Abstract
This is a rapidly emerging field. The application of knowledge regarding the relationship between neural and immune systems in order to gain a better understanding of human conditions has been slow. In this discussion we describe how the brain and microbiota interact, and try to bring this into a context that is clinically relevant. We begin by describing established facts pertaining to the gut–brain axis and the role of gut bacteria. We then focus upon emerging data that will contribute to the generation of a new conceptual framework about the microbiota–gut–brain axis. In the final section we anticipate future directions of this field.
Keywords: antibiotics, behaviour, inflammation, irritable bowel syndrome, metabolomics
What is established knowledge in this field?
The gut–brain axis (GBA) is a bi-directional communication system through which the brain modulates gastrointestinal function and through which gut function is monitored by the brain. Neural, endocrine and immunological mechanisms underlie gut–brain interactions.
In man, hepatic failure, including deep coma, responds to the administration of laxatives and oral antibiotics, indicating a communication between the intestinal microbiota and the brain, albeit under pathological conditions.
The GBA has been implicated in primary psychiatric and in functional and inflammatory gastrointestinal (GI) diseases. Psychiatric co-morbidity occurs in up to 60% of these GI disorders [1].
The intestinal microbiota imprint the immunological and physiological systems at birth or shortly thereafter, and influence these systems throughout life. This includes the enteric nervous system, the endocrine system [hypothalamic–pituitry–adrenal (HPA) axis] and other determinants of gastrointestinal physiology. Germfree (GF) rodents display differences in the expression of genes that encode function in several systems, including the nervous system; these changes in gene expression are normalized upon bacterial colonization [2] depending upon the postnatal maturity of the host.
The brain is aware of the introduction of pathogenic microbes into the gastrointestinal tract. This results in brain stem nuclei becoming activated and, in some instances, associated with the development of anxiety-like behaviour [3,4]. These studies were conducted in mice or rats and involved measurements of brain nuclei activity and behaviour that occurred within hours of the introduction of the pathogens at a subclinical threshold. The involvement of the nucleus of the solitary tract and the lateral parabrachial nucleus suggest that these changes are mediated, at least in part, by the vagus nerve.
What is emerging from the field?
Psychiatric diseases such as depression may have an inflammatory basis, but the role of the microbiota has not yet been examined
There is increasing evidence that the immune system and the HPA axis play a role in the pathogenesis of major affective disorders such as depression (for reviews see [5,6]). In this model, both psychological factors and immune activation outside the brain are seen as stressors. This view has been extended to incorporate infection and the hygiene hypothesis in the pathogenesis of depression [6]. In the literature supporting this model, there are demonstrations of changes in autonomic function (including parasympathetic impairment), changes in corticotrophin-releasing hormone (CRH) and cortisol levels, as well as elevations in inflammatory markers such as C-reactive protein and some cytokines. In terms of relating this to the intestinal microbiota, awareness is drawn to the fact that psychiatric morbidity such as depression occurs in up to 60% of patients who suffer from the two main categories of gastrointestinal illness in our society; namely, functional disorders such as irritable bowel syndrome (IBS) and chronic inflammatory bowel disease such as Crohn's disease [1]. There is emerging evidence of changes in the microbiota in patients with IBS, and probiotics may be helpful in treating this condition. Crohn's disease is regarded as a breakdown in the immunological accommodation of the intestinal microbiota and in some studies there are differences in the microbiota profile between IBD patients and controls. It is unclear from this literature whether the intestinal microbiota play a role in the development of the psychiatric co-morbidity seen in these conditions. To date, in most studies treatment with probiotics may provide improvement in gastrointestinal symptoms, but their effects on psychiatric co-morbidity have yet to be determined. There are some studies that use breath analysis of gases such as hydrogen or methane to demonstrate abnormalities in fermentation by intestinal bacteria, and these are accompanied by reports suggesting that restriction of certain carbohydrates such as sucrose or fructose improves behaviour in patients with depression. These studies are controversial. Similarly, behavioural conditions such as autism have been linked with abnormalities in the intestinal microbiota (based on culture or molecular techniques), and there are reports of at least transient benefit following antibiotic treatment. Again, these results are controversial and have not been confirmed.
One recent study claims that anxiety in patients with chronic fatigue syndrome was diminished in a randomized double-blind controlled study of a probiotic [7]. This is virtually the only indication in the clinical literature suggesting that gut microbiota may influence behaviour.
Animal-based studies have described changes in the intestinal microbiota following psychological stress. Unfortunately, most of these studies either used culture-based techniques to assess the intestinal flora or used stressors that, directly or indirectly, influence the microbiota. These stressors include changes in bedding, and separation of pups from dams during the first few weeks of life when initial colonization is occurring and at a time when the immune and physiological systems of the newborn are being imprinted. There are no studies to our knowledge that examine the intestinal microbiota in models in which behavioural disturbances are induced later in life, when the above-described processes are stable.
Depression is associated with an increased vulnerability to inflammatory stimuli in the gastrointestinal tract
Clinical observational studies have correlated stress and depression with the activity of both functional and inflammatory bowel diseases. From a clinical standpoint, little attention is paid to the influence of the nervous system on the inflammatory process and the role of antidepressants in the management of IBD has received no attention (attention is usually focused upon the evaluation of anti-inflammatory or immune-modulatory biological agents in the management of these conditions). These observations, however, prompted studies in mice regarding the relationship between behaviour and inflammation in the gastrointestinal tract. Recent studies in mice have examined the effects of depression on the susceptibility of the gastrointestinal tract to inflammatory stimuli. These studies used a model of intracerebroventricularly (ICV) administered reserpine to induce depression-like behaviour in adult mice and found that this behaviour was accompanied by susceptibility to acute experimental colitis [8]. In addition, depression reactivated colitis that had been induced in the chronic manner and allowed to become quiescent prior to the induction of depression. The study showed that tricyclic antidepressants could prevent the vulnerability to inflammation and that this effect was seen only in depressed mice. Furthermore, the action of the antidepressant was dependent upon the integrity of the vagus nerve and the presence of macrophages in the intestine. The vulnerability to reactivation of colitis by depression could be transferred adoptively into naive mice using macrophages isolated from depressed mice. The mechanism by which depression induces this vulnerability has been elucidated, and involves the loss of vagal modulation of inflammatory cytokine production by the macrophage via the alpha seven subunit of the nicotinic acetylcholine receptor. The extent to which the microbiota are involved in this depression-induced vulnerability remains to be determined, but it is known that stress alters mucosal barrier function and mucin production in experimental animals, and similar findings may occur in mice with depression-like behaviour. If so, these changes would be expected to alter the habitat of the microbiota and change the bacterial composition of the gut.
Interactions between gut bacteria and the enteric nervous system
We speculate that there is a bi-directional interaction between gut bacteria and the enteric nervous system; our evidence is based exclusively on experimental basic scientific work. Recent work suggests that, directly or indirectly, the feeding of a single commensal bacterium to rats enhances the excitability of a particular subset of neurones in the enteric nervous system by inhibiting the opening of a calcium-dependent potassium channel [9].
The local release of biological amines such as noradrenalin by the host may influence the composition of the intestinal microbiota, as this neurotransmitter has been shown to stimulate the growth of pathogenic and non-pathogenic Escherichia coli in vitro, and to influence their adherence to the mucosa [10]. Intestinal bacteria can also produce substances such as serotonin, melatonin, gamma aminobutyric acid (GABA), catecholamines, histamine and acetylcholine, which can act on the host's enteric nervous system. In addition, the microbiome synthesizes and influences the synthesis of several gases (carbon monoxide, hydrogen sulphide and nitric oxide), shown to be involved in neurotransmission in the peripheral (enteric) and central nervous systems (for review see Forsythe et al.[11]). Other substances produced by intestinal bacteria include putrescine, spermidine, spermine and cadaverine, all of which have been shown to be involved in the central nervous system responses to stress. Quorum sensing among bacteria is mediated by substances secreted by bacteria and these, too, have been implicated in neuronal functioning [12]. Thus, it is likely that the brain and commensal bacteria of the gut communicate with each other, and we speculate that this may be part of homeostasis that maintains not only stability within the microbiota of the intestine, but also potentially modulates brain function and behaviour.
The enteric nervous system may also play a role in the presentation of gut microbes to the immune system via Peyer's patches or dendritic cells. A study using porcine intestinal tissue showed that the uptake of bacteria, including Escherichia coli and Salmonella species, by the gut from the lumen, was critically dependent upon adrenergic nerves and could be blocked by phentolamine. Given the proximity of the noradrenalin transporter to the domes of Peyer's patches, the authors concluded that adrenergic nerves affect gut sampling of bacteria and facilitate their uptake, and subsequent presentation to the mucosal immune system [13].
The intestinal microbiota modulate pain responses in the gastrointestinal tract
Abdominal pain is a common feature of many gastrointestinal diseases, but is particularly prominent in functional gastrointestinal disorders. A previous study has shown that normal pain perception in the gastrointestinal tract of mice required the integrity of the mucosal immune system; the absence of CD4 cells resulted in hyperalgesia, which could be normalized by restoring CD4 cells [14]. As the intestinal microbiota imprint and educate the mucosal immune system, the question arose as to whether the microbiota influence pain perception in the gut. Using oral antibiotics to perturb the microbiota (resulting in a profound production of lactobacilli), it was found that levels of the sensory neurotransmitter substance P were increased in the myenteric plexus. This was accompanied by an increase in pain perception following colonic distension. These antibiotic-induced changes could be prevented by treating the mice with either Lactobacillus or dexamethasone [15]. These results imply that the intestinal microbiota can modulate function in the enteric nervous system and influence pain perception by a mechanism that involves the immune system. Use of a mutant bacterium lacking D alanine in lipoteichoic acid in its cell wall has supported the combined involvement of the immune and nervous system in visceral pain perception. The mutant which is effective as an inducer of an immune regulatory anti-inflammatory cytokine interleukin (IL)-10, which is also antinociceptive, was much more effective than the wild-type bacterium in inhibition of pain [16]. This suggests that cell wall composition may be important in communication with the nervous system. Probiotic ingestion by normal healthy rats induced inhibition of the cardiac reflection of visceral pain due to colorectal distension, and this inhibition was mirrored by the decrease in afferent signalling in single fibres of the dorsal root ganglia (DRG) [17], and also prevented the hyperexcitability in dorsal root ganglion cells which accompanies this type of noxious stimulus [18]. Thus we may conclude that both immune and nervous pathways are involved intimately in visceral pain perception and that intestinal microbiota can modulate this.
While probiotic effects in pain modulation were confined to visceral pain, GF animals perceive somatic pain with a higher threshold than conventional mice [19]. Indeed, conventionalization of the GF microbiome induced a lowering of the threshold for somatic pain, suggesting a complex role for microbiota on the differential sensation of visceral and somatic pain.
The impact of the intestinal microbiota on the central nervous system
The impact of chronic infection and inflammation on behaviour
There is already considerable interest in an inflammatory basis for depression [5], and this has been extended to include a possible role for infection and inflammation [20] Here, we discuss recent animal-based research that supports the view that intestinal infection and inflammation influence the brain and behaviour. Gastric infection with the bacterium Helicobacter pylori is associated with peptic ulcer disease and gastric cancer in genetically predisposed individuals. In others, Helicobacter infection can be completely asymptomatic. It has been associated with functional GI disorders such as functional dyspepsia, although this relationship is controversial because eradication of the bacterium does not readily improve symptoms. Thus, Helicobacter falls into a grey area of being a benign commensal in some individuals, the cause of ulcers and cancer in some, and a possible cause of functional disorders in others. Studies in a mouse model of H. pylori chronic infection reveal that most mice develop chronic gastritis and gastric dysfunction (slow gastric emptying) and exhibit an abnormal pattern of feeding behaviour characterized by the ingestion of small meals; there is no accompanying weight loss, as the mice compensate by eating more frequently [21]. There is no malaise or anorexia that one might associate with an inflammatory process. Abnormal feeding behaviour was accompanied by elevated plasma ghrelin and postprandial cholecystokinin (CCK), higher tumour necrosis factor (TNF)-alpha mRNA in the median eminence and lower proopiomelanocortin (POMC) mRNA in the arcuate nucleus of the brain compared to uninfected controls. Interestingly, following successful eradication of the Helicobacter, gastric physiology normalized but the abnormal feeding behaviour and changes in brain mRNA expression persisted. Furthermore, these changes were normalized following treatment with two probiotics, Lactobacillus rhamnosus R0011 and L. helveticus R0052 [22]. The mechanism underlying the action of the probiotics is unclear from these experiments. Subsequent work showed that Helicobacter-infected mice also exhibit anxiety-like behaviour. Intriguingly, the probiotics that improved the feeding behaviour did not influence the anxiety-like behaviour and the latter was corrected by another probiotic, Bifidobacterium. These probiotics each possess anti-inflammatory properties but quite different effects on brain and behaviour. Putative mechanisms include immune activation, bacterial-induced interactions between the enteric and central nervous systems and the elaboration of chemicals that interact directly with the relevant areas of the brain [21,22]. It is of interest to recall that from a clinical perspective, Helicobacter infection does not fulfill Koch's postulates as a cause of functional dyspepsia as its eradication does not improve symptoms readily. However, given the above-described findings, this bacterium remains a putative trigger for this condition, as it produces consistent changes in the brain resulting in feeding behaviour that is reminiscent of the increased satiety which is characteristic of the syndrome.
The intestinal microbiota influence behaviour in adult mice
The long-standing observation that deterioration in brain function can be reversed by oral antibiotics in patients with uncompensated liver disease provides a medically relevant basis for examining the role of the microbiota in maintaining normal brain function and behaviour. Using a strategy similar to that described above in the context of a modulation of pain [15], oral antibiotics produced anxiety-like behaviour in mice [23]. The antibiotic-induced behavioural changes were transient, and normal behaviour returned once the normal flora had been restored following cessation of the antibiotic therapy. The behavioural changes were not attributed to a direct effect of the antibiotics, as no changes were seen if these antibiotics were administered systemically. The changes in behaviour were accompanied by an increase in brain-derived neurotrophic factor (BDNF) in the hippocampus and amygdala and normalized following discontinuation of the antibiotics (for review see Collins and Bercik [23]). Another study used dietary manipulation to affect the flora and assess changes in behaviour in 5-week-old mice. Mice were fed normal chow or a beef-enriched diet, and this produced greater bacterial diversity in the beef-fed mice. These mice showed a reduction in anxiety and better memory compared to the normal chow-fed mice [24]. Taken together, these observations provide early experimental evidence in support of the notion that the intestinal microbiota contribute to behaviour under normal conditions and that perturbation of the normal flora may result in behavioural change. If functional and inflammatory gastrointestinal diseases result in a change in commensal bacteria, it is possible that the alteration of microbiota could contribute to the behavioural changes (and psychiatric co-morbidity) seen in these disorders.
The intestinal microbiota influence the development of the hypothalamic–pituitary response to stress and the development of normal cognitive function in mice
Studies involving germ-free mice have shown that the development of the hypothalamic–pituitary axis is influenced by commensal bacteria early in life [25]. Mild restraint stress was applied to young germ-free mice and resulted in an exaggerated adrenocorticotrophic hormone (ACTH) and corticosterone responses. This hyper-responsiveness of the HPA axis was also reversed by mono-association of mice with a single organism, Bifidobacterium infantis, which is a predominant bacterium in the infant gut and a commonly used probiotic organism. The reversibility of the exaggerated stress response was evident only if colonization occurred before 6 weeks of age. The fact that a single bacterium B. infantis completely reversed the hyper-responsiveness of the HPA, whereas colonization with specific pathogen-free (SPF) commensal bacteria only partially reversed the changes, indicates that commensal bacteria have a selective effect on the development of the setpoint for the HPA axis early in life. Interestingly, levels of BDNF, noradrenalin and 5-hydroxytryptamine (5-HT) were significantly lower in the cortex and hippocampus of germ-free mice compared to SPF colonized mice. More recent work has shown that germ-free mice exhibit anxiolytic behaviour which is accompanied by increased expression of transcripts for BDNF in the dentate gyrus. These changes could not be reversed by colonization when the mice reached adulthood [26].
Potential mechanisms underlying behavioural modification by gut bacteria
It is likely that the immune system is important in the development and normal functioning of the brain. A study by Kipnis et al.[27] showed that cognitive function and normal behaviour was impaired in mice deprived of mature T cells, and that both these functions could be restored following adoptive transfer of CD4 lymphocytes from immunocompetent mice.
It is known that the vagus nerve plays an important role conveying information from the brain to the microbiota and vice versa. There is also accumulating evidence that intestinal bacteria elaborate substances that can affect the host nervous system, and we present a few examples here. A recent study showed that treatment of rats with a Bifidobacterium produced changes in dopamine chemistry. These rats exhibited a marked increase in plasma concentrations of the serotonin precursor tryptophan and degradation products such as kynurenic acid compared to controls [28]. Lactobacilli have been shown to increase the activity of indoleamine 2, 3 dioxygenase (IDO), an enzyme involved in catabolism of tryptophan and formation of the neuroactive compounds kynurenic and quinolinic acid. GABA, an important neurotransmitter, is one of many examples of the consequences of bacterial synthesis of neuroactive molecules which remain to be explored (for review see Forsythe et al.[11]). The products of anaerobic bacterial fermentation include short-chain fatty acids such as butyrate, which when administered systemically produced anti-depressant-like effects in mice associated with increased BDNF transcripts in the frontal cortex [29]. The intraventricular infusions of propionic acid in mice induced irreversible behavioural changes that are similar to autism [30]. Clearly, these observations are provocative, but it is unclear whether the doses used in these experiments bear resemblance to the concentrations provided to the host by the metabolism of its commensal bacteria.
While the mechanisms involved in microbiome–brain interactions remain to be clarified, recent metabolomic studies of plasma from GF and conventionalized mice provide significant support for conclusions that these occur. Wikoff et al.[31] have shown that conventionalization resulted in two- to eightfold increases in serotonin and major changes in levels of tryptophan and certain neuroactive metabolites.
What is not known and future directions
There are clearly a number of questions that present themselves from the data we have outlined above. The information presented generates a schematic diagram to illustrate the bi-directional interactions between microbiota and the nervous system, and these are shown in Fig. 1. Although mechanisms underlying this gut–brain axis are unknown, we have presented schematically in Fig. 2 what would appear to us to be the most likely pathways and mediators. The demonstration that perturbation of the intestinal microbiota produces anxiety-like behaviour [23] suggests that the host's normal behaviour receives an input from the commensal bacteria. If this is indeed correct, then it is possible to construct models of disease in which the gut–brain axis has been implicated, such as the psychiatric co-morbidity that occurs in irritable bowel syndrome and other functional disorders as well as in IBD patients with psychiatric co-morbidity. It is unclear from the evidence presented that primary psychiatric diseases, such as depression, autism or schizophrenia, have their origins in the intestinal microbiota. A modulatory contribution from the microbiota in these conditions is possible based on evidence presented here, but future studies need to evaluate carefully the microbiota of patients with primary psychiatric disease before the field can advance. These findings also support an evaluation of the use of pre- or probiotics in the treatment of primary psychiatric illness.
Fig. 1.
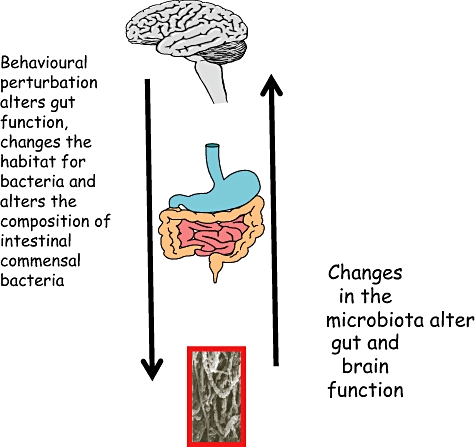
The bi-directional microbiota–gut–brain axis. Changes in the microbiota alter gut and brain function. Behavioural perturbation alters gut function, changes the habitat for bacteria and alters the composition of intestinal commensal bacteria.
Fig. 2.
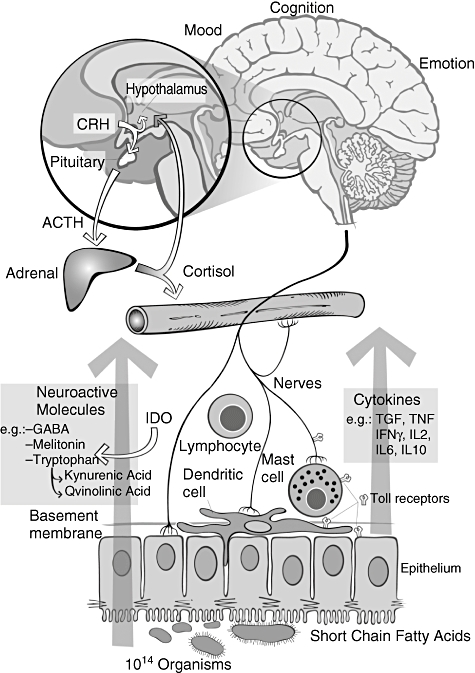
Putative mechanisms underlying microbiota–brain and nervous system interactions (see [11]).
With respect to gastrointestinal disease, one can be confident that emerging evidence supports a role for the intestinal microbiota in diseases such as Crohn's disease and ulcerative colitis, as well as in the increasingly common antibiotic-induced colitis (Clostridium difficile). There is good evidence in IBD that there is a breakdown of immunological accommodation of the normal intestinal microbiota and that the microbial balance present in this condition is itself altered. The extent to which this is a primary or secondary phenomenon remains to be determined.
Functional gastrointestinal disorders remain an enigma for clinicians. To date, the lack of a conceptual model that embraces both behavioural and gut manifestations has hampered rational drug development for these conditions. It also leaves both the physician and the patient without a plausible explanation for symptoms, generating frustration and sometimes resentment in both parties. Emerging research provides clear demonstrations that shifts in the microbiota can change gut physiology, including visceral pain perception and motility, which may also influence symptomatology in functional disorders. There is also emerging evidence to support the existence of alterations in the diversity and stability of the microbiota in IBS patients. Thus, it is possible to envisage that this common category of disease may be consequent to an event such as infection or course of antibiotics which will perturb the flora and change function in the gut. Changes in gut physiology are an integral part of these disorders and generate instability in the intestinal habitat, which in turn contributes to the unstable microbiota seen in these patients. A vicious cycle is generated in which an unstable microbiota perturbs gut physiology which generates further instability of the flora; the instability of the flora may then contribute to the psychiatric co-morbidity seen in these conditions. This provides a basis for the use of either pre- or probiotic therapies aimed at stabilizing the intestinal microbiota in these disorders.
Disclosure
Dr SM Collins receives an unrestricted grant-in-aid from Nestle Research Institute.
References
- 1.Whitehead WE, Palsson O, Jones KR. Systematic review of the comorbidity of irritable bowel syndrome with other disorders: what are the causes and implications? Gastroenterology. 2002;122:1140–56. doi: 10.1053/gast.2002.32392. [DOI] [PubMed] [Google Scholar]
- 2.Hooper LV, Gordon JI. Commensal host–bacterial relationships in the gut. Science. 2001;292:1115–18. doi: 10.1126/science.1058709. [DOI] [PubMed] [Google Scholar]
- 3.Gaykema RP, Goehler LE, Lyte M. Brain response to cecal infection with Campylobacter jejuni: analysis with Fos immunohistochemistry. Brain Behav Immun. 2004;18:238–45. doi: 10.1016/j.bbi.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Lyte M, Li W, Opitz N, et al. Induction of anxiety-like behaviour in mice during the initial stages of infection with the agent of murine colonic hyperplasia Citrobacter rodentium. Physiol Behav. 2006;89:350–7. doi: 10.1016/j.physbeh.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 5.Anisman H. Cascading effects of stressors and inflammatory immune system activation: implications for major depressive disorder. J Psychiatry Neurosci. 2009;34:4–20. [PMC free article] [PubMed] [Google Scholar]
- 6.Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends Neurosci. 2008;31:464–8. doi: 10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 7.Rao AV, Bested AC, Beaulne TM, et al. A randomized, double-blind, placebo-controlled pilot study of a probiotic in emotional symptoms of chronic fatigue syndrome. Gut Pathog. 2009;1:6. doi: 10.1186/1757-4749-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghia JE, Blennerhassett P, Collins SM. Impaired parasympathetic function increases susceptibility to inflammatory bowel disease in a mouse model of depression. J Clin Invest. 2008;118:2209–18. doi: 10.1172/JCI32849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kunze W, Mao YK, Wang B, et al. Lactobacillus reuteri enhances excitability of colonic AH neurons by inhibiting calcium dependent potassium channel opening. J Cell Mol Med. 2009;13(8B):2261–70. doi: 10.1111/j.1582-4934.2009.00686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freestone PP, Sandrini SM, Haigh RD, et al. Microbial endocrinology: how stress influences susceptibility to infection. Trends Microbiol. 2008;16:55–64. doi: 10.1016/j.tim.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Forsythe P, Sudo N, Dinan T, et al. Mood and gut feelings. Brain Behav Immun. 2009;24:9–16. doi: 10.1016/j.bbi.2009.05.058. [DOI] [PubMed] [Google Scholar]
- 12.Hughes DT, Sperandio V. Inter-kingdom signalling: communication between bacteria and their hosts. Nat Rev Microbiol. 2008;6:111–20. doi: 10.1038/nrmicro1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green BT, Lyte M, Kulkarni-Narla A, et al. Neuromodulation of enteropathogen internalization in Peyer's patches from porcine jejunum. J Neuroimmunol. 2003;141:74–82. doi: 10.1016/s0165-5728(03)00225-x. [DOI] [PubMed] [Google Scholar]
- 14.Verma-Gandhu M, Bercik P, Motomura Y, et al. CD4+ T-cell modulation of visceral nociception in mice. Gastroenterology. 2006;130:1721–8. doi: 10.1053/j.gastro.2006.01.045. [DOI] [PubMed] [Google Scholar]
- 15.Verdu EF, Bercik P, Verma-Gandhu M, et al. Specific probiotic therapy attenuates antibiotic induced visceral hypersensitivity in mice. Gut. 2006;55:182–90. doi: 10.1136/gut.2005.066100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duncker SC, Wang L, Hols P, et al. The D-alanine content of lipoteichoic acid is crucial for Lactobacillus plantarum-mediated protection from visceral pain perception in a rat colorectal distension model. Neurogastroenterol Motil. 2008;20:843–50. doi: 10.1111/j.1365-2982.2008.01085.x. [DOI] [PubMed] [Google Scholar]
- 17.Kamiya T, Wang L, Forsythe P, et al. Inhibitory effects of Lactobacillus reuteri on visceral pain induced by colorectal distension in Sprague–Dawley rats. Gut. 2006;55:191–6. doi: 10.1136/gut.2005.070987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma X, Mao YK, Wang B, et al. Lactobacillus reuteri ingestion prevents hyperexcitability of colonic DRG neurons induced by noxious stimuli. Am J Physiol Gastrointest Liver Physiol. 2009;296:G868–75. doi: 10.1152/ajpgi.90511.2008. [DOI] [PubMed] [Google Scholar]
- 19.Amaral FA, Sachs D, Costa VV, et al. Commensal microbiota is fundamental for the development of inflammatory pain. Proc Natl Acad Sci USA. 2008;105:2193–7. doi: 10.1073/pnas.0711891105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rook GA. Review series on helminths, immune modulation and the hygiene hypothesis: the broader implications of the hygiene hypothesis. Immunology. 2009;126:3–11. doi: 10.1111/j.1365-2567.2008.03007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bercik P, Verdu EF, Foster JA, et al. Role of gut–brain axis in persistent abnormal feeding behavior in mice following eradication of Helicobacter pylori infection. Am J Physiol Regul Integr Comp Physiol. 2009;296:R587–94. doi: 10.1152/ajpregu.90752.2008. [DOI] [PubMed] [Google Scholar]
- 22.Verdu EF, Bercik P, Huang XX, et al. The role of luminal factors in the recovery of gastric function and behavioral changes after chronic Helicobacter pylori infection. Am J Physiol Gastrointest Liver Physiol. 2008;295:G664–70. doi: 10.1152/ajpgi.90316.2008. [DOI] [PubMed] [Google Scholar]
- 23.Collins SM, Bercik P. The relationship between intestinal microbiota and the central nervous system in normal gastrointestinal function and disease. Gastroenterology. 2009;136:2003–14. doi: 10.1053/j.gastro.2009.01.075. [DOI] [PubMed] [Google Scholar]
- 24.Li W, Dowd SE, Scurlock B, et al. Memory and learning behavior in mice is temporally associated with diet-induced alterations in gut bacteria. Physiol Behav. 2009;96:557–67. doi: 10.1016/j.physbeh.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 25.Sudo N, Chida Y, Aiba Y, et al. Postnatal microbial colonization programs the hypothalamic–pituitary–adrenal system for stress response in mice. J Physiol. 2004;558:263–75. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neufeld KM, Bienenstock J, Foster JA. The impact of intestinal microbiota on anxiety-like behaviour. Neurogastroenterol Motil. 2008;20:125. [Google Scholar]
- 27.Kipnis J, Cohen H, Cardon M, et al. T cell deficiency leads to cognitive dysfunction: implications for therapeutic vaccination for schizophrenia and other psychiatric conditions. Proc Natl Acad Sci USA. 2004;101:8180–5. doi: 10.1073/pnas.0402268101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Desbonnet L, Garrett L, Clarke G, et al. The probiotic Bifidobacteria infantis: an assessment of potential antidepressant properties in the rat. J Psychiatr Res. 2008;43:164–74. doi: 10.1016/j.jpsychires.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 29.Schroeder FA, Lin CL, Crusio WE, et al. Antidepressant-like effects of the histone deacetylase inhibitor, sodium butyrate, in the mouse. Biol Psychiatry. 2007;62:55–64. doi: 10.1016/j.biopsych.2006.06.036. [DOI] [PubMed] [Google Scholar]
- 30.Shultz SR, Macfabe DF, Martin S, et al. Intracerebroventricular injections of the enteric bacterial metabolic product propionic acid impair cognition and sensorimotor ability in the Long–Evans rat: further development of a rodent model of autism. Behav Brain Res. 2009;200:33–41. doi: 10.1016/j.bbr.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 31.Wikoff WR, Anfora AT, Liu J, et al. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci USA. 2009;106:3698–703. doi: 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]


