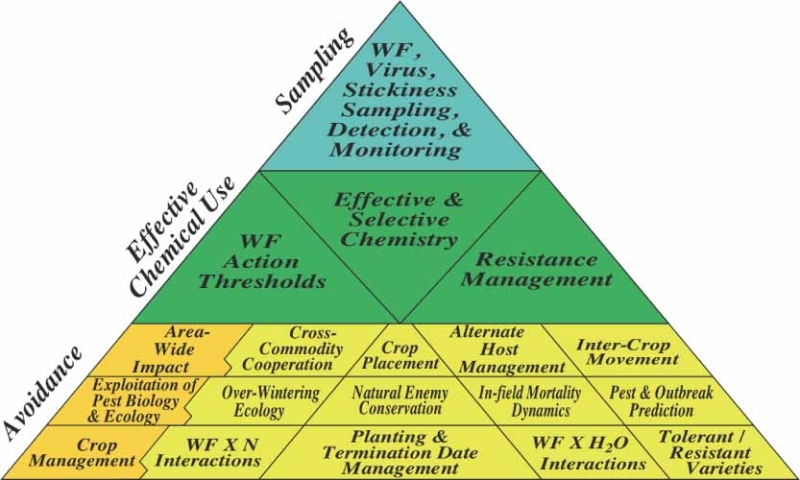
Conceptual diagram of IPM showing the main interacting management tactics arranged in an inherently stable pyramid where elements build upon one another resulting in a sustainable management strategy. Adapted with permission from Elsevier68.
Pest population dynamics and demography
Many of the biological qualities of B. tabaci enable the insect to thrive in the low desert production areas of Arizona and southern California. The lack of any quiescence period allows the insect to develop and reproduce year round, limited only by temperature, the availability of suitable host plants and natural control elements in the environment. Watson et al.33 outlined the seasonal cycle of B. tabaci in an Arizona agricultural setting (e.g. Fig. 2). Populations persist at very low densities during winter months on winter vegetables, weeds and perennial hosts such as alfalfa and ornamentals within urban and rural landscapes, build on spring-planted crops such as cantaloupe (a favored host), reach peak densities during summer months on crops such as cotton and then decline during the fall. B. tabaci populations frequently exceed established threshold levels (see Section 4.1.1) in Arizona cotton (Fig. 3), although the magnitude and extent of these outbreaks is highly variable both over years and across the cotton production region of the state, much as depicted in the insightful schematic of Stern et al. (p. 90).1 Overall, the seasonal cycle is enabled by the insect's ready ability to disperse within the environment and continually exploit new hosts.34–36 These qualities make B. tabaci not just a single crop pest but an ecosystem pest, and this ultimately drives the formulation of pest management strategies on both local and regional scales.
Figure 2.
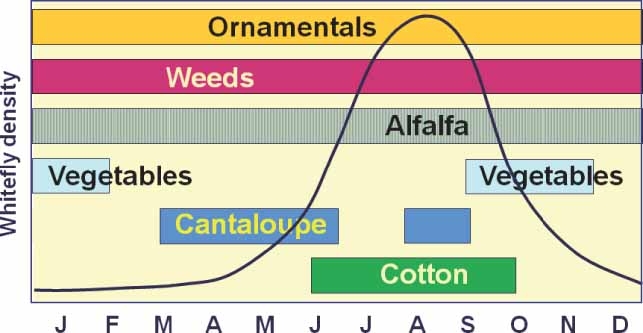
Depiction of a typical seasonal cycle for B. tabaci in an Arizona agroecosystem. The line shows the general pest population density over time.
Figure 3.
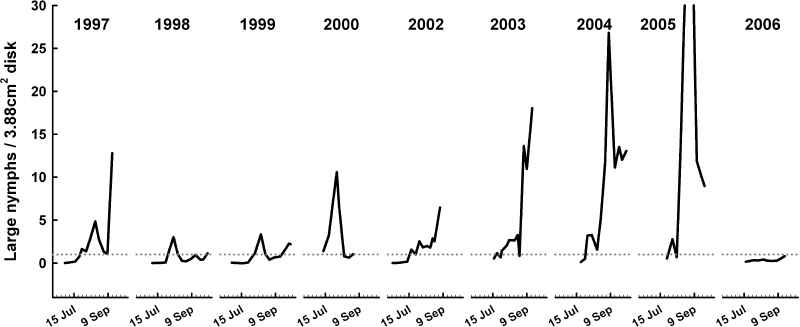
Population dynamics of B. tabaci over a ten year period in unsprayed fields at the University of Arizona, Maricopa Agricultural Center in south-central Arizona. The dotted horizontal line represents an action threshold of 1 large nymph (3rd or 4th instar) per quarter-sized leaf disk (3.88 cm2).
Somewhat counterbalancing the huge biotic potential of B. tabaci has been the discovery that populations of this pest are subject to high levels of natural mortality (>93%), both in the cotton system37 (Fig. 4) and in a variety of crops and host plants that comprise the seasonal cycle of this insect.38 The sessile nature of the immature stages of this insect afforded the opportunity to directly and accurately measure rates of mortality by various factors via life table studies in the field. As detailed below, predation is the key factor determining intergenerational variation in B. tabaci mortality, and this mortality factor also contributes the highest level of irreplaceable mortality.37 Further life table studies on other crops and host plants of B. tabaci showed that predators also provide high levels of mortality of B. tabaci on ornamental lantana, alfalfa, spring and fall cantaloupe and various annual weeds.38 One key finding from this life table work in non-cotton host plants has been the discovery that total generational mortality in spring cantaloupes is relatively low (≈65%), which likely acts as a biological release valve during the late spring, precipitating large influxes of whiteflies into temporally and spatially adjacent crops such as cotton. The role of migration and dispersal in the population dynamics of B. tabaci has been acknowledged by researchers39–43 for many years, but there has yet to be any direct or quantitative measure of the phenomenon. However, comparison of simulated population dynamics, based on life table mortality rates to generate endogenous population growth of B. tabaci, with actual population densities in cotton hints at the magnitude of early-season immigration and late-season emigration37 (Fig. 5). This pattern of immigration into cotton plays a key role in the management strategy for this pest that is detailed later.
Figure 4.
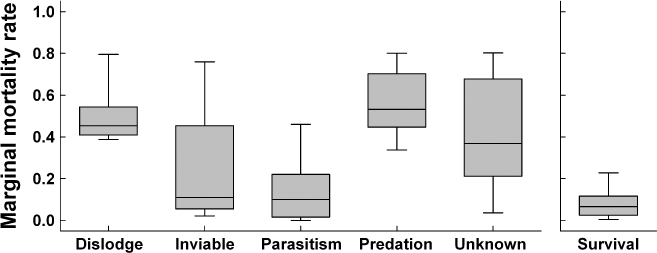
Box plots showing levels of marginal mortality of immature B. tabaci by various factors and overall generational survival in unsprayed cotton fields over 14 generations. The line within each box represents the median, the box bounds the 25th and 75th percentiles, and the whiskers denote the 10th and 90th percentiles. Adapted with permission from Wiley InterScience37.
Figure 5.
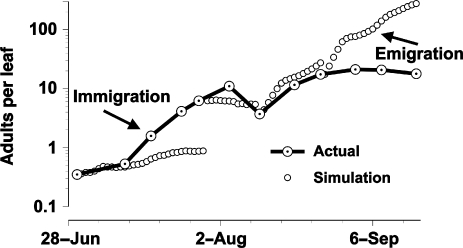
Comparison of simulated and actual population trajectories for adult B. tabaci in cotton. Simulation is based on a temperature-dependent, stage-structured model that was initiated with actual insect stage densities and observed rates of immature mortality from life tables. Adapted with permission from Wiley InterScience37.
THE MANAGEMENT SYSTEM
Sampling and thresholds
Sampling is a fundamental tool for the study of population dynamics and the formulation of decision aids in a pest management program. This was one of the first tools developed upon the invasion of the new B. tabaci biotype into Arizona. Guided in part by prior work,44–46 basic information was developed on within- and between-plant distributions of immature stages, optimal sampling units were selected on the basis of variability and costs and fixed-precision sampling models and plans were developed and validated.47,48 Equivalent studies were conducted for the adult stage, with the added element of exploring different methods for estimating density of this mobile stage.48–50 The result of this work was the development of simple plans based on counting immature and adult stages on the underside of cotton main stem leaves located at the fifth node below the terminal (Fig. 6) that reflect densities on the entire plant. These sampling plans were instrumental in the study of basic population dynamics, in conducting experiments to test and evaluate various control tactics (e.g. references 51 to 54) and ultimately in the development of thresholds.55,56
Figure 6.
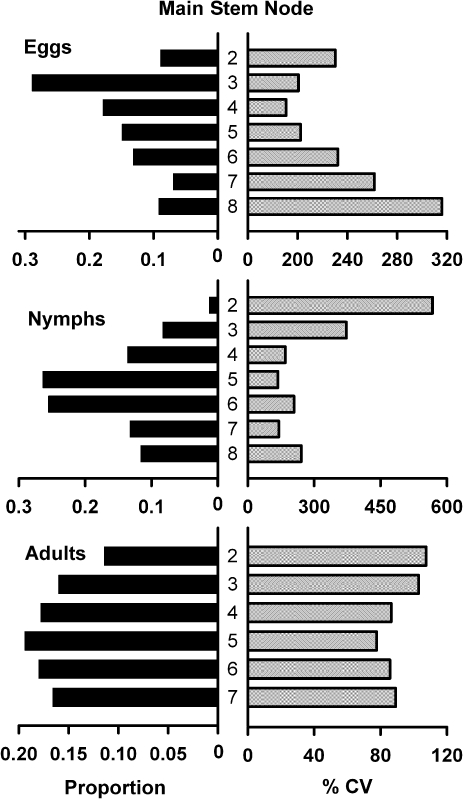
Distribution and variation of immature and adult stages of B. tabaci on mainstem node leaves of cotton. Based on these patterns and the accuracy of leaf-based counts to predict whole plant abundance, the 5th node leaf was chosen as the optimal sample unit for estimating population density. Adapted from47,49.
Evolution and validation of decision aids
Perhaps the most critical and long-standing concept introduced by Stern and colleagues1 was that of the EIL, ‘the lowest population density that will cause economic damage’, and the associated ET, ‘the density at which control measures should be determined to prevent an increasing pest population from reaching the economic injury level’. Although these have since been defined in more specific mathematical terms amenable to experimentation and development,57,58 they remain the guiding principle of modern pest management based on prescriptive control. Stone and Pedigo59 were the first to develop an EIL, and since that time hundreds of EILs and ETs have been developed for a range of agricultural and horticultural commodities.60
The initial invasion of B. tabaci biotype B into Arizona happened suddenly, requiring a rapid response to provide producers with some basic tools for management. In addition to the development of sampling tools, several concurrent studies attempted to derive usable EILs and thresholds for decision-making.51,55 A multistate project was initiated in 1993 to develop action thresholds for B. tabaci in cotton for California, Arizona and Texas.56 The main chemical tools available were pyrethroids synergized primarily with organophosphates, and the target of decision-making was the adult stage. The EIL research pointed to levels of around 5–15 adults per leaf, based on cotton prices and control costs of the time.55 Field experiments based on the deployment of a series of candidate, predetermined threshold levels pointed to action threshold levels of around 5–10 adults per leaf.51,56 Further experience with typical population trajectories and concern about lint stickiness from honeydew contamination led to the selection of an economic threshold of five adults per leaf in Arizona and the desert valleys of California. This level preserved yield and lint quality while also permitting effective suppression with the chemical arsenal available at that time. In the San Joaquin Valley of California, where B. tabaci was less problematic, a threshold of ten adults per leaf was adopted.
Stern et al.1 further pointed to the critical need for sampling plans that were rapid and simple enough to use, so that consultants and growers would readily adopt them. Since the inception of ICC, sampling theory and practice have grown considerably,61–63 and hundreds of sampling plans have been developed for making treatment decisions relative to critical densities.
Based on the newly defined threshold, a simple binomial sampling plan for classifying the density of B. tabaci adults was developed,64 validated on over 3200 ha of commercial cotton,48 and delivered and taught to growers and pest control advisors through intensive and extensive workshops throughout the state.65–67 Although the original binomial model was developed as a sequential sampling plan, based on user input, it was delivered as a fixed-sample-size plan requiring that 30 leaves be examined per field (15 each at two sites in a field) before determining the need for control action. The whole decision process took about 5 min to execute and was very accurate, leading to the correct decision nearly 88% of the time.48
The decision protocol enabled a version of supervised control, was enthusiastically adopted by growers and helped them maintain profitability in the face of severe B. tabaci outbreaks in the early 1990s.24,68 However, this fell short of IC, because the broad-spectrum insecticides in use severely disrupted natural enemy populations and biological control played essentially no role.69 Not unexpectedly, overreliance on synergized pyrethroids led to rapid evolution in loss of susceptibility in B. tabaci populations by 1995,29,70 precipitating the need for a new strategy centered around buprofezin and pyriproxyfen, two insect growth regulators (IGRs) that had been successfully used in Israel for suppression of B. tabaci in cotton and greenhouse systems for many years71,72 (see Section 4.2.2). Both IGRs were shown to have low vertebrate toxicity,73,74 and numerous laboratory studies pointed to their putative selectivity in the field,75–81 which was later verified in the Arizona cotton-whitefly system82,83 (see Section 4.3).
Because these IGRs are mainly active on the immature stages of B. tabaci, the focus of the decision protocol was expanded to include both adult and immature stages. A provisional threshold of 0.5–1 large nymphs (third and fourth instars) per quarter-sized leaf disk was determined on the basis of experience with the IGRs in Israeli cotton (Horowitz AR, private communication, 1995) and patterns of seasonal population structure and growth in Arizona cotton. Additional experience in the field showed that densities of around 3–5 adults per leaf, coupled with evidence of in-field reproduction (via large nymphs), denoting the leading edge of an upward trajectory of population growth, represented the optimal time to deploy these IGRs. The objective was to initiate control with IGRs when nymphal populations were relatively low but at an inflection point leading to inevitable upward growth. A fixed-sample-size binomial sampling plan was developed for large nymphs using the leaf disk (3.88 cm2) as the sampling unit to facilitate efficiency.84 The dual-stage decision protocol was delivered with a matrix of decision outcomes depending on adult and nymphal densities.66 For example, if neither threshold was exceeded, resampling in 3–7 days was recommended, but, if nymphal densities exceeded the threshold and adults did not, then the decision was to resample in 3 days or apply buprofezin because of its effect only on nymphal stages. This matrix approach allowed some flexibility in decisions when growth of the two stages might be asynchronous owing to atypical immigration events (see above) or previous insecticide use. A commercial-scale study served to validate the decision protocol85 and showed that the nymphal sampling plan was accurate, leading to the correct decision with regards to insecticide applications nearly 89% of the time.84 The dual-stage sample plan and threshold takes about 7 min to execute in the field with a sample size of 30 leaves and remains the underlying strategy for decision-making while supporting additional selective and partially selective insecticide options that have since been identified and added86 (see Section 4.2.2).
Chemical and biological control
Stern et al.1 articulated a clear vision for how IC could be accomplished: ‘Integrated control is most successful when sound economic thresholds have been established, rapid sampling methods have been devised, and selective insecticides are available’. At the time of the spotted alfalfa aphid outbreak in 1954, there was little understanding of, or value placed on, selective insecticides. Much like the invasion of B. tabaci in the early 1990s, the dimension of the crisis was such that immediate relief was needed in the form of effective chemical controls. Stern et al.87 stated: ‘There is little doubt that the insecticides parathion, malathion, and TEPP prevented a widespread devastation of California's alfalfa industry following the 1954 appearance of the spotted alfalfa aphid Therioaphis maculata (Buckton), but the materials also proved to be toxic to insect enemies of the aphid’.
Stern and his colleagues conducted a wide array of field tests in commercial alfalfa fields over a short period of time in the southern valleys of California.87–89 Their findings helped them identify demeton (Systox) as a compound with potential selectivity in their system. Even though parathion and other chemistries were at times nearly 100% effective, the occurrences of pest resurgence and potential for secondary pest outbreaks convinced them that the strategic use of a selective compound like demeton would better complement the existing biological controls present.87–89
These field studies provided the empirical basis for the ICC, and their views on the role of chemical control. They wrote: ‘with adequate understanding [chemical and biological control] could be made to augment one another’.1 More fundamentally, they defined a selective insecticide as one that, while killing the pest individuals, ‘spares much or most of the other fauna, including beneficial species, either through differential toxic action or through the manner in which the insecticide is utilized (formulation, dosage, timing, etc.)’.
Ecosystem services and conservation biological control
Sparing fauna of any kind in agricultural fields was not a common theme among pest management practitioners of the time. However, these themes pervade the IPM literature of today and are now guiding a new generation of ecological theory in the realm of ecosystem services. Daily90 defines ecosystem services as ‘the conditions and processes through which natural ecosystems, and the species that make them up, sustain and fulfill human life’. This concept has since been expanded to include managed systems and organized around four basic categories of ecosystem services: supporting, provisioning, regulating and cultural (e.g. reference 91). Stern et al.1 saw the importance of ‘regulating services’ in conservation biological control and stated that ‘… these regulating factors actually keep thousands of potentially harmful arthropod species permanently below economic thresholds’.
Conservation biological control is widely recognized as a key ecosystem service that provides target and secondary pest control while preventing pest outbreaks and resurgences. Many studies today are dedicated to manipulating the environment in strategic ways so as to maximize this regulating service to natural and managed ecosystems. Much effort has focused on conservation biological control enhancement via the use of alternative food sources including artificial sprays, provision of shelter, exploitation of semiochemicals and other aspects of habitat management (see reference 92 for a review). And, in spite of considerable research on defining toxicity of insecticides to natural enemies (e.g. reference 93), relatively little attention has been paid to manipulation of chemical controls towards compatibility with biological controls in the field, a foundational element of ICC. Arguably, chemical control is the principal tactic in use by most pest managers. The Millennium Ecosystem Assessment91 concluded that pesticide use around the world was diminishing these regulating services and in fact replacing pest control with natural enemies. It further concluded that pesticide use itself was degrading the ability of agroecosystems to provide for pest control.
Selectivity as the basis for integrated control
Like demeton 50 years ago in alfalfa, pyriproxyfen and buprofezin were the key chemical controls tested in the mid-1990s that provided an opportunity to more strategically manage B. tabaci in Arizona cotton (e.g. references 53 and 94 to 96). These IGRs had no previous registrations in the USA. Starting in 1996 under EPA Section 18 emergency exemption, growers had simultaneous access to these two compounds in response to the emergency need and outbreak of 1995. Large-scale testing, including aerial applications, was conducted,68,85,97 and new guidelines were developed and deployed66,98,99 (see Section 4.1).
Pyriproxyfen is a juvenoid with activity on developing eggs and metamorphosing nymphs. Its translaminar activity permits extended control even from aerial applications, in spite of very dense plant canopies and pest feeding behavior concentrated on the abaxial surface of leaves. Buprofezin is a chitin synthesis agonist with activity on B. tabaci nymphs. Its vapor activity is also important in the redistribution of residues within the plant canopy. Later, acetamiprid, a neonicotinoid with excellent acropetal movement in the plant, was registered in 2002 for Arizona cotton. Spiromesifen, a lipid biosynthesis inhibiting ketoenol, was registered in 2005. These four compounds have become the current-day chemical arsenal used to augment B. tabaci control in Arizona cotton.
The extension guidelines in use today86,100 are the product of progressive refinement of the original guidelines66 and a better understanding of the role of chemical and biological control in the Arizona agroecosystem. Bemisia tabaci chemical controls are divided into three stages defined not only by their efficacy but also by their selectivity attributes. Stage I contains all the fully selective compounds (pyriproxyfen, buprofezin and low rates of spiromesifen). Stage II contains partially selective compounds like acetamiprid and other neonicotinoids, or higher rates of spiromesifen. Stage III is reserved for the synergized pyrethroids that were once the mainstay of the chemical control program in the early 1990s. Today, these broad-spectrum options are recommended for use only in the late season, if needed, to assist in the control of B. tabaci along with other pest insects. These guidelines do not mandate a specific sequence or rotation among chemical use stages, but teach growers that more selective approaches will create more effective ecosystem services that will provide regulation of all pest species. The benefits of IC are regularly taught to growers and pest control advisors (PCAs), the licensed professionals who prescribe pesticide use in Arizona. Conditions are identified when greatest benefits may be gained by deploying stage-I chemistry first [e.g. typical build-up and balance (nymphs and adults) of populations (see Sections 3.1 and 4.1.1); use of transgenic Bt cotton for selective control of caterpillar pests and with no prior sprays for other insect pests; sufficient time to the end of the season to permit the functioning of the IGRs]. However, stage-I compounds in current use have no strong adulticidal action. Therefore, in scenarios of large immigrating populations, growers are encouraged to make use of the adult active neonicotinoids, especially acetamiprid, which has longer residual and better systemic characteristics. Landmark grower agreements and guidelines are also in place to manage resistance and to share chemistry among multiple crops, depending on cropping complexity.29,100,101
The importance of ecological context
The ecological context is critical to understanding the selective potential of any approach. While laboratory toxicological studies are valuable (e.g. references 102 and 103), they do not adequately address the milieu in which the candidate agent is to be used or the manner in which biological control agents interact with that environment. Initially alarming reports of centuries-old biological control being disrupted by new chemistry (e.g. cottony cushion scale controlled by Vedalia beetle in Californian citrus) have since been quelled by the more careful timing and application of pyriproxyfen in those systems.104,105 Nevertheless, the potential toxicological impact of these IGRs, particularly pyriproxyfen, on non-target coccinellids should be reconciled with the ecological context in which the materials are to be used.106–108 Thus, pyriproxyfen and buprofezin have proven quite selective in the Arizona cotton–B. tabaci system,66,82,83,109 but pyriproxyfen has shown potential conflict with biological control in citrus systems in California,103,105 Australia108 and South Africa.107 Even then, compounds with potential toxicity to natural enemies may fail to reach them in sufficient dosages to cause harm (e.g. only 15% of labeled pyriproxyfen reached Podisus maculiventris Say from its intoxicated prey).102,108
As an organophosphate, demeton was a broadly toxic compound with much potential for harm against certain natural enemies. However, Stern and colleagues' approach was to test as low a rate as possible to accomplish sufficient pest control with the greatest safety to beneficial organisms (for example, see Fig. 16). Even Smith et al.108 concluded that very low rates of pyriproxyfen in Australian citrus would control citrus red scale with some margin of safety for Vedalia and other coccinellid beetles. In another example, novaluron, a chitin-inhibiting IGR, has shown great potential for B. tabaci and other pest control in Israeli cotton.110 However, recent testing in the Arizona cotton system has shown it to be as destructive to natural enemies as the negative control, acephate, and more likely to contribute to pest resurgence of B. tabaci (Ellsworth PC and Naranjo SE, unpublished). As a consequence, this product is not recommended for use in cotton in Arizona, in spite of its availability since 2004.
Figure 16.
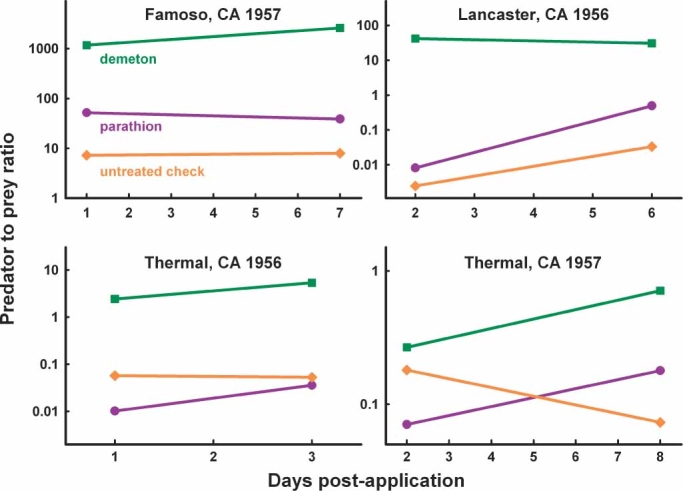
Predator–prey dynamics (as ratio of total counts of predators using various methods to spotted alfalfa aphids per stem) for two different aphid control strategies and an untreated check in alfalfa, 1956–1957, California, USA. Demeton use was associated with dramatic increases in the ratios, up to 3 orders of magnitude larger than the conventional standard, parathion. Data derived from published reports87,89.
In a remarkable statement for its time, Stern et al.1 warned that ‘… failure to recognize that control of arthropod populations is a complex ecological problem … leads to the error of imposing insecticides on the ecosystem, rather than fitting them into it’. For Arizona cotton, this suggests that novaluron is poorly suited for present needs because of its toxic effects on hemiptera and the reliance on generalist hemipteran predators for biological control (see Section 4.3). In comparison, pyriproxyfen used indiscriminately in a citrus system for scale control may disrupt biological control processes dependent on coccinellids there. However, lower dosages (<2 mg L−1)108 and more properly timed applications of pyriproxyfen in Californian citrus orchards have effected long-term control of citrus red scale and other scale insects without disruption of coccinellids and with better safety to hymenopteran parasitoids than the alternatives.104 Furthermore, the Arizona cotton system is not typically dependent on the ecosystem services provided by Coccinellidae, nor have negative effects been measured on this group of insects. On the contrary, Coccinellidae have generally been conserved in Arizona cotton compared with the conventional alternatives.83
Pesticides poison the concept?
Those familiar with the original ICC, or later its expansion to IPM, have often been frustrated by the course that these concepts have taken; so much so that alternative monikers have been developed over the last 20 years. There have been calls for biologically or ecologically intensive IPM, organic or sustainable pest management and intriguing spin-offs such as integrated biodiversity management (IBM).111 There are also indictments that IPM has become nothing more than integrated pesticide management,112 leading to modifiers like ‘real’ or ‘true’ being added to IPM. At the core of many of these critical shifts is a genuine frustration with the use of pesticides in IPM. However, the ICC was genius in this regard by acknowledging the role that chemical control plays in systems and noting that agricultural production of any scale is a distortion of natural processes and therefore unlikely to be sustained by regulating ecosystem services alone. Furthermore, ‘provisioning’, or the production of food and fiber for society, is a key ecosystem service that needs to be put in balance and perspective with other ecosystem services. Denial of this fundamental ecosystem service (i.e. provisioning) will only serve to alienate and confuse the practitioner and producer who simply wish to control pest populations in the least disruptive manner possible while producing a safe and abundant food supply.
The role that chemical control plays in modern-day IPM is subject to both scientific skepticism and overzealous marketing. Suppliers of pesticides are very savvy and have capitalized on the conceptual discord present by selling nearly everything as reduced-risk, biorational (see reference 113) or ‘soft’ on beneficials. Cleverly placed lacewings or other beneficial archetypes adjacent to a chemical jug or plant have now become the norm in the marketing of any new insecticide. At some level, this supports the idea that the ICC has penetrated the agricultural industry psyche; however, the claims made for these chemicals are rarely, if ever, subject to the important research that is needed to validate their selectivity within the relevant agroecosystem context.
Bioresidual, biorational and validated integrated control
Experiences with the IGRs in large-scale replicated and unreplicated experiments since 1996 have shown a very consistent pattern: pest population increase to and through the economic threshold (see Fig. 3), an IGR spray is applied, continued short-term growth in the population ensues, drastic population collapse occurs and eventual long-term suppression and subeconomic levels of B. tabaci are achieved (Figs. 7 and 8). This extended suppressive interval made possible by the use of a selective insecticide was coined ‘bioresidual’.68,114 It is defined as the ‘combined contribution of all natural mortality factors … that allow for lowering of the general equilibrium position of the target pest and long-term pest control following the use of selective insecticides that is otherwise absent with most broad-spectrum insecticide use’.109 The goal in developing this term was better to communicate to growers the potential selectivity benefits of their control chemistry and to accommodate all the mortality processes present in a selective system, especially those related to conservation biological control. Stern et al.1 defined natural control as the combined actions of abiotic and biotic elements of the environment. Bioresidual is simply that natural control possible when a selective insecticide is used in a well-timed and effective manner, providing extended suppression that has been measured up to 12 weeks in the cotton/whitefly ecosystem. This regulating ecosystem service helps form the foundation our IPM system (Fig. 1).
Figure 7.
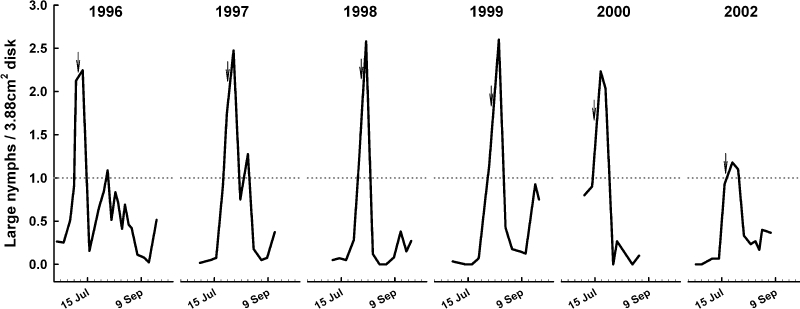
Population dynamics of B. tabaci over a six year period in IGR-treated fields at the University of Arizona, Maricopa Agricultural Center in south-central Arizona. The dotted horizontal line represents an action threshold of 1 large nymph (3rd or 4th instar) per quarter-sized leaf disk (3.88 cm2). Populations increased past threshold, were treated once with an IGR, and after a short delay, collapsed and remained sub-economic in most years (also see Fig. 3). Arrows denote the timing of insecticide application.
Figure 8.
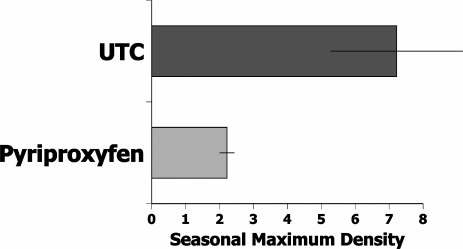
Seasonal average maximal density ( ± SEM) for large nymphs (3rd or 4th instar) per quarter-sized leaf disk (3.88 cm2) over five years of replicated, large-scale assessments of IGRs in comparison to the UTC in cotton, Maricopa, Arizona, USA (P = 0.02). The properly timed usage of IGR sprays significantly lowered the maximal seasonal density of whiteflies compared to the UTC. Severe economic loss (reductions in yield and quality) is common at levels in excess of 4 large nymphs per disk86.
Designing and labeling a product as selective or ‘biorational’ is not sufficient, however, to establish its selectivity.113 Some effort must be made to validate the candidate approach or product in the system of interest, because, as noted, the control dynamic is embedded in an ecological context. A product may be fully selective in one environment and catastrophically disruptive in another. Laboratory and other bioassays without an ecological context are inadequate for establishing insecticide selectivity in a system.
There are several ways to verify and validate an approach as being selective. General observation can establish the presence and function of natural enemies. Natural enemy densities can be examined in comparative systems using innovative community ordination methods (e.g. principal response curves).115,116 The functional role of natural enemies and other mortality factors can further be inferred from predator–prey dynamics and demography. The Arizona system is based on this kind of careful research and validation, which are key to the development and implementation of IC, the unique interplay between chemical and biological control that is fully maximized by a validated decision support system (Fig. 1).
Early work carried out in the 1950s, even before the availability of putative selective insecticides, showed that selectivity could be accomplished in part through effective timing of insecticide applications. Naranjo et al.69 showed that some changes in adult thresholds for conventional materials could have relatively minor effects on B. tabaci control dynamics, yet relatively large impacts on the associated predator dynamics (Fig. 9). They concluded that more potential for biological control was possible if producers deferred chemical controls until ten adults per leaf instead of five adults per leaf. Lower thresholds were too expensive to maintain, and higher ones were insufficient to prevent economic loss (see Section 4.1). They further concluded that the date of first spray was a key factor in predator dynamics in a system dependent on broad-spectrum chemistry.
Figure 9.
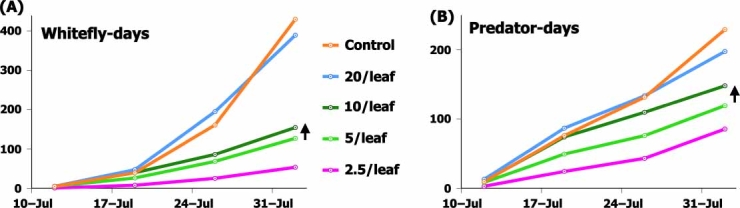
(A) B. tabaci population dynamics in cumulative whitefly-days (adults per leaf) and (B) predator population dynamics in cumulative predator-days (total counts of 12 species of predators per 25 sweeps), for five nominal action thresholds (adult whiteflies per 5th mainstem leaf) for conventional chemistry and an UTC in cotton, 1994, Maricopa, Arizona, USA. There were significant treatment effects on every date (P < 0.05). Arrows show small and somewhat larger increases in whitefly and predator densities, respectively, for two candidate thresholds, suggesting benefits in conservation biological control by deferring treatments until 10 adults per leaf. Re-drawn from69.
Natural enemy community responses
Principal response curves (PRCs), a multivariate, time-dependent, analytic approach,115,116 present a powerful way to visualize and understand whole-community responses to external treatments via canonical coefficients and species weights. Canonical coefficients are a scaled measure of species densities relative to some standard (usually an untreated check), while species weights denote the correspondence of each species to the overall community pattern. Species with weights greater than 0.5 are generally considered most influential and most reflective of the estimated PRC pattern. Species with weights lower than −0.5 are also considered to influence the trends shown, but in the opposite direction. When a broad-spectrum insecticide is used, for example, dramatic and immediate reductions in natural enemy densities occur compared with an untreated check (UTC)83 (Fig. 10). Likewise, effects from a single broad-spectrum spray in cotton to control L. hesperus had significant negative consequences for the beneficial arthropod community for up to 7 weeks.
Figure 10.
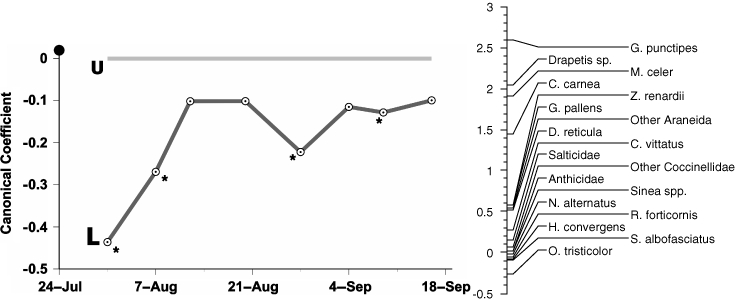
Principal response curves (PRC) showing the long-term negative effects possible from a single broad spectrum Lygus insecticide [(L) = acephate spray] on the predatory arthropod community comprising of ca. 20 taxa in cotton, 1997, Maricopa, Arizona, USA. Species weights indicate strength of the trend for each species or species group, with values > 0.50 generally reflecting the trends shown. Weights < −0.50 indicate a negative association or inverse of the trends depicted. The product of species weight and the canonical coefficient (y-axis) for a given treatment and time equals the natural log change in density of that species relative to the control. Dot at top of y-axis denotes timing of an acephate spray; Stars, denote significant differences between the acephate treatment and the UTC (y = 0) by date (P < 0.05). The PRC analysis over all dates was significant (P < 0.01) based on an F-type permutation test. Adapted with permission from Elsevier83.
As part of a very large, commercial-scale evaluation, Naranjo et al.82 compared IGRs with conventional rotations of broad-spectrum chemistry commonly used at the time. Regimes initiated with either IGR generally supported more natural enemies than the conventional control, with a crab spider, Misumenops celer Hertz, among the top influencers of that PRC (Fig. 11). The only departure from the overall trend was when a commercially required spray for L. hesperus was needed over the entire experiment and a monsoon-associated storm impacted on the study area. Fewer sprays were needed in the IGR regimes for comparable control as a result of what was later learned to be the bioresidual present.
Figure 11.
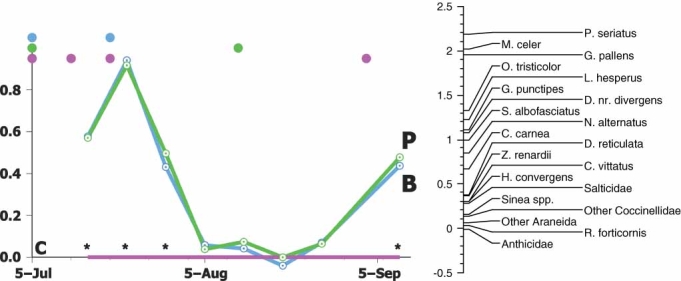
Principal response curves (PRC) showing the effects of different whitefly control strategies on the predatory arthropod community comprising of 20 taxa in cotton, 1996, Maricopa, Arizona, USA. Species weights indicate strength of the trend for each species or species group, with values > 0.50 generally reflecting the trends shown. Weights < −0.50 indicate a negative association or inverse of the trends depicted. The product of species weight and the canonical coefficient (y-axis) for a given treatment and time equals the natural log change in density of that species relative to the control. C = a rotation of conventional chemistry (control); P = pyriproxyfen-initiated rotation of IGRs; B = buprofezin-initiated rotation of IGRs; Dots denote timing of sprays of chemistry with corresponding color; Stars, denote significant differences between either IGR treatment and the rotation of conventional insecticides (y = 0) by date (P < 0.05). The PRC analysis over all dates was significant (P < 0.01) based on an F-type permutation test. Adapted with permission from Taylor & Francis82.
Subsequent large-plot replicated assessments, including UTCs, showed similar trends;83 only the major drivers of the relationships changed. In 1997, the sucking predators Orius tristicolor White and Geocoris punctipes Say were the predominant species involved (Fig. 12). In 1998, an empidid fly, Drapetis nr. divergens Loew, had the largest species weight (Fig. 13). This small fly preys on adult B. tabaci as an adult.117 While not a specialist, strictly speaking, it is often associated with the presence of adult B. tabaci in cotton. A comparable species is present in Israeli cotton and may play a similar role there, where B. tabaci became a key pest in the mid-1980s (D. subaenescens Collin).118 The 1999 study was driven by a combination of the species already mentioned (Fig. 14). Some other predators also play significant roles in these analyses. Chewing predators like Collops vittatus Say were important in two of the four years examined (1996–1999). Coccinellids are not typically present in mid-summer cotton in large numbers in Arizona, but a complex of species was important in one of the four years (Fig. 14). Chrysoperla carnea s.l. Stephens also had species weights greater than 0.5 in each year studied, but generally lower than those of the other sucking predators in the system. The larger predators present (e.g. Zelus renardii Kolenarti in 1997, Nabis alternatus Parshley in 1996, and spiders in 1997 and 1999) (Figs 11 to 14) may function more as intraguild predators on the primary predators of B. tabaci in the system.
Figure 12.
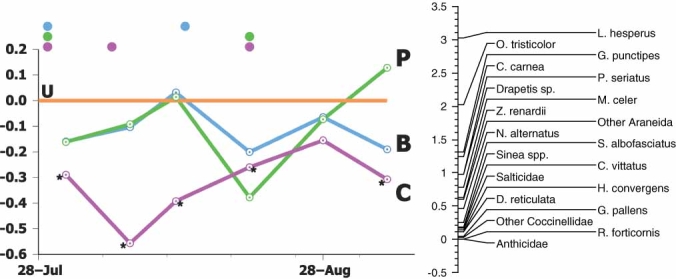
Principal response curves (PRC) showing the effects of different whitefly control strategies on the predatory arthropod community comprising of ca. 20 taxa in cotton, 1997, Maricopa, Arizona, USA. Species weights indicate strength of the trend for each species or species group, with values > 0.50 generally reflecting the trends shown. Weights < −0.50 indicate a negative association or inverse of the trends depicted. The product of species weight and the canonical coefficient (y-axis) for a given treatment and time equals the natural log change in density of that species relative to the control. U = UTC (control); C = a rotation of conventional chemistry; P = pyriproxyfen-initiated rotation of IGRs; B = buprofezin-initiated rotation of IGRs; Dots denote timing of sprays of chemistry with corresponding color; Stars, denote significant differences between adjacent treatment and the UTC (y = 0) by date (P < 0.05). The PRC analysis over all dates was significant (P < 0.01) based on an F-type permutation test. Adapted with permission from Elsevier83.
Figure 13.
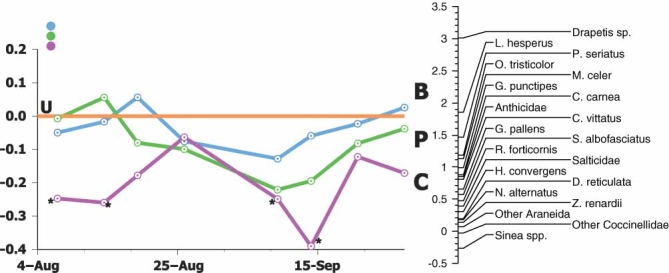
Principal response curves (PRC) showing the effects of different whitefly control strategies on the predatory arthropod community comprising of ca. 20 taxa in cotton, 1998, Maricopa, Arizona, USA. Species weights indicate strength of the trend for each species or species group, with values > 0.50 generally reflecting the trends shown. Weights < −0.50 indicate a negative association or inverse of the trends depicted. The product of species weight and the canonical coefficient (y-axis) for a given treatment and time equals the natural log change in density of that species relative to the control. U = UTC (control); C = a rotation of conventional chemistry; P = pyriproxyfen-initiated rotation of IGRs; B = buprofezin-initiated rotation of IGRs; Dots denote timing of sprays of chemistry with corresponding color; Stars, denote significant differences between adjacent treatment and the UTC (y = 0) by date (P < 0.05). The PRC analysis over all dates was significant (P < 0.01) based on an F-type permutation test. Adapted with permission from Elsevier83.
Figure 14.
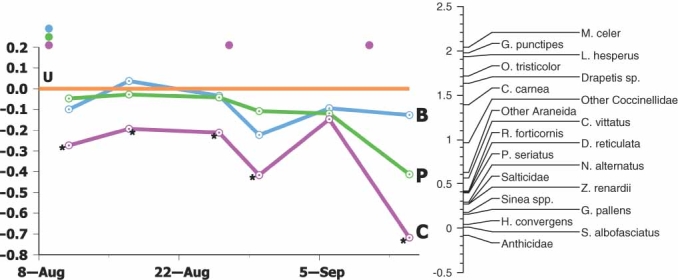
Principal response curves (PRC) showing the effects of different whitefly control strategies on the predatory arthropod community comprising of ca. 20 taxa in cotton, 1999, Maricopa, Arizona, USA. Species weights indicate strength of the trend for each species or species group, with values > 0.50 generally reflecting the trends shown. Weights < −0.50 indicate a negative association or inverse of the trends depicted. The product of species weight and the canonical coefficient (y-axis) for a given treatment and time equals the natural log change in density of that species relative to the control. U = UTC (control); C = a rotation of conventional chemistry; P = pyriproxyfen-initiated rotation of IGRs; B = buprofezin-initiated rotation of IGRs; Dots denote timing of sprays of chemistry with corresponding color; Stars, denote significant differences between adjacent treatment and the UTC (y = 0) by date (P < 0.05). The PRC analysis over all dates was significant (P < 0.01) based on an F-type permutation test. Adapted with permission from Elsevier83.
The idea that different species dominate the community analyses in different years or locations in Arizona cotton is a remarkable testament to the complexity and plasticity of the cotton arthropod food web. Certain conditions may favor certain pathways in certain years and other pathways in other years. Using a species weight > 1 and omitting the primarily plant-feeding mirids (Lygus hesperus, Pseudatomoscelis seriatus Reuter), four, three, three and five species dominated the food webs in 1996–1999 respectively. These were the sucking predators Geocoris spp. (usually punctipes, but also pallens), O. tristicolor and C. carnea, the empidid D. nr. divergens, and the crab spider M. celer.
Biological control function
The function of this complex of predators can also be inferred by their relative abundance in these otherwise well-controlled field experiments. For example, the marginal decline in overall predator abundance, as depicted in PRCs, in the IGR-initiated regimes relative to UTCs is suggestive of some weak density dependence in relation to the most abundant prey83 (Figs. 11 to 14). Predator to prey ratios are another comparative method for inferring predator function. Naranjo et al.83 constructed ratios based on all predators captured in 50 sweeps and all B. tabaci per leaf found in cotton. The ratio increased initially in the UTC cotton, but then plateaued in what was an outbreak population of B. tabaci (Fig. 15). When conventional chemistry was used repeatedly to maintain control of B. tabaci populations, the predator:prey ratios were similar to, but lower than, the UTC, suggesting that both predator and prey were reduced equally. However, after initially similar ratios, the IGR-initiated regimes eventually resulted in higher predator:prey ratios 3–5 weeks after application. This suggests that the chemical residual of the selective IGRs was providing suppressive control initially, but that natural enemies were playing a large role in the bioresidual, thereby providing effective pest control extending to the end of the season.
Figure 15.
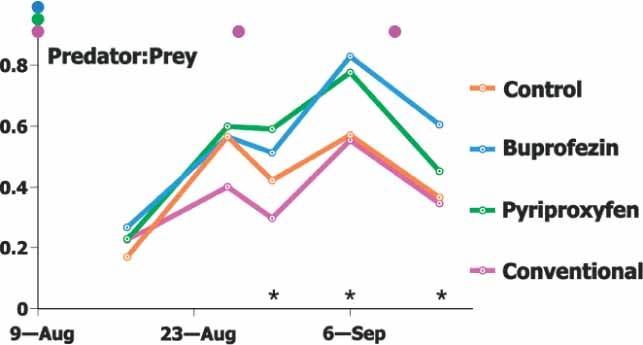
Predator–prey dynamics (as ratio of total counts of ca. 20 species of predators per 50 sweeps to total whiteflies per leaf) for three different whitefly control strategies and an UTC (Control) in cotton, 1999, Maricopa, Arizona, USA. Dots denote timing of sprays of chemistry with corresponding color; Stars, denote significant treatment effects by date (P < 0.05). IGRs increase the ratios favoring more efficient pest control. Adapted with permission from Elsevier83.
Predator:prey ratios that favor pest control are very much in keeping with the vision of Stern et al.;1 ‘The ideal material is not one that eliminates all individuals of the pest species … [It] is the one that shifts the balance back in favor of natural enemies’. Interestingly, in spite of all their work with spotted alfalfa aphid and their many commercial evaluations of predator and prey abundances, Stern and coworkers never analyzed the basic relationship of predator to prey ratios. There was a huge advantage to the use of demeton compared with either parathion or the UTC (Fig. 16). The UTC supported outbreak levels of aphids. Both parathion and demeton effectively controlled the pest, but the latter was less harmful to the natural enemies, sometimes leading to a situation where abundant coccinellids had insufficient prey and even resorted to cannibalism.89 Nonetheless, demeton-treated areas consistently supported much higher predator:prey ratios and achieved better compatibility between chemical and biological controls.
Like parathion in the alfalfa system of 50 years ago, conventional sprays lowered prey densities as well as predator densities in the Arizona system. Furthermore, like Stern's demeton, the IGRs not only reduced prey numbers effectively, they also conserved existing predator numbers and created a more favorable balance of predators to prey. This more efficient control system should create collateral benefits in regulation of other pests in the system.
Validating biological control function through demography
To gain a better understanding of how survivorship of B. tabaci was changing in IGR-treated systems, it was necessary to use techniques of demography, specifically life tables. Naranjo and Ellsworth37,109 tracked 14 summer generations of treated and untreated B. tabaci over multiple years. The resulting survivorship curves for the two systems are striking in how similar the endpoints appear (Fig. 17). Few B. tabaci survive in cotton, even when not treated with insecticides, on average just ca 4.4%. However, when IGRs are used, not only is the shape of the curve changed, i.e. more B. tabaci die sooner in their life cycle, but fewer than 1% on average survive. Thus, pest managers are trying to leverage, on average, only about 4% absolute change in survivorship by using insecticides.109
Figure 17.
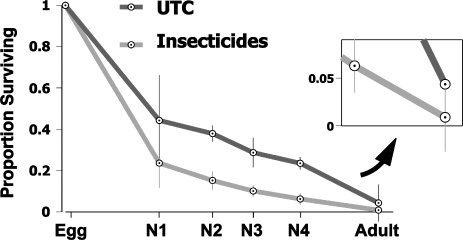
Average survivorship ( ± SEM) for 14 summer field generations of B. tabaci for IGR-initiated regimes (Insecticides) and an UTC (Untreated) in cotton, 1997–1999, Maricopa, Arizona, USA. Inset, blown-up view of survivorship to the adult stage. Only ca. 4% absolute difference in survivorship to the adult stage separates outbreak levels of whiteflies from well-controlled populations37,109.
Life table data can be used to calculate marginal mortality rates and, from this, irreplaceable or indispensable mortality, which is that portion of the total generational mortality that would not occur if a given mortality factor were eliminated.119 What happens when a mortality source fails to function? Is it replaced with contemporaneous factors? The Millennium Ecosystem Assessment91 concluded: ‘In many agricultural areas, pest control provided by natural enemies has been replaced by pesticides’. This is the antithesis of IC, where the two tactics fail to augment each other.
In untreated cotton, Naranjo and Ellsworth37 calculated the largest net reproduction in B. tabaci when predation or dislodgement (a factor related in part to predation and in part to weather) was excluded from their analyses of irreplaceable mortality (see Fig. 4). They concluded that conserving predators and managing immigration of adult B. tabaci best achieved efficient pest management (see Fig. 5). Similar life table studies over 3 years in untreated cotton embedded in a multihost design showed very similar trends (Fig. 18) (Naranjo SE, Ellsworth PC and Cañas L, unpublished), with predation providing the largest proportion of irreplaceable mortality in B. tabaci populations. In all cases (7 years in total), parasitism has not been influential in B. tabaci control in cotton, in spite of rather significant shifts in the parasitoid fauna in Arizona.120
Figure 18.
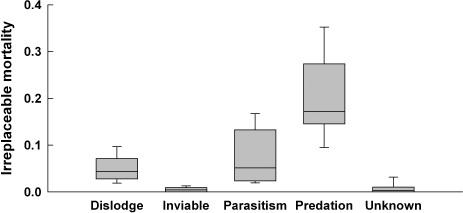
Box plots showing levels of irreplaceable mortality of immature B. tabaci by various factors in unsprayed cotton fields over 20 generations, 2000–2003, Maricopa, Arizona, USA (Naranjo SE, Ellsworth PC and Cañas L, unpublished). The line within each box represents the median, the box bounds the 25th and 75th percentiles, and the whiskers denote the 10th and 90th percentiles. Predation is the major mortality factor operating in untreated cotton.
When insecticides are used in cotton, the relative importance of mortality factors is similar, but the relationship between insecticide-induced mortality and predation changes over time.109 Life tables constructed from the generation of B. tabaci present during the initiation of chemical controls were contrasted to life tables constructed from a subsequent generation, 3–6 weeks after the initial use of either IGR or conventional insecticides (Fig. 19). During the first generation of exposure, irreplaceable mortality supplied by insecticides or predation is similar no matter what compound is used. Either regime kills B. tabaci and is significantly different from the UTC. This shows how an effective insecticide supplants the irreplaceable mortality contribution of predation (Fig. 19A). Looking at the next time course (Fig. 19B), there is still significant, but much lower, insecticide mortality. Irreplaceable mortality due to predation, however, increased significantly where IGRs were used, now similar to the UTC. This shows mechanistically how one component of bioresidual, predation, is fostered by the use of selective insecticides.
Figure 19.
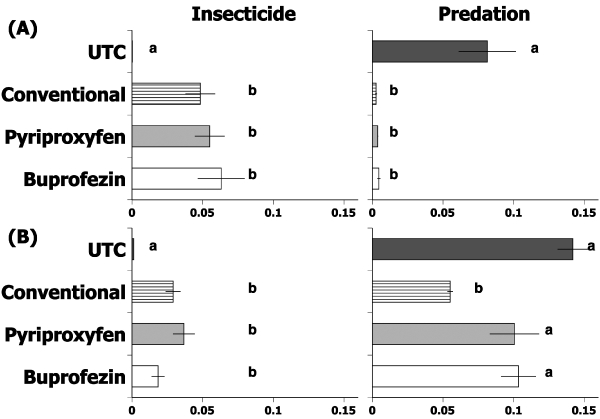
Irreplaceable mortality ( ± SEM; N = 12) of immature B. tabaci by insecticides or predation for three different whitefly control strategies and an UTC during (A) the first generation post-spray and (B) the second generation post-spray, 1997–1999, Maricopa, Arizona, USA. IGRs enable conservation biological control in the generation following application comparable to what is present in the UTC and contribute to their bioresidual. Adapted with permission from Elsevier109.
Validation by disabling biological control
The final step in verifying that IC is functioning is to attempt to disable it. One obvious way to test this is to remove the chemical control agent and measure what happens. In years of testing, the UTC typically suffers serious deposits of whitefly-excreted sugars that make the marketing of the cotton impossible, and sometimes cause yield losses. However, this fails to demonstrate the compatibility of chemical and biological controls; only that chemical control is necessary to accomplish commercial production. Thus, the approach must involve disabling the biological control agents present in the system, usually through chemical exclusion.
In a recent study of irrigation and natural enemy effects, our graduate student excluded natural enemies in replicated plots using acephate on a biweekly basis for a total of four sprays (Asiimwe P, Ellsworth PC and Naranjo SE, unpublished). Acephate has poor efficacy against B. tabaci in Arizona cotton (Ellsworth, unpublished data), but is broadly toxic to a wide range of natural enemies and routinely used for L. hesperus control in Arizona.121 No other sprays were made. In this field experiment, regardless of irrigation regime, the acephate-treated plots sustained heavy damage from B. tabaci, including excess sugars, extensive sooty mold development and premature defoliation and plant death (Fig. 20). The UTC sustained much lower B. tabaci populations and the plants continued to grow normally.
Figure 20.
Photos of representative plots on two dates, 16-Sep (A, B) and 30-Sep (C, D) showing progressive damage resulting in premature defoliation and plant death in cotton by whiteflies released from control by four bi-weekly sprays of a broad spectrum insecticide (acephate; B, D) commonly used for Lygus control in comparison to UTCs (A, C), 2008, Maricopa, Arizona, USA.
In another series of studies to investigate candidate chemistry for L. hesperus control, biweekly sprays of acephate, in contrast to metaflumizone and flonicamid, were implemented for a total of three sprays in cotton. Community analyses showed severe and season-long depression of ca 20 non-target species where acephate was used. Furthermore, during years (2006–2007) when populations were at historically low levels otherwise, B. tabaci outbreaks were evident in the acephate-treated plots after the second application. No B. tabaci chemical controls were necessary in the other plots, and flonicamid and metaflumizone appear to be excellent selective controls for L. hesperus (Ellsworth PC and Naranjo SE, unpublished). This discovery fueled the widespread adoption of flonicamid, once it became commercialized and widely available, starting in 2007. More than half of all L. hesperus sprays in 2007 and more than 91% of all fields treated for L. hesperus in 2008 were sprayed at least once with flonicamid in central Arizona cotton (Ellsworth PC, unpublished data).
The relationship between control of B. tabaci and control of L. hesperus is a not a new one, as L. hesperus has been the number-one, yield-limiting pest of cotton since 1997 in Arizona.121,122 Ironically, it is in part because of the success of other selective controls for B. tabaci control (IGRs) and for lepidopteran control (Bt cotton) that L. hesperus has gained in prominence. Furthermore, the idea that chemical controls for one pest could interfere with the biological control of another pest is well known by practitioners. Stern and colleagues1 wrote: ‘Where prophylactic treatments are proved to be necessary for a perennial pest, selective materials must be developed and utilized to foster biological control both of other pests and of the pest of direct concern at other times’.
Impact and outlook for IC and IPM
Some might say that IPM as a concept has left IC behind, and introduced new depths of complexity that the ICC did not readily accommodate (e.g. reference 123). However, given that arguably most IPM programs depend on some remedial chemical controls, IC could and should still exist at the center of the IPM concept (e.g. Fig. 1). Recalling the ecosystem nature of the Arizona system and its pests, the multidimensional elements of the Arizona IPM system owes its success to pest-source reduction and cross-commodity agreements in cotton, vegetables and melons,29,100 an unprecedented scale of host plant resistance in widespread transgenic Bt cotton deployment for pink bollworm control, IC of B. tabaci using fully or partially selective insecticides and most recently the availability and widespread usage of selective insecticides for L. hesperus control.
The results have been impressive and include an areawide change in the agroecosystem. As noted, Arizona's B. tabaci are shared among cotton, melon and vegetable crops that serve as year-round crop islands (Fig. 2). The cotton situation here has been discussed, but the outbreak of 1991/1992 nearly wiped out the fall melon and vegetable industry in Arizona and southern California. Emergency exemptions were sought for the soil use of imidacloprid. Palumbo (unpublished data) set up a series of replicated studies of soil use in commercial lettuce fields and compared B. tabaci dynamics in treated and untreated plots. The control was impressive, but the immigrating pressure was nearly overwhelming in 1993, and the UTC contained > 8 large nymphs cm−2. The industry adopted this soil usage more widely in 1994–1995, and areawide pressure in the UTC was lowered by an order of magnitude (ca 1–1.6 large nymphs cm−2), implicating a broad-area regional effect on B. tabaci population dynamics. Once the IGRs were deployed in 1996 in the cotton system, another order of magnitude lowering of B. tabaci densities was observed in the UTC over nearly a decade (ca 0.1 large nymphs cm−2). The combination of source reduction in fall melons and vegetables and IC of B. tabaci in cotton helped produce a decade-long period of areawide suppression of B. tabaci densities in the agroecosystem. Notable are new concerns (2006–2008) about lost performance in the imidacloprid–B. tabaci system where fall vegetables are grown (ca 50% reduction) (Palumbo JC, unpublished data).
The impact on grower practices, economics, health and environmental risks has also been historic. As part of an ongoing cotton survey process, the number and costs of cotton insect control in Arizona are determined annually.124 Cotton growers in Arizona were accustomed to spraying 5–10 times per season, starting in the 1990s (Fig. 21). Bemisia tabaci broke out statewide in 1992, and the majority of the control was focused on this pest using very broad-spectrum pyrethroids, organophosphates and endosulfan. The following year (1993) was filled with uncertainty, and growers curtailed their season by more than 30 days in order to minimize the damage caused by B. tabaci and the costs for control. The synergized pyrethroids became the control standards of the time (1993–1995), but, as noted, resistance threatened the continued use of these broad-spectrum mixtures and helped create outbreak conditions in 1995. In 1996, the IGRs were introduced, along with an extensive educational program to support the usage of these novel chemistries with decision support tools like sampling and thresholds.68 Transgenic Bt cotton for pink bollworm control was also introduced that year, but adopted on a minority of acreage. Adoption of Bt cotton increased to 60–80% over the next decade, until a pink bollworm eradication program was implemented and stimulated near-complete adoption starting in 2006 (95–98.3%). In 2008 there were no grower-initiated foliar insecticide sprays to control pink bollworm for the first time since 1965. Eradication program sprays against pink bollworm were made on fewer than 200 ha statewide (Antilla L, private communication, 2008). Selective chemistry for L. hesperus (i.e. flonicamid) became widely available in 2007 and led to acephate falling from its decade-long position as one of the top two most frequently used insecticides in Arizona cotton (Ellsworth PC, unpublished).
Figure 21.
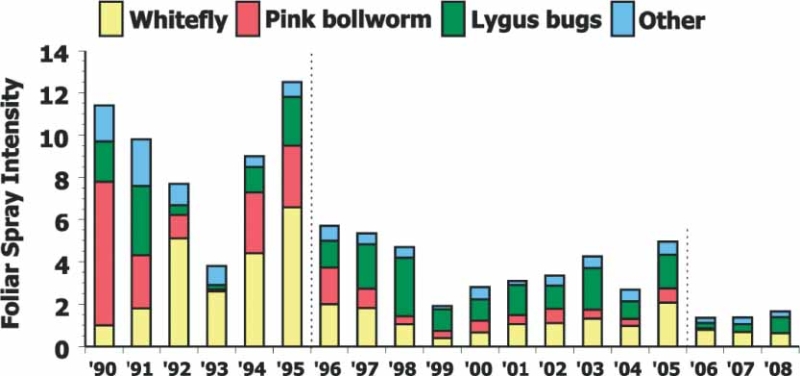
Statewide average foliar insecticide intensity (∼ sprays) made in cotton for key pests over time, 1990–2008, Arizona, USA. The IGRs, pyriproxyfen and buprofezin, and transgenic Bt cotton were introduced in 1996; pink bollworm eradication program efforts began in 2006 and flonicamid for Lygus control became available in 2006–2007 (derived from124).
The number of sprays for B. tabaci declined to ca one spray per season shortly after the introduction of the IGRs. Environmental conditions and other factors in 2005 led to outbreak conditions for B. tabaci once again, but sprays and control costs were still relatively restrained. Growers sprayed, on average, 4.1 times to control B. tabaci prior to the introduction of the IGRs and after the invasion of the B-biotype of B. tabaci (5 years, 1991–1995). In contrast, growers sprayed 1.18 and 1.25 times in the 5 and 10 year post-IGR introduction intervals respectively. Even wider deployment of Bt cotton, starting in 2006 with pink bollworm eradication efforts and introduction and adoption of flonicamid as the principal L. hesperus control agent, has driven foliar insecticide usage to 30 year lows, averaging just 0.60 sprays for B. tabaci control and 1.46 sprays for all arthropod pests during the last 3 years.124
IC was critical to the activation of this remarkable trend, which represents a reduction of ca 70% in foliar insecticide use compared with pre-IGR deployment, and enabled significant economic and environmental savings as well. The authors estimate cumulative post-deployment savings in control costs and insect-related yield loss prevention of over $ 201 599 000 in the last 14 years (derived from data in reference 124). Furthermore, in spite of a general trend of more expensive per application costs, growers invested a 30 year record low in insecticides in 2007 (<$69 ha−1 versus record high 1995 levels of >$749 ha−1) (Fig. 22). The insecticide load on the environment creates untold savings to non-target organisms and in risks to human health. After a decade's high usage of insecticides in 1995, there has been about a 0.77 million kg reduction in insecticides used annually, starting in 2006.
Figure 22.
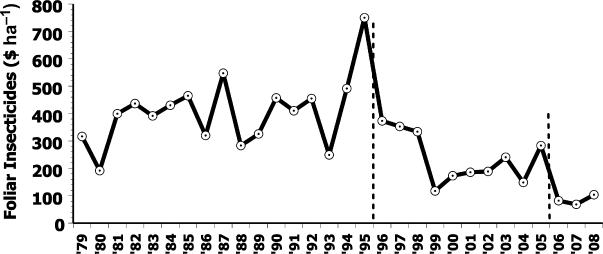
Statewide average foliar insecticide costs including application costs (in $ US per hectare) invested by growers to control all arthropod pests, adjusted for inflation, 1979–2008, Arizona, USA (derived from124). IGRs, transgenic Bt cotton, and the Arizona IPM plan for cotton was introduced in 1996. Pink bollworm eradication activities began in 2006 and flonicamid for Lygus control became available in 2006–2007.
The detailed synthesis here provides for a rare documented case of IC, where the interplay between biological and chemical control is explicitly measured and exploited for augmented pest control. With the added and coincident significant benefits of Bt cotton (1996–) and later eradication efforts (2006–) for the pink bollworm, and more selective alternatives to acephate for L. hesperus control, growers are now able better to exploit and build upon IC of B. tabaci. This effort has engendered a culture of respect for the in-field mortality dynamics and cross-commodity interactions that govern the B. tabaci system, and has led to large IPM subscription rates by the agricultural community in Arizona. Broad-spectrum chemistry still plays a role, but is relegated to the option of last resort and generally deferred to the final seasonal spray, if needed at all. The result has been an unprecedented stability of ecosystem services and major economic and environmental gains in Arizona cotton that has extended to benefit the entire agroecosystem of the region.
Acknowledgments
The program under development in Arizona required significant research and extension resources. Many dedicated staff were involved, too many to list completely. However, the authors wish to give special thanks to V Barkley, D Meade, J Diehl, L Cañas, D Sieglaff, R Burke and G Owens. They also wish to thank the following agencies who supported this enterprise through in-house funds and competitive grants over the last 17 years, in addition to the many industry gifts and grants provided to examine and assess whitefly control chemistry: USDA-ARS (1992-2008), USDA-ES IPM Special Projects (1992-1995), National and Western Regional Pesticide Impact Assessment Program (1993-1994, 1996-1997), Arizona Cotton Growers Association (1994, 2006-2008), Arizona Cotton Research and Protection Council (1995), Cotton Incorporated (1996-1999, 2000-2002, 2006-2008), USDA-Pest Management Alternatives (1996, 2000-2003), Western Regional IPM Competitive Grants Program (1996, 1999-2002), Western IPM Center Working Groups (2003-2008) and Arizona Pest Management Center (2003-2008).
REFERENCES
- 1.Stern VM, Smith RF, van den Bosch R, Hagen KS. The integrated control concept. Hilgardia. 1959;29:81–101. [Google Scholar]
- 2.Trumble JT, Morse JP. Economics of integrating the predaceous mite Phytoseilus persimilis (Acari: Phytoseiidae) with pesticides in strawberries. J Econ Entomol. 1993;86:879–885. [Google Scholar]
- 3.Furlong MJ, Shi ZH, Liu YQ, Guo SJ, Lu YB, Liu SS, et al. Experimental analysis of the influence of pest management practice on the efficacy of an endemic arthropod natural enemy complex of the diamondback moth. J Econ Entomol. 2004;97:1814–1827. doi: 10.1093/jee/97.6.1814. [DOI] [PubMed] [Google Scholar]
- 4.Hamilton AJ, Schellhorn NA, Endersby NM, Ridland PM, Ward SA. A dynamic binomial sequential sampling plan for Plutella xylostella (Lepidoptera: Plutellidae) on broccoli and cauliflower in Australia. J Econ Entomol. 2004;97:127–135. doi: 10.1093/jee/97.1.127. [DOI] [PubMed] [Google Scholar]
- 5.Musser FR, Nyrop JP, Shelton AM. Integrating biological and chemical controls in decision making: European corn borer (Lepidoptera: Crambidae) control in sweet corn as an example. J Econ Entomol. 2006;99:1538–1549. doi: 10.1603/0022-0493-99.5.1538. [DOI] [PubMed] [Google Scholar]
- 6. Crop Information. [Online]. National Cotton Council. Available: http://www.cotton.org/econ/cropinfo/index.cfm [accessed: 21 September 2009]
- 7.Naranjo SE, Ruberson JR, Sharma HC, Wilson L. The present and future role of insect-resistant genetically modified cotton in IPM. In: Romeis J, Shelton AM, Wu KM, editors; Kennedy GG, editor. Integration of Insect-Resistant Genetically Modified Crops with IPM Systems. Berlin, Germany: Springer; 2008. pp. 159–194. [Google Scholar]
- 8.Naranjo SE. Cotton arthropod IPM. In: Radcliff EB, Hutchison WD, Luttrell RG, editors; Cancelado RE, editor. Integrated Pest Management. Cambridge, UK: Cambridge University Press; 2009. pp. 324–340. [Google Scholar]
- 9.Smith RF, Huffaker CB. Integrated control strategy in the United States and its practical implementation. OEPP/EEPO Bull. 1973;3:31–49. [Google Scholar]
- 10.Cotton—the crop and its pesticide market. Pestic News. 1995;30:1. [Google Scholar]
- 11.Carruth LA, Moore L. Cotton scouting and pesticide use in eastern Arizona. J Econ Entomol. 1973;66:187–190. doi: 10.1093/jee/66.1.187. [DOI] [PubMed] [Google Scholar]
- 12.Gennadius P. Disease of tobacco plantations in the Trikonia. The aleurodid of tobacco. Ellenike Georgia. 1889;5:1–3. [Google Scholar]
- 13.Mound LA, Halsey SH. A Systematic Catalogue of the Aleyrodidae (Homoptera) with Host Plant and Natural Enemy Data. Chichester, UK: British Museum (Natural History), London, and John Wiley & Sons, Ltd; 1978. Whitefly of the world. [Google Scholar]
- 14.Greathead AH. Host plants. In: Cock MJW, editor. Bemisia tabaci—a Literature Survey on the Cotton Whitefly with an Annotated Bibliography. Ascot, UK: CAB International Institute of Biological Control; 1986. pp. 17–25. [Google Scholar]
- 15.Simmons AM, Harrison HF, Ling KS. Forty-nine new host plant species for Bemisia tabaci (Hemiptera: Aleyrodidae) J Entomol Sci. 2008;11:385–390. [Google Scholar]
- 16.Jones DR. Plant viruses transmitted by whiteflies. Eur J Plant Pathol. 2003;109:195–219. [Google Scholar]
- 17.Oliveira MRV, Henneberry TJ, Anderson P. History, current status, and collaborative research projects for Bemisia tabaci. Crop Prot. 2001;20:709–723. [Google Scholar]
- 18.Hequet EF, Henneberry TJ, Nichols RL, editors. 2007. in Sticky cotton—causes, impacts and prevention, United States Department of Agriculture, Agricultural Research Service Technical Bulletin No. 1915.
- 19.Russell LM. Collection records of Bemisia tabaci (Gennadius) in the United States. USDA Coop Econ Insect Rep. 1975;25:229–230. [Google Scholar]
- 20.Allen RM, Tucker H, Nelson RA. Leaf crumple disease of cotton in Arizona. Plant Dis Rep. 1960;44:246–250. [Google Scholar]
- 21.Butler GD., Jr . Bemisia tabaci as a cotton pest in the desert cotton-growing areas of the southwestern United States. In: Brown JM, Henneberry TJ, editors. Proc Beltwide Cotton Prod Res Conf. Memphis, TN: National Cotton Council; 1984. pp. 195–197. [Google Scholar]
- 22.Brown JK, Frohlich DR, Rosell RC. The sweetpotato or silverleaf whiteflies: biotypes of Bemisia tabaci or a species complex? Annu Rev Entomol. 1995;40:511–534. [Google Scholar]
- 23.Perring TM. The Bemisia tabaci species complex. Crop Prot. 2001;20:725–737. [Google Scholar]
- 24.Ellsworth PC. in Arizona: a decade of research, implementation and education. In: Dugger P, Jones JS, Cotton IPM, editors; Richter D, editor. Proc Beltwide Cotton Conf. Memphis, TN: National Cotton Council; 2001. pp. 1088–1096. [Google Scholar]
- 25.Watson TF. Cotton, a College of Agriculture Report, Series P-94. Tucson, AZ: University of Arizona; 1993. Chemical control of the sweetpotato whitefly in cotton; pp. 221–239. [Google Scholar]
- 26.Dennehy TJ, Ellsworth PC, Nichols RL. 1995. p. 4. Whitefly management in Arizona cotton 1995, IPM Series No. 3, University of Arizona, Cooperative Extension.
- 27.Dennehy TJ, Ellsworth PC, Watson TF. 1995. p. 2. Whiteflies in Arizona: pocket guide, University of Arizona, Cooperative Extension, Publ. 195009.
- 28.Ellsworth PC, Watson TF. Pocket Guide ‘96. Whiteflies in Arizona (No. 7). [Online]. University of Arizona, College of Agriculture and Life Sciences, Cooperative Extension, Tucson, AZ, 1 p. Available: http://cals.arizona.edu/crops/cotton/insects/wf/wfly796.pdf [accessed: 21 September 2009]
- 29.Palumbo JC, Horowitz AR, Prabhaker N. Insecticidal control and resistance management for Bemisia tabaci. Crop Prot. 2001;20:739–765. [Google Scholar]
- 30.Horowitz AR. Chemical control of Bemisia—management and application. In: Gerling D, Ishaaya I, Mayer RT, editors. Bemisia: 1995 Taxonomy, Biology, Damage, Control and Management. Andover, Hants, UK: Intercept Ltd; 1996. pp. 537–556. [Google Scholar]
- 31.Henneberry TJ, Toscano NC, Perring TM, Faust RM. 1997. p. 268. Silverleaf whitefly, 1997 supplement to the 5-Year National Research and Action Plan: progress review, technology transfer, and new research and action plan (1997–2001), US Dept Agric., Agric. Res. Serv., February 1997.
- 32.Henneberry TJ, Faust RM, Jones WA, Perring TM. 2002. p. 438. Silverleaf Whitefly National Research, Action, and Technology Transfer Plan, 1997–2001: fourth annual review of the second 5-year plan and final report for 1992–2002, US Dept Agric., Agric. Res. Serv., June 2002.
- 33.Watson TF, Silvertooth JC, Tellez A, Lastra L. Seasonal dynamics of sweetpotato whitefly in Arizona. Southwest Entomol. 1992;17:149–167. [Google Scholar]
- 34.Blackmer JL, Byrne DN. Flight behaviour of Bemisia tabaci in a vertical flight chamber: effect of time of day, sex, age and host quality. Physiol Entomol. 1993;18:223–232. [Google Scholar]
- 35.Blackmer JL, Byrne DN, Tu Z. Behavioral, morphological, physiological traits associated with migratory Bemisia tabaci (Homoptera: Aleyrodidae) J Insect Behav. 1995;8:251–267. [Google Scholar]
- 36.Byrne DN. Migration and dispersal by the sweet potato whitefly, Bemisia tabaci. Agric Forest Meterol. 1999;97:309–316. [Google Scholar]
- 37.Naranjo SE, Ellsworth PC. Mortality dynamics and population regulation in Bemisia tabaci. Entomol Exp Appl. 2005;116:93–108. [Google Scholar]
- 38.Naranjo SE, Ellsworth PC. Mortality and population dynamics of Bemisia tabaci within a multi-crop system. In: Mason PG, Gillespie DR, Cañas L, Vincent CD, editors. Proc 3rd Internat Symp on Biological Control of Arthropods, Christchurch, New Zealand. USDA Forest Service; 2009. pp. 202–207. Publ. FHTET-2008-06. [Google Scholar]
- 39.Husain MA, Trehan KK. Observations on the life-history, bionomics and control of the white-fly of cotton (Bemisia gossypiperda M. & L.) Indian J Agric Sci. 1933;3:701–753. [Google Scholar]
- 40.Khalifa A, El Khidir E. Biological study on Trialeurodes lubia and Bemisia tabaci (Aleyrodidae) Bull Entomol Soc Egypt. 1964;48:115–129. [Google Scholar]
- 41.Costa AS. Increase in the populational density of Bemisia tabaci, a threat of widespread virus infection of legume crops in Brazil. In: Bird J, Maramorosch K, editors. Tropical Diseases of Legumes. New York, NY: Academic Press; 1975. pp. 27–29. [Google Scholar]
- 42.Naresh JS, Nene YL. Host range, host preference for oviposition and development and the dispersal of Bemisia tabaci Gennadius, a vector of several plant viruses. Indian J Agric Sci. 1980;50:620–623. [Google Scholar]
- 43.Gerling D, Horowitz AR. Yellow traps for evaluating the population levels and dispersal patterns of Bemisia tabaci (Homoptera: Aleyrodidae) Ann Entomol Soc Am. 1984;77:753–759. [Google Scholar]
- 44.Mabbett T, Nachapong M, Mekdaeng J. The within-canopy distribution of adult cotton whitefly (Bemisia tabaci Gennadius) incorporating economic thresholds and meteorological conditions. Thai J Agric Sci. 1980;13:97–108. [Google Scholar]
- 45.von Arx RV, Baumgärtner J, Delucchi V. Sampling of Bemisia tabaci (Genn.) (Sternorrhyncha: Aleyrodidae) in Sudanese cotton fields. J Econ Entomol. 1984;77:1130–1136. [Google Scholar]
- 46.Ohnesorge B, Rapp G. Methods for estimating the density of whitefly nymphs (Bemisia tabaci Genn.) in cotton. Trop Pest Manag. 1986;32:207–211. [Google Scholar]
- 47.Naranjo SE, Flint HM. Spatial distribution of preimaginal Bemisia tabaci (Homoptera: Aleyrodidae) in cotton and development of fixed-precision sequential sampling plans. Environ Entomol. 1994;23:254–266. [Google Scholar]
- 48.Naranjo SE, Diehl JW, Ellsworth PC. Sampling whiteflies in cotton: validation and analysis of enumerative and binomial plans. Environ Entomol. 1997;26:777–788. [Google Scholar]
- 49.Naranjo SE, Flint HM. Spatial distribution of adult Bemisia tabaci (Homoptera: Aleyrodidae) in cotton and development and validation of fixed-precision sampling plans for estimating population density. Environ Entomol. 1995;24:261–270. [Google Scholar]
- 50.Naranjo SE, Flint HM, Henneberry TJ. Comparative analysis of selected sampling methods for adult Bemisia tabaci (Homoptera: Aleyrodidae) in cotton. J Econ Entomol. 1995;88:1666–1678. [Google Scholar]
- 51.Ellsworth PC. Action thresholds for whiteflies in Arizona. In: Herber DJ, Meade DL, Richter DA, editors. Proc Beltwide Cotton Conf. Memphis, TN: National Cotton Council; 1994. pp. 878–881. [Google Scholar]
- 52.Ellsworth PC, Meade DL. 1994. pp. 346–351. Novel pyrethroid combinations for control of sweetpotato whitefly and their impact on Lygus, in Cotton, a College of Agriculture Report, Series P-96, University of Arizona, Tucson, AZ.
- 53.Ellsworth PC, Meade DL, Odom P. 1994. pp. 363–367. Preliminary field evaluation of an insect growth regulator, buprofezin, for control of the sweetpotato whitefly, Bemisia tabaci, in Cotton, a College of Agriculture Report, Series P-96, University of Arizona, Tucson, AZ.
- 54.Flint HM, Naranjo SE, Leggett JE, Henneberry TJ. Cotton water stress, arthropod dynamics, and management of Bemisia tabaci (Homoptera: Aleyrodidae) J Econ Entomol. 1996;89:1288–1300. [Google Scholar]
- 55.Naranjo SE, Chu CC, Henneberry TJ. Economic injury levels for Bemisia tabaci (Homoptera: Aleyrodidae) in cotton: impact of crop price, control costs, and efficacy of control. Crop Prot. 1996;15:779–788. [Google Scholar]
- 56.Naranjo SE, Ellsworth PC, Chu CC, Henneberry TJ, Riley DG, Watson TF, et al. Action thresholds for the management of Bemisia tabaci (Homoptera: Aleyrodidae) in cotton. J Econ Entomol. 1998;91:1415–1426. [Google Scholar]
- 57.Norton GA. Analysis of decision making in crop protection. Agro-Ecosystems (Now Agric Ecosystems Environ) 1976;3:27–44. [Google Scholar]
- 58.Pedigo LP, Hutchins SH, Higley LG. Economic injury levels in theory and practice. Annu Rev Entomol. 1986;31:341–368. [Google Scholar]
- 59.Stone JD, Pedigo LP. Development and economic-injury level of the green cloverworm on soybean in Iowa. J Econ Entomol. 1972;65:197–201. [Google Scholar]
- 60.Higley LG, Pedigo LP, editors. Economic Thresholds for Integrated Pest Management. Lincoln, NE: University of Nebraska Press; 1997. [Google Scholar]
- 61.Pedigo LP, Buntin GD, editors. Handbook of Sampling Methods for Arthropods in Agriculture. Boca Raton, FL: CRC Press; 1994. [Google Scholar]
- 62.Naranjo SE, Hutchison WD. Validation of arthropod sampling plans using a resampling approach: software and analysis. Am Entomol. 1997;43:48–57. [Google Scholar]
- 63.Binns MR, Nyrop JP, van der Werf W. Sampling and Monitoring in Crop Protection: the Theoretical Basis for Developing Practical Decision Guides. Wallingford, Oxon, UK: CABI Publishing; 2000. [Google Scholar]
- 64.Naranjo SE, Flint HM, Henneberry TJ. Binomial sampling plans for estimating and classifying population density of adult Bemisia tabaci in cotton. Entomol Exp Appl. 1996;80:343–353. [Google Scholar]
- 65.Ellsworth P, Diehl J, Dennehy T, Naranjo S. Sampling Sweetpotato Whiteflies in Cotton. [Online]. IPM Series No. 2, Publ. No. 194023 (Rev. 11/2000), University of Arizona, Cooperative Extension, Tucson, AZ. Available: http://cals.arizona.edu/crops/cotton/insects/wf/wfsampl.html [accessed: 21 September 2009]
- 66.Ellsworth PC, Diehl JW, Naranjo SE. Sampling Sweetpotato Whitefly Nymphs in Cotton. [Online]. IPM Series No. 6, University of Arizona, Cooperative Extension, Tucson, AZ, Publ. 196006. Available: http://cals.arizona.edu/crops/cotton/insects/wf/ipm6.html [accessed: 21 September 2009]
- 67.Ellsworth PC, Diehl JW. Establishment of integrated pest management infrastructure: a community-based action program for Bemisia management. In: Gerling D, Husman WH, Mayer RT, editors. Bemisia: 1995 Taxonomy, Biology, Damage, Control and Management. Andover, Hants, UK: Intercept Ltd; 1996. pp. 681–693. [Google Scholar]
- 68.Ellsworth PC, Martinez-Carrillo JL. IPM for Bemisia tabaci: a case study from North America. Crop Prot. 2001;20:853–869. [Google Scholar]
- 69.Naranjo SE, Ellsworth PC, Chu CC, Henneberry TJ. Conservation of predatory arthropods in cotton: role of action thresholds for Bemisia tabaci (Homoptera: Aleyrodidae) J Econ Entomol. 2002;95:682–691. doi: 10.1603/0022-0493-95.4.682. [DOI] [PubMed] [Google Scholar]
- 70.Dennehy TJ, Williams L. Management of resistance in Bemisia in Arizona cotton. Pestic Sci. 1997;51:398–406. [Google Scholar]
- 71.Ishaaya I, Mendelson Z, Melamed-Madjar V. Effect of buprofezin on embryogenesis and progeny formation of sweetpotato whitefly (Homoptera: Aleyrodidae) J Econ Entomol. 1988;81:781–784. [Google Scholar]
- 72.Ishaaya I, Horowitz AR. Novel phenoxy juvenile hormone analog (pyriproxyfen) suppresses embryogenesis and adult emergence of sweetpotato whitefly (Homoptera, Aleyrodidae) J Econ Entomol. 1992;85:2113–2117. [Google Scholar]
- 73.Dhadialla TS, Carlson GR, Le DP. New insecticides with ecdysteroidal and juvenile hormone activity. Annu Rev Entomol. 1998;43:545–569. doi: 10.1146/annurev.ento.43.1.545. [DOI] [PubMed] [Google Scholar]
- 74.Pener MP. Insect growth regulators. In: Pimentel D, editor. Encyclopedia of Pest Management. New York, NY: Marcel Dekker; 2002. pp. 398–401. [Google Scholar]
- 75.Peleg BA. Effect of a new phenoxy juvenile hormone analog on California red scale, Florida wax scale and the ectoparasite Aphytis holoxanthus. J Econ Entomol. 1988;81:88–92. [Google Scholar]
- 76.Balasubramani V. Safety of the insect growth regulator buprofezin to the egg parasitoid Trichogramma chilonus and the predator Chrysoperla carnea. In: Goel SC, Regupathy A, editors. Insect and Environment. Vol. 5. Biological Control of Insect Pests. India: Uttar Pradesh Zoological Society; 1994. pp. 177–180. [Google Scholar]
- 77.Jones WA, Wolfenbarger DA, Kirk AA. Response of adult parasitoids of Bemisia tabaci to leaf residues of selected cotton insecticides. Entomophaga. 1995;40:153–162. [Google Scholar]
- 78.Castane C, Arino J, Arno J. Toxicity of some insecticides and acaricides to the predatory bug Dicyphus tamaninii. Entomophaga. 1996;41:211–216. [Google Scholar]
- 79.Delbeke F, Vercruysse P, Tirry L, DeClercq P, Degheele D. Toxicity of diflubenzuron, pyriproxyfen, imidacloprid and diafenthiuron to the predatory bug Orius laevigatus (Het.: Anthocoridae) Entomophaga. 1997;42:349–358. [Google Scholar]
- 80.Liu TX, Stansly PA. Effects of pyriproxyfen on three species of Encarsia (Hymenoptera: Aphelinidae), endoparasitoids of Bemisia argentifolii (Homoptera: Aleyrodidae) J Econ Entomol. 1997;90:404–411. [Google Scholar]
- 81.Hoddle MS, van Driesche RG, Lyon SM, Sanderson JP. Compatibility of insect growth regulators with Eretmocerus eremicus for whitefly control on poinsettias: I. Laboratory assays. Biol Control. 2001;20:122–131. [Google Scholar]
- 82.Naranjo SE, Hagler JR, Ellsworth PC. Improved conservation of natural enemies with selective management systems for Bemisia tabaci (Homoptera: Aleyrodidae) in cotton. Biocontrol Sci Technol. 2003;13:571–587. [Google Scholar]
- 83.Naranjo SE, Ellsworth PC, Hagler JR. Conservation of natural enemies in cotton: role of insect growth regulators in management of Bemisia tabaci. Biol Control. 2004;30:52–72. [Google Scholar]
- 84.Diehl JW, Ellsworth PC. Bemisia growth regulators: a field sampling-protocol for nymphs. In: Dugger P, Naranjo SE, Richter D, editors. Proc Beltwide Cotton Conf. Memphis, TN: National Cotton Council; 1997. pp. 929–931. [Google Scholar]
- 85.Ellsworth PC, Diehl JW, Kirk IW. Bemisia growth regulators: large-scale evaluation. In: Dugger P, Henneberry TJ, Richter D, editors. Proc Beltwide Cotton Conf. Memphis, TN: National Cotton Council; 1997. pp. 922–929. [Google Scholar]
- 86.Ellsworth PC, Palumbo JC, Naranjo SE, Dennehy TJ, Nichols RL. Whitefly Management in Arizona Cotton 2006. [Online]. Cooperative Extension, IPM Series No. 18, The University of Arizona, Tucson, AZ. Available: http://cals.arizona.edu/pubs/insects/az1404.pdf [accessed: 21 September 2009]
- 87.Stern VM, van den Bosch R, Born D. New control for alfalfa aphid. Calif Agric. 1958;12(1):4–5. 13. [Google Scholar]
- 88.Smith RF, Hagen KS. Impact of commercial insecticide treatments. Hilgardia. 1959;29:131–154. [Google Scholar]
- 89.Stern VM, van den Bosch R. Field experiments on the effects of insecticides. Hilgardia. 1959;29:103–130. [Google Scholar]
- 90.Daily GE. Nature’s Services—Societal Dependence on Natural Ecosystems. Washington, DC: Island Press; 1997. [Google Scholar]
- 91.Millennium Ecosystem Assessment, Ecosystems and Human Well-being: Synthesis. Washington, DC: Island Press; 2005. [Online]. Available: http://www.millenniumassessment.org/documents/document.356.aspx.pdf. [Google Scholar]
- 92.Jonsson M, Wratten SD, Landis DA, Gurr GM. Recent advances in conservation biological control of arthropods by arthropods. Biol Control. 2008;45:172–175. [Google Scholar]
- 93.Croft BA. Arthropod Biological Control Agents and Pesticides. New York, NY: John Wiley & Sons, Inc.; 1990. [Google Scholar]
- 94.Ellsworth PC, Meade DL. 1993. pp. 280–282. Performance of selected insecticides against the sweetpotato whitefly and cotton aphid, in Cotton, a College of Agriculture Report, Series P-94, University of Arizona, Tucson, AZ.
- 95.Ellsworth P, Meade D. 1995. p. 72. Field evaluation of novel chemistries for control of the sweetpotato whitefly, in Silverleaf Whitefly (Formerly Sweetpotato Whitefly, Strain B): 1995 Supplement to the 5-Year National Research and Action Plan, US Dept Agric., Agric. Res. Serv., February 1995, p.
- 96.Nichols R, Ellsworth P. Insect growth regulators for whitefly management. In: Dugger P, Dennehy T, Richter DA, editors. Proc Beltwide Cotton Conf. Memphis, TN: National Cotton Council; 1996. p. 153. [Google Scholar]
- 97.Ellsworth PC, Diehl JW. Whiteflies in Arizona No. 6: Commercial-scale Trial 1995 (rev. 5/97). [Online]. The University of Arizona, Cooperative Extension, Tucson, Arizona, AZ, 4 pp. Available: http://ag.arizona.edu/crops/cotton/insects/wf/wfly6.pdf) [accessed: 21 September 2009]
- 98.Ellsworth PC, Dennehy TJ, Nichols RL. 1996. p. 2. Whitefly management in Arizona cotton 1996, IPM Series No. 3, University of Arizona, Cooperative Extension, Tucson, AZ, Publ. 196004.
- 99.Ellsworth P, Dennehy T, Diehl J, Ansolobehere M, Antilla L, Howell D, et al. 1997. p. 195. Statewide training sessions for use of insect growth regulators in Arizona, in Silverleaf Whitefly, 1997 Supplement to the 5-Year National Research and Action Plan: Progress Review, Technology Transfer, and New Research and Action Plan (1997–2001), US Dept Agric., Agric. Res. Serv., February 1997, p.
- 100.Palumbo JC, Ellsworth PC, Dennehy TJ, Nichols RL. Cross-commodity Guidelines for Neonicotinoid Insecticides in Arizona. [Online]. IPM Series 17, Publ. No. AZ1319, University of Arizona, College of Agriculture and Life Sciences, Cooperative Extension, Tucson, AZ, 4 pp. Available: http://cals.arizona.edu/pubs/insects/az1319.pdf [accessed: 21 September 2009]
- 101.Head G. Adapting insect resistance management programs to local needs. In: Onstad DW, Savinelli C, editors. Insect Resistance Management: Biology, Economics, and Prediction. London, UK: Academic Press; 2008. pp. 89–106. [Google Scholar]
- 102.Declercq P, Vinuela E, Smagghe G, Degheele D. Transport and kinetics of diflubenzuron and pyriproxyfen in the beet armyworm Spodoptera exigua and its predator Podisus maculiventris. Entomol Exp Appl. 1995;76:189–194. [Google Scholar]
- 103.GraftonCardwell EE, Gu P. Conserving vedalia beetle, Rodolia cardinalis (Mulsant) (Coleoptera: Coccinellidae), in citrus: a continuing challenge as new insecticides gain registration. J Econ Entomol. 2003;96:1388–1398. doi: 10.1093/jee/96.5.1388. [DOI] [PubMed] [Google Scholar]
- 104.Grafton-Cardwell EE, Godfrey LD, Chaney WE, Bentley WJ. Various novel insecticides are less toxic to humans, more specific to key pests. Calif Agric. 2005;59:29–34. [Google Scholar]
- 105.Grafton-Cardwell EE, Lee JE, Stewart JR, Olsen KD. Role of two insect growth regulators in integrated pest management of citrus scales. J Econ Entomol. 2006;99:733–744. doi: 10.1603/0022-0493-99.3.733. [DOI] [PubMed] [Google Scholar]
- 106.Hattingh V, Tate B. Effects of field-weathered residues of insect growth regulators on some Coccinellidae (Coleoptera) of economic importance as biocontrol agents. Bull Entomol Res. 1995;85:489–493. [Google Scholar]
- 107.Hattingh V. The use of insect growth regulators—implications for IPM with citrus in southern Africa as an example. Entomophaga. 1996;41:513–518. [Google Scholar]
- 108.Smith KM, Smith D, Lisle AT. Effect of field-weathered residues of pyriproxyfen on the predatory coccinellids Chilocorus circumdatus and Cryptolaemus montrouzieri. Aust J Exp Agric. 1999;39:995–1000. [Google Scholar]
- 109.Naranjo SE, Ellsworth PC. The contribution of conservation biological control to integrated management of Bemisia tabaci in cotton. Biol Control. 2009 (in press). DOI: 10.1016/j.biocontrol.2009.08.006. [Google Scholar]
- 110.Ishaaya I, Horowitz AR, Tirry L, Barazani A. Novaluron (Rimon), a novel IGR-mechanism, selectivity and importance in IPM programs. Med Fac Landbouww Univ Gent. 2002;67:617–626. [PubMed] [Google Scholar]
- 111.Kiritani K. Integrated biodiversity management in paddy fields: shift of paradigm from IPM toward IBM. Integrated Pest Manag Rev. 2000;5:175–183. [Google Scholar]
- 112.Ehler LE. Integrated pest management (IPM): definition, historical development and implementation, and the other IPM. Pest Manag Sci. 2006;62:787–789. doi: 10.1002/ps.1247. [DOI] [PubMed] [Google Scholar]
- 113.Horowitz AR, Ellsworth PC. Biorational pest control - An overview. In: Ishaaya I, Ishaaya I, Horowitz AR, editors. Biorational Control of Arthropod Pests: Application and Resistance Management. Berlin, Germany: Springer; 2009. pp. 1–20. [Google Scholar]
- 114.Naranjo SE. Conservation and evaluation of natural enemies in IPM systems for Bemisia tabaci. Crop Prot. 2001;20:835–852. [Google Scholar]
- 115.Van den Brink PJ, Ter Braak CJF. Multivariate analysis of stress in experimental ecosystems by principal response curves and similarity analysis. Aquatic Ecol. 1998;32:163–178. [Google Scholar]
- 116.Van den Brink PJ, Ter Braak CJF. Principal response curves: analysis of time-dependent multivariate responses of biological communities to stress. Environ Toxicol Chem. 1999;18:138–148. [Google Scholar]
- 117.Hagler JR. Foraging behavior, host stage selection and gut content analysis of field collected Drapetis nr. divergens: a predatory fly of Bemisia argentifolii. Southwest Entomol. 2002;27:241–249. [Google Scholar]
- 118.Freidberg A, Susman I, Kaplan F. Cotton Insects of Israel. Tel Aviv, Israel: The Cotton Production and Marketing Board Ltd; 1989. (in Hebrew) [Google Scholar]
- 119.Southwood TRE. Ecological Methods. 2nd edition. London, UK: Chapman and Hall; 1978. [Google Scholar]
- 120.Naranjo SE. Establishment and impact of exotic aphelinid parasitoids in Arizona: a life table approach. J Insect Sci (Tucson) 2008;8:36. [Google Scholar]
- 121.Fournier A, Ellsworth PC, Barkley V. 2007. Economic Impact of Lygus in Arizona Cotton: a Comparative Approach, 2007 Cotton Report. [Online]. College of Agriculture and Life Sciences, University of Arizona, Tucson, AZ. Available: http://cals.arizona.edu/pubs/crops/az1437/az14374a.pdf [accessed: 21 September 2009]
- 122.Ellsworth PC, Barkley V. Transitioning Lygus chemical controls to more selective options for Arizona cotton. Cotton, a College of Agriculture Report, Series P-142. 2005:165–178. [Online]. University of Arizona, Tucson, AZ, pp. Available: http://cals.arizona.edu/pubs/crops/az1366/az13665.pdf. [accessed: 21 September 2009] [Google Scholar]
- 123.Kogan M. Integrated pest management: historical perspectives and contemporary developments. Annu Rev Entomol. 1998;43:243–270. doi: 10.1146/annurev.ento.43.1.243. [DOI] [PubMed] [Google Scholar]
- 124.Ellsworth PC, Fournier A, Smith TD. Arizona Cotton Insect Losses. [Online]. University of Arizona, Cooperative Extension, Tucson, AZ, Publ. No. AZ1183 (Rev. 9/08). Available: http://cals.arizona.edu/crops/cotton/insects/cil/cil.html [accessed: 21 September 2009]



