Abstract
The ‘hygiene hypothesis’ proposes that the epidemic of allergic and autoimmune diseases is due to changes in the interactions between humans and the microbes of their ecosystem. This theory apparently does not explain (i) why allergic asthma is rising in ‘unhygienic’ American inner cities; (ii) why allergic diseases are less prevalent among migrants' children living in European big cities; (iii) why infections with airborne viruses do not ‘protect’ from allergic sensitization; (iv) why the inverse association between some infections (e.g. hepatitis A virus) and allergic diseases has been reproduced in some populations, but not in others; and (v) why probiotics are not effective in the prevention and therapy of allergic diseases. These challenging questions are useful starting points to improve our understanding of the hypothesis, and to identify among the infectious agents those really responsible for a protective influence against atopic and autoimmune diseases.
Keywords: allergy, asthma, autoimmunity, hygiene hypothesis, infections
Introduction
The ‘hygiene hypothesis’, in its more recent formulation, proposes that the epidemic of allergic and autoimmune diseases is due to changes in the interactions between humans and the microbes of their ecosystem [1]. This theory, unlike others (pollution, increased allergen exposure), explains the epidemic in evolutionistic terms. Microbes that co-evolved with mammals influenced the evolution of their immune structures and functions along the zootic scale up to primates. Partially deprived of these microbial stimuli, some components of the human immune system are no longer regulated adequately. Therefore, in modern societies, allergies and autoimmune diseases spread among new cohorts according to gradients dictated by hygiene and by the individual degree of genetic predisposition to atopy and autoimmunity [2–6].
However, the hygiene hypothesis has been challenged by conflicting epidemiological data which apparently undermine its basis. These arguments include, for example, the frequent occurrence of severe allergic asthma in unhygienic American inner cities, the low prevalence of allergies among migrant children living in big European cities, the lack of a preventive effect of probiotics on allergic disease inception, and many others. This review offers an incomplete list of critical questions and proposes some tentative answers which may reconcile the apparently conflicting epidemiological evidence with the hypothesis itself.
How does the hygiene hypothesis explain the rise of allergic asthma in ‘unhygienic’ American inner cities?
The hygiene hypothesis seems consistent with the epidemiology of allergy and asthma in the United States until the 1960s. Nevertheless, the main epidemiological development of the last three decades in the United States, i.e. the emergence of ‘inner-city asthma’, has provided arguments against the hygiene hypothesis [7,8]. Most cases of asthma in inner cities affect allergic subjects, and a major triggering role is provided by sensitization to cockroaches [9,10], thus making the case that ‘lack of cleanliness’ is not sufficient per se to protect from the development of allergic sensitization [7].
However, this apparent conceptual conflict between the hygiene hypothesis and inner-city asthma should be reviewed in a dynamic model (Fig. 1) [11]. When income and education improve dramatically, as has been the case in western Europe in the wake of the Second World War), the borders of low socio-economic status remain fluid and relative. Historically, the least affluent of one generation end up adopting the lifestyle of the middle classes of the previous one. The rising trend in respiratory allergies among the poorest could therefore be seen as the natural continuation of epiphenomena that affected the richest socio-economic strata of the United States population in the first half of the 20th century [12] to reach the middle classes in the 1950s and 1960s [13], and eventually cascading down to affect the least-advantaged Americans in the inner cities from the 1970s onwards.
Fig. 1.

Hypothetical model (not to scale) of the spread of hay fever and allergic asthma in the United States according to socio-economic status (y-axis), westernization level and exposure to relevant infections protecting from allergy. (1) Before the post-industrial revolution, lifestyle factors that protect from allergy (e.g. persistent exposure to food-borne and orofaecal pathogens, under the hygiene hypothesis) were so common in the whole of society that allergy was rare. (2) With the slow progress of westernization, these factors were gradually lost by the highest-income groups downwards in the course of the 19th century. This correlated with the emergence of hay fever among the more affluent classes. (3) Further progress toward westernization and more hygienic conditions in the whole of society led to the decline of the same protective factors among the more numerous, middle-income groups during the first seven decades of the 20th century. This reinforced the epidemic trend of hay fever and allergic asthma that spared the lowest socio-economic classes, still exposed at that time to sufficient levels of protective factors. Indeed, even though unsanitary environmental conditions have always plagued inner cities (overcrowding, damp, cockroaches, suboptimal health care, etc.), allergic asthma prevalence was low in the inner cities until the 1970s. (4) As a consequence of further westernization (e.g. improvement of hygiene and further decline of infections, mainly food-borne and orofaecal), protective factors were removed even from the inner cities. At this point, exposure to overcrowding, damp, cockroaches, suboptimal health care, etc. worsened the primary effects of increasing atopy susceptibility and made new asthma cases more severe in this population (reproduced from [11]– This figure was published in Annals of Allergy Asthma Annals of Allergy, Asthma & Immunology, Vol. 89(S1). Matricardi PM, Bouygue GR, Tripodi S. Inner-city asthma and the hygiene hypothesis, pp. 69–74. Copyright Elsevier 2002, with permission.).
Poverty in Europe coincided generally with rural origins and a traditional lifestyle with daily exposure to animals and their waste, and thus to a high turnover of orofaecal and food-borne infections. It is this kind of relative poverty that has been associated with protection from the atopic phenotype. By contrast, environmental risk factors aggravating asthma and typical of inner cities include smoking, overcrowding, poor ventilation, inadequate heating or faulty air conditioning and cooking gases [14]. Psychosocial risk factors also include unawareness of asthma, dysfunctional families and inadequate social support [15]. ‘Poverty’ in the American inner cities therefore entails living in an urban environment where exposure to indoor allergens (e.g. cockroaches, rodent urine) has become chronic but where food-borne and orofaecal infections may be relatively much less or even not relevant.
It can be speculated that the primary cause of the epidemic of allergic asthma in American inner cities is the consequence of the deprivation infections which confer protection from atopy and airways allergic inflammation. Emerging cases of allergic asthma among the least affluent African American and Hispanic communities are probably more severe because atopic susceptibility is combined with concurrent, chronic exposure to secondary risk factors typical at any time of poor urban environments (high exposure to cockroaches, smoking, damp and inadequate access to health care). Inner-city asthma may thus be the final stage of a class-driven urbanization and westernization that started two centuries ago in the United States and that is only now coming full circle (Fig. 1) [11].
Children of migrants are protected from allergic diseases, yet they live in big cities and not in a farming environment
Turkish migrants' children living in European metropolises tend to be protected from allergic diseases [16]. This protection is associated inversely with the level of adaptation to the host country culture (e.g. if parents do not speak the host country's language) and it is not explained by any of the known common risk factors or protective factors for allergies (e.g. older siblings, pet ownership, education level, etc.) [17]. Migrant families live in typical flats in the same urban environment as the native children. They are not exposed to cows, cattle, livestock or any other component typical of a farming environment. Migrant children and their families therefore preserve some protective factors of the traditional lifestyle which exert their influence even if children are exposed to many other environmental factors typical of the host country. The ‘migrant model’ is therefore complementary to the ‘farming children’ model. On a traditional farm, a huge list of putative protective factors is combined (high exposure to endotoxins, ingestion of unpasteurized milk, exposure to cattle, rural environment, traditional diet, etc.) [18]; in such an intricate mix, the search for the major protective factor has often been compared to the classical search for ‘a needle in a haystack’. By contrast, research on the ‘migrant model’ can be focused upon those few factors not shared with the native, ‘westernized’ peers: diet and contacts with relatives coming from or living in the native land. One interesting hypothesis is that migrant children and their families preserve a ‘protective’ microbial ecosystem typical of the country of origin. If confirmed, such a hypothesis would greatly facilitate the way towards the identification of protective infections and towards successful intervention strategies.
Airborne viral infections are not protective for allergic diseases. On the contrary, some of them are an important co-factor of asthma. Which infections then prevent immune disorders?
A preventive role against allergy and asthma was attributed initially to respiratory viruses, whereas infections of the gastrointestinal tract were thought to protect from food allergies. In contrast, atopy was related inversely to hepatitis A virus (HAV) [19,20] and other orofaecal/food-borne infections, but not to airborne viruses [21]. In these studies, exposure to HAV was considered a reliable proxy of being reared in an environment characterized by faecal contamination and high exposure to orofaecal/food-borne microbes [19,21]. Interestingly, in addition the studies performed in traditional farms confirmed that exposure to cattle and livestock (i.e. to a faecally contaminated environment) [22,23] and ingestion of unpasteurized milk (i.e. exposure to food-borne microbes) [23,24] protect against allergic disease. Similar conclusions were obtained by experimental studies in animal models [25]. This converging trend of totally independent research lines is making increasingly clear that gastrointestinal infections are probably more important in the context of hygiene hypothesis than other infections (Table 1 and Fig. 2).
Table 1.
Converging results of research lines in the hygiene hypothesis.
| (A)Immunoepidemiological studies |
| 1.Sibship size and birth order was shown to directly affect the IgE response against common allergens, and not only disease manifestation and/or their reporting [26] |
| 2.This ‘effect’ was shown to be dependent upon the level of exposure to hepatitis A virus (HAV), considered initially a marker of poor hygiene and faecal contamination of food and environment [19] |
| 3.HAV was then shown to be a more specific marker of exposure to GI, food-borne, faecal–oral infections: such as TG, HP and HAV. The link between these infections and atopy has been confirmed repeatedly in several populations undergoing epidemiological transition [21] |
| 4.A birth cohort study showed that commensals of the GI tract probably do not have an atopy protective influence [27] |
| 5.A longitudinal although retrospective study showed that Salmonellae prevent the development of respiratory allergies [28] |
| (B)Studies in the farming environment |
| 1.First it has been shown that children raised in a traditional farming environment are protected from allergies [29] |
| 2.This ‘effect’ was shown to be linked to many factors, but especially to stables and livestock, i.e. to faecal contamination of the environment [23] |
| 3.The stronger protective factor in and outside a farming environment is the ingestion of unpasteurized milk, i.e. a source of food-borne infections [24] |
| (C)Studies in animal models |
| 1.Several studies in animal models have shown that the ingestion of microbial products and the stimulation of GALT are able to prevent or even suppress atopic sensitization and its consequences [25]. |
| 2.Most of the microbes (‘old friends’) [30] which play a major atopy protective role in animal models are mild intracellular pathogens. |
IgE: immunoglobulin E; GI: gastrointestinal; TG: Toxoplasma gondii; HP: Helicobacter pylori; GALT: gut-associated lymphoid tissue.
Fig. 2.
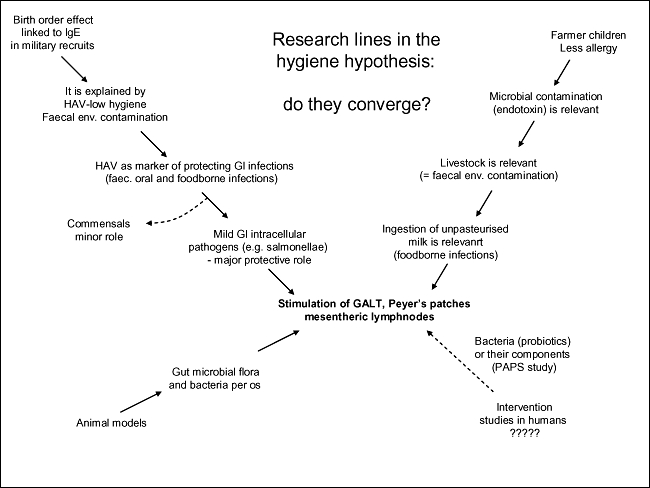
Totally independent research lines in the hygiene hypothesis converge on gastrointestinal infections with mild intracellular pathogens (transmitted through contaminated food and the faceal–oral route) as players of a protective influence against allergy. They also converge on gut-associated lymphoid tissue (GALT), Peyer's patches and mesenteric lymph nodes as the sites where immune regulation is generated.
The inverse association of a positive serology for HAV and allergy has been confirmed in some populations, but not in others. Why?
In the late 1990s it was reported that atopic diseases are related inversely to the acquisition of HAV infection [19]. Some years later, a mechanistic explanation for this association proposed on the basis of epidemiogenetic and experimental studies [31]. The original epidemiological observation has been confirmed in several studies [32,33], but not in others [34]. This inconsistency is explained using a historical perspective. In fact, the relationship between atopic diseases and HAV serology changes in a given population before, during and after its epidemiological transition from a traditional to a westernized lifestyle. These changes can be summarized in a sequence of three consecutive patterns (Fig. 3), as follows.
Fig. 3.
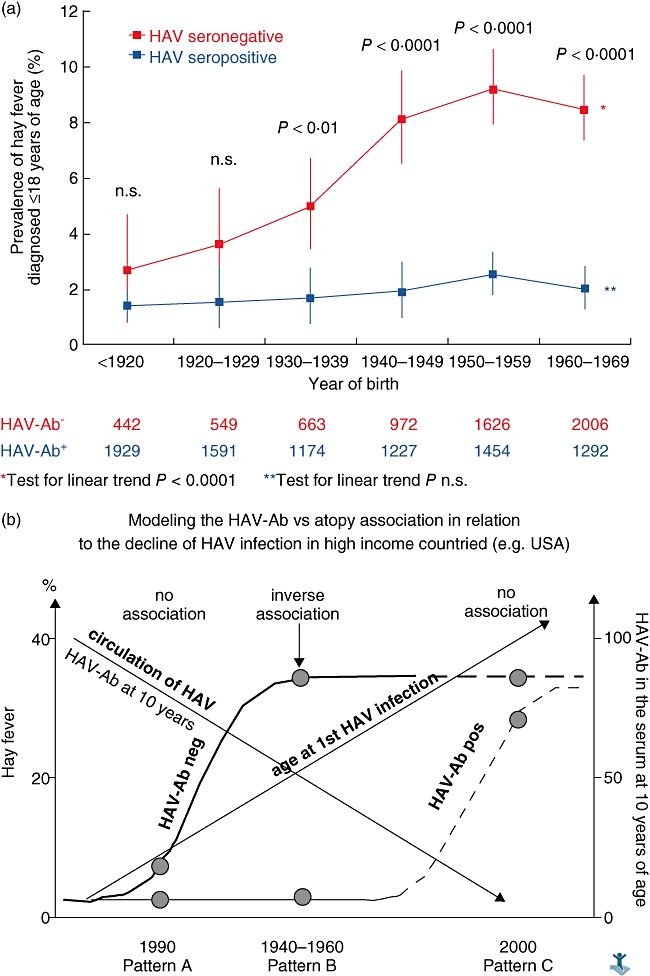
(a) Trends of hay fever in childhood among American citizens, by birth year and hepatitis A virus (HAV) serology (reprinted from [33], Journal of Allergy and Clinical Immunology, Vol. 110. Matricardi PM, Rosmini F, Panetta V., et al. Hay fever and asthma in relation to markers of infections in the United States, pp. 381–7. Copyright 2002, with permission from Elsevier). (b). A model showing variations of the relationship between hay fever and a positive HAV serology during the ‘westernization’ process.
Pattern A (‘before transition’). This pattern is observed among subjects born in the first decades of the 20th century in the United States and Western Europe. In these populations, the prevalence of a positive HAV serology was high and atopic sensitization was low, in both HAV seronegative and seropositive subjects. HAV infection is acquired early in childhood in all the socio-economic strata. In this condition there is low variability in both HAV serology and atopic diseases, and no inverse association can be observed.
Pattern B (‘during transition’). This pattern is typical of subjects born after the 1940s in the United States and in western Europe. In this period the incidence of food-borne and faecal–oral infections, but not that of airborne viral infections, decline because of hygiene. In these populations the prevalence of a positive serology for HAV in young adults is between 20 and 60%. HAV is acquired in most cases during childhood, because of overcrowding, poor hygiene and traditional lifestyle. In this condition a strong inverse association between a positive HAV serology and atopy can be observed (pattern B: ‘during transition’).
Pattern C (‘after transition’). This pattern is typical of modern, westernized populations with a very low circulation of HAV. In these populations the prevalence of atopy is high and is not related to a positive HAV serology, which is simply a consequence of occasional exposure (e.g. travel to endemic areas) and not a marker of a higher exposure to faecal–oral or food-borne infections. HAV infection is acquired in adulthood (= no association).
These three patterns can be observed simultaneously nowadays in countries/regions/cities in different stages of development. With a great degree of approximation, pattern A is today typical of rural areas of low- and middle-income countries, pattern B of urban areas of middle-income countries and pattern C is typical of high-income countries.
If the hygiene hypothesis holds true, why are probiotics not clearly effective in the prevention and therapy of allergic diseases?
The hygiene hypothesis has been also used as a rationale to test probiotics such as lactobacilli or bifidobacteria [35] for the prevention and therapy of allergic diseases. Initial studies, although promising [36], have been criticized for their inadequate design or other weaknesses in data handling or data interpretation [37]. The evidence provided was then considered insufficient to advise the use of probiotics for primary prevention or therapy of allergies, and this approach was considered an experimental one by a Task Force of the European Academy of Allergology and Clinical Immunology (EAACI) [38], the Global Initiative for Asthma (GINA) group [39] and individual opinion leaders [40]. Since then, further trials using probiotics in the prevention or therapy of allergic diseases have been published. A Finnish intervention trial in more than 900 infants demonstrated that probiotic treatment containing Lactobacillus rhamnosus GG (LGG) plus other three probiotic strains had no effect on the incidence of all allergic diseases by age 2 years, but prevented eczema and especially atopic eczema significantly [41]. By contrast, an Australian intervention trial in more than 170 Australian infants demonstrated that early probiotic supplementation with L. acidophilus did not reduce the risk of atopic dermatitis (AD) in high-risk infants, but was associated with increased allergen sensitization in infants receiving probiotics [42]. A combination of L. rhamnosus and L. reuteri did not improve significantly the SCORAD (SCORing Atopic Dermatitis) index of AD patients compared to placebo; nevertheless, the treatment was considered ‘beneficial’ on the basis of the patients' subjective evaluation during intervention [43]. No differences were observed in infants with atopic eczema/dermatitis syndrome (AEDS) and suspected cow's milk allergy who were treated with LGG, compared to placebo [44]. No therapeutic effect was observed in a double-blind placebo-controlled trial of LGG in German infants with moderate AD [45]. There was no significant difference in the improvement of SCORAD values between the placebo and treatment group in a trial studying the effect of L. fermentum in Australian infants with AD [46]. Finally, no clinical or immunological effect of LGG was observed in Dutch infants with AD compared to placebo [47]. A case report showed that contamination of probiotic preparations with milk allergens can cause anaphylaxis in children with cow's milk allergy [48]; the same report warned that two out of three probiotics used widely in France contain significant amounts of beta-lactoglobulin [48]. The more recent literature therefore reinforced the negative conclusions reached by the EAACI Task Force, the GINA group and individual opinion leaders on the basis of trials published before 2003.
How can this failure be reconciled with the hygiene hypothesis? The ‘gut commensal’ hypothesis, based initially on very small epidemiological studies [49,50], has been tested more thoroughly through a large birth cohort study performed in three European cities. The qualitative and quantitative composition of the faecal microflora of more than 300 infants born in London, Rome and Gothenburg was monitored seven times throughout the first year of age and related to the appearance of sensitization against food allergens and atopic eczema at 18 months of age [27]. Neither atopic eczema nor food-specific immunoglobulin (IgE) by 18 months of age were associated with time of acquisition of any particular bacterial group. The authors concluded that their study did not support the hypothesis that sensitization to foods or atopic eczema in European infants in early life is associated with the lack or presence of any particular culturable intestinal commensal bacteria, including so-called ‘probiotic’ bacteria (bifidobacteria and lactobacilli) [27]. Moreover, there is no direct evidence that lactobacilli or bifidobacteria explain the protective effect of the ‘traditional’ lifestyle of a farming environment. The epidemiological basis of the ‘gut commensals’ hypothesis is therefore a somewhat weak one, and this lack of rationale may explain the failure of the trials with probiotics in allergic diseases.
Is there any suggestion regarding which microbes might prevent atopic sensitization?
A crucial point in the hygiene hypothesis is whether only dangerous microbes exert a protective effect. If this were true, it would be difficult to translate the hygiene hypothesis into effective intervention strategies. In this respect, we may identify at least three possibilities assigning a role to: commensal, not dangerous bacteria; mild pathogens; and potentially fatal pathogens. From an evolutionistic viewpoint, it is unlikely that a protective effect is dependent upon highly virulent, potentially fatal infectious agents (e.g. poxviruses, vibrio cholerae). Conversely, it is also unlikely that a few species of non-pathogenic commensals play an allergy protective role (see above). Two further hypotheses have been proposed, as follows.
(1) The ‘high turnover and diversity’ hypothesis. In the late 1990s, it was proposed that a high turnover and diversity of appropriate bacteria at mucosal level [nasal-associated lymphoid tissue (NALT), bronchus-associated lymphoid tissue (BALT), gut-associated lymphoid tissue (GALT)], rather than specific, stable colonization by certain species, provides the continuous immune stimulation necessary to prevent atopy and atopic diseases [51,52]. A somewhat small nested case–control study of the same European birth cohort study mentioned above [27,53] disclosed that the median number of peaks, Shannon–Wiener index and Simpson index of diversity were significantly less for infants with atopic eczema than for infants remaining healthy in the whole group. The authors hypothesize that a reduced diversity in the early faecal microbiota of infants in the first week of life may be linked causally with atopic eczema appearing during the first 18 months of life [53]. The study population was small, however, and further studies are necessary to test the hypothesis.
(2) The ‘old friends’ and the ‘intracellular mild pathogen’ hypotheses. Since 1998, Graham Rook has proposed his ‘old friends’ hypothesis [6]. According to this theory, which may be considered the strongest one at present providing an explanation for the hygiene hypothesis, a regulating influence on the immune system would be exerted mainly by bacteria which induce a strong immune response, and not by commensals. The most typical example of this category is represented by mycobacteria which contaminate the soil, water and food. These bacteria exert an influence on the immune system which is only transient. Therefore, a provocative suggestion is that humans should be exposed continuously, mainly through ingestion, to the beneficial influence of these bacteria from food and environmental sources [30].
The somewhat broad range of ‘old friends’ could be restricted further to infections which are able to survive in the antigen-presenting cells until they induce protective T helper type 1 (Th1) responses. These intracellular mild pathogens include not only mycobacteria, but also listeria, salmonellae, Toxoplasma gondii, etc. These infections are susceptible to hygienic measures and declined dramatically in the last century during the westernization process. A strong support to the old friend hypothesis came from the observation that infection by Salmonella early in life is associated with a lower risk of hay fever and asthma [28]. Interestingly, salmonellae and mycobacteria share many characteristics in the way they stimulate the immune system. Salmonellae, exactly as the mycobacteria, grow into the endosomes of macrophages and induce a strong activation of CD4+ Th1 lymphocytes, which is required to enhance intracellular killing and clearance, and T regulatory functions. The immunological basis of this hypothesis is reported elsewhere in this issue [54].
Can novel therapeutic methods be found to balance the deleterious effects of hygiene without any side effects?
Many studies have shown that exposure to endotoxin and other bacterial molecules are an important proxy of an allergy-preventing environment. In addition, the use of LPS derivatives and of immunostimulatory sequencies of oligodeoxynucleotides (ISS-ODN) as adjuvants for allergen specific immunotherapy have given encouraging results in experimental models [55] and clinical trials [56]. The use of oral bacterial lysates in the prevention and therapy of allergic diseases is not recommended [38]. However, a new large trial of an extract of Escherichia coli and Enterococcus faecalis in the prevention of atopic eczema is in progress, and results are awaited for 2010 (Ulrich Wahn, personal communication).
Can we make a better use of the epidemiological evidence? It has been shown consistently that consumption of unpasteurized milk is the most relevant atopy protective factor in the farming environment [24]. Could this information be useful for intervention studies? Unfortunately, ‘unpasteurized’ milk is neither a standardized nor a totally ‘safe’ product, and intervention trials with unpasteurized milk from traditional farms would pose ethical issues and safety concerns [57]. However, if we consider that farmers' children ‘protected’ by the ingestion of unpasteurized milk grow up as healthy as their peers, we may also hope to find novel and safe therapeutic methods to mimic the effect of unpasteurized milk in a standardized and safe way. Studies investigating which microbial components or nutrients contained in the unpasteurized milk are useful are in progress, and these types of study are crucial for the development of future translational research originated from the hygiene hypothesis.
Conclusions
The hygiene hypothesis remains a fascinating scientific adventure and an incredibly broad and productive field of investigation. Thanks to the debate around conflicting data and continuing research, our ideas are nowadays more specific and clear. One promising mechanistic explanation is centred on the protective role of intracellular mild pathogens ingested through the faecally contaminated environment and through contaminated soil, water and food. Such infections include microbes which are able to colonize antigen-presenting cells for a long time, and in this way affect the future development of immune responses. This knowledge is a good basis to identify the protective infections and to develop translational research targeted to identify successful preventive and therapeutic intervention strategies against allergic diseases.
Disclosure
The author has no conflict of interest to declare.
References
- 1.Okada H, Kuhn C, Feillet H, Bach J-F. The ‘hygiene hypothesis’ for autoimmune and allergic diseases: an update. Clin Exp Immunol. 2010;160:1–9. doi: 10.1111/j.1365-2249.2010.04139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strachan DP. Family size, infection and atopy: the first decade of the ‘hygiene hypothesis’. Thorax. 2000;55:S2–S10. doi: 10.1136/thorax.55.suppl_1.s2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matricardi PM. Infections preventing atopy: facts and new questions. Allergy. 1997;52:879–82. doi: 10.1111/j.1398-9995.1997.tb01246.x. [DOI] [PubMed] [Google Scholar]
- 4.Martinez FD, Holt PG. Role of microbial burden in aetiology of allergy and asthma. Lancet. 1999;354(sII):12–5. doi: 10.1016/s0140-6736(99)90437-3. [DOI] [PubMed] [Google Scholar]
- 5.Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. 2002;347:911–20. doi: 10.1056/NEJMra020100. [DOI] [PubMed] [Google Scholar]
- 6.Rook GA, Stanford JL. Give us this day our daily germs. Immunol Today. 1998;19:113–6. doi: 10.1016/s0167-5699(97)01204-8. [DOI] [PubMed] [Google Scholar]
- 7.Platts-Mills TAE, Woodfolk JA, Sporik RB. The increase in asthma cannot be ascribed to cleanliness. Am J Respir Crit Care Med. 2001;164:1107–8. doi: 10.1164/ajrccm.164.7.2107130b. [DOI] [PubMed] [Google Scholar]
- 8.Marder D, Targonski P, Orris P, et al. Effect of racial and socioeconomic factors on asthma mortality in Chicago. Chest. 1992;101:S426–9. doi: 10.1378/chest.101.6_supplement.426s. [DOI] [PubMed] [Google Scholar]
- 9.Rosenstreich DL, Eggleston P, Kattan M, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med. 1997;336:1356–63. doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]
- 10.Leaderer BP, Belanger K, Triche E, et al. Dust mite, cockroach, cat, and dog allergen concentrations in homes of asthmatic children in the northeastern United States: impact of socioeconomic factors and population density. Environ Health Perspect. 2002;110:419–25. doi: 10.1289/ehp.02110419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matricardi PM, Bouygue GR, Tripodi S. Inner-city asthma and the hygiene hypothesis. Ann Allergy Asthma Immunol. 2002;89:69–74. doi: 10.1016/s1081-1206(10)62127-8. [DOI] [PubMed] [Google Scholar]
- 12.Beard GM. Hay fever; or, summer catarrh: its nature and treatment. New York: Harper & Bros; 1876. p. 15.p. 44. National Public Library, Stuart Collection, #13446-7. [Google Scholar]
- 13.Platts-Mills TAE. Changes in the 20th century environment: do they explain the increase in asthma? Proceedings of the 54th Annual Meeting of the American Academy of Allergy Asthma and Immunology. Washington, DC: AAAAI; 1998. [Google Scholar]
- 14.Weitzman M, Gortmaker S, Sobol A. Racial, social and environmental risk for childhood asthma. Am J Dis Child. 1990;144:1189–94. doi: 10.1001/archpedi.1990.02150350021016. [DOI] [PubMed] [Google Scholar]
- 15.Sly RM. Changing prevalence of allergic rhinitis and asthma. Ann Allergy Asthma Immunol. 1999;82:233–52. doi: 10.1016/S1081-1206(10)62603-8. [DOI] [PubMed] [Google Scholar]
- 16.Kabesch M, Schaal W, Nicolai T, et al. Lower prevalence of asthma and atopy in Turkish children living in Germany. Eur Respir J. 1999;13:577–82. doi: 10.1183/09031936.99.13357799. [DOI] [PubMed] [Google Scholar]
- 17.Grüber C, Illi S, Plieth A, Sommerfeld C, Wahn U. Cultural adaptation is associated with atopy and wheezing among children of Turkish origin living in Germany. Clin Exp Allergy. 2002;32:526–31. doi: 10.1046/j.0954-7894.2002.01331.x. [DOI] [PubMed] [Google Scholar]
- 18.von Mutius E. Asthma and allergies in rural areas of Europe. Proc Am Thorac Soc. 2007;4:212–6. doi: 10.1513/pats.200701-028AW. [DOI] [PubMed] [Google Scholar]
- 19.Matricardi PM, Rosmini F, Ferrigno L, et al. Cross-sectional retrospective study of prevalence of atopy among Italian military students with antibodies against hepatitis A virus. BMJ. 1997;314:999–1003. doi: 10.1136/bmj.314.7086.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Umetsu DT, DeKruyff RH. 99th Dahlem Conference on Infection, Inflammation and Chronic Inflammatory Disorders: Microbes, apoptosis and TIM-1 in the development of asthma. Clin Exp Immunol. 2010;160:125–9. doi: 10.1111/j.1365-2249.2010.04136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matricardi PM, Rosmini F, Riondino S, et al. Exposure to foodborne and orofaecal microbes versus airborne viruses in relation to atopy and allergic asthma: epidemiological study. BMJ. 2000;320:412–7. doi: 10.1136/bmj.320.7232.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kilpelainen M, Terho EO, Helenius H, et al. Farm environment in childhood prevents the development of allergies. Clin Exp Allergy. 2000;30:201–8. doi: 10.1046/j.1365-2222.2000.00800.x. [DOI] [PubMed] [Google Scholar]
- 23.Riedler J, Braun-Fahrlander C, Eder W, et al. Exposure to farming in early life and development of asthma and allergy: a crosssectional survey. Lancet. 2001;358:1129–33. doi: 10.1016/S0140-6736(01)06252-3. [DOI] [PubMed] [Google Scholar]
- 24.Perkin MR, Strachan DP. Which aspects of the farming lifestyle explain the inverse association with childhood allergy? J Allergy Clin Immunol. 2006;117:1374–81. doi: 10.1016/j.jaci.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 25.Renz H, Blümer N, Virna S, et al. The immunological basis of the hygiene hypothesis. Chem Immunol Allergy. 2006;91:30–48. doi: 10.1159/000090228. [DOI] [PubMed] [Google Scholar]
- 26.Matricardi PM, Franzinelli F, Franco A, et al. Sibship size, birth order, and atopy in 11,371 Italian young men. J Allergy Clin Immunol. 1998;101:439–44. doi: 10.1016/s0091-6749(98)70350-1. [DOI] [PubMed] [Google Scholar]
- 27.Adlerberth I, Strachan DP, Matricardi PM, et al. Gut microbiota and development of atopic eczema in 3 European birth cohorts. J Allergy Clin Immunol. 2007;120:343–50. doi: 10.1016/j.jaci.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 28.Pelosi U, Porcedda G, Tiddia F, et al. The inverse association of salmonellosis in infancy with allergic rhinoconjunctivitis and asthma at school-age: a longitudinal study. Allergy. 2005;60:626–30. doi: 10.1111/j.1398-9995.2005.00747.x. [DOI] [PubMed] [Google Scholar]
- 29.Braun-Fahrländer C, Gassner M, Grize L, et al. Prevalence of hay fever and allergic sensitization in farmer's children and their peers living in the same rural community. SCARPOL team. Swiss Study on Childhood Allergy and Respiratory Symptoms with Respect to Air Pollution. Clin Exp Allergy. 1999;29:28–34. doi: 10.1046/j.1365-2222.1999.00479.x. [DOI] [PubMed] [Google Scholar]
- 30.Rook GA, Brunet LR. Old friends for breakfast. Clin Exp Allergy. 2005;35:841–2. doi: 10.1111/j.1365-2222.2005.02112.x. [DOI] [PubMed] [Google Scholar]
- 31.McIntire JJ, Umetsu DT, DeKruyff RH. TIM-1, a novel allergy and asthma susceptibility gene. Springer Semin Immunopathol. 2004;25:335–48. doi: 10.1007/s00281-003-0141-3. [DOI] [PubMed] [Google Scholar]
- 32.Linneberg A, Ostergaard C, Tvede M, et al. IgG antibodies against microorganisms and atopic disease in Danish adults: the Copenhagen Allergy Study. J Allergy Clin Immunol. 2003;111:847–53. doi: 10.1067/mai.2003.1335. [DOI] [PubMed] [Google Scholar]
- 33.Matricardi PM, Rosmini F, Panetta V, et al. Hay fever and asthma in relation to markers of infection in the United States. J Allergy Clin Immunol. 2002;110:381–7. doi: 10.1067/mai.2002.126658. [DOI] [PubMed] [Google Scholar]
- 34.Gonzalez-Quintela A, Gude F, Boquete O, et al. Association of hepatitis A virus infection with allergic sensitization in a population with high prevalence of hepatitis A virus exposure. Allergy. 2005;60:98–103. doi: 10.1111/j.1398-9995.2005.00682.x. [DOI] [PubMed] [Google Scholar]
- 35.Kalliomäki M, Isolauri E. Pandemic of atopic diseases – a lack of microbial exposure in early infancy? Curr Drug Targets Infect Disord. 2002;2:193–9. doi: 10.2174/1568005023342452. [DOI] [PubMed] [Google Scholar]
- 36.Kalliomäki M, Salminen S, Arvilommi H, et al. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet. 2001;357:1076–9. doi: 10.1016/S0140-6736(00)04259-8. [DOI] [PubMed] [Google Scholar]
- 37.Matricardi PM. Probiotics against allergy: data, doubts, and perspectives. Allergy. 2002;57:185–7. doi: 10.1034/j.1398-9995.2002.1a3299.x. [DOI] [PubMed] [Google Scholar]
- 38.Matricardi PM, Bjorksten B, Bonini S, et al. Microbial products in allergy prevention and therapy. Allergy. 2003;58:461–71. doi: 10.1034/j.1398-9995.2003.00175.x. [DOI] [PubMed] [Google Scholar]
- 39.GINA Workshop Report 2002: Global Strategy for Asthma Management and Prevention. NHLBI/WHO Workshop Report. Bethesda: National Institutes of Health, National Heart, Lung, and Blood Institute; 2002. p. 99. Publication No. 02-3659. p. [Google Scholar]
- 40.Flohr C, Pascoe D, Williams HC. Atopic dermatitis and the ‘hygiene hypothesis’: too clean to be true? Br J Dermatol. 2005;152:202–16. doi: 10.1111/j.1365-2133.2004.06436.x. [DOI] [PubMed] [Google Scholar]
- 41.Kukkonen K, Savilahti E, Haahtela T, et al. Probiotics and prebiotic galacto-oligosaccharides in the prevention of allergic diseases: a randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2007;119:192–8. doi: 10.1016/j.jaci.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 42.Taylor AL, Dunstan JA, Prescott SL. Probiotic supplementation for the first 6 months of life fails to reduce the risk of atopic dermatitis and increases the risk of allergen sensitization in high-risk children: a randomized controlled trial. J Allergy Clin Immunol. 2007;119:184–91. doi: 10.1016/j.jaci.2006.08.036. [DOI] [PubMed] [Google Scholar]
- 43.Rosenfeldt V, Benfeldt E, Nielsen SD, et al. Effect of probiotic Lactobacillus strains in children with atopic dermatitis. J Allergy Clin Immunol. 2003;111:389–95. doi: 10.1067/mai.2003.389. [DOI] [PubMed] [Google Scholar]
- 44.Viljanen M, Savilahti E, Haahtela T, et al. Probiotics in the treatment of atopic eczema/dermatitis syndrome in infants: a double-blind placebo-controlled trial. Allergy. 2005;60:494–500. doi: 10.1111/j.1398-9995.2004.00514.x. [DOI] [PubMed] [Google Scholar]
- 45.Grüber C, Wendt M, Lau S, et al. Randomized placebo-controlled trial of Lactobacillus rhamnosus GG as treatment of mild to moderate atopic dermatitis in infancy. J Allergy Clin Immunol. 2005;117:239. doi: 10.1111/j.1398-9995.2007.01543.x. [DOI] [PubMed] [Google Scholar]
- 46.Weston S, Halbert A, Richmond P, et al. Effects of probiotics on atopic dermatitis: a randomised controlled trial. Arch Dis Child. 2005;90:892–7. doi: 10.1136/adc.2004.060673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brouwer ML, Wolt-Plompen SA, Dubois AE, et al. No effects of probiotics on atopic dermatitis in infancy: a randomized placebo-controlled trial. Clin Exp Allergy. 2006;36:899–906. doi: 10.1111/j.1365-2222.2006.02513.x. [DOI] [PubMed] [Google Scholar]
- 48.Lee TT, Morisset M, Astier C, et al. Contamination of probiotic preparations with milk allergens can cause anaphylaxis in children with cow's milk allergy. J Allergy Clin Immunol. 2007;119:746–7. doi: 10.1016/j.jaci.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 49.Björkstén B, Naaber P, Sepp E, et al. The intestinal microflora in allergic Estonian and Swedish 2-year-old children. Clin Exp Allergy. 1999;29:342–6. doi: 10.1046/j.1365-2222.1999.00560.x. [DOI] [PubMed] [Google Scholar]
- 50.Kalliomaki M, Kirjavainen P, Eerola E, et al. Distinct patterns of neonatal gut microflora in infants in whom atopy was and was not developing. J Allergy Clin Immunol. 2001;107:129–34. doi: 10.1067/mai.2001.111237. [DOI] [PubMed] [Google Scholar]
- 51.Wold AE. The hygiene hypothesis revised: is the rising frequency of allergy due to changes in the intestinal flora? Allergy. 1998;53:20–5. doi: 10.1111/j.1398-9995.1998.tb04953.x. [DOI] [PubMed] [Google Scholar]
- 52.Matricardi PM, Bonini S. High microbial turnover rate preventing atopy: a solution to inconsistencies impinging on the hygiene hypothesis? Clin Exp Allergy. 2000;30:1506–10. doi: 10.1046/j.1365-2222.2000.00994.x. [DOI] [PubMed] [Google Scholar]
- 53.Wang M, Karlsson C, Olsson C, et al. Reduced diversity in the early faecal microbiota of infants with atopic eczema. J Allergy Clin Immunol. 2008;121:129–34. doi: 10.1016/j.jaci.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 54.Rook GAW. 99th Dahlem Conference on Infection, Inflammation and Chronic Inflammatory Disorders: Darwinian medicine and the ‘hygiene’ or ‘old friends’ hypothesis. Clin Exp Immunol. 160:70–9. doi: 10.1111/j.1365-2249.2010.04133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Raz E, Tighe H, Sato Y, et al. Preferential induction of a Th1 immune response and inhibition of specific IgE antibody formation by plasmid DNA immunization. Proc Natl Acad Sci USA. 1996;93:5141–5. doi: 10.1073/pnas.93.10.5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Creticos PS, Schroeder JT, Hamilton RG, et al. Immunotherapy with a ragweed–Toll-like receptor 9 agonist vaccine for allergic rhinitis. N Engl J Med. 2006;355:1445–55. doi: 10.1056/NEJMoa052916. [DOI] [PubMed] [Google Scholar]
- 57.Perkin MR. Unpasteurized milk: health or hazard? Clin Exp Allergy. 2007;37:627–30. doi: 10.1111/j.1365-2222.2007.02715.x. [DOI] [PubMed] [Google Scholar]


